Abstract
Background/Aims
Urine microscopy is a useful diagnostic tool; however, the manner in which nephrologists prepare and examine urinary sediment is variable. We developed an acute kidney injury (AKI) cast scoring index (CSI) in order to standardize urinary microscopy. Further, we sought to assess the precision of this scoring system.
Methods
Urine from 30 patients with AKI consistent with the syndrome of acute tubular necrosis were collected. Sample preparation was uniform and standardized. A panel of 3 nephrologists blinded to the sample preparation were instructed to grade each slide using the AKI CSI. Subsequently, the AKI CSI was then tested in another 18 patients with AKI to determine if this score could predict nonrenal recovery.
Results
The inter-observer agreement index was 99.80%, with a coefficient of variation of 1.24%. Of the 90 paired observations, 98.8% fell within 2 standard deviations of the mean, signifying good agreement. The receiver operator characteristic area under the curve for AKI CSI to predict nonrenal recovery was 0.79.
Conclusions
AKI CSI is a simple, novel, reliable scoring system to grade the degree of epithelial cell and granular casts present on urine microscopy. A standardized AKI CSI has the potential to incorporate urinary cast analysis into the advancing field of AKI diagnostics. These preliminary data endorse the notion that the AKI CSI may be useful in predicting renal outcomes.
Key Words: Epithelial cell casts, Acute kidney injury, Renal replacement therapy, Granular casts
Introduction
Acute kidney injury (AKI) is common among hospitalized patients, and it is associated with increased mortality [1]. The most common causes of AKI in hospitalized patients are prerenal disorders and acute tubular necrosis (ATN) [2, 3]. The diagnosis of AKI is typically made in conjunction with the clinical history, physical exam, blood and urine laboratory values, along with urine microscopy [4,5,6]. Tsai et al. [7] have shown that nephrologists are superior to standard hospital laboratories in the preparation and interpretation of urine microscopy for the diagnosis of AKI. In their study in hospitalized patients, ATN was the most common form of AKI consistent with previous reports [7]. The presence of granular casts and renal epithelial cell (RTE) casts are consistent with the diagnosis of ATN [8, 9]. However, the manner in which nephrologists prepare and examine urine sediment is quite variable. We conducted a straw poll via email among our staff and among AKI experts from around the USA about the manner in which they prepare and analyze urine sediment (fig. 1). We were surprised to find that there was a great deal of variability in the responses. The variability in the responses occurred with the amount of urine to be spun (5–15 ml), the duration of centrifugation (3–15 min), the speed at which centrifugation should be conducted (1,500–3,500 rpm), how the urine pellet should be resuspended (manually vs. with a pipette) and the volume in which the pellet should be resuspended (0.1–1.0 ml). Moreover, we could not find any consensus on how many granular casts or RTE cell casts are required to make the diagnosis of ATN given a clinical history consistent with ATN.
Fig. 1.
Answers provided by 4 AKI experts in a straw poll on urine sediment processing methods. A = Expert from Pittsburgh, Pa.; B = expert from Birmingham, Ala.; C = expert from Charlottesville, N.C.; D = expert from Indianapolis, Ind.
There is general agreement of the value of utilizing urine microscopy in the diagnosis of AKI [5]. However, current diagnostics for AKI are insufficient and often nonspecific. The need for improved AKI diagnostics has led to the emerging field of AKI biomarkers. Multiple biomarkers have been shown to have diagnostic potential (e.g. KIM-1, NGAL, IL-18, Fetuin-A) [10,11,12,13]. We believe that these biomarkers will have greater discriminatory power if coupled with clinicopathologic measures such as urine microscopy and/or diagnostic radiology studies. Further, the sensitivity and specificity of urine microscopy in conjunction with AKI biomarkers has never been studied. However, urine microscopy has significant limitations. Because urinary casts are vulnerable to variations in pH and temperature, frozen samples are not amenable to urine microscopy, and so sediment must be evaluated from ‘fresh’ samples [14]. Typically, studies involving urinary biomarkers for AKI are frozen and batched for analysis at a later date. These logistical challenges make the assessment of urinary biomarkers in conjunction with urinary sediment difficult because they cannot be assessed simultaneously.
In past studies, casts have been either quantified as an average number per low-power field, or authors have used the Addis method to calculate the rate of excretion of formed elements of the urine over a 12-hour period [8, 9, 15]. In this study, we set out to develop a simple AKI cast scoring index (CSI) that could be used to grade the level of RTE casts and granular casts in urine sediment so that urine sediment could be evaluated in a uniform fashion. Our goal was to develop a simple, reproducible score with good precision. We believe that if a precise AKI CSI can be validated, that would allow clinicians to investigate the accuracy of an AKI CSI with or without biomarkers in future investigations.
Methods
Part 1
A total of 30 urine samples were collected from 30 patients at the George Washington University Hospital in Washington, D.C., USA. Because a definitive diagnosis of ATN can only be made with a renal biopsy, patients were included if they had clinical syndrome consistent with ATN as determined by the renal consult service. Patients were excluded if they had known kidney disease that was not consistent with ATN (e.g. glomerular nephritis, interstitial nephritis, pyelonephritis, urinary obstruction, etc.) or hyperbilirubinemia (serum bilirubin >2.0 mg/dl). Fresh urine samples were taken and the identities of the patients from who they came were hidden before they were handed to a research assistant who prepared the urine sample for sediment analysis. The research assistant was not aware of the patient history or laboratory data. A waiver of informed consent and a Health Insurance Portability and Accountability Act waiver were obtained from the institutional review board for this study.
All samples were prepared in an identical fashion. The sample preparation was based on a combination of recommendations from various AKI experts. Each sample was measured to a volume of 10 ml. Urine aliquots were centrifuged for 5 min at 2,000 rpm. The supernatant was subsequently decanted, leaving a residual of 0.5 ml, and thus yielding a concentration factor of 20. To resuspend the pellet, the test tubes were agitated gently by hand. A pipette was used to dispense 1 drop of sediment to a standard size glass slide. A 24 × 30-mm coverslip was gently applied. Samples were excluded from the study if they were not viewed by all 3 nephrologists within 2 h of collection. When the slides were not actively under review by 1 of the judges, the slides were stored in a covered opaque plastic box at room temperature. An individual slide was viewed by all the participating nephrologists within a 30-min period. The nephrologists were blinded to each other's scores. The 3 nephrologists were also blinded to the identity of the source of the urine sample.
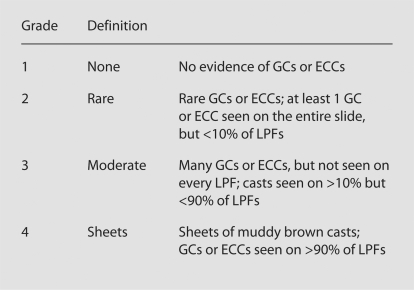
The nephrologists were instructed to view the entire slide under bright light microscopy at low power (×10) to search for granular and RTE casts. All nephrologists used the same microscope. High power (×40) was available to confirm the type of cast. Nephrologists A, B and C were instructed to grade each slide using the AKI CSI (fig. 2), which was displayed alongside the microscope.
Fig. 2.
Granular cast index. GC = Granular casts; ECC = epithelial cell casts; LPF = low-power field (×10). The approach to viewing slide was to search for casts (GCs or ECCs), then view the entire slide.
Part 2
After validation of the repeatability of the AKI CSI, we tested its capacity to predict clinically relevant outcomes. Patients with a clinical syndrome consistent with ATN from nephrotoxic, ischemic and multifactorial causes were prospectively enrolled. The diagnosis of ATN was determined by the renal consult service's assessment of the patient, based on their clinical history, a physical exam, radiologic investigation, laboratory investigation of the blood and urine and the urine microscopy. The protocol was approved by the George Washington University Medical Center institutional review board. Urine sediments of all patients were assessed and graded as described above within 72 h of their initial increase in serum creatinine. Demographic, clinical, and laboratory data were collected, and patients were followed for the duration of their hospitalization. Based on the renal consultation and chart review, ATN was characterized as ischemic, nephrotoxic or multifactorial. The primary endpoint was nonrenal recovery. Nonrenal recovery was defined as: (1) the need for renal replacement therapy, or (2) death while the serum creatinine trend was still rising. Renal recovery was defined as: (1) the serum creatinine returning to baseline levels, or (2) death while the serum creatinine was improving as compared to the peak creatinine after study entry.
Statistical Analysis
The interobserver agreements were calculated according to the statistical methods proposed by Bland and Altman [16]. The assessments of the urinary specimens made by the 3 nephrologists were converted into pairs of observations and then transformed into an equivalent percentage scale of interobserver agreement, according to the following formula:
in which xa and xb are the grade given to a sample by 2 different observers. In this fashion, nephrologist A's grades were compared to those from nephrologist B for each sample, yielding 30 pairs. Similarly, nephrologist A was compared to nephrologist C, and nephrologist B was compared to nephrologist C. This process yielded 90 paired values.
The coefficient of variation was determined by 2 standard deviations of
In addition, a scatter plot was generated in order to further validate the data. The above technique has been described by others for similar interobserver analyses [17].
The capacity of the CSI to predict nonrenal recovery was assessed by receiver operator characteristic (ROC) analysis, and the sensitivity and specificity for each CSI was assessed. Mean CSI was compared using the Mann-Whitney U test (SPSS 13.0, Chicago, Ill., USA).
Results
Part 1
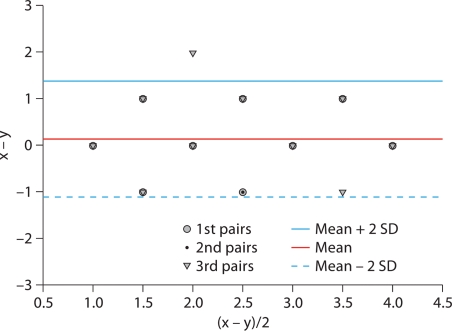
In the set of 30 patients wherein the ATN CSI was assessed for precision, the interobserver index was 99.8 ± 0.29%. The coefficient of variation was 1.24%. A Bland-Altman scatter plot (fig. 3) shows that all but 1 of the paired samples fall within 2 standard deviations of the mean, and within 1 grade of one another.
Fig. 3.
Bland-Altman scatter plot.
Part 2
Of the 18 patients with ATN whose urinary sediment was assessed for outcome, the mean age was 61.9 ± 18.7 years, 72% of the patients were male, 66.6% of the subjects were African American, while the remainder (33.3%) were European American. The baseline serum creatinine was 1.1 ± 0.5 mg/dl. The rate of nonrenal recovery was 61.1%, and the mean AKI CSI was 2.2. The patients with nonrenal recovery had a higher CSI as compared to those patients that did recover renal function (2.55 ± 0.93 vs. 1.57 ± 0.79, p = 0.04) ROC area under the curve for CSI to diagnose nonrenal recovery was 0.79.
Discussion
We attempted to design a simple, easily reproduced scoring system for urinary casts associated with ATN. We modeled our system after the New York Heart Association Congestive Heart Failure Grading system, wherein 4 different categories separate various levels of heart failure, which has proven to be a simple and durable classification system [18]. We have shown that a simple granular/epithelial cell cast score can be reproduced between different reviewers to within 1 degree, based on the AKI CSI that we proposed. In our study, the interobserver index was 99.8%, signifying good agreement. In addition, the Bland-Altman scatter plot shows that all but 1 assessment fell within 2 standard deviations of the mean. Our grading system is simple and the sample preparation is easily reproducible in any hospital where urine sediment is part of the clinical work-up of kidney disease. Similarly, our system separates different levels of urinary epithelial and granular casts on standard urine sediment analysis.
In addition to validating a standard technique to analyze urinary sediment for patients with a clinical syndrome suggestive of ATN, we assessed the capacity of this score to predict nonrenal recovery. The ROC area under the curve was 0.79, which is comparable to urinary KIM-1 (ROC area under the curve = 0.61) and NAG (ROC area under the curve = 0.71) [19]. While this value is not adequate to be the sole diagnostic measure for renal outcomes, the ROC area under the curve value does suggest the score has potential for diagnostic use. Clinicians currently use urinary sediment as an aid to diagnose the etiology of renal disease. We propose that the AKI CSI might have diagnostic utility for renal outcomes in combination with other relevant clinical and biologic measures. A point scoring system that incorporates the AKI CSI may improve current and/or future AKI diagnostic measures. If validated, an AKI CSI could be used in conjunction with serum and urinary biomarkers to help improve the diagnosis of AKI. For instance, a twofold increase in KIM-1 may not prove to be diagnostic of AKI as compared to a fourfold increase, but a twofold increase in KIM-1 with evidence of granular or RTE casts may be specific for AKI. Also, an AKI CSI could be useful in the prognosis of severity and duration of kidney injury. By standardizing the urine sediment analysis, studies done at different centers using urine sediment analysis can be compared to one another.
Previously, investigators have assessed the diagnostic and predictive value of urine sediment analysis [8, 9]. In a study of 51 patients with acute renal failure, Marcussen et al. [8] found that patients with ATN had a higher total number of casts than acute renal failure patients without ATN, and the 12 patients that required dialysis had a higher number of different types of casts. The authors demonstrated that cytodiagnostic urinalysis may be valuable in establishing a diagnosis and predicting the severity of acute renal failure [8].
Schentag et al. [9] conducted a study which showed that the number of urinary casts were able to identify early aminoglycoside nephrotoxicity, which endorses the notion that the quantification of urinary casts has diagnostic utility. In this study, a urinary cast count was performed over an aggregated 5-day period utilizing a modification of the Addis method [9]. In contrast to our study, the study was conducted in patients undergoing treatment with aminoglycosides and did not assess their renal recovery or need for renal replacement therapy. Further, current urinary sediment analysis differs significantly from the methods used by the investigators in this study and would not be as easily reproducible compared to our methodology.
There are several limitations to our study. First, we did not rigorously control for urine osmolarity and pH, which can both affect the formation and stability of urinary casts. However, our goal in this first portion of this study was not to assess the diagnostic accuracy of urinary casts, as we only sought to determine if different observers could arrive at the same grade for a given slide. These effects may have led to a decrease in the observed sensitivity of our analysis. In addition, we did not assess the samples in each patient at the same time of the day, which could have also affected the urine sediment. Future studies will need to control for urine pH and osmolarity. A second limitation is the possibility that the slides may have deteriorated between viewings by graders. However, we took great pains to make sure that there was minimum deterioration in slide quality between assessments by standardizing the processing of our samples and maintaining viewing times to within short intervals (<30 min). Third, the number of reviewers and the variation in the reviewer's training was relatively limited. Ideally, we would have involved more reviewers. In addition, all 3 reviewers trained in the same program. Further validation of the ATN CSI will need to include a greater number of reviewers with more heterogeneous backgrounds, who have been trained at different centers to decrease training bias. Fourth, urinary electrolyte measurements were not available for all patients. Knowledge of the urinary electrolyte concentrations would have been desirable in order to place the urinary sediment findings into a broader context. Fifth, given that this is a pilot study, the sample size is quite modest, and will need to be validated in larger cohorts with more diverse causes of AKI.
In conclusion, the AKI CSI that we propose is a novel, simple, and reliable scoring system to grade the degree of urinary casts present on urine microscopy. A standardized AKI CSI has the potential to incorporate urinary cast analysis into the advancing field of AKI diagnostics. Our preliminary data endorse the notion that the AKI CSI may be useful in predicting renal outcomes.
Acknowledgement
This paper was supported by a Satellite Research: Norman S. Coplon Extramural Research Grant and by the National Institutes of Health, NIDDK, RO1-DK080234-01. We would like to thank Arnold M. Schwartz, MD, for his assistance in preparing the manuscript.
References
- 1.Nash K, Hafeez A, Hou S. Hospital-acquired renal insufficiency. Am J Kidney Dis. 2002;39:930–936. doi: 10.1053/ajkd.2002.32766. [DOI] [PubMed] [Google Scholar]
- 2.Liano F, Pascual J. Epidemiology of acute renal failure: a prospective, multicenter, community-based study. Madrid Acute Renal Failure Study Group. Kidney Int. 1996;50:811–818. doi: 10.1038/ki.1996.380. [DOI] [PubMed] [Google Scholar]
- 3.Mehta RL, Pascual MT, Soroko S, Savage BR, Himmelfarb J, Ikizler TA, Paganini EP, Chertow GM. Spectrum of acute renal failure in the intensive care unit: the PICARD experience. Kidney Int. 2004;66:1613–1621. doi: 10.1111/j.1523-1755.2004.00927.x. [DOI] [PubMed] [Google Scholar]
- 4.Geyer SJ. Urinalysis and urinary sediment in patients with renal disease. Clin Lab Med. 1993;13:13–20. [PubMed] [Google Scholar]
- 5.Rabb H. Evaluation of urinary markers in acute renal failure. Curr Opin Nephrol Hypertens. 1998;7:681–685. doi: 10.1097/00041552-199811000-00010. [DOI] [PubMed] [Google Scholar]
- 6.Szwed JJ. Urinalysis and clinical renal disease. Am J Med Technol. 1980;46:720–725. [PubMed] [Google Scholar]
- 7.Tsai JJ, Yeun JY, Kumar VA, Don BR. Comparison and interpretation of urinalysis performed by a nephrologist versus a hospital-based clinical laboratory. Am J Kidney Dis. 2005;46:820–829. doi: 10.1053/j.ajkd.2005.07.039. [DOI] [PubMed] [Google Scholar]
- 8.Marcussen N, Schumann J, Campbell P, Kjellstrand C. Cytodiagnostic urinalysis is very useful in the differential diagnosis of acute renal failure and can predict the severity. Ren Fail. 1995;17:721–729. doi: 10.3109/08860229509037640. [DOI] [PubMed] [Google Scholar]
- 9.Schentag JJ, Gengo FM, Plaut ME, Danner D, Mangione A, Jusko WJ. Urinary casts as an indicator of renal tubular damage in patients receiving aminoglycosides. Antimicrob Agents Chemother. 1979;16:468–474. doi: 10.1128/aac.16.4.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou H, Pisitkun T, Aponte A, Yuen PS, Hoffert JD, Yasuda H, Hu X, Chawla L, Shen RF, Knepper MA, Star RA. Exosomal Fetuin-A identified by proteomics: a novel urinary biomarker for detecting acute kidney injury. Kidney Int. 2006;70:1847–1857. doi: 10.1038/sj.ki.5001874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vaidya VS, Ramirez V, Ichimura T, Bobadilla NA, Bonventre JV. Urinary kidney injury molecule-1: a sensitive quantitative biomarker for early detection of kidney tubular injury. Am J Physiol Renal Physiol. 2006;290:F517–F529. doi: 10.1152/ajprenal.00291.2005. [DOI] [PubMed] [Google Scholar]
- 12.Parikh CR, Mishra J, Thiessen-Philbrook H, Dursun B, Ma Q, Kelly C, Dent C, Devarajan P, Edelstein CL. Urinary IL-18 is an early predictive biomarker of acute kidney injury after cardiac surgery. Kidney Int. 2006;70:199–203. doi: 10.1038/sj.ki.5001527. [DOI] [PubMed] [Google Scholar]
- 13.Mishra J, Dent C, Tarabishi R, Mitsnefes MM, Ma Q, Kelly C, Ruff SM, Zahedi K, Shao M, Bean J, Mori K, Barasch J, Devarajan P. Neutrophil gelatinase-associated lipocalin (NGAL) as a biomarker for acute renal injury after cardiac surgery. Lancet. 2005;365:1231–1238. doi: 10.1016/S0140-6736(05)74811-X. [DOI] [PubMed] [Google Scholar]
- 14.Henry JB, Dayey FR, Herman CJ, McPherson RA, Pincus MR, Threatte GA, Yasuda H. Clinical Diagnosis and Management by Laboratory Methods. ed 20. Philadelphia: Saunders; 2001. [Google Scholar]
- 15.Addis T. The number of formed elements in the urinary sediment of normal individuals. J Clin Invest. 1926;2:409–415. doi: 10.1172/JCI100055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bland JM, Altman DJ. Regression analysis. Lancet. 1986;i:908–909. doi: 10.1016/s0140-6736(86)91008-1. [DOI] [PubMed] [Google Scholar]
- 17.Filippi M, Horsfield MA, Rovaris M, Yousry T, Rocca MA, Baratti C, Bressi S, Comi G. Intraobserver and interobserver variability in schemes for estimating volume of brain lesions on MR images in multiple sclerosis. AJNR Am J Neuroradiol. 1998;19:239–244. [PMC free article] [PubMed] [Google Scholar]
- 18.Guyatt GH, Devereaux PJ. A review of heart failure treatment. Mt Sinai J Med. 2004;71:47–54. [PubMed] [Google Scholar]
- 19.Liangos O, Perianayagam MC, Vaidya VS, Han WK, Wald R, Tighiouart H, MacKinnon RW, Li L, Balakrishnan VS, Pereira BJ, Bonventre JV, Jaber BL. Urinary N-acetyl-beta-(D)-glucosaminidase activity and kidney injury molecule-1 level are associated with adverse outcomes in acute renal failure. J Am Soc Nephrol. 2007;18:904–912. doi: 10.1681/ASN.2006030221. [DOI] [PubMed] [Google Scholar]