Abstract
We compared the effects of mode of delivery of neuroactive agents and the effects of Dimethyl sulfoxide (DMSO), a vehicle for dissolving neuroactive agents, on locomotor-like activity in vitro. By superfusion, D-glutamate (0.3 – 0.9 mM) produced robust walking-like activity at superfusion rates 10–25 ml/min. In contrast, bolus application of the same or higher doses of glutamate (0.1–1.5 mM) failed to induce any rhythmic activity. Superfusion with AP-5, a NMDA receptor antagonist, produced dose-dependent inhibition of the ongoing walking-like activity induced by D-glutamate and completely blocked the activity at 20 µM. In contrast, bolus application of AP-5 did not block the walking-like activity at concentrations up to 120 µM. Similarly, superfusion of AP-5 inhibited the initiation of walking-like activity and completely blocked the initiation at 20 µM, while bolus application of AP-5 failed to do so at concentrations up to 120 µM. Superfusion of strychnine, a glycine receptor antagonist, blocked the walking-like activity at concentrations of 3–5 µM, while its bolus application altered NMDA-induced, but not glutamate-induced, walking-like activity to a synchronized pattern. DMSO significantly affected the walking-like activity in a dose-dependent manner at concentrations ranging 1–10% (v/v). These results demonstrate that the way by which the neuroactive agents are applied is a significant factor that determines the outcome of experiments on the neural control of locomotion. Also, the dose-dependent effects of DMSO on the activity of neural networks for locomotion should be taken into account in data interpretation.
Keywords: Drug application, Locomotion, Central pattern generator, Spinal cord, Mudpuppy, NMDA, Glutamate, Strychnine, DMSO, Neuromodulation
INTRODUCTION
Much of the knowledge about the neural control of rhythmic behaviors has been gained by the utilization of applying neuroactive agents into the bath of in vitro spinal cord or brainstem preparations. Locomotor-like activities were induced by bolus application of N-methyl-D-aspartic acid (NMDA) in in vitro preparations from the chick embryo (Barry and O’Donovan, 1987), frog embryo and tadpole (Roberts et al., 1983), neonatal rat (Kudo and Yamada, 1987; Smith and Feldman, 1987), neonatal mouse (Hernandez et al., 1991; Bonnot et al., 1998; Jiang et al., 1999), lamprey (Grillner et al., 1981), and the mudpuppy (Wheatley and Stein 1992; Wheatley et al,. 1992; Wheatley et al., 1994; Jovanovic et al., 1996; Cheng et al. 1998, 2002, Fok and Stein, 2002; Igor and Cheng 2004). Bath application of 5-HT has also induced locomotor-like activities in preparations from the lamprey (Grillner et al., 1991) and the neonatal rat (Cowley and Schmidt, 1997; Kiehn and Kjaerulff, 1996). For all these studies, bolus application was sufficient to produce stable locomotor-like activities. However, although bolus application of D-glutamate, L-glutamate or DL-homocysteate produced fictive locomotion in lamprey (Poon 1980; Cohen and Wallén, 1980) and chick embryo (Barry and O’Donovan, 1987), such application has not been successful in inducing locomotor-like activity in the mudpuppy and other preparations, whereas the same substance can induce robust walking-like pattern when applied to the bath with superfusion (Brodin and Grillner, 1985 Lavrov and Cheng, 2004). Clearly, the means by which the neuroactive agents are delivered can be an important determinant in the outcomes of locomotor behavior. We thus compared effects of continuous superfusion of the agonists and antagonists of the excitatory and inhibitory neurotransmitter receptors on the initiation and maintenance of locomotor-like activity in comparison to bolus applications of these agents.
A second issue concerns the use of DMSO as a vehicle to facilitate the application of neuroactive agents for medicine and experimental practices, as many of the agents are poorly water-soluble (Jacob and Herschler, 1986; Bralow et al., 1973). An ideal vehicle should be inert, highly penetratable through biological membranes, and have no biological action on the nervous and muscular systems. However, such vehicles rarely exist. DMSO, a vehicle commonly used for dissolving water insoluble substances, may have a wide range of actions on different tissues (Bralow et al., 1973; Jacob and Herschler, 1986; North and Mark, 1989; Sams and Carroll, 1966; Jourdon et al., 1986; Winmill and Hedrick, 2003; Hedric and Morales, 1999). For instance, effects of DMSO were noted on the rhythmicity of the heart (Kramer et al., 1995; Bazil et al., 1993) and respiration (de la Torre et al., 1974, 1975). Superfusion of 1%DMSO enhanced the duration and amplitude of burst complex without affecting the rhythmicity of respiration (Hedric and Moralis, 1999). It is therefore important to quantify the effects of DMSO on the locomotor behavior for a better understanding of its impact on the study of neural control of locomotion. We thus investigated the effects of DMSO on the walking-like activity induced by NMDA or Glutamate in the mudpuppy. Part of this study was published in an abstract (Cheng and Lavrov 2004).
MATERIALS AND METHODS
Experiments used 40 adult mudpuppies (body length 20–30 cm). The experimental protocols were approved by the Animal Care and Use Committee of the University of Louisville.
The spinal cord-forelimb preparation
The dissection was performed as described in detail elsewhere (Wheatley et al. 1992). Briefly, animals were first anesthetized with application of 3-aminobenzoic acid ethyl ester (1–1.5 g/l) (Sigma, St. Louis, MO) to the water in which mudpuppy was placed. A longitudinal incision was made and paravertebral muscles were removed. A dorsal Laminectomy was performed from the first to the fifth cervical segments, which are then isolated along with the brachial nerve plexuses and the forelimbs. The preparation is placed in a Petri dish containing 100% oxygenate Ringer’s solution (NaCl 115mM, KCl 2mM, CaCl 2mM, MgCl2 1.8mM, HEPES 5mM and glucose 1 gm/l, pH 7.35). While in the Petri dish, the brachial plexus was exposed, the paraspinal muscles were removed, and the dura mater covering the spinal cord was opened. The dissection took about 45 min to complete. After dissection, the preparation was transferred to a recording chamber (120 ml) and perfused with cooled (15°C) and oxygenated Ringer’s solution throughout the experiment at a flow rate of 4–5 ml/min. The spinal cord and forelimbs were stabilized by the pinning the vertebral column to the Sylgard resin (Dow Corning) coating the base of the bath. Teflon-coated silver wires (75µm) were inserted into the elbow flexor (Brahialis) and extensor (Extensor ulnae) muscles for electromyography (EMG) recording, which was amplified, high-pass (10 Hz) and low-pass (300 Hz) filtered, and stored on a computer’s hard disk. Commercially available programs (Axon Instruments/Molecular Devices, Sunnyvale, CA; Origin, Northampton, MA and SPSS, Chicago, IL) were used for recording and processing. After a recovery period of 1 hour, all preparations showed a withdrawal reflex to pinching of the limbs.
Application of neuroactive agents
The neuroactive agents were applied either directly to the bath as a bolus or by continuous superfusion. The spinal cord compartment of the recording chamber contains 15 ml of artificial CSF solution and the superfusion rates were 4–5ml/min when bolus application is used and 5–35 ml/min when continuous superfusion with neuroactive agents is intended. Induction of walking-like activity was attempted by bolus bath application of D-glutamate (0.3–0.9 mmol/L, Sigma, St. Louis, MO) together with D-serine (10 µmol/L, Sigma, St. Louis, MO) or by continuous superfusion of known concentrations of D-glutamate. D-serine was co-applied with D-glutamate for more stable walking-like activity, presumably through saturating the coagonist glycine binding site (Wheatly et al. 1992). However, it was not required as application of D-glutamate alone induced robust walking-like activity in all 4 preparations tested. A 3-tank system interconnected by a 3-way stopcock was used for (i) washout with Ringer' s solution, (ii) delivery of a glutamate receptor agonist, or (iii) simultaneous delivery of a glutamate receptor agonist and an antagonist. Only 1 of the 3 solutions was delivered to the preparation at any given time. The agonist was either D-glutamate or NMDA (20–80 µmol/L, Sigma, St. Louis, MO) and the antagonist was AP-5 (1–120 µmol/L), CNQX (1–100 µmol/L), Strychnine (1–120 µM) (Sigma, St. Louis, MO). All drugs were dissolved in 100–200 mL of Ringer's solution immediately before use. Each application was followed by a period of washing-out with Ringer's solution. The walking-like activity was monitored, recorded, and analyzed with the use of Axoscope 8.1 (Axon Instruments). Measurements of cycle duration of flexor and extensor bursts were made before and after each application of agonist and antagonist and quantified as means ± SE of at least 20 cycles. DMSO (1–10% v/v, Sigma, St. Louis, MO) was applied through continuous superfusion in the presence of NMDA or glutamate.
Dorsal root reflex testing
To test effects of neuroactive and DMSO on reflex amplitude, the dorsal root (C3) was stimulated with constant cathode current of 0.5 ms pulse duration through a stimulator (S88; Grass-Telefactor, Astro-Med Inc, West Warwick, RI, USA) and stimulus isolation unit (PSIU6; Grass-Telefactor, Astro-Med Inc, West Warwick, RI, USA). The stimulus intensity was up to 1.5 times the motor threshold. The amplitude of the short latency reflex was evaluated from Extensor ulnae.
Analysis
Measurements of cycle duration of flexor and extensor bursts were made of greater that 20 cycles and averaged. The amplitude of EMG bursts was calculated as area of rectified EMG and reported as percentage of the control for each preparation. All data are reported as mean ± SEM. Statistically significant differences were determined using a one-way repeated measures analysis of variance (ANOVA). The confidence level of statistical significance was 95% for all comparisons.
RESULTS
Initiation and maintenance of walking-like activity
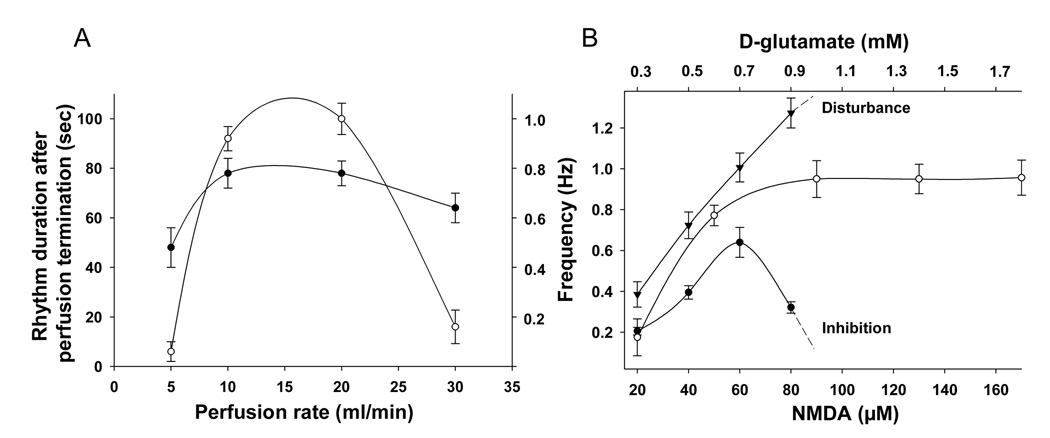
Bolus application of D-glutamate produced tonic activity in a wide range of concentrations (0.5–100 mM). Short bouts of irregular rhythmic pattern (5–10 sec) were observed in 2 out of 12 animals. In contrast, stable and long-lasting walking-like activity was induced by continuous superfusion of glutamate (0.5 mM) at a rate of 20 ml/min for all tested animals. The walking-like pattern became unstable and irregular and came to a termination when the superfusion rate was reduced to < 5ml/min. Strong tonic activity was induced at superfusion at rates > 30 ml/min and the rhythmic activity was terminated shortly after such rate was reached. Upon termination of glutamate application, rhythmic walking-like activity continued for a period of 5–100 seconds. The duration of this period was superfusion rate dependent with the longest duration (100 seconds) obtained at superfusion rates of 10–20 ml/min, as shown in Figure 1A. Also shown in this Figure is the relationship between the frequency of the walking-like activity and the superfusion rate. Clearly, the frequency was also dependent on the superfusion rate, albeit to a lesser extent. The highest frequency (0.8 Hz) was achieved at superfusion rates of 10– 20 ml/min.
Figure 1. Optimal superfusion flow rate and comparisons of delivery modes.
A: Frequency (●) and duration (○) of walking-like activity were dependent on the superfusion flow rate of D-glutamate (0.5 mM) (mean ± SEM, n=5). The optimal range of superfusion rate was between 10 and 20 ml/min. In this range, peak frequency (0.8 Hz) was achieved and the du-ration of the activity upon termination of the superfusion was longest (90–100 seconds).
B: Effects of mode of delivery on the walking-like activity (mean ± SEM, n=12). Superfusion of D-glutamate (▼) induced stable walking-like activity, the frequency of which was dose-dependent in the range of 0.3–0.9 mM. Beyond 0.9 mM, superfusion of glutamate caused tonic activity of flexor and extensor muscles. Superfusion of NMDA (●) induced walking-like activity in a small effective range of concentrations (40–80 µM), with the highest frequency of 0.6 Hz at a concentration of 60µM. Beyond 80 µM, superfusion of NMDA induced tonic activities. Bolus application of NMDA (○) was effective in a much wider range of concentrations (50–300 µM). The peak frequency was higher than that induced by superfusion.
Both bolus application and continuous superfusion NMDA produced robust, long-lasting (> 30 min) rhythmic patterns in all preparations, beginning within a few seconds after NMDA was applied (Figure 1B). Bolus application induced walking-like activity at a wide range of concentrations. In contrast, continuous superfusion showed a window of concentrations (around 60 µM) at which the best locomotor-like pattern was achieved. Beyond this window of concentrations, NMDA lost its efficacy in producing locomotor-like activity. Also shown in this panel is the linear relationship between the frequency of the rhythmic activity and the concentration of glutamate superfusion solution within the range of 0.3 to 0.9 mM (established at a superfusion rate of 20ml/min). The rhythmic activity was disrupted with tonic activity at glutamate concentrations greater than 0.9 mM. In comparison, NMDA bolus application produced stable walking-like activity at concentrations ranging 20–300 µM (data not shown).
Inhibitory effects of AP-5 and strychnine applied as a bolus or with superfusion
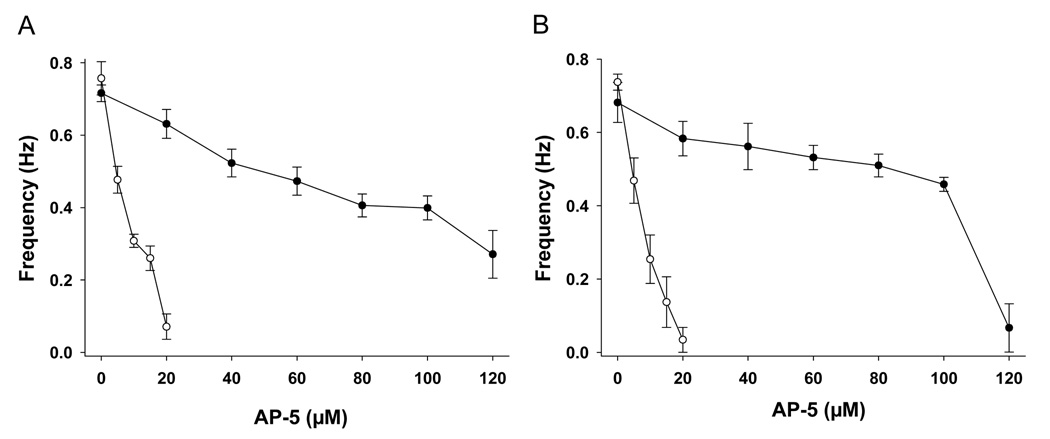
Both bolus application and continuous superfusion of AP-5 reduced the frequency of walking-like activity induced by D-glutamate in a dose-dependent manner (Figure 2). However, much lower concentrations of AP-5 (5–20 µM) were required for this inhibitory effect when applied as continuous superfusion (open circles), in contrast to bolus application (20–120 µM, closed circles) (Figure 2A). Similar effects were observed on the initiation phase of walking-like activity induced by glutamate superfusion (Figure 2B). In contrast to bolus application (20–120 µM, closed circles), continuous superfusion of much lower concentrations of AP-5 (5–20 µM) was required to suppress the frequency of the locomotor-like activity. Complete block of the activity was achieved at 20 µM AP-5 in 86% of preparations (6/7).
Figure 2. Mode of application of AP-5 affects the efficacy of its inhibitory effects.
A. Compared to bolus application (●), continuous superfusion (○) of AP-5 was more effective in inhibiting the ongoing walking-like activity induced glutamate (mean ± SEM, n=7).
B: Continuous superfusion of AP-5 (○) was similarly more effective in inhibiting the initiation of walking-like activity by glutamate, compared to bolus application (●) (mean ± SEM, n=7).
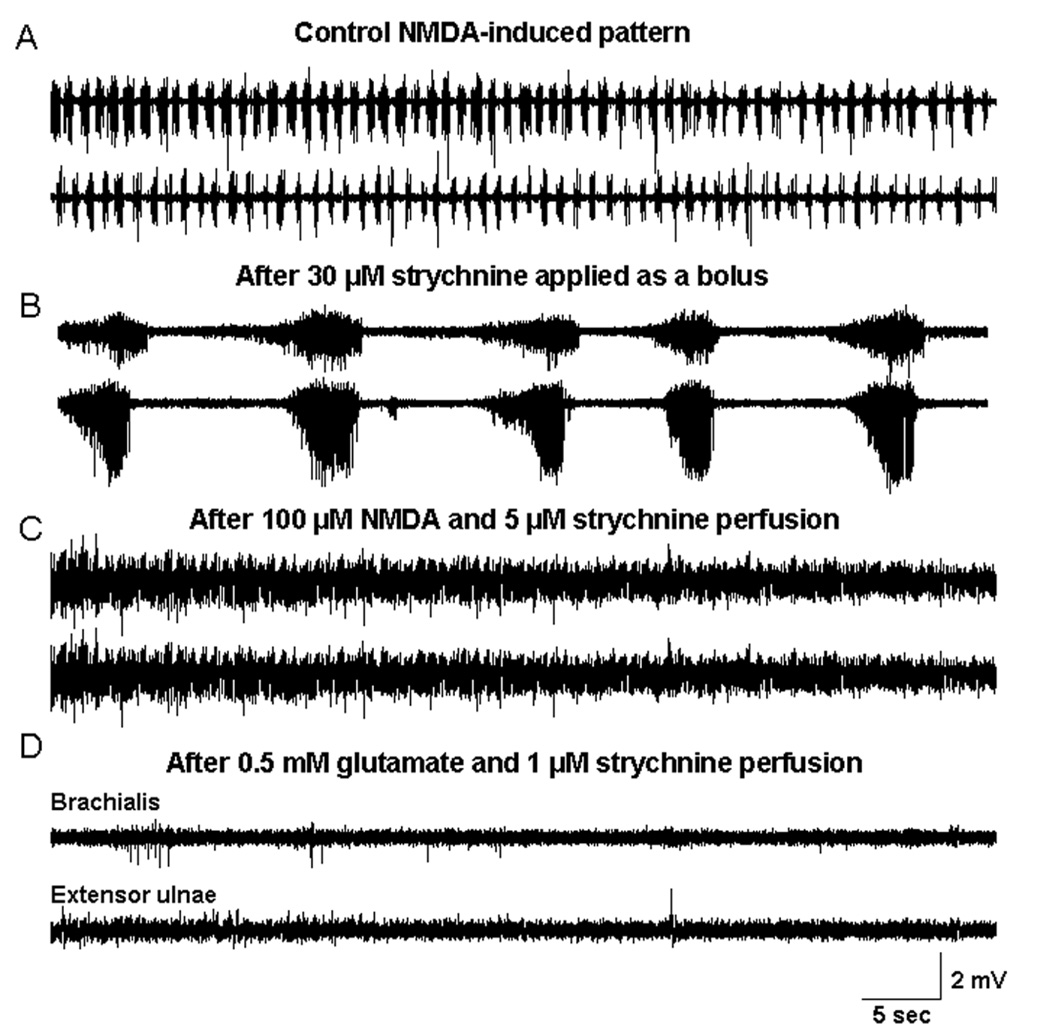
Bolus application of glycine receptor antagonist strychnine had profound effects on the walking-like activity (Figure 3). At low concentrations (1–20 µM), strychnine increased its frequency without affecting the alternating patterns of flexor-extensor activation (data not shown). At higher concentrations (20–100 µM), in contrast, Strychnine converted the alternating pattern to a synchronized pattern and reduced the frequency dramatically (Figure 3A, B). The slow, synchronized rhythm was long-lasting (>30 minutes) and was stable without additional NMDA administration. Restoration of the regular patterns was only possible after prolonged washout (2–3 h) or did not occur at all in 3 out of 8 preparations. In contrast, continuous superfusion of strychnine (3–5 µM) completely blocked the walking-like activity induced by NMDA (50 µM) in all preparations (n=8) (Figure 3C). A slow and synchronous pattern, similar to that produced by bolus strychnine application, emerged within 10–15 minutes after termination of the superfusion. Continuous superfusion of NMDA converted this synchronous pattern to tonic activities and abolished the rhythmicity. The synchronous rhythm was also terminated with superfusion of Ringer’s solution at rates 15–25 ml/min and reappeared after the superfusion was stopped.
Figure 3. Inhibitory effects of strychnine on walking-like activity.
Bolus application of strychnine changed the rhythmic, alternating the pattern of walking-like activity (A) to a slow and synchronized pattern (B). In contrast, superfusion of strychnine blocked completely the walking-like activity induced by NMDA (C) or glutamate (D).
The walking-like activity induced by glutamate superfusion was completely terminated by strychnine application either as a boluses or by continuous superfusion at 1–2 µM (Figure 3D). In comparison with NMDA-induced rhythm, even prolonged washout (> 9 hours) did not restore the ability to produce rhythmic activity with glutamate superfusion.
Effects of DMSO on walking-like activity
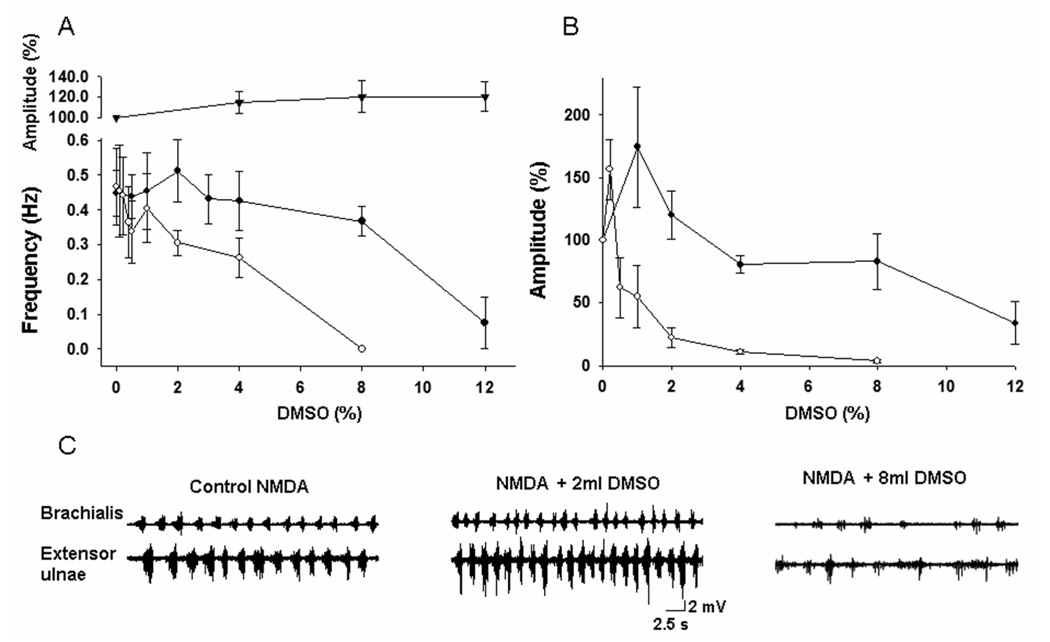
Dose-dependent effects of DMSO on the walking-like activity were observed. The frequency and amplitude of the locomotor-like activity induced by NMDA were not significantly affected by DMSO at concentrations up to 8% (v/v) (Figure 4A, B). The activity was depressed in both frequency and amplitude when DMSO concentration reached 12%. In contrast, the locomotor-like activity induced by glutamate was more sensitive to DMSO. The frequency and amplitude were depressed when the concentrations of DMSO were as low as 2%. The glutamate induced locomotor-like activity was completely blocked at DMSO concentration of 8% while the NMDA induced pattern was depressed, but not blocked, at 12% of DMSO. In higher concentrations (> 14%), DMSO produced complete block in the first seconds of superfusion. The inhibitory effects of DMSO were reversible with washout (data not shown). However, it took 6 hours of washout before locomotor-like activity could be evoked in 4 of 5 preparations and higher concentrations of NMDA or glutamate were required. In contrast to the locomotor-like activity, the amplitude of the dorsal root reflex was not affected significantly by DMSO at concentrations up to 12% (p < 0.05; n=8) (Figure 4A). In a few preparations (3/8), an excitatory effect of DMSO was observed as the frequency and amplitude of the walking-like activity were increased when 1–2% of DMSO was applied. On average, however, this effect did not reach statistical significance.
Figure 4. Effects of DMSO on locomotor-like activity.
A: The mean frequency of the walking-like activity was decreased dose-dependently with superfusion of DMSO (mean ± SEM, n=8). The activity induced by glutamate (○) was more sensitive to the inhibitory effects of DMSO than that induced by NMDA (●). In contrast, the amplitudes of the dorsal root reflexes were not significantly affected by DMSO (top curve, mean ± SEM, n=8).
B: The mean amplitude of the walking-like active was more profoundly depressed in a dose-dependent manner. Again, the activity induced by glutamate was more sensitive to the inhibition compared to that induced by NMDA (mean ± SEM, n=8).
C: Examples of dose-dependent inhibition of the walking-like activity by DMSO. Note that depression of the amplitude was more profound than the inhibition of the frequency, consistent with the pooled data shown in panels A and B.
DISCUSSION
The in vitro spinal cord-forelimb preparation from the mudpuppy allows neuropharmacological and electrophysiological studies of locomotion in a mature spinal cord. (Wheatley et al., 1992, 1994; Jovanovic et al., 1996; Shik 1997; Jovanovic et al., 1999; Cheng et al. 1998, 2002; Fok and Stein, 2002; Lavrov and Cheng 2004). Bath application of neuroactive agents (neurotransmitters and neuromodulators) was able to produce long-lasting and stable locomotor-like patterns. In this study, we systematically investigated the methodological factors that affect locomotor-like activity in the mudpuppy and identified parameters that are useful to optimize the experimental conditions for future studies.
Consistent with our earlier report (Lavrov and Cheng, 2004), the results of this study demonstrated that glutamate is only effective in inducing locomotor-like activity when it is applied by superfusion. In contrast to bolus application of NMDA, glutamate delivered as boluses failed to induce rhythmic walking-like activity regardless of the concentrations used. This difference may be accounted for, at least in part, by pharmacodynamics of drug-receptor interactions. The mode of applications may be less important for agents with high affinity, such as NMDA, to activate sufficient number of receptors. In contrast, bolus application may be not adequate for agents with low affinity, such as glutamate, to activate sufficient receptors. This is particularly relevant when rapid uptake of amino acid neurotransmitters takes place in the spinal cord, as suggested by experiments with amino acid uptake inhibitors in the lamprey (Brodin and Grillner, 1985). Other potential disadvantages of bolus application include an unequal distribution of the neuroactive agent in the chamber, rapid desensitization of neurotransmitter receptors, and inability to determine the exact concentrations for the initiation and maintenance of locomotor-like activity.
The optimal superfusion rates for glutamate-induced walking-like activity are around 10– 20 ml/per minutes (Figure 1). At this range, the frequency of the walking-like activity was highest (0.8 Hz) and the duration of the activity upon termination of glutamate superfusion was longest (9–100 seconds) (Figure 1A). It is note worthy that this optimal superfusion rate was determined at a glutamate concentration of 0.5mM. Clearly, the frequency of the walking-like activity was dependent on the concentration within the range of 0.3–0.9 (Figure 1B). The optimal superfusion rate may shift slightly if a different concentration of glutamate is used. High doses of glutamate were able to induce fictive locomotion without fluid circulation in the lamprey (Brodin and Grillner, 1985), while superfusion rate is critical even with high concentrations of glutamate in the mudpuppy (Lavrov and Cheng, 2004).
In contrast to glutamate, bolus application of NMDA is more effective than NMDA superfusion in the induction of walking-like activity. The effective range of NMDA concentrations (20–300 µM) was much wider when applied as boluses, compared to application by superfusion (20–80 µM). Faster and more robust walking-like activity was induced when NMDA was applied as boluses at any given concentrations, compared to superfusion (Figure 1B). Superfusion of NMDA at concentrations greater than 60 µM depressed the locomotor-like activity. These data suggest that bolus application is a preferred means of inducing locomotor-like activity when NMDA is used.
NMDA receptor antagonist AP-5 produced dose-dependent inhibition of the initiation and maintenance of the locomotor-like activities (Figure 2). Notably, superfusion of AP-5 produced more profound inhibition of the frequency of walking-like activity induced by glutamate, compared to bolus application. This applies to both the initiation phase and the maintenance phase of the walking-like activity. The alternating pattern and the amplitude of the activity were not significantly changed by either mode of AP-5 application.
Interestingly, superfusion of glycine receptor antagonist strychnine produced complete and irreversible block of the walking-like activity, while bolus application changed the alternating pattern to a slow and synchronized pattern (Figure 3). The inhibitory effects of strychnine applied as boluses have been studied in vitro preparations from neonatal rats (Cowley and Schmidt, 1995; McClellan, 1996; Kremer and Lev-Tov 1997) and mudpuppies (Jovanovic et al., 1999). A consistent finding is a change from a reciprocal to a synchronized pattern between the flexor and extensor muscles. The present data showed that application of strychnine by superfusion can not reproduce the results of bolus application, regardless the concentrations employed. This result was not changed whether the walking-like activity was induced by NMDA or by glutamate.
We demonstrated for the first time that DMSO has a profound impact on the activity of neural networks for locomotion. DMSO caused dose-dependent inhibition of the walking-like activity both in frequency and amplitude (Figure 4). The activity was blocked at concentrations above 8% (v/v). The inhibitory effects were reversible with adequate washout. The block can not be explained by non-specific toxic effects of DMSO as the dorsal root reflexes were not blocked at concentrations up to 12% (v/v). A further observation is that the walking-like activity induced by glutamate was more sensitive to the inhibitory effects of DMSO, compared to that induced by NMDA. The mechanisms of DMSO action on the nervous system may be related to its effects on cell membrane ion channels and neurotransmitter receptors. It has been shown that the repolarizing phase of the action potentials is prolonged by 8% DMSO as it blocks the active increase in potassium permeability (Sawada and Sato, 1975). This change suppresses the high frequency discharge, because the refractory period of each spike is increased. In concentrations less than 20%, DMSO may make neurons more excitable by depolarizing resting membrane potentials through inhibiting neuronal membrane permeability to potassium and chloride ions channels (Jourdon et al., 1986). DMSO in concentrations less than 1% may facilitate cholinergic transmission via blocking cholinesterase activity (Sawada and Sato, 1975). It may also produce dose-dependent inhibition of GABA-induced inward current at concentrations of 0.3–3% (Nakahiro et al., 1992). Both inhibitory and facilitate effects have been observed at concentrations of 1–10% (McLarnon, 1986; Sawada and Sato, 1975). In cholinergic synapses, excitatory transmission is more susceptible to DMSO than inhibitory transmission. This is because the activity of the excitatory receptor is blocked more readily than that of the inhibitory receptor. The depressing effects of DMSO are not specific to the cholinergic system and the activities of GABA and glutamate receptors are similarly depressed (Sawada and Sato, 1975). Our data suggest that the rhythmicity of the locomotor-like behavior is largely uninterrupted when the concentration of DMSO is below 4% and that the amplitude is essentially stable when the concentration of DMSO is below 2% in the mudpuppy. The walking-like activity induced NMDA is more resistant to the effects of DMSO than that induced by glutamate.
Taken together, this study demonstrates that way by which neuroactive agents are delivered may have a profound impact on the results of in vitro neurophysiological experiments. This principle applies to both excitatory and inhibitory neurotransmitters and their antagonists. The vehicle for dissolving neuroactive agents, DMSO, can also have significant effects on the activities of neural networks for locomotion. It is therefore essential to take into account of these factors when interpreting data of this kind of experiments.
Acknowledgments
This work was supported by grants to Dr. Cheng (grant # 0–4) from the Kentucky Spinal Cord and Head Injury Research Trust (KSCHIRT) and NIH/NINDS 1 R01 NS052372-03.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Barry MJ, O'Donovan MJ. The effects of excitatory amino acids and their antagonists on the generation of motor activity in the isolated chick spinal cord. Brain Res. 1987;433:271–276. doi: 10.1016/0165-3806(87)90030-7. [DOI] [PubMed] [Google Scholar]
- Bazil MK, Krulan C, Webb RL. Telemetric monitoring of cardiovascular parameters in conscious spontaneously hypertensive rats. J Cardiovasc Pharmacol. 1993;22:897–905. doi: 10.1097/00005344-199312000-00019. [DOI] [PubMed] [Google Scholar]
- Bonnot A, Morin D, Viala D. Genesis of spontaneous rhythmic motor patterns in the lumbosacral spinal cord of neonate mouse. Brain Res Dev Brain Res. 1998;108:89–99. doi: 10.1016/s0165-3806(98)00033-9. [DOI] [PubMed] [Google Scholar]
- Bralow SP, Gruenstein M, Hitanant S, Johnson W. Effect of dimethyl sulfoxide (DMSO) on gastric secretion in rats. Digestion. 1973;8:120–129. doi: 10.1159/000197307. [DOI] [PubMed] [Google Scholar]
- Brodin L, Grillner S. The role of Putativre excitatory amino acid neurostransmitters in the initiation of locomotion in the lamprey spinal cord. II. The effect of amino acid uptake inhibitors. Brain Res. 1985;360:149–158. doi: 10.1016/0006-8993(85)91230-2. [DOI] [PubMed] [Google Scholar]
- Cheng J, Stein RB, Jovanovic K, Yoshida K, Bennett DJ, Han Y. Identification, localization, and modulation of neural networks for walking in the mudpuppy (Necturus maculatus) spinal cord. J Neurosci. 1998;18:4295–4304. doi: 10.1523/JNEUROSCI.18-11-04295.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng J, Jovanovic K, Aoyagi Y, Bennett DJ, Han Y, Stein RB. Differential distribution of interneurons in the neural networks that control walking in the mudpuppy (Necturus maculatus) spinal cord. Exp Brain Res. 2002;145:190–198. doi: 10.1007/s00221-002-1102-0. [DOI] [PubMed] [Google Scholar]
- Cohen AH, Wallen P. The neuronal correlate of locomotion in fish. "Fictive swimming" induced in an in vitro preparation of the lamprey spinal cord. Exp Brain Res. 1980;41:11–18. doi: 10.1007/BF00236674. [DOI] [PubMed] [Google Scholar]
- Cowley KC, Schmidt BJ. Effects of inhibitory amino acid antagonists on reciprocal inhibitory interactions during rhythmic motor activity in the in vitro neonatal rat spinal cord. J Neurophysiol. 1995;74:1109–1117. doi: 10.1152/jn.1995.74.3.1109. [DOI] [PubMed] [Google Scholar]
- Cowley KC, Schmidt BJ. Regional distribution of the locomotor pattern-generating network in the neonatel rat spinal cord. J Neurophysiol. 1997;77:247–259. doi: 10.1152/jn.1997.77.1.247. [DOI] [PubMed] [Google Scholar]
- De la Torre JC, Rowed DW. DMSO: a new respiratory stimulant? J Clin Pharmacol. 1974;14:345–353. doi: 10.1002/j.1552-4604.1974.tb01410.x. [DOI] [PubMed] [Google Scholar]
- De la Torre JC, Kawanaga HM, Johnson CM, Goode DJ, Kajihara K, Mullan S. Dimethyl sulfoxide in central nervous system trauma. Ann N Y Acad Sci. 1975;243:362–389. doi: 10.1111/j.1749-6632.1975.tb25377.x. [DOI] [PubMed] [Google Scholar]
- Fok M, Stein RB. Effects of cholinergic and noradrenergic agents on locomotion in the mudpuppy (Necturus maculatus) Exp Brain Res. 2002;145:498–504. doi: 10.1007/s00221-002-1125-6. [DOI] [PubMed] [Google Scholar]
- Grillner S, McClellan A, Sigvardt K, Wallen P, Wilen M. Activation of NMDA-receptors elicits "fictive locomotion" in lamprey spinal cord in vitro. Acta Physiol Scand. 1981;113:549–551. doi: 10.1111/j.1748-1716.1981.tb06937.x. [DOI] [PubMed] [Google Scholar]
- Grillner S, Wallen P, Brodin L, Lansner A. Neuronal network generating locomotor behavior in lamprey: circuitry, transmitters, membrane properties, and simulation. Annu Rev Neurosci. 1991;14:169–199. doi: 10.1146/annurev.ne.14.030191.001125. [DOI] [PubMed] [Google Scholar]
- Hedrick MS, Morales RD. Nitric oxide as a modulator of central respiratory rhythm in the isolated brainstem of the bullfrog (Rana catesbeiana) Comp Biochem Physiol A Mol Integr Physiol. 1999;124:243–251. doi: 10.1016/s1095-6433(99)00115-4. [DOI] [PubMed] [Google Scholar]
- Hernandez P, Elbert K, Droge MH. Spontaneous and NMDA evoked motor rhythms in the neonatal mouse spinal cord: an in vitro study with comparisons to in situ activity. Exp Brain Res. 1991;85:66–74. doi: 10.1007/BF00229987. [DOI] [PubMed] [Google Scholar]
- Jacob SW, Herschler R. Pharmacology of DMSO. Cryobiology. 1986;23:14–27. doi: 10.1016/0011-2240(86)90014-3. [DOI] [PubMed] [Google Scholar]
- Jourdon P, Berwald-Netter Y, Dubois JM. Effects of dimethylsulfoxide on membrane currents of neuroblastoma x glioma hybrid cell. Biochim et Biophys Acta. 1986;856:399–402. doi: 10.1016/0005-2736(86)90053-2. [DOI] [PubMed] [Google Scholar]
- Jiang Z, Carlin KP, Brownstone RM. An in vitro functionally mature mouse spinal cord preparation for the study of spinal motor networks. Brain Res. 1999;816:493–499. doi: 10.1016/s0006-8993(98)01199-8. [DOI] [PubMed] [Google Scholar]
- Jovanovic K, Petrov T, Greer JJ, Stein RB. Serotonergic modulation of the mudpuppy (Necturus maculatus) locomotor pattern in vitro. Exp Brain Res. 1996;111:57–67. doi: 10.1007/BF00229556. [DOI] [PubMed] [Google Scholar]
- Jovanovic K, Petrov T, Stein RB. Effects of inhibitory neurotransmitters on the mudpuppy (Necturus maculates) locomotor pattern in vitro. Exp Brain Res. 1999;129:172–184. doi: 10.1007/s002210050887. [DOI] [PubMed] [Google Scholar]
- Kiehn O, Kjaerulff O. Spatiotemporal characteristics of 5-HT and dopamine-induced rhythmic hindlimb activity in the in vitro neonatal rat. J Neurophysiol. 1996;75:1472–1482. doi: 10.1152/jn.1996.75.4.1472. [DOI] [PubMed] [Google Scholar]
- Kremer E, Lev-Tov A. Localization of the spinal network associated with generation of hindlimb locomotion in the neonatal rat and organization of its transverse coupling system. J Neurophysiol. 1997;77:1155–1170. doi: 10.1152/jn.1997.77.3.1155. [DOI] [PubMed] [Google Scholar]
- Kramer K, Van Acker SA, Grimbergen JA, Van den Berg DJ, Van der Vijgh WJ, Bast A. Effect of dimethyl sulfoxide (DMSO) on the electrocardiogram (ECG) in freely moving male Balb/c mice. Gen Pharmacol. 1995;26:1403–1407. doi: 10.1016/0306-3623(94)00300-c. [DOI] [PubMed] [Google Scholar]
- Kudo N, Yamada T. N-methyl-D,L-aspartate-induced locomotor activity in a spinal cord-hindlimb muscles preparation of the newborn rat studied in vitro. Neurosci Lett. 1987;75:43–48. doi: 10.1016/0304-3940(87)90072-3. [DOI] [PubMed] [Google Scholar]
- Lavrov I, Cheng J. Activation of NMDA receptors is required for the initiation and maintenance of walking in mudpuppy (Necturus Maculatus) Can J Physiol Pharmacol. 2004;82:637–644. doi: 10.1139/y04-044. [DOI] [PubMed] [Google Scholar]
- McClellan AD. Organization of spinal locomotor networks: contribution from model system. Comments Theor Biol. 1996;4:63–91. [Google Scholar]
- McLarnon JG, Saint DA, Quastel DM. The actions of dimethyl sulfoxide on neuromuscular transmission. Molecular Pharmacol. 1986;30:631–638. [PubMed] [Google Scholar]
- Nakahiro M, Arakawa O, Narahashi T, Ukai S, Kato Y, Nishinuma K, Nishimura T. Dimethyl sulfoxide (DMSO) blocks GABA-induced current in rat dorsal root ganglion neurons. Neurosci Lett. 1992;138:5–8. doi: 10.1016/0304-3940(92)90459-k. [DOI] [PubMed] [Google Scholar]
- North PE, Mrak RE. Synaptosomal uptake of choline and of gamma-aminobutyric acid: effects of ethanol and of dimethylsulfoxide. Neurotoxicology. 1989;10:569–576. [PubMed] [Google Scholar]
- Poon MLT. Induction of swimming in lamprey by L-dopa and amino acids. J. Comp. Physiol. 1980;136:337–344. [Google Scholar]
- Roberts A, Soffe SR, Clarke JD, Dale N. Initiation and control of swimming in amphibian embryos. Symp Soc Exp Biol. 1983;37:261–284. [PubMed] [Google Scholar]
- Sams WM, Jr, Carroll NV. Cholinesterase inhibitory property of dimethyl sulphoxide. Nature. 1966;212:405. doi: 10.1038/212405a0. [DOI] [PubMed] [Google Scholar]
- Sawada M, Sato M. The effect of dimethyl sulfoxide on the neuronal excitability and cholinergic transmission in Aplysia ganglion cells. Ann N Y Acad of Sci. 1975;243:337–357. doi: 10.1111/j.1749-6632.1975.tb25375.x. [DOI] [PubMed] [Google Scholar]
- Shik ML. Locomotor patterns elicited by electrical stimulation of the brain stem in the mudpuppy. Motor Control. 1997;1:354–368. [Google Scholar]
- Smith JC, Feldman JL. In vitro brainstem-spinal cord preparations for study of motor systems for mammalian respiration and locomotion. J Neurosci Methods. 1987;21:321–333. doi: 10.1016/0165-0270(87)90126-9. [DOI] [PubMed] [Google Scholar]
- Wheatley M, Stein RB. An in vitro preparation of the mudpuppy for simultaneous intracellular and electromyographic recording during locomotion. J Neurosci Methods. 1992;42:129–137. doi: 10.1016/0165-0270(92)90143-2. [DOI] [PubMed] [Google Scholar]
- Wheatley M, Edamura M, Stein RB. A comparison of intact and in-vitro locomotion in an adult amphibian. Exp Brain Res. 1992;88:609–614. doi: 10.1007/BF00228189. [DOI] [PubMed] [Google Scholar]
- Wheatley M, Jovanovic K, Stein RB, Lawson V. The activity of interneurons during locomotion in the in vitro necturus spinal cord. J Neurophysiol. 1994;71:2025–2032. doi: 10.1152/jn.1994.71.6.2025. [DOI] [PubMed] [Google Scholar]
- Winmill RE, Hedrick MS. Gap junction blockade with carbenoxolone differentially affects fictive breathing in larval and adult bullfrogs. Resp Physiol Neurobiol. 2003;138:239–251. doi: 10.1016/j.resp.2003.08.005. [DOI] [PubMed] [Google Scholar]