Abstract
A key feature of the immune system is the capacity to monitor and control infections from non-self pathogens while maintaining tolerance to self-antigens. Primary Immunodeficiencies (PID) are characterized by an increased susceptibility to infections, often associated with aberrant inflammatory responses and a concomitant high prevalence of autoimmunity. Autoimmunity in PID raises a conundrum: How can an immune system fail to respond to non-self pathogens while reacting vigorously to self-antigens? Recent advances from studies of PID patients and related animal models have revealed the critical role of Aire-induced expression of self-antigens for deletion of autoreactive T cells in the thymus (central tolerance). Moreover, lessons from PID have provided unequivocal evidence for the essential role of regulatory T cells in suppressing autoreactive T cells in the periphery. Finally, findings from PID have broadened our understanding of how homeostatic proliferation and increased load or decreased clearance of apoptotic cells and non-self pathogens can lead to breakdown of peripheral tolerance.
INTRODUCTION
The immune system has evolved to efficiently clear non-self pathogens and to prevent attack on self-antigens. The establishment and maintenance of self-tolerance is an intrinsic requirement of adaptive immunity. Central tolerance induces deletion of self-reactive T cells during development in the thymus. Peripheral tolerance ensures that self-reactive T cells that escape central tolerance checkpoints remain innocuous in peripheral organs. Breakdown of either central or peripheral tolerance can lead to autoimmunity. Primary immunodeficiencies (PID) are genetic disorders in which part of the host’s immune system is lacking or dysfunctional. Careful analysis of the clinical features associated with various forms of PID has shown that these disorders are often characterized by aberrant inflammatory responses and autoimmunity [1]. Recent findings in several monogenic human immune disorders where animal models exist have provided evidence for how the reduced capacity of the immune system can provoke breakdown of central and peripheral tolerance and consequently the development of autoimmune disease. In this review we will focus on recent studies of five well characterized PID (APECED, OS, IPEX, WAS, and ALPS, Table 1) that have directly contributed to our understanding of the role of central and peripheral T cell tolerance in preventing autoimmunity. PID that are caused primarily by antibody deficiencies and frequently associated with autoimmunity, including CVID and XLA, is not covered in this review since a role for alterations in T cell tolerance has not yet been clearly defined [1].
Table 1.
Autoimmunity in Selected T-cell associated Primary Immunodeficiences
| Autoimmunity | APECED | OS | IPEX | WAS | ALPS | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mutated Gene | Aire | Rag1/2, Artemis Ligase4 | Foxp3 | WASP | Fas, FasL Caspase 8, 10 NRAS | |||||
| Autoimmune manifestations | Hypoparathyroidism, Addison disease, Primary ovarian failure, IDDM, Hepatitis [2] | Erythrodermia, Enteropathy, Multiple organs lymphoid infiltration [6] | Enteropathy, IDDM, Eczema, Autoimmune Polyendocrinopathy [15] | AHA, Vasculitis, Arthritis, Renal disease, IBD [23] | AHA, Cytopenias [43,44] | |||||
| Frequency of autoimmunity | almost 100% | 100% | 100% | 40–72% | more than 80% | |||||
| Patients/Mouse Models | Patients | Mice | Patients | Mice | Patients | Mice | Patients | Mice | Patients | Mice |
| Autoreactive T cells | ↑ [2] | ↑ [3,4] | ↑ [9] | ↑ [7,8] | ↑ [14,15] | ↑ [13] | ↑ [23] | ↑ [26] | ↑ [43,44] | ↑ [43,44] |
| Autoantibodies | ↑ [15] | ↑ [3,4] | na | na | ↑ [15] | ↑ [15] | ↑ [24] | ↑ [28] | ↑ [43,44] | ↑ [43,49] |
| Oligoclonal T repertoire | yes Tregs [22] | skewed [4] | yes [6,42] | yes [7,8] | na | na | yes [41] | na | na | na |
| Expression of Aire | ↓ [2] | ↓ [3,4] | ↓ [9] | ↓ [8] | na | na | na | na | na | na |
| Treg cell number | normal [22] | normal [31] | na | ↓ [8] | ↓ to normal [14,15] | ↓ [13] | normal [27,30] | ↓ to normal [28,29] | na | na |
| Treg cell function | ↓ [22] | normal [31] | na | na | ↓ [14,15] | ↓ [13] | ↓ [27,30] | ↓ [27–29] | na | na |
| Lymphopenia | na | na | yes [6,42] | yes [7,8] | na | na | yes [36] | yes [25,32] | na | na |
| Homeostatic Proliferation | na | normal [4] | (↑) [42] | ↑ [7] | na | na | (↑) [41] | na | na | na |
| Apoptosis | na | na | na | na | na | na | ↑ [55,56] | na | na | ↓ [43,44] |
| Apoptotic cell uptake | na | na | na | na | na | na | na | ↓ [48] | na | ↓ [47] |
Abbreviations: AHA, autoimmune haemolytic anaemia; Aire, autoimmune response; ALPS, autoimmune lymphoproliferative syndrome; APECED, autoimmune polyendrocrinopathy candidiasis ectodermal dystrophy; FasL, Fas ligand; Foxp3, forkhead box P3; IDDM, insulin-dependent diabetes mellitus; IBD, inflammatory bowel disease; IPEX, immunodysregulation polyendocrinopathy enteropathy X-linked; na, not addressed; OS, Omenn syndrome; Rag, Recombination activating protein; Treg, regulatory T; WAS, Wiskott-Aldrich syndrome, WASP, WAS protein
CENTRAL TOLERANCE
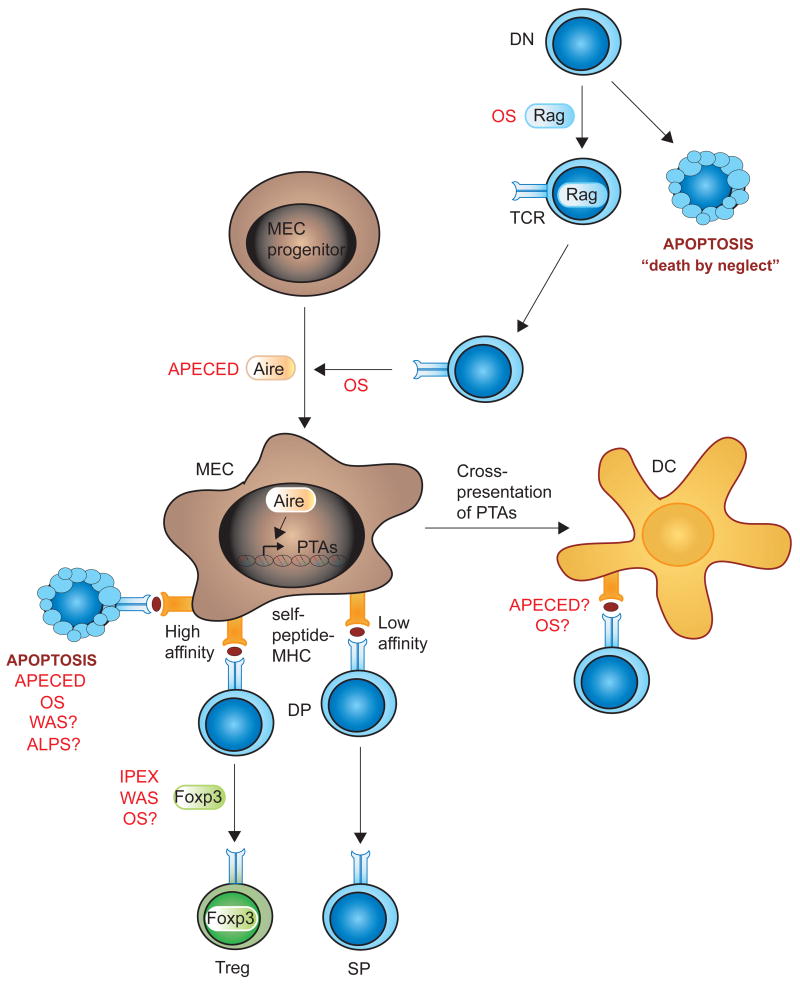
Central tolerance ensures that the vast majority of autoreactive T cells are deleted in the thymus. During development in the thymus, immature CD4+CD8+ double-positive (DP) thymocytes that have successfully assembled a T cell receptor (TCR) are selected based on the TCR affinity for self-peptides presented on major histocompatibility class molecules (MHC) by medullary epithelial cells (MECs) and dendritic cells (DCs). Thymocytes expressing TCRs that fail to recognize any self-peptide-MHC die from “neglect”, whereas strong recognition of self-peptide-MHC leads to thymocyte death or lineage deviation, removing self-reactive cells from the T cell repertoire (negative selection). Weak recognition of self-peptide-MHC complexes by the TCR and co-receptors results in the development of mature CD4+ or CD8+ single-positive (SP) T cells that egress to the periphery. Figure 1 illustrates critical steps for central tolerance in the thymus. The importance of TCR affinity for self-peptide-MHC during thymocyte development is well recognized although the mechanism(s) responsible for the expression of a wide array of peripheral-tissue antigens (PTA) in the thymus remain an enigma. Recent literature has defined the critical role of the autoimmune regulator (Aire) for expression of self-antigens in the thymus and studies of Aire-deficient patients and animals have revealed the importance of negative selection for establishment of central tolerance [2].
Figure 1. Central tolerance and PIDs.
Developing thymocytes undergo specific developmental checkpoints. DN cells upregulate the Rag proteins to initiate V(D)J recombination of the TCRbeta gene. DN cells that fail to express a pre-TCR that recognizes peptide-MHC molecules in the thymic cortex die through apoptosis. DN cells that express a functional TCR are selected and upregulate CD4 and CD8 thereby becoming DP. MEC progenitor cells that upregulate Aire become mature MECs in the thymic medulla and express a wide array of PTAs. DP cells expressing TCRs with low affinity for self-peptide-MHC molecules develop into naïve CD4 or CD8 SP T cells and egress from the thymus to the periphery. DP cells that express TCRs with high affinity for self-peptide-MHC molecules are negatively selected and die by apoptosis or deviate to the Treg (and other) lineage(s). MECs can cross-present PTAs to thymic DCs. PID discussed in this review are indicated in red where they are known to interfere with central tolerance mechanisms, and followed by a question mark when a possible interference is suggested.
Abbreviations: Aire, autoimmune response; ALPS, autoimmune lymphoproliferative syndrome; APECED, autoimmune polyendrocrinopathy candidiasis ectodermal dystrophy; DC, dendritic cell; DN, (CD4 and CD8) double-negative cell; DP, (CD4 and CD8) double-positive; Foxp3, forkhead box P3; IPEX, immunodysregulation polyendocrinopathy enteropathy X-linked; MEC, medullary epithelial cell; MHC, major histocompatibility molecule; OS, Omenn syndrome; PTA, peripheral tissue antigen; Rag, recombination activating protein; SP, (CD4 or CD8) single-positive; TCR, T cell receptor; Treg, regulatory T cell; WAS, Wiskott-Aldrich syndrome
APECED: Expression of Aire
Patients with mutations in the Aire gene suffer from the autoimmune disease APECED (autoimmune, polyendrocrinopathy, candidiasis, ectodermal dystrophy) that is typically characterized by a triad of chronic mucocutaneous candidiasis, hypoparathyroidism, and adrenal insufficiency [2]. Expression of Aire is highly enriched in MECs that have the remarkable ability to ‘promiscuously’ express a wide array of PTA, including insulin, thyroglobulin, myelin basic protein, retinal antigen [2]. The generation of Aire-deficient mice that suffer from multiple organ autoimmunity and high titers of autoantibodies revealed that Aire activity in MECs is critical for the expression of PTA [3,4]. In view of the critical role of Aire for expression of a wide array of PTA in mTECs it is not intuitively self-evident that the autoimmune disease in APECED patients and Aire-deficient mice would be restricted to certain organs, in particular endocrine organs. The expression of other known PTA, such as those encoding C-reactive protein and glutamic-acid decarboxylase of 67kDa (GAD67), is Aire-independent and suggests that other ‘Aire-like’ factors probably exist and remain to be identified [3,5].
OS: Autoreactive T cells due to decreased expression of Aire?
In the developing thymus, cross-talk between T cells and epithelial cells is crucial for induction of normal thymic compartments [6]. The molecular mechanism for development of Omenn syndrome (OS) has provided new insight into the critical role of cross-talk between T cells and MECs. OS is characterized by the presence of activated T cells that infiltrate target organs such as the skin and gut [6]. The most common mutations in OS leads to hypomorphic (decreased) activity of the recombination activated (Rag) 1 and 2 proteins [6], that are essential for V(D)J recombination leading to expression of the B cell and T cell receptors. Only thymocytes that successfully rearrange and express TCRbeta are positively selected at the double-negative 3 stage of thymic development. Mutations in other proteins involved in V(D)J recombination and thymocyte development, including Artemis and Ligase4, causes OS [6]. Two animal models for OS were recently described. The memory mutant (MM) mouse has a spontaneous mutation in Rag1 (R972Q) and this mutation was also found in an OS patient [7]. The rag2R229Q/R229Q animal harbors the hypomorphic R229Q mutation in Rag2 identified in a subset of OS patients [8]. Both murine models have an atrophic thymus in which thymocyte development is predominantly arrested at the double-negative 3 stage, due to the decreased activity of either Rag1 or Rag2 proteins. TCRbeta chains are virtually undetectable, implying severe deficiency in pre-TCR expression and signaling. The few single-positive T cells that escape infiltrate target organs and express an oligoclonal, autoimmune TCR repertoire. It remains elusive why hypomorphic Rag activity induces autoreactive T cells. However, one plausible explanation comes from recent data from the rag2R229Q/R229Q mice that is associated with reduced thymic expression of Aire [8]. Similarly, Aire expression is markedly reduced in thymic medulla of OS patients [9].
It is now well established that MECs are critical for Aire-regulated expression of PTA, though the role of MECs for antigen presentation to developing thymocytes is less explored. In fact, dendritic cells (DC) can capture PTA from MECs and delete autoreactive T cells via cross-presentation [10]. Considering the superior role of DCs in antigen presentation due to high expression of co-stimulatory molecules, the function of DCs in negative selection of thymocytes needs to be examined in more detail. The mouse models for APECED and OS [3,4,7,8] will be useful to determine which antigenic peptides are presented by MECs and DCs in the thymus in the absence of Aire and how altered peptide presentation may influence the negative selection of autoreactive T cells.
PERIPHERAL TOLERANCE
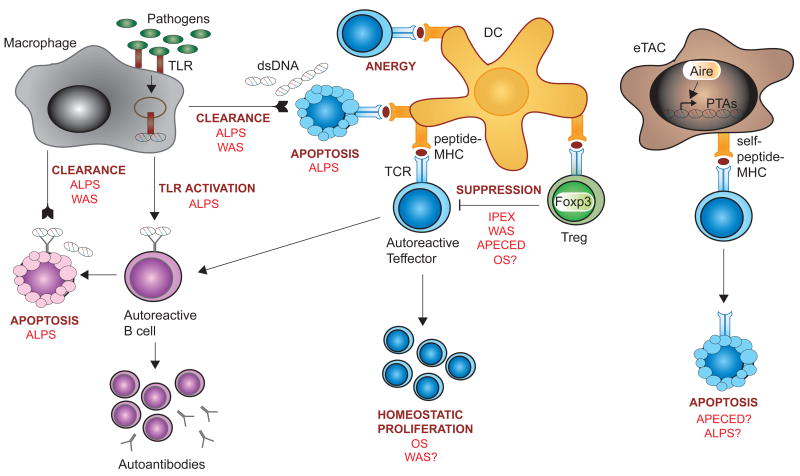
A dominant role for central tolerance predicts that the majority of autoreactive lymphocytes are deleted. However, autoreactive B and T cells are present in healthy individuals in the periphery but remain quiescent due to peripheral tolerance. Breakdown of peripheral tolerance can be caused by several mechanisms; reduced fitness of regulatory T cells (Tregs), homeostatic proliferation of autoreactive lymphocytes, altered apoptosis of lymphocytes, and decreased clearance and cross-presentation of self antigens. Figure 2 illustrates critical mechanisms for peripheral tolerance.
Figure 2. Peripheral tolerance and PIDs.
In peripheral lymphoid organs, several mechanisms exist to maintain autoreactive T cells quiescent. DCs presenting peptide-MHC molecules regulate the activity of autoreactive Teffector cells by inducing anergy or apoptosis. Treg cells serve a key function in peripheral tolerance by suppressing harmful activation of Teffector cells and, consequently, activation of autoreactive B cells. Homeostatic proliferation ensures an appropriate pool of peripheral T cells that express a diverse TCR repertoire, but can lead to expansion of autoreactive T cells in immunodeficient hosts. Macrophages clear apoptotic cell debris including dsDNA and take up non-self pathogens. The promiscuous recognition of bacterial antigens and dsDNA by TLRs may lead to TLR-dependent activation of autoreactive B cells in hosts that have increased load of bacterial antigen and/or dsDNA. eTACs are peripheral stromal cells that present a wide array of Aire-induced PTAs that largely differ from those presented by MECs in the thymus. eTACs participate in deletion of autoreactive T cells in the periphery. PID discussed in this review are indicated in red where they are known to interfere with peripheral tolerance mechanisms, and followed by a question mark when a possible interference is suggested.
Abbreviations: Aire, autoimmune response; ALPS, autoimmune lymphoproliferative syndrome; APECED, autoimmune polyendrocrinopathy candidiasis ectodermal dystrophy; DC, dendritic cell; eTAC, extra-thymic Aire-expressing cell; Foxp3, forkhead box P3; dsDNA, double-stranded DNA; IPEX, immunodysregulation polyendocrinopathy enteropathy X-linked; MHC, major histocompatibility molecule; MEC, medullary epithelial cell; OS, Omenn syndrome; PTA, peripheral tissue antigen; Rag, recombination activating protein; TCR, T cell receptor; TLR, Toll-like receptor; Treg, regulatory T cell; WAS, Wiskott-Aldrich syndrome
IPEX, OS, APECED, and WAS: Reduced fitness of regulatory T cells
Recent research has revealed the essential role of Tregs in maintenance of peripheral tolerance. Tregs regulate effector T cells by suppressing their activity [11]. The transcription factor forkhead box P3 (Foxp3) is a master regulator of both Treg cell development and function [11]. IPEX (immunodysregulation, polyendocrinopathy, enteropathy, X-linked syndrome) patients and scurfy mice lack expression of the Foxp3 gene and have absent or severely reduced number of Tregs and consequently develop multi-organ autoimmunity with hyperactive CD4+ T cells, overproduction of inflammatory cytokines and autoantibodies [12–15]. Ectopic expression of Foxp3 is sufficient to impart suppressive function to conventional (CD4+CD25−) T cells [11]. Recent data suggest that Foxp3 may serve a more complex role in delineating Treg cell generation [16,17] by regulating gene expression through association with other transcription factors such as NFAT, NF-kappaB and Runx1 [18]. The TCR repertoire of Tregs is highly diverse having high affinity both for self and non-self antigens and includes TCRs dominantly expressed by naïve T cells [19]. However, many TCRs expressed by Tregs are unique and not expressed by conventional CD4+ T cells [19,20]. Both TCR specificity and the cytokine milieu (primarily IL-2) have been proposed to divert thymocytes into Treg cell lineage commitment although the specific mechanism for Treg cell development remains largely unknown. The CD25hiCD4+Foxp3− thymic subset contains Treg cell precursors and TCR-dependent signaling results in the expression of proximal IL-2 signaling components facilitating cytokine-mediated induction of Foxp3 [21]. The discovery of Foxp3 loss-of-function mutations in IPEX patients and scurfy mice have provided unequivocal evidence for the essential role of Tregs in peripheral immune tolerance and highlight the fact that autoreactive T cells indeed escape central tolerance checkpoints in a normal host [11].
Reduced number of Treg cells may contribute to autoimmunity in OS since the hypomorphic rag2R229Q/R229Q mice, in addition to having oligoclonal peripheral T cells, also have dramatically reduced frequency of Tregs in the thymus and spleen [8]. It is unknown how hypomorphic mutations in the Rag proteins influence the development of Treg cells. It has been suggested that reduced Rag protein activity, leading to an oligoclonal TCR repertoire, restricts the unique TCR repertoire of Tregs thereby inhibiting Treg cell development [6,8]. Decreased suppressive activity of Tregs has been implicated in APECED patients and, interestingly, Tregs from APECED patients express a less diverse TCR repertoire [22].
Recent data from Wiskott-Aldrich syndrome (WAS) patients and WAS protein (WASP)-deficient mice provide compelling evidence that reduced number and decreased suppressive capacity of Tregs contributes to autoimmune development in WAS. Patients with WAS exhibit high prevalence of autoimmune disease with up to 70% of patients suffering from at least one autoimmune disorder (hemolytic anemia, neutropenia, arthritis, skin vasculitis, glomerulonephritis, or inflammatory bowel disease) [23]. Autoimmune manifestations lead to a poorer clinical prognosis [23] and even patients with otherwise mild WAS disease (thrombocytopenia only) can develop life-threatening autoimmune disease with high autoantibody titers [24]. Similar to the enteropathy seen in Foxp3-deficient mice [12,13], the majority of WASP-deficient mice develop colitis [25,26]. WASP is required for Treg cell-dependent suppression in vitro and in vivo [27–30]. IL-2 produced by activated CD4+CD25− effector T cells is critical for Treg cell activation in periphery [31]. Many studies incriminate decreased IL-2 in WASP deficiency [25,27,29,32], however, ectopic IL-2 can not rescue peripheral expansion of WASP-deficient Tregs in mice [28], suggesting that other signaling molecules and mechanisms may contribute to Treg cell dysfunction in WASP deficiency. Tregs form long-lasting conjugates (synapses) with antigen-primed DCs in vivo and compete with effector T cells for DC interaction [33]. WASP is essential for optimal signaling through the TCR and stabilizes the immune synapse between T cells and antigen-presenting cells [34]. A recent report using in vivo imaging of DC-T cell interactions in lymph nodes show that WASP-deficient DCs have reduced ability to form and stabilize conjugates with naive CD8+ T cells [35]. The role of WASP during conjugate formation of Tregs and DC in vivo remains to be defined.
OS, WAS, and ALPS: Homeostatic proliferation and apoptosis of lymphocytes
In a normal host, the pool of peripheral lymphocytes is maintained in part by homeostatic mechanisms that ensure an appropriate balance of T cells through new generation, peripheral expansion (homeostatic proliferation), and turnover by programmed cell death (apoptosis). Lymphopenia is common in PID associated with autoimmunity, including OS and WAS [6,36]. In lymphocyte-depleted animals, interaction of peripheral T cells with self-peptide-MHC molecules on antigen-presenting cells will induce homeostatic proliferation [37] and can induce expansion of autoreactive T cells that express an autoimmune TCR repertoire [38,39]. T cells that express TCRs with higher affinity for self-peptide-MHC molecules will be favored in competition with T cells expressing lower affinity TCRs and this limits the diversity of the T cell repertoire induced by homeostatic proliferation [40]. Increased homeostatic proliferation of T cells has been suggested as a mechanism for expansion of severely oligoclonal T cell repertoires in OS and WAS patients and in the memory mutant mouse model for OS [7,41,42].
A striking example of the essential role for apoptosis in peripheral homeostasis comes from ALPS (autoimmune lymphoproliferative syndrome) patients. Due to genetic mutations in major apoptotic inducers, including Fas, and more rarely FasL, caspase 8 and caspase 10, proliferating and activated lymphocytes fail to undergo programmed cell death [43,44]. ALPS patients suffer from autoantibody-mediated cytopenia, lymphadenopathy, splenomegaly, and are at high risk for the development of lymphoproliferative disease. MRL.Faslpr/lpr mice that have reduced expression of the Fas gene spontaneously develop systemic autoimmune disease resembling human systemic lupus erythematosus (SLE). Studies of ALPS patients and MRL.Faslpr/lpr mice have provided evidence for the essential role of Fas-mediated cell death in normal homeostasis of lymphocytes. A characteristic feature of both ALPS patients and MRL.Faslpr/lpr mice is a high percentage of TCRalphabeta+CD4−CD8− double-negative T cells in blood and peripheral organs [43,44]. TCRalphabeta+CD4−CD8− T cells express a TCR repertoire with high affinity for self-peptide MHC and have been suggested to represent T cells destined for Fas-mediated apoptosis [43,44]. Notably, ALPS patients have reduced rather than accumulated number of lymphocytes after acute infection, suggesting that ALPS lymphocytes can undergo apoptosis in response to cytokine (IL-2) withdrawal [43,44]. Moreover, a recent report describes that defects in IL-2 withdrawal-induced apoptosis can cause ALPS in a patient that expresses an activating mutation in NRAS [45]. Together, these studies imply that IL-2 exhaustion caused by the lymphoproliferative disease in ALPS leads to altered lymphocyte homeostasis and increased load of dying cells [43–46].
ALPS and WAS: Decreased clearance and cross-presentation of self antigens
Pathogen challenge initiates proliferation of antigen-specific lymphocytes that leads to clearance of the pathogens followed by lymphocyte apoptosis to restore normal immune cell homeostasis. Mammalian chromosomal DNA released from apoptotic cells constitutes a major autoantigen and apoptotic cell debris needs to be cleared from the inflamed tissue. An increased load of dying and apoptotic cells in ALPS patients and MRL.Faslpr/lpr mice, due to the lymphoproliferative disease, causes insufficient clearance by macrophages [47]. Similarly, autoimmunity in WAS may be caused by an increased load of apoptotic cells due to impaired uptake of apoptotic cells by WASP-deficient macrophages [48].
A hallmark of PID is the failure to clear major bacterial infections leading to an increased load of bacterial antigens. Toll-like receptor (TLR) 3 and 9 recognize double-stranded RNA and CpG DNA from pathogens. However, TLR3 and TLR9 can also recognize and cross-present mammalian chromosomal DNA, released by dying cells [49]. Evidence for the involvement of TLR9 in autoantibody production was provided upon crossing TLR9-deficient mice to the MRL.Faslpr/lpr background. The high serum titers of anti-double-stranded DNA and anti-chromatin antibodies normally present in MRL.Faslpr/lpr animals were completely abolished in the absence of TLR9 [49], suggesting that TLR9-dependent co-stimulation triggers production of anti-DNA antibodies. TLR activation triggers survival and pro-inflammatory pathways [50] and may in immuno-compromised hosts favor expansion of autoreactive cells.
CONCLUSIONS
The combined studies of PID patients and related animal models have increased our understanding of how the immune system fails to respond to non-self pathogens while still reacting vigorously to self-antigens. While much progress has been made in recent years, many important questions remain to be addressed.
What is the hierarchy of events mediating autoimmunity, i.e. what is the relative role of specific effector cells such as T, B or myeloid cells? To address the contribution of a specific cell type or developmental stage in multifactorial PIDs, including OS, WAS, and ALPS, conditionally-targeted animal models in which a PID-associated gene can be deleted in a spatial or temporal manner would be desirable. In this regard, it was recently described that conditional deletion of Foxp3 in adult mice leads to more severe autoimmunity as compared to when Foxp3 is deleted at the neonatal stage, highlighting the importance for continuous suppression of autoreactive T cells by Tregs [51].
Is Aire the master regulator for expression of self-peptides? Recently, two studies reveal that extra-thymic expression of Aire in stromal-type epithelial cells in lymph nodes and spleen leads to expression of PTAs [52,53]. Importantly, these PTAs differ from those expressed by MECs in the thymus suggesting that Aire-induced peripheral PTAs serve as a “safety net” to delete autoreactive T cells that have escaped negative selection in the thymus [53]. The role of peripheral-expressed Aire for development of autoimmune T cells and for Treg cell activity in APECED and OS needs further assessment.
How can the clinical spectrum of autoimmunity be explained, i.e. why do specific genetic defects lead to a defined organ-specific autoimmunity, such as the autoimmunity typically affecting endocrine organs seen in APECED patients? Since expression of some PTAs are independent of Aire other factors or tolerogenic mechanisms may exist that remain to be identified [3,5].
What is the role of extrinsic factors such as pathogen-induced TLR signaling? Considering that PID are associated with severe and chronic infections and that homeostatic proliferation of naïve T cells in immunodeficient hosts can be abolished if the animals are kept under germfree conditions [54], implies that pathogen-provoked signaling may induce homeostatic proliferation of autoreactive T cells in immunocompromised hosts.
Defining the answers to these questions will be important for development of more specific therapeutic intervention, including gene therapy.
Acknowledgments
We appreciate the critical reading of this manuscript by Drs. Luigi Notarangelo, Charlotte Cunningham-Rundles, Deanna Nguyen, and Agnès Gardet. This work was supported by a postdoctoral fellowship from the Swedish Society for Medical Research to L.S.W., and by grants (DFG SFB738) to C.K. and (NIH AI050950 and HL59561) to S.B.S.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES AND RECOMMENDED READING
Papers of particular interest published within the period of review have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Carneiro-Sampaio M, Coutinho A. Tolerance and autoimmunity: lessons at the bedside of primary immunodeficiencies. Adv Immunol. 2007;95:51–82. doi: 10.1016/S0065-2776(07)95002-6. [DOI] [PubMed] [Google Scholar]
- 2.Mathis D, Benoist C. A decade of AIRE. Nat Rev Immunol. 2007;7:645–650. doi: 10.1038/nri2136. [DOI] [PubMed] [Google Scholar]
- 3•.Anderson MS, Venanzi ES, Klein L, Chen Z, Berzins SP, Turley SJ, von Boehmer H, Bronson R, Dierich A, Benoist C, et al. Projection of an immunological self shadow within the thymus by the aire protein. Science. 2002;298:1395–1401. doi: 10.1126/science.1075958. See comment for [4•] [DOI] [PubMed] [Google Scholar]
- 4•.Ramsey C, Winqvist O, Puhakka L, Halonen M, Moro A, Kampe O, Eskelin P, Pelto-Huikko M, Peltonen L. Aire deficient mice develop multiple features of APECED phenotype and show altered immune response. Hum Mol Genet. 2002;11:397–409. doi: 10.1093/hmg/11.4.397. This paper together with [3•] describes the first mouse models for APECED and defines that Aire is required for expression of self-antigens in the thymus. [DOI] [PubMed] [Google Scholar]
- 5•.Derbinski J, Gabler J, Brors B, Tierling S, Jonnakuty S, Hergenhahn M, Peltonen L, Walter J, Kyewski B. Promiscuous gene expression in thymic epithelial cells is regulated at multiple levels. J Exp Med. 2005;202:33–45. doi: 10.1084/jem.20050471. This paper describes that the expression of specific self-antigens occurs in the absence of Aire. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Villa A, Marrella V, Rucci F, Notarangelo LD. Genetically determined lymphopenia and autoimmune manifestations. Curr Opin Immunol. 2008;20:318–324. doi: 10.1016/j.coi.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 7•.Khiong K, Murakami M, Kitabayashi C, Ueda N, Sawa S, Sakamoto A, Kotzin BL, Rozzo SJ, Ishihara K, Verella-Garcia M, et al. Homeostatically proliferating CD4 T cells are involved in the pathogenesis of an Omenn syndrome murine model. J Clin Invest. 2007;117:1270–1281. doi: 10.1172/JCI30513. See comment for [8•] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8•.Marrella V, Poliani PL, Casati A, Rucci F, Frascoli L, Gougeon ML, Lemercier B, Bosticardo M, Ravanini M, Battaglia M, et al. A hypomorphic R229Q Rag2 mouse mutant recapitulates human Omenn syndrome. J Clin Invest. 2007;117:1260–1269. doi: 10.1172/JCI30928. This paper together with [7•] describes the first mouse models for OS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9••.Cavadini P, Vermi W, Facchetti F, Fontana S, Nagafuchi S, Mazzolari E, Sediva A, Marrella V, Villa A, Fischer A, et al. AIRE deficiency in thymus of 2 patients with Omenn syndrome. J Clin Invest. 2005;115:728–732. doi: 10.1172/JCI23087. This paper describes reduced Aire expression in the thymus of two patients with OS, thereby providing a mechanism for the presence of peripheral autoreactive T cells in OS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gallegos AM, Bevan MJ. Central tolerance to tissue-specific antigens mediated by direct and indirect antigen presentation. J Exp Med. 2004;200:1039–1049. doi: 10.1084/jem.20041457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133:775–787. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 12•.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. See comment for [13•] [PubMed] [Google Scholar]
- 13•.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–336. doi: 10.1038/ni904. This paper together with [12•] defines the key role of Foxp3 for generation of Treg cells using Foxp3-deficient mice. [DOI] [PubMed] [Google Scholar]
- 14.Bacchetta R, Passerini L, Gambineri E, Dai M, Allan SE, Perroni L, Dagna-Bricarelli F, Sartirana C, Matthes-Martin S, Lawitschka A, et al. Defective regulatory and effector T cell functions in patients with FOXP3 mutations. J Clin Invest. 2006;116:1713–1722. doi: 10.1172/JCI25112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Torgerson TR, Ochs HD. Immune dysregulation, polyendocrinopathy, enteropathy, X-linked: forkhead box protein 3 mutations and lack of regulatory T cells. J Allergy Clin Immunol. 2007;120:744–750. doi: 10.1016/j.jaci.2007.08.044. quiz 751–742. [DOI] [PubMed] [Google Scholar]
- 16.Lin W, Haribhai D, Relland LM, Truong N, Carlson MR, Williams CB, Chatila TA. Regulatory T cell development in the absence of functional Foxp3. Nat Immunol. 2007;8:359–368. doi: 10.1038/ni1445. [DOI] [PubMed] [Google Scholar]
- 17.Hill JA, Feuerer M, Tash K, Haxhinasto S, Perez J, Melamed R, Mathis D, Benoist C. Foxp3 transcription-factor-dependent and -independent regulation of the regulatory T cell transcriptional signature. Immunity. 2007;27:786–800. doi: 10.1016/j.immuni.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 18.Hu H, Djuretic I, Sundrud MS, Rao A. Transcriptional partners in regulatory T cells: Foxp3, Runx and NFAT. Trends Immunol. 2007;28:329–332. doi: 10.1016/j.it.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 19.Pacholczyk R, Kern J, Singh N, Iwashima M, Kraj P, Ignatowicz L. Nonself-antigens are the cognate specificities of Foxp3+ regulatory T cells. Immunity. 2007;27:493–504. doi: 10.1016/j.immuni.2007.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hsieh CS, Zheng Y, Liang Y, Fontenot JD, Rudensky AY. An intersection between the self-reactive regulatory and nonregulatory T cell receptor repertoires. Nat Immunol. 2006;7:401–410. doi: 10.1038/ni1318. [DOI] [PubMed] [Google Scholar]
- 21.Lio CW, Hsieh CS. A two-step process for thymic regulatory T cell development. Immunity. 2008;28:100–111. doi: 10.1016/j.immuni.2007.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22•.Kekalainen E, Tuovinen H, Joensuu J, Gylling M, Franssila R, Pontynen N, Talvensaari K, Perheentupa J, Miettinen A, Arstila TP. A defect of regulatory T cells in patients with autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy. J Immunol. 2007;178:1208–1215. doi: 10.4049/jimmunol.178.2.1208. This paper defines decreased generation and function of Tregs in APECED patients. [DOI] [PubMed] [Google Scholar]
- 23.Dupuis-Girod S, Medioni J, Haddad E, Quartier P, Cavazzana-Calvo M, Le Deist F, de Saint Basile G, Delaunay J, Schwarz K, Casanova JL, et al. Autoimmunity in Wiskott-Aldrich syndrome: risk factors, clinical features, and outcome in a single-center cohort of 55 patients. Pediatrics. 2003;111:e622–627. doi: 10.1542/peds.111.5.e622. [DOI] [PubMed] [Google Scholar]
- 24.Imai K, Morio T, Zhu Y, Jin Y, Itoh S, Kajiwara M, Yata J, Mizutani S, Ochs HD, Nonoyama S. Clinical course of patients with WASP gene mutations. Blood. 2004;103:456–464. doi: 10.1182/blood-2003-05-1480. [DOI] [PubMed] [Google Scholar]
- 25.Snapper SB, Rosen FS, Mizoguchi E, Cohen P, Khan W, Liu CH, Hagemann TL, Kwan SP, Ferrini R, Davidson L, et al. Wiskott-Aldrich syndrome protein-deficient mice reveal a role for WASP in T but not B cell activation. Immunity. 1998;9:81–91. doi: 10.1016/s1074-7613(00)80590-7. [DOI] [PubMed] [Google Scholar]
- 26.Nguyen DD, Maillard MH, Cotta-de-Almeida V, Mizoguchi E, Klein C, Fuss I, Nagler C, Mizoguchi A, Bhan AK, Snapper SB. Lymphocyte-dependent and Th2 cytokine-associated colitis in mice deficient in Wiskott-Aldrich syndrome protein. Gastroenterology. 2007;133:1188–1197. doi: 10.1053/j.gastro.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27•.Adriani M, Aoki J, Horai R, Thornton AM, Konno A, Kirby M, Anderson SM, Siegel RM, Candotti F, Schwartzberg PL. Impaired in vitro regulatory T cell function associated with Wiskott-Aldrich syndrome. Clin Immunol. 2007;124:41–48. doi: 10.1016/j.clim.2007.02.001. See comment for [30•] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28•.Humblet-Baron S, Sather B, Anover S, Becker-Herman S, Kasprowicz DJ, Khim S, Nguyen T, Hudkins-Loya K, Alpers CE, Ziegler SF, et al. Wiskott-Aldrich syndrome protein is required for regulatory T cell homeostasis. J Clin Invest. 2007;117:407–418. doi: 10.1172/JCI29539. See comment for [30•] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29•.Maillard MH, Cotta-de-Almeida V, Takeshima F, Nguyen DD, Michetti P, Nagler C, Bhan AK, Snapper SB. The Wiskott-Aldrich syndrome protein is required for the function of CD4(+)CD25(+)Foxp3(+) regulatory T cells. J Exp Med. 2007;204:381–391. doi: 10.1084/jem.20061338. See comment for [30•] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30•.Marangoni F, Trifari S, Scaramuzza S, Panaroni C, Martino S, Notarangelo LD, Baz Z, Metin A, Cattaneo F, Villa A, et al. WASP regulates suppressor activity of human and murine CD4(+)CD25(+)FOXP3(+) natural regulatory T cells. J Exp Med. 2007;204:369–380. doi: 10.1084/jem.20061334. This paper together with [27•], [28•], and [29•] describes deficient Treg cell suppression in WAS patients and WASP-deficient mice. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fontenot JD, Rasmussen JP, Gavin MA, Rudensky AY. A function for interleukin 2 in Foxp3-expressing regulatory T cells. Nat Immunol. 2005;6:1142–1151. doi: 10.1038/ni1263. [DOI] [PubMed] [Google Scholar]
- 32.Zhang J, Shehabeldin A, da Cruz LA, Butler J, Somani AK, McGavin M, Kozieradzki I, dos Santos AO, Nagy A, Grinstein S, et al. Antigen receptor-induced activation and cytoskeletal rearrangement are impaired in Wiskott-Aldrich syndrome protein-deficient lymphocytes. J Exp Med. 1999;190:1329–1342. doi: 10.1084/jem.190.9.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tang Q, Adams JY, Tooley AJ, Bi M, Fife BT, Serra P, Santamaria P, Locksley RM, Krummel MF, Bluestone JA. Visualizing regulatory T cell control of autoimmune responses in nonobese diabetic mice. Nat Immunol. 2006;7:83–92. doi: 10.1038/ni1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sims TN, Soos TJ, Xenias HS, Dubin-Thaler B, Hofman JM, Waite JC, Cameron TO, Thomas VK, Varma R, Wiggins CH, et al. Opposing effects of PKCtheta and WASp on symmetry breaking and relocation of the immunological synapse. Cell. 2007;129:773–785. doi: 10.1016/j.cell.2007.03.037. [DOI] [PubMed] [Google Scholar]
- 35.Pulecio J, Tagliani E, Scholer A, Prete F, Fetler L, Burrone OR, Benvenuti F. Expression of Wiskott-Aldrich syndrome protein in dendritic cells regulates synapse formation and activation of naive CD8+ T cells. J Immunol. 2008;181:1135–1142. doi: 10.4049/jimmunol.181.2.1135. [DOI] [PubMed] [Google Scholar]
- 36.Park JY, Kob M, Prodeus AP, Rosen FS, Shcherbina A, Remold-O’Donnell E. Early deficit of lymphocytes in Wiskott-Aldrich syndrome: possible role of WASP in human lymphocyte maturation. Clin Exp Immunol. 2004;136:104–110. doi: 10.1111/j.1365-2249.2004.02409.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Krupica T, Jr, Fry TJ, Mackall CL. Autoimmunity during lymphopenia: a two-hit model. Clin Immunol. 2006;120:121–128. doi: 10.1016/j.clim.2006.04.569. [DOI] [PubMed] [Google Scholar]
- 38•.King C, Ilic A, Koelsch K, Sarvetnick N. Homeostatic expansion of T cells during immune insufficiency generates autoimmunity. Cell. 2004;117:265–277. doi: 10.1016/s0092-8674(04)00335-6. See comment for [39•] [DOI] [PubMed] [Google Scholar]
- 39•.Milner JD, Ward JM, Keane-Myers A, Paul WE. Lymphopenic mice reconstituted with limited repertoire T cells develop severe, multiorgan, Th2-associated inflammatory disease. Proc Natl Acad Sci U S A. 2007;104:576–581. doi: 10.1073/pnas.0610289104. This paper together with [38•] describes how homeostatic proliferation leads to expansion of autoreactive T cells in lymphopenic hosts. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ge Q, Rao VP, Cho BK, Eisen HN, Chen J. Dependence of lymphopenia-induced T cell proliferation on the abundance of peptide/MHC epitopes and strength of their interaction with T cell receptors. Proc Natl Acad Sci U S A. 2001;98:1728–1733. doi: 10.1073/pnas.98.4.1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wada T, Schurman SH, Garabedian EK, Yachie A, Candotti F. Analysis of T-cell repertoire diversity in Wiskott-Aldrich syndrome. Blood. 2005;106:3895–3897. doi: 10.1182/blood-2005-06-2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wada T, Toma T, Okamoto H, Kasahara Y, Koizumi S, Agematsu K, Kimura H, Shimada A, Hayashi Y, Kato M, et al. Oligoclonal expansion of T lymphocytes with multiple second-site mutations leads to Omenn syndrome in a patient with RAG1-deficient severe combined immunodeficiency. Blood. 2005;106:2099–2101. doi: 10.1182/blood-2005-03-0936. [DOI] [PubMed] [Google Scholar]
- 43.Worth A, Thrasher AJ, Gaspar HB. Autoimmune lymphoproliferative syndrome: molecular basis of disease and clinical phenotype. Br J Haematol. 2006;133:124–140. doi: 10.1111/j.1365-2141.2006.05993.x. [DOI] [PubMed] [Google Scholar]
- 44.Fleisher TA. The autoimmune lymphoproliferative syndrome: an experiment of nature involving lymphocyte apoptosis. Immunol Res. 2008;40:87–92. doi: 10.1007/s12026-007-8001-1. [DOI] [PubMed] [Google Scholar]
- 45.Oliveira JB, Bidere N, Niemela JE, Zheng L, Sakai K, Nix CP, Danner RL, Barb J, Munson PJ, Puck JM, et al. NRAS mutation causes a human autoimmune lymphoproliferative syndrome. Proc Natl Acad Sci U S A. 2007;104:8953–8958. doi: 10.1073/pnas.0702975104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Strasser A, Pellegrini M. T-lymphocyte death during shutdown of an immune response. Trends Immunol. 2004;25:610–615. doi: 10.1016/j.it.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 47.Potter PK, Cortes-Hernandez J, Quartier P, Botto M, Walport MJ. Lupus-prone mice have an abnormal response to thioglycolate and an impaired clearance of apoptotic cells. J Immunol. 2003;170:3223–3232. doi: 10.4049/jimmunol.170.6.3223. [DOI] [PubMed] [Google Scholar]
- 48.Leverrier Y, Lorenzi R, Blundell MP, Brickell P, Kinnon C, Ridley AJ, Thrasher AJ. Cutting edge: the Wiskott-Aldrich syndrome protein is required for efficient phagocytosis of apoptotic cells. J Immunol. 2001;166:4831–4834. doi: 10.4049/jimmunol.166.8.4831. [DOI] [PubMed] [Google Scholar]
- 49•.Leadbetter EA, Rifkin IR, Hohlbaum AM, Beaudette BC, Shlomchik MJ, Marshak-Rothstein A. Chromatin-IgG complexes activate B cells by dual engagement of IgM and Toll-like receptors. Nature. 2002;416:603–607. doi: 10.1038/416603a. This paper describes how TLR signaling contributes to development of anti-chromatin autoantibodies in autoimmune prone animals. [DOI] [PubMed] [Google Scholar]
- 50.Medzhitov R. Recognition of microorganisms and activation of the immune response. Nature. 2007;449:819–826. doi: 10.1038/nature06246. [DOI] [PubMed] [Google Scholar]
- 51.Kim JM, Rasmussen JP, Rudensky AY. Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice. Nat Immunol. 2007;8:191–197. doi: 10.1038/ni1428. [DOI] [PubMed] [Google Scholar]
- 52••.Lee JW, Epardaud M, Sun J, Becker JE, Cheng AC, Yonekura AR, Heath JK, Turley SJ. Peripheral antigen display by lymph node stroma promotes T cell tolerance to intestinal self. Nat Immunol. 2007;8:181–190. doi: 10.1038/ni1427. See comment for [53••] [DOI] [PubMed] [Google Scholar]
- 53••.Gardner JM, Devoss JJ, Friedman RS, Wong DJ, Tan YX, Zhou X, Johannes KP, Su MA, Chang HY, Krummel MF, et al. Deletional tolerance mediated by extrathymic Aire-expressing cells. Science. 2008;321:843–847. doi: 10.1126/science.1159407. This paper together with [52••] reveals that extra-thymic expression of Aire in stromal-type epithelial cells in lymph nodes and spleen leads to expression of peripheral self-antigens. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kieper WC, Troy A, Burghardt JT, Ramsey C, Lee JY, Jiang HQ, Dummer W, Shen H, Cebra JJ, Surh CD. Recent immune status determines the source of antigens that drive homeostatic T cell expansion. J Immunol. 2005;174:3158–3163. doi: 10.4049/jimmunol.174.6.3158. [DOI] [PubMed] [Google Scholar]
- 55.Rawlings SL, Crooks GM, Bockstoce D, Barsky LW, Parkman R, Weinberg KI. Spontaneous apoptosis in lymphocytes from patients with Wiskott-Aldrich syndrome: correlation of accelerated cell death and attenuated bcl-2 expression. Blood. 1999;94:3872–3882. [PubMed] [Google Scholar]
- 56.Rengan R, Ochs HD, Sweet LI, Keil ML, Gunning WT, Lachant NA, Boxer LA, Omann GM. Actin cytoskeletal function is spared, but apoptosis is increased, in WAS patient hematopoietic cells. Blood. 2000;95:1283–1292. [PubMed] [Google Scholar]