Abstract
Objective
The patency of prosthetic grafts is limited, in part due to incomplete endothelial cell (EC) coverage and development of anastomotic intimal hyperplasia. The goal of this study was to determine the effect of elevated cholesterol on prosthetic graft healing and the ability of α-tocopherol to improve healing.
Methods
Rabbits were placed on one of four diets: chow, chow plus 1% cholesterol, chow plus α-tocopherol, or chow plus 1% cholesterol and α-tocopherol. After two weeks, 12 cm long, 4 mm internal diameter expanded tetrafluoroethylene grafts were implanted in the abdominal aorta. Grafts were removed after 6 weeks and analyzed for cholesterol and α-tocopherol content, endothelial coverage, anastomotic intimal thickness, and cellular composition of the neointima.
Results
At the time of graft implantation, plasma cholesterol was 34±4 mg/dl in the chow group and 689±30 mg/dl in the 1% cholesterol group (P<.05). Grafts removed from hypercholesterolemic rabbits had marked intimal thickening with an intima:graft thickness ratio of 0.76±0.29 compared with 0.14±0.06 in chow animals (P<.05). Macrophage infiltrate was increased to 45±11 macrophages per 0.625 mm2 in high cholesterol grafts compared with 0±0.4 in controls (P<.05). Endothelialization of grafts was lower in hypercholesterolemic rabbits than the chow group, endothelial cells covering 46±7% and 62±7% of the graft surface, respectively (P=.05). When α-tocopherol was added to the 1% cholesterol diet the macrophage count decreased to 12±8 and the intimal:graft thickness ratio to 0.17±0.09, and endothelial coverage increased to 70±7% (P<.05 compared to the high cholesterol group).
Conclusions
Anastomotic intimal hyperplasia is dramatically increased and endothelialization is reduced in rabbits on a high cholesterol diet, but α-tocopherol supplementation blocks the augmented neointimal thickening and improves EC coverage.
Clinical Relevance
Elevated cholesterol is associated with an increased inflammatory response and development of intimal hyperplasia and reduced endothelialization following stent or prosthetic graft placement in animal models and decreased graft patency in clinical studies. α-Tocopherol or other anti-oxidant, anti-inflammatory agents may be effective in lessening this pathologic response.
Keywords: endothelial, smooth muscle, intimal hyperplasia, α-tocopherol, prosthetic vascular graft
Long-term patency of prosthetic grafts is limited by the thrombogenicity of the synthetic material, the development of intimal hyperplasia at the anastomosis, and the progression of atherosclerotic disease in the inflow or outflow arteries. Although the use of an autologous conduit is preferred, lower extremity vascular reconstructive surgery using prosthetic grafts may be necessary because of the failure of endovascular techniques, an inadequate vein, or previous use of the vein conduit. Implantation of a prosthetic graft is followed by macrophage infiltration, lipid deposition, endothelial cell (EC) ingrowth, smooth muscle cell (SMC) accumulation, and matrix deposition that may progress to intimal hyperplasia and graft failure. ECs migrate onto the graft from the adjacent artery and circulating endothelial progenitor cells are deposited on the graft,1 but ECs fail to cover the entire graft in humans. SMCs migrate from the adjacent artery and proliferate at elevated rates at the anastomosis long after graft placement 2. Lipids and lipoproteins are deposited in prosthetic grafts, particularly at the anastomoses.3,4 Macrophages are activated as part of the inflammatory response to graft placement and produce reactive oxygen species (ROS) that can oxidize these lipids. Lipid oxidation products accumulate in vascular grafts,5 and in vitro studies show that lipid oxidation products, but not native lipids or lipoproteins, cause cellular dysfunction including inhibition of EC migration.6 Oxidized low density lipoprotein (oxLDL) also stimulates SMC growth factor production by ECs,7 is chemotactic for SMCs,8 stimulates SMC proliferation,9 and enhances SMC production of collagen.10 These properties would adversely affects vascular graft healing.
Lipid deposition and atherosclerotic changes have been documented in vascular grafts in clinical studies,11,12 and the same risk factors that contribute to atherogenesis also hasten graft occlusion.13,14 Aggressive lipid lowering promotes the patency of coronary artery bypass grafts,15 and statin therapy is associated with improved patency of autogenous infrainguial bypass grafts.16 The mechanism responsible remains speculative buy may be a reflection of the lipid lowering, antioxidant, and anti-inflammatory activity of statins.
α-Tocopherol, the major lipid-soluble antioxidant in human plasma and most potent antioxidant form of vitamin E,, may have a beneficial effect on prosthetic graft healing. In vitro studies show that it restores migration of ECs incubated with cell-oxidized LDL,17 and inhibits oxidized LDL-induced SMC proliferation..18 Studies in hypercholesterolemic animals suggest that α-tocopherol attenuates intimal hyperplasia and restenosis after balloon injury.18,19 We postulate that high levels of cholesterol would adversely affect prosthetic graft healing in vivo by inhibiting EC migration and stimulating smooth muscle cell proliferation, and that α-tocopherol would lessen these adverse effects. In the present study the effect of hypercholesterolemia on EC coverage and anastomotic intimal hyperplasia, and the ability of α-tocopherol to ameliorate the adverse effects of hyperlipidemia were evaluated.
METHODS
Graft implantation and removal
Adult New Zealand white rabbits (3.5 to 4.0 kg, Covance Research Products Inc., Denver, PA,) were randomized to one of four dietary groups: Chow diet NIH-09 containing 2.4% fat (Chow; Zeigler Brothers Inc., Gardners, PA), high-cholesterol (HC) diet consisting of NIH-09 supplemented with 1.0% (wt/wt) cholesterol, α-tocopherol (AT) diet consisting of NIH-09 supplemented with 1500 IU α-tocopherol/kg, and high cholesterol plus α-tocopherol (HC+AT) diet consisting of NIH-09 supplemented with 1.0% cholesterol and 1500 IU/kg α-tocopherol. The protocol for animal studies was approved by the Institutional Animal Care and Use Committee. All procedures and care complied with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publication No. 85-23, 1996).
Rabbits were placed on the assigned diet two weeks prior to graft placement. For surgery, rabbits were anesthetized using a mixture of ketamine hydrochloride (40 mg/kg) and xylazine (20 mg/kg), intubated, mechanically ventilated, and maintained on 1.25% isoflurane throughout the procedure. Rabbits were anticoagulated with 150 U/kg of sodium heparin. Expanded polytetrafluoroethylene (ePTFE) grafts (W.L. Gore & Associates, Flagstaff, AZ), 4 mm internal diameter, 12 cm long, were anastomosed end-to-end to the abdominal aorta between the celiac axis and superior mesenteric artery and end-to-side to the infrarenal aorta.
Rabbits were maintained on the assigned diet for six weeks after graft implantation. Then rabbits were anesthetized and systemically anticoagulated using heparin 200 U/kg. Graft and aorta were flushed with 500 mL of Dulbecco’s Modified Eagle’s Medium through an infusion catheter inserted into the proximal descending aorta. In half of the animals in each group, the aorta and graft were perfusion-fixed using 4% paraformaldehyde for 30 min. In the other animals, the aorta and graft were removed for lipid extraction and analysis.
Plasma biochemical assays
Plasma was obtained from blood collected at the time of the implant surgery and at the conclusion of the study. Butylated hydroxytoluene (BHT) was added to each sample for a final concentration of 227 µmol/L. Plasma samples were stored in cryogenic vials under N2 at −80° C until analyzed. Total cholesterol was determined using a cholesterol oxidase method (Infinity Cholesterol Reagent, Thermo Fisher Scientific Inc., Waltham, MA).
Tissue lipid extraction
Graft and aorta were carefully isolated from surrounding tissue, and immersed in a cold solution of 0.15 mol/L NaCl, 0.1 mmol/L BHT and 1 mmol/L EDTA. Vessels were cut into segments as follows: descending thoracic aorta, proximal anastomosis including 5 mm of aorta and 5 mm of graft, proximal half of the graft, distal half of the graft, distal anastomosis including 5 mm graft and 5 mm aorta, distal abdominal aorta. Each segment was sectioned in half longitudinally and the wet weight recorded. Samples were stored under N2 at −80° C until analysis. Tissue was then minced and lipids extracted using chloroform, methanol and water in a ratio of 2:2:1.8,20 allowed to separate into phases, the lower chloroform layer removed, and the sample dried under N2.
Tissue cholesterol content
An HPLC method was used to quantify free and total cholesterol.21 Briefly, extracted samples were re-suspended in ethanol and 10 µL aliquots were added to 200 µL of PBS and containing β-sitosterol (5 µg; Sigma, St. Louis, MO) which served as an internal standard. Free cholesterol was determined by adding 0.1 U of cholesterol oxidase (from Streptomyces) in 50 µL of reaction mixture (150 mmol/L NaPO4, 30 mmol/L sodium taurocholate, 1 mmol/L polyethylene glycol). Total cholesterol was determined by including 0.1 U of cholesterol esterase (from Pseudomonas fluorescens) in the reaction mixture. Sterols were extracted using 250 µL of 4 mol/L NaCl and 500 µL of acetonitrile, allowing separation into phases, centrifuging, and analyzing the supernatant using reverse-phase HPLC (Nova-Pak C18 3.9 × 75 mm, Waters Corp., Milford, MA). Methanol:acetonitrile (1:1) was used as the eluent at a flow rate of 1 mL/min with detection at 235 nm.
Determination of α-tocopherol in plasma and tissue
α-Tocopherol was quantitated using an HPLC method. Lipid extracts were re-suspended in 300 µL of hexane. A 100 µL aliquot was dried under N2, α-tocopherol acetate (2.5 µg) added as an internal standard, and acetonitrile (400 µL) added to resuspend the tocopherol. Tocopherol was extracted by adding 100 µL of PBS and 300 µL of 4 mol/L NaCl, vortexing, allowing separation into phases, and centrifuging. α-Tocopherol was quantified by HPLC using a Waters Symmetry C18 column (4.6× 150 mm, 5 µmol/L). Methanol:water (98:2) was used as the eluent at a flow rate of 1.5 mL/min with detection at 290 nm. The efficiency of the extraction procedure was confirmed by comparison of extracted and non-extracted samples.
Morphologic Assessment of Grafts
After removal, graft and aortic segments were fixed in 4% paraformaldehyde at 4° C for 24 hours. Grafts were divided longitudinally with one half used for scanning electron microscopy (SEM) and the other for immunohistochemistry. SEM samples were dehydrated with graded alcohols, dried in hexamethyldisilazine, sputter-coated with gold, and examined using JSM-6930 microscope (JEOL, Peabody, MA). The extent of endothelial coverage of ePTFE samples was analyzed using SEM as previously described.22 The entire luminal graft surface was imaged at low (x15) magnification to determine the extent of endothelialization and high (x1500) magnification to confirm the surface structure. The graft’s luminal surface area and endothelialized area were quantitated using Scion Image (Scion Corp., Frederick, MD). Corresponding sections immunostained with antibody to von Willebrand Factor (1:100, DAKO, Glostrup, Denmark) were examined to confirm findings on SEM. Endothelialization was reported as the percentage of endothelial coverage (endothelialized surface area/total luminal surface area × 100).
For light microscopy tissue segments were embedded in paraffin, sectioned longitudinally, and stained with hematoxylin and eosin. Images of the anastomotic region were captured and analyzed using Scion Image. To avoid the distortion at the suture line, the thickness of the intima was measured at the thickest point between 1.5 and 2.5 mm from the end of the graft. Results were reported as the ratio of the thickness of neointima (I) to ePTFE graft (G) (I/G ratio) at the proximal anastomosis.
Immunostaining for Macrophages and Nitrotyrosine
To assess the presence of macrophages or nitrotyrosine, sections were prepared for immunohistochemistry. Antibodies to RAM11 (1:100, DAKO, Glostrup, Denmark) or human nitrotyrosine (1:200, Cayman Chemical, Ann Arbor, MI) were used to identify macrophages or nitrotyrosine, respectively. Avidin-conjugated secondary antibody (ABC Kit, Vector Laboratories, Burlingame, CA) was visualized using diaminobenzidine (DAB Kit, Vector Laboratories).
Macrophage accumulation was quantitated by identifying cells positive for RAM11 within graft or neointima. Positively-stained cells and non-stained cells were counted in three 250 µm × 250 µm fields in two distinct regions of the graft by two observers blinded as to dietary group. One region was within 2 mm of the anastomosis and the other was mid graft.
Nitrotyrosine in proteins, that results from peroxynitrite formation in vivo, was assessed as a qualitative measure of oxidative stress. Tissue sections from all dietary groups were processed simultaneously for nitrotyrosine determination to minimize variations in staining technique. Nitrotyrosine staining was scored on a scale from 1 to 5 by two observers blinded to dietary group, and scores were averaged.
Statistical Analysis
Results were represented as the mean ± standard error (SE) of the mean. Statistical analysis was evaluated by student t-test or analysis of variance (ANOVA) followed by Fisher’s posthoc multiple comparison using Stat-View (SAS Institute Inc. Cary, NC), and P<.05 was considered statistically significant.
RESULTS
A total of 61 rabbits were allocated to four dietary groups two weeks prior to graft implantation, and maintained on these diets until graft removal 6 weeks after placement. In the Chow group (n=18), surgical mortality was 22% with one rabbit euthanized because it was paralyzed, two euthanized because they did not regain normal dietary intake postoperatively and had excessive weight loss, and one dying 1 month postoperatively of unknown cause. In the HC group (n=18), operative mortality was 22% with one intraoperative death, two rabbits euthanized for paralysis, and one euthanized for failure to thrive postoperatively. In the AT group (n=13), operative mortality was 15% with two rabbits euthanized for paralysis. In the HC+AT group (n=12), operative mortality was 33% with one rabbit euthanized for paralysis, one euthanized for failure to eat postoperatively, and two dying postoperatively of unknown cause. Although the HC+AT group had a higher operative mortality than other groups, this did not reach statistical significance. One rabbit in the Chow, HC, and AT groups were excluded from analysis because inadequate fixation resulted in loss of all cellular components from the inner surface of the graft.
The pre- and post-operative data for each group of rabbits is summarized in Table 1. The age and weight of rabbits were similar between the groups. The 1% cholesterol diet increased plasma cholesterol levels over a 20-fold compared to the control diet. The addition of α-tocopherol to the diet significantly increased plasma levels, and at 8 weeks reduced plasma cholesterol in the HC+AT group compared with the HC group. α-Tocopherol levels were slightly higher in the HC group compared to the Chow group. This difference, attributed to the higher level of plasma lipids allowing retention of more lipid-soluble α-tocopherol, was not statistically significant.
Table 1.
Rabbit Data (Mean ± SE)
| Group | Age | Weight (kg) | Plasma Cholesterol (mg/dl) | α-Tocopherol (ng/mL) | ||
|---|---|---|---|---|---|---|
| (months) | At implant | 6 wks post op | At implant | 6 wks post op | 6 wks post op | |
| Chow Control | 8.1±0.5 | 3.8±0.1 | 3.9±0.1 | 34.0±4.4a,b | 18.2±3.0a,b | 2.1±0.3b |
| (n=13) | ||||||
| High Cholesterol | 8.4±0.4 | 4.0±0.1c | 3.9±0.1 | 689.4±30.2c | 1087.7±116.5c,d | 10.94±2.9d |
| (n=13) | ||||||
| High Cholesterol | ||||||
| +α-Tocopherol | 8.7±0.3 | 3.9±0.1 | 3.9±0.1 | 700.2±108.2e | 848.5±102.0e | 56.3±7.3e |
| (n=8) | ||||||
| Chow | ||||||
| +α-Tocopherol | 9.0±0.4 | 3.7±0.1 | 3.8±0.1 | 56.7±21.3 | 34.3±18.2 | 8.2±0.8 |
| (n=10) | ||||||
One-way ANOVA was used for comparison of the four groups and significant difference (p<0.05) designated as:
between Chow Control group and High Cholesterol group
between Chow Control group and High Cholesterol+α-Tocopherol group
between High Cholesterol group and α -Tocopherol group
between High Cholesterol group and High Cholesterol+ α-Tocopherol group
between High Cholesterol+ α-Tocopherol group and α-Tocopherol group
Tissue cholesterol content
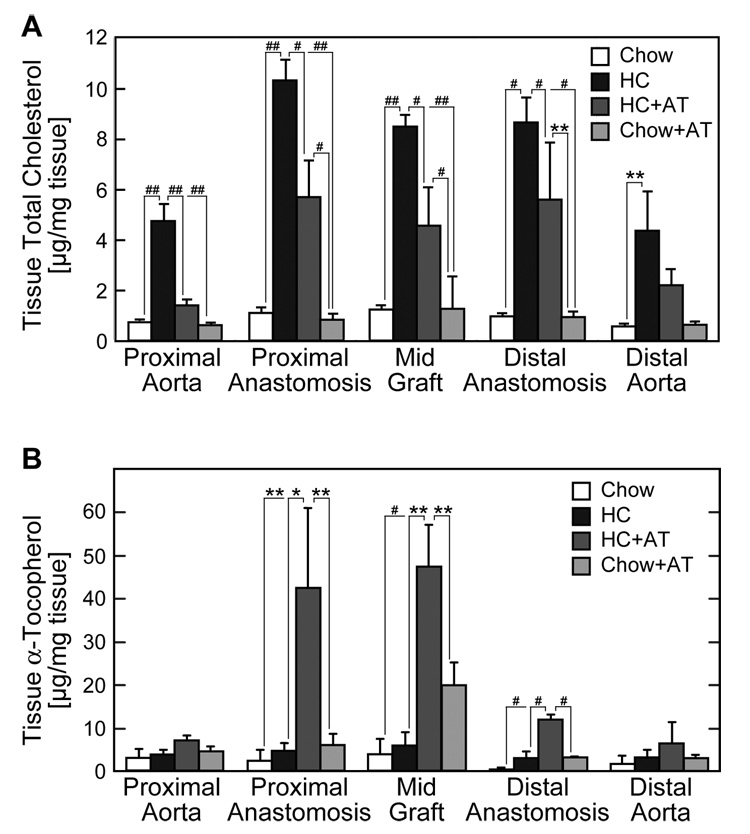
Cholesterol content in aorta and graft was increased in animals on a high cholesterol diet. Cholesterol accumulation was significantly greater in the graft, especially at the proximal anastomosis, compared to the aorta (Figure 1A). Cholesterol content in proximal anastomotic tissue was twice that in proximal aortic tissue. Addition of α-tocopherol to NIH-09 diet did not alter the tissue cholesterol content, however, α-tocopherol supplementation of a high cholesterol diet suppressed cholesterol accumulation in both aortic tissue (70%) and graft material (40%) (Figure 1A).
Fig. 1.
Tissue cholesterol and α-tocopherol content. Lipids were extracted from aortic and graft samples. Cholesterol (A) or α-tocopherol (B) was determined using HPLC and was reported as the ng/mg tissue (wet weight). Values are expressed as the mean ± SE for each tissue segment and dietary group. (Chow: n=3 rabbits; HC: n=3 rabbits; HC+AT: n=3 rabbits; and AT: n=5 rabbits) *P<.05, **P<.01, #P<.005, ##P<.0001.
Tissue α-tocopherol content
α-Tocopherol (standardized to wet weight of tissue) was elevated in mid graft tissue in rabbits on the HC+AT or AT diet (Figure 1B). When α-tocopherol content was corrected for changes in free cholesterol (a measure of cellular cholesterol) relative increases were observed in the proximal aorta, proximal anastomosis, and mid graft in animals on diets supplemented with α-tocopherol (data not shown).
Endothelialization of ePTFE grafts
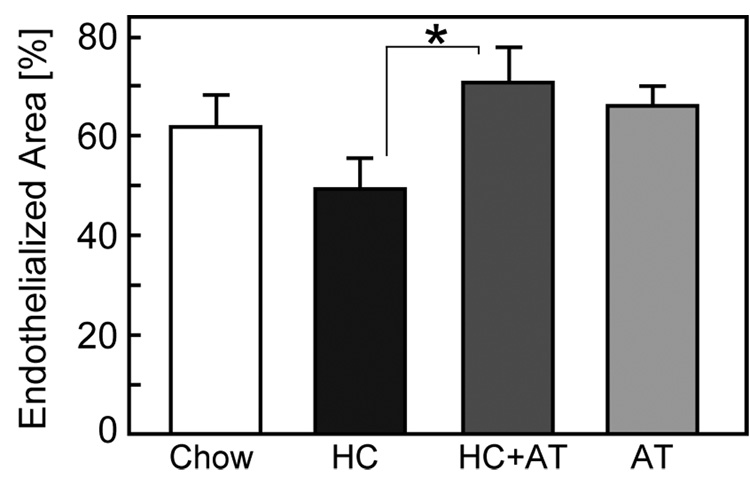
EC coverage on ePTFE grafts implanted for 6 weeks was determined using SEM. Endothelialized areas appeared as shiny, dark gray areas under low power magnification (x15), while nonendothelialized areas were nonreflective, light gray areas. To verify surface coverage, each area was also examined under high power magnification (x1500). Endothelium had a characteristic smooth surface with microvilli and an oval nucleus. Non-endothelialized areas had a nonuniform surface covered with fibrin, white blood cells, and platelets. Endothelialization of grafts in the HC group was lower than that in the Chow group, 46±7% compared with 62±7%, respectively (P=.05). α-Tocopherol supplementation significantly improved endothelialization of ePTFE grafts in hypercholesterolemic rabbits, 70±7% in HC+AT rabbits compared with 46±7% in HC rabbits, P<.05 (Figure 2).
Fig. 2.
Endothelialization of ePTFE grafts. The endothelialized area and total luminal area of the graft were determined by SEM using the Scion Image analysis program. Endothelialization of ePTFE graft was reported as the percentage of vascular graft covered by endothelium relative to the total luminal area of the graft and expressed as the mean ± SE for each of the four dietary groups. (Chow: n=10 rabbits; HC: n=10 rabbits; HC+AT: n=5 rabbits; AT: n=5 rabbits) *P<.05.
Anastomotic intimal hyperplasia
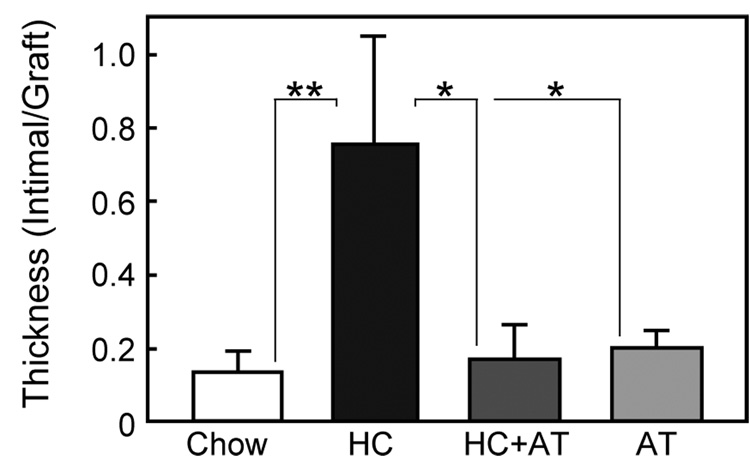
The I/G ratio of the proximal anastomosis was increased in hypercholesterolemic rabbits (Figure 3). In the Chow group the I/G ratio was 0.14±0.06, but in the HC group the ratio was significantly higher at 0.76±0.29 (P<.02). Although α-tocopherol had no effect on intimal thickening in animals with normal cholesterol levels (I/G ratio 0.20±0.05), it significantly reduced intimal thickening in hypercholesterolemic animals (I/G ration 0.17±0.09, P<.05 compared with HC).
Fig. 3.
Intimal hyperplasia at proximal anastomosis. Anastomotic Intimal hyperplasia was measured on histologic sections between 1.5 mm and 2.5 mm from graft end and expressed as the mean ± SE for each of the four dietary group. (Chow: n=7 rabbits; HC: n=4 rabbits; HC+AT: n=3 rabbits; AT: n=5 rabbits) *P<.05, **P<.01.
Macrophage accumulation
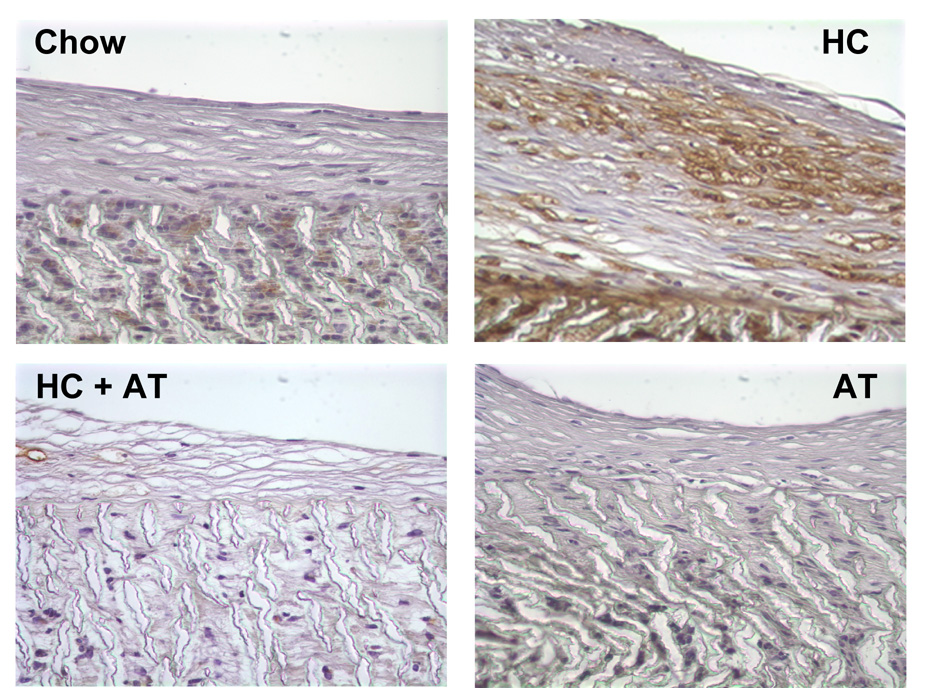
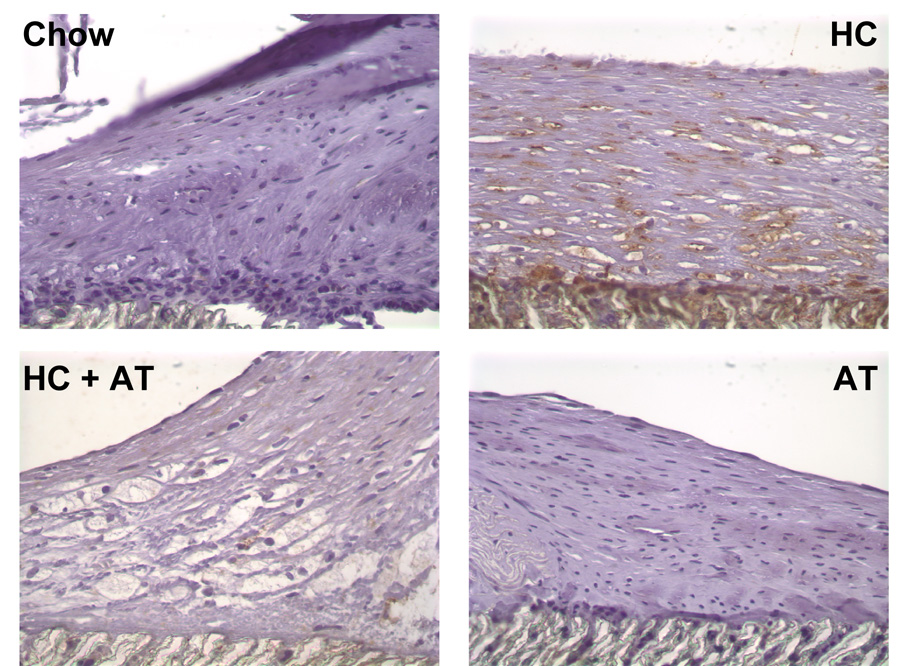
The total number of cells in a 0.0625 mm2 area of graft or neointima was not significantly different between groups (Table 2). The number of macrophages, as demonstrated by positive staining for RAM, was significantly higher in the HC group than in other groups. The addition of α-tocopherol to a HC diet markedly reduced the number of macrophages (Figure 4).
Table 2.
Cellularity and Macrophage Accumulation (Mean ± SE)
| Group | Neointima | Graft | ||
|---|---|---|---|---|
| Cells/ | Macrophage/ | Cells/ | Macrophage/ | |
| 0.625 mm2 | 0.625 mm2 | 0.625 mm2 | 0.625 mm2 | |
| Chow Control (n=6) | 163±19 | 0±0.1a | 146±30 | 0±0.4a |
| High Cholesterol (n=5) | 195±20 | 50±19 | 159±28 | 45±11b |
| High Cholesterol+α Tocopherol (n=5) | 253±66 | 9±6b | 161±30 | 12±8 |
| Chow+ α-Tocopherol (n=5) | 149±49 | 0±0.3c | 170±40 | 0±0.3c |
significant difference between Chow Control group and High Cholesterol group (P<.05)
significant difference between High Cholesterol group and High Cholesterol+α-Tocopherol group (P<.05)
significant difference between High Cholesterol group and Chow+α-Tocopherol group (P<.05)
Fig. 4.
Macrophage staining. Grafts were perfusion-fixed with 4% paraformaldehyde and processed for immunohistochemistry using mouse anti-rabbit macrophage antibody (RAM-11, DAKO). Representative sections are shown from Chow rabbits (n=4), HC rabbits (n=4), HC+AT rabbits (n=2), and AT rabbits (n=2). Original magnification × 100.
Nitrotyrosine staining
Tissue samples from animals fed a high cholesterol diet had increased staining for nitrotyrosine, suggesting increased oxidative stress, compared with other groups (Figure 5). The graft nitrotyrosine score averaged 3.0±0.3, 3.7±0.5, 2.6±0.5, and 2.7±0.3 in rabbits on a chow, HC, HC+AT, and AT diet, respectively (P≤.05 for HC compared with Chow or HC+AT). A similar pattern was seen in the neointimal nitrotyrosine scores. α-Tocopherol supplementation of a high cholesterol diet reduced the relative amount of nitrotyrosine present in graft material.
Fig. 5.
Nitrotyrosine staining. Grafts were perfusion-fixed with 4% paraformaldehyde and processed for immunohistochemistry using mouse anti-human nitrotyrosine antibody (Cayman). Representative sections are shown from Chow rabbits (n=4), HC rabbits (n=4), HC+AT rabbits (n=2), and AT rabbits (n=2). Original magnification × 100.
DISCUSSION
The adverse effects of oxidized lipids on SMC proliferation and EC migration, as well as the ability of α-tocopherol to counteract these effects, have been demonstrated in vitro. The purpose of this study was to determine if in vivo graft healing reflected these in vitro findings. Elevated cholesterol adversely impacts on ePTFE graft healing in a rabbit model.4,23 Hypercholesterolemia is associated with increased anastomotic intimal thickening, ranging from a doubling in rabbits on a 0.25% cholesterol diet to a 5-fold increase in rabbits on 1% cholesterol diets at 10–12 weeks after graft placement. Our study documents a 3-fold increase at 6 weeks in rabbits on a 1% cholesterol diet compared to a chow diet. In agreement with a previous study,4 the majority of cells in the neointimal are SMCs and the cellularity is similar in normocholesterolemic and hypercholesterolemic animals. In the present study, more macrophages are present in grafts from hypercholesterolemic than normaocholesterolemic rabbits, but Baumann et al. report no difference.4 This discrepancy may reflect of the shorter time (6 versus 10 weeks) or the higher dietary cholesterol (1% versus 0.25%) in the present study compared with Baumann’s study.
Hypercholesterolemia may adversely effect endothelial healing of arterial injuries or prosthetic grafts. Lipid oxidation products inhibit EC migration in vitro,6 and hypercholesterolemia disrupts aortic EC alignment with flow and "distorts" EC growth and regeneration after arterial injury in rabbits.24,25 In our study, graft endothelialization is lower in rabbits on a high cholesterol diet than a chow diet. Interestingly, the adverse effect of hypercholesterolemia on endothelialization of grafts was not seen when the 1% cholesterol diet was supplemented with α-tocopherol. In fact, graft endothelial coverage was greater in the HC+AT group than in any other dietary group and significantly higher than in the 1% cholesterol diet group. The source of EC is not addressed in this study, but may be a combination of direct migration from the adjacent artery and circulating endothelial progenitor cells. In a rat hindlimb ischemia model, capillary formation and homing capacity of EC progenitor cells is increased by metabolic treatment, including vitamin E.26
Healing after arterial injury or graft placement is aided by α-tocopherol. It inhibits SMC proliferation in vitro and attenuates intimal hyperplasia and restenosis after balloon injury in hypercholesterolemic rabbits.18,27 In the present study, α-tocopherol decreases anastomotic hyperplastic lesion thickness and inflammatory cell infiltrate. In addition, α-tocopherol significantly increases endothelialization of prosthetic grafts. Multiple mechanisms may play a role in α-tocopherol’s improvement of graft healing in hypercholesterolemic rabbits. α-Tocopherol acts as a free radical scavenger, breaks the chain reaction associated with lipid peroxidation, and decreases monocyte production of superoxide by inhibiting the assembly of NADPH oxidase in the cell membrane.28 The nitrotyrosine data suggests that α-tocopherol has an antioxidant effect in our model. This may contribute to the reduction of intimal hyperplasia because ROS stimulate SMC proliferation and collagen production.10,29 Furthermore, limiting formation of oxLDL could improve graft healing because oxLDL inhibits EC migration,6 is chemotactic for monocytes and SMCs,8,30 stimulates SMC proliferation,9 and enhances SMC production of collagen.10 Probucol, an antioxidant that also has mild lipid lowering effects, reduces the cellularity but does not alter the degree of anastomotic intimal thickening of ePTFE grafts in hypercholesterolemic rabbits.31 Whether the limited effectiveness of probucol compared with α-tocopherol reflects differences in study duration or the importance of non-antioxidant properties of α-tocopherol is not clear.
Several non-antioxidant activities of α-tocopherol may contribute to the control of intimal hyperplasia. α-Tocopherol raises plasma HDL and reverse cholesterol transport,32 which may account for lower tissue cholesterol in rabbits on the HC+AT diet compared with the HC diet (Figure 2). α-Tocopherol can block protein kinase C activation by oxLDL,33 with subsequent inhibition of SMC proliferation,34 which would limit the development of intimal hyperplasia. α-Tocopherol suppresses macrophage accumulation, decreasing the cells available to generate a variety of cytokines and growth factors. α-Tocopherol also inhibits EC expression of adhesion molecules and monocyte production of cytokines,35,36 thus limiting the inflammatory response to a prosthetic graft as is evident in our study. α-Tocopherol intercalates into membranes and prevents changes in fluidity induced by cell-oxidized LDL in vitro, preserving EC migration.17 These properties of α-tocopherol could improve EC migration from the adjacent artery, transinterstitial EC ingrowth, and the spreading of circulating endothelial progenitor cells that are deposited onto the graft’s luminal surface. Together these effects of α-tocopherol could account for the increased graft endothelialization in rabbits on the HC+AT diet compared with the HC diet.
The current study shows a clear benefit of α-tocopherol on prosthetic graft healing with improved endothelialization, decreased anastomotic intimal hyperplasia, and decreased macrophage accumulation. Attempted extrapolation of these findings to graft healing in humans, however, is not prudent. Use of 1% cholesterol diet in rabbits is a standard method for rapid induction of atherosclerotic changes, but this does not equate to sustained, lower levels of hypercholesterolemia in humans. Diet-induced hypercholesterolemia in rabbits is characterized by a marked increase in LDL and VLDL cholesterol with a small increase in HDL cholesterol.24 The LDL and VLDL from hypercholesterolemic rabbits are more susceptible to oxidation than LDL from normal rabbits.37 Thus, α-tocopherol’s antioxidant activity may produce a greater effect in the HC+AT group than the AT rabbits. Although increased vitamin E consumption correlates with primary prevention of cardiovascular disease in observational studies,38,39 it does not improve mortality in prospective randomized studies most of which address secondary prevention.40 The reason for the discrepancy is unknown, but may reflect the limited antioxidant effect of vitamin E especially in smokers and diabetic patients. A better understanding of the mechanisms involved in α-tocopherol’s favorable effects will allow development of strategies to minimize EC and SMC dysfunction to promote endothelialization and control intimal hyperplasia at angioplasty sites or in vascular grafts.
Acknowledgments
This work was supported by grants from the NIH/NHLBI (Grant No. HL41178 and HL64357)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competition of interest: nil
References
- 1.Shi Q, Rafii S, Wu MHD, et al. Evidence for circulating bone marrow-derived endothelial cells. Blood. 1998;92:362–367. [PubMed] [Google Scholar]
- 2.Clowes AW, Kirkman TR, Clowes MM. Mechanisms of arterial graft failure. II. Chronic endothelial and smooth muscle cell proliferation in healing polytetrafluoroethylene prostheses. J Vasc Surg. 1986;3:877–884. [PubMed] [Google Scholar]
- 3.Miller A, Schoen FJ, Lees AM, Fallon JT, Strauss HW, Lees RS. Low density lipoprotein accumulation by PTFE grafts in the rabbit aorta. Autoradiographic-morphologic correlations. Asaio Transactions. 1987;33:489–493. [PubMed] [Google Scholar]
- 4.Baumann DS, Doblas M, Daugherty A, Sicard G, Schonfeld G. The role of cholesterol accumulation in prosthetic vascular graft anastomotic intimal hyperplasia. J Vasc Surg. 1994;19:435–445. doi: 10.1016/s0741-5214(94)70070-2. [DOI] [PubMed] [Google Scholar]
- 5.van Aalst JA, Fox PL, Graham LM. Lipid oxidation products in implanted vascular grafts. Surg Forum. 1998;49:324–326. [Google Scholar]
- 6.Murugesan G, Chisolm GM, Fox PL. Oxidized low density lipoprotein inhibits the migration of aortic endothelial cells in vitro. J Cell Biol. 1993;120:1011–1019. doi: 10.1083/jcb.120.4.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kume N, Gimbrone MA. Lysophosphatidylcholine transcriptionally induces growth factor gene expression in cultured human vascular endothelial cells. J Clin Invest. 1994;93:907–911. doi: 10.1172/JCI117047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Autio I, Jaakkola O, Solakivi T, Nikkari T. Oxidized low-density lipoprotein is chemotactic for arterial smooth muscle cells in culture. FEBS Lett. 1990;277:247–249. doi: 10.1016/0014-5793(90)80857-f. [DOI] [PubMed] [Google Scholar]
- 9.Chatterjee S. Role of oxidized human plasma low density lipoproteins in atherosclerosis: effects on smooth muscle cell proliferation. Mol Cell Biochem. 1992;111:143–147. doi: 10.1007/BF00229586. [DOI] [PubMed] [Google Scholar]
- 10.Absood A, Furutani A, Kawamura T, Graham LM. A comparison of oxidized LDL-induced collagen secretion by graft and aortic SMCs: role of PDGF. Am J Physiol Heart Circ Physiol. 2004;287:H1200–H1206. doi: 10.1152/ajpheart.00228.2004. [DOI] [PubMed] [Google Scholar]
- 11.Walton KW, Slaney G, Ashton F. Atherosclerosis in vascular grafts for peripheral vascular disease. Part 2. Synthetic arterial prostheses. Atherosclerosis. 1986;61:155–167. doi: 10.1016/0021-9150(86)90076-6. [DOI] [PubMed] [Google Scholar]
- 12.Wagner E, Guidoin R, Marois M, et al. Histopathologic finding in synthetic and biologic explanted grafts used in peripheral arterial reconstruction. asaio J. 1994;40:M279–M283. doi: 10.1097/00002480-199407000-00008. [DOI] [PubMed] [Google Scholar]
- 13.Campeau L, Enjalbert M, Lespérance J, et al. The relation of risk factors to the development of atherosclerosis in saphenous-vein bypass grafts and the progression of disease in the native circulation. N Engl J Med. 1984;311:1329–1332. doi: 10.1056/NEJM198411223112101. [DOI] [PubMed] [Google Scholar]
- 14.AbuRahma AF, Robinson PA, Stuart SP, Witsberger TA, Stewart WA, Boland JP. Polytetrafluoroethylene grafts in infrainguinal arterial revascularization. Factors affecting outcome. Arch Surg. 1993;128:417–422. doi: 10.1001/archsurg.1993.01420160055008. [DOI] [PubMed] [Google Scholar]
- 15.The Post Coronary Artery Bypass Graft Trial Investigators. The effect of aggressive lowering of low-density lipoprotein cholesterol levels and low-dose anticoagulation on obstructive changes in saphenous vein coronary-artery bypass grafts. The Post Coronary Artery Bypass Graft Trial Investigators. N Engl J Med. 1997;336:153–162. doi: 10.1056/NEJM199701163360301. [DOI] [PubMed] [Google Scholar]
- 16.Abbruzzese TA, Havens J, Belkin M, et al. Statin therapy is associated with improved patency of autogenous infrainguinal bypass grafts. J Vasc Surg. 2004;39:1178–1185. doi: 10.1016/j.jvs.2003.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Aalst JA, Burmeister W, Fox PL, Graham LM. α-Tocopherol preserves endothelial cell migration in the presence of cell-oxidized LDL by inhibiting changes in cell membrane fluidity. J Vasc Surg. 2004;39:229–237. doi: 10.1016/s0741-5214(03)01038-3. [DOI] [PubMed] [Google Scholar]
- 18.Lafont AM, Chai Y-C, Cornhill JF, Whitlow PL, Howe PH, Chisolm GM. Effect of alpha-tocopherol on restenosis after angioplasty in a model of experimental atherosclerosis. J Clin Invest. 1995;95:1018–1025. doi: 10.1172/JCI117746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen MF, Hsu HC, Liau CS, Lee YT. Vitamin E supplementation attenuates myointimal proliferation of the abdominal aorta after balloon injury in diet-induced hypercholesterolemic rabbits. Prostaglandins. 1998;56:219–238. doi: 10.1016/s0090-6980(98)00057-4. [DOI] [PubMed] [Google Scholar]
- 20.Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 21.Goh EH, Krauth DK, Colles SM. Analysis of cholesterol and desmosterol in cultured cells without organic solvent extraction. Lipids. 1990;25:738–741. doi: 10.1007/BF02544043. [DOI] [PubMed] [Google Scholar]
- 22.Kaufman BR, DeLuca DJ, Folsom DL, et al. Elevated platelet-derived growth factor production by aortic grafts implanted on a long-term basis in a canine model. J Vasc Surg. 1992;15:806–816. [PubMed] [Google Scholar]
- 23.O-hara M, Esato K, Harada M, et al. Eicosapentanoic acid suppresses intimal hyperplasia after expanded polytetrafluoroethylene grafting in rabbits fed a high cholesterol diet. J Vasc Surg. 1991;13:480–486. [PubMed] [Google Scholar]
- 24.Keaney JF, Jr., Gaziano JM, Xu A, et al. Low-dose α-tocopherol improves and high-dose α-tocoperol worsens endothelial vasodilator function in cholesterol-fed rabbits. J Clin Invest. 1994;93:844–851. doi: 10.1172/JCI117039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reidy MA, Bowyer DE. Distortion of endothelial repair. The effect of hypercholesterolaemia on regeneration of aortic endothelium following injury by endotoxin. A scanning electron microscope study. Atherosclerosis. 1978;29:459–466. doi: 10.1016/0021-9150(78)90174-0. [DOI] [PubMed] [Google Scholar]
- 26.De Nigris F, Balestrieri ML, Williams-Ignarro S, et al. Therapeutic effects of autologous bone marrow cells and metabolic intervention in the ischemic hindlimb of spontaneously hypertensive rats involve reduced cell senescence and CXCR4/Akt/eNOS pathways. J Cardiovasc Pharmacol. 2007;50:424–433. doi: 10.1097/FJC.0b013e31812564e4. [DOI] [PubMed] [Google Scholar]
- 27.Azzi A, Boscoboinik D, Clément S, et al. α-tocopherol as a modulator of smooth muscle cell proliferation. Prostaglandins Leukot Essent Fatty Acids. 1997;57:507–514. doi: 10.1016/s0952-3278(97)90436-1. [DOI] [PubMed] [Google Scholar]
- 28.Rueckschloss U, Galle J, Holtz J, Zerkowski HR, Morawietz H. Induction of NAD(P)H oxidase by oxidized low-density lipoprotein in human endothelial cells: antioxidative potential of hydroxymethylglutaryl coenzyme A reductase inhibitor therapy. Circulation. 2001;104:1767–1772. doi: 10.1161/hc4001.097056. [DOI] [PubMed] [Google Scholar]
- 29.Griendling KK, Ushio-Fukai M. Redox control of vascular smooth muscle proliferation. J Lab Clin Med. 1998;132:9–15. doi: 10.1016/s0022-2143(98)90019-1. [DOI] [PubMed] [Google Scholar]
- 30.Quinn MT, Parthasarathy S, Fong LG, Steinberg D. Oxidatively modified low density lipoproteins: a potential role in recruitment and retention of monocyte/macrophages during atherogenesis. Proc Natl Acad Sci USA. 1987;84:2995–2998. doi: 10.1073/pnas.84.9.2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baumann DS, Doblas M, Schonfeld G, Sicard GA, Daugherty A. Probucol reduces the cellularity of aortic intimal thickening at anastomotic regions adjacent to prosthetic grafts in cholesterol-fed rabbits. Arterioscler Thromb. 1994;14:162–167. doi: 10.1161/01.atv.14.1.162. [DOI] [PubMed] [Google Scholar]
- 32.Jeon S-M, Park YB, Kwon O-S, et al. Vitamin E supplementation alters HDL-cholesterol concentration and paraoxonase activity in rabbits fed high-cholesterol diet: comparison with probucol. J Biochem Mol Toxicol. 2005;19:336–346. doi: 10.1002/jbt.20098. [DOI] [PubMed] [Google Scholar]
- 33.Keaney JF, Jr., Guo Y, Cunningham D, Shwaery GT, Xu AM, Vita JA. Vascular incorporation of α-tocopherol prevents endothelial dysfunction due to oxidized LDL by inhibiting protein kinase C stimulation. J Clin Invest. 1996;98:386–394. doi: 10.1172/JCI118804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Özer NK, Palozza P, Boscoboinik D, Azzi A. d-α-tocopherol inhibits low density lipoprotein induced proliferation and protein kinase C activity in vascular smooth muscle cells. FEBS Lett. 1993;322:307–310. doi: 10.1016/0014-5793(93)81592-n. [DOI] [PubMed] [Google Scholar]
- 35.Faruqi R, De la Motte C, DiCorleto PE. α-tocopherol inhibits agonist-induced monocytic cell adhesion to cultured human endothelial cells. J Clin Invest. 1994;94:592–600. doi: 10.1172/JCI117374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Devaraj S, Jialal I. α-Tocopherol decreases interleukin-1β release from activated human monocytes by inhibition of 5-lipoxygenase. Arterioscler Thromb Vasc Biol. 1999;19:1125–1133. doi: 10.1161/01.atv.19.4.1125. [DOI] [PubMed] [Google Scholar]
- 37.Nenseter MS, Gudmundsen O, Malterud KE, Berg T, Drevon CA. Effect of cholesterol feeding on the susceptibility of lipoproteins to oxidative modification. Biochim Biophys Acta. 1994;1213:207–214. doi: 10.1016/0005-2760(94)90028-0. [DOI] [PubMed] [Google Scholar]
- 38.Rimm EB, Stampfer MJ, Ascherio A, Giovanucci E, Colditz GA, Willett WC, Vitamin E. consumption and the risk of coronary heart disease in men. N Engl J Med. 1993;328:1450–1456. doi: 10.1056/NEJM199305203282004. [DOI] [PubMed] [Google Scholar]
- 39.Stampfer MJ, Hennekens CH, Manson JE, Colditz GA, Rosner B, Willett WC, Vitamin E. consumption and the risk of coronary disease in women. N Engl J Med. 1993;328:1444–1449. doi: 10.1056/NEJM199305203282003. [DOI] [PubMed] [Google Scholar]
- 40.Vivekananthan DP, Penn MS, Sapp SK, Hsu A, Topol EJ. Use of antioxidant vitamins for the prevention of cardiovascular disease: meta-analysis of randomised trials. Lancet. 2003;361:2017–2023. doi: 10.1016/S0140-6736(03)13637-9. [DOI] [PubMed] [Google Scholar]