Abstract
The adaptive immune response of ovarian cancer patients has been linked to survival, and is known to be impaired in the tumor microenvironment. Little is known about relationships between biobehavioral factors such as depressed mood and anxiety and the adaptive immune response in ovarian cancer. Thirty-seven patients with epithelial ovarian cancer and 14 patients with benign neoplasms completed psychosocial questionnaires pre-surgery. Lymphocytes from peripheral blood, tumor, and ascites (fluid around the tumor), were obtained on the day of surgery. Expression of the Type-1 cytokine interferon gamma (IFNγ), and the Type-2 cytokine interleukin-4 (IL-4) by T-helper (CD4+) and T-cytotoxic (CD8+) cells was measured under autologous tumor-stimulated, polyclonally-stimulated, or unstimulated conditions. Links with mood were examined. Among cancer patients, marked elevations in unstimulated and tumor-stimulated Type-2 responses were seen, particularly in ascites and tumor-infiltrating lymphocytes (P values < 0.01). With polyclonal stimulation, lymphocytes from all compartments expressed elevated Type-1 cytokines (P values <0.014). Depressed and anxious mood were both associated with significantly lower ratios of polyclonally-stimulated CD4+ cells producing IFNγ (TH1 cells) vs. IL-4 (TH2 cells) in all compartments (depressed mood: P = 0.012; anxiety: P = 0.038) and depressed mood was also related to lower ratios of polyclonally-stimulated CD8+ cells producing IFNγ (TC1) vs. IL-4 (TC2) (P =0.035). Although effects of polyclonal stimulation should be generalized with caution to the in vivo immune response, findings suggest that depressed and anxious mood are associated with greater impairment of adaptive immunity in peripheral blood and in the tumor microenvironment among ovarian cancer patients.
Keywords: Ovarian cancer; Cytokines; Depressed mood; anxiety; Psychoneuroimmunology; T-cell response, distress, tumor microenvironment
1. Introduction
Although ovarian cancer has the second highest incidence among gynecologic cancers, more women die annually from ovarian cancer than from cancers of all other gynecologic sites combined (American Cancer Society, 2007). Presence of tumor infiltrating CD3+ T-lymphocytes is linked to an approximately three-fold increase in overall survival, highlighting the importance of the adaptive immune response in ovarian cancer (Zhang et al., 2003). In a second study, tumor infiltrating T-cells were associated with disease-free interval, survival, and improved response to chemotherapy (Raspollini et al., 2005). The poor prognosis in ovarian cancer is thought in part to reflect local immunosuppression coupled with a well-developed capacity for immune escape (Santin et al., 2004). Considerable impairment of local anti-tumor effector mechanisms has been observed in lymphocytes derived from malignant ascites (fluid surrounding the tumor, largely composed of secretions by tumor cells) and in tumor infiltrating lymphocytes (TIL) as compared to peripheral blood mononuclear cells (PBMC) (Badger et al., 1981; Berek et al., 1984; Rabinowich et al., 1996; Santin et al., 2004). T-cells from ovarian ascites show decreased expression and function of signaling molecules, defects in the T-cell receptor and transmembrane receptors, decreased ability to produce interleukin-2 (IL-2) and interferon-gamma (IFNγ) (Lai et al., 1996), and decreased activation markers, even in the presence of stimulatory cytokines (Chen et al., 1999). Although tumor-specific cytotoxic T-lymphocytes (CTL) have been observed in ascites and tumor of ovarian cancer patients (Yamada et al., 1999) and are capable of recognizing multiple antigens on ovarian tumors, their functional capacity is largely impaired (Ioannides et al., 1991).
The elimination of tumor cells by immune mechanisms is generally thought to involve cytokine-mediated up-regulation of immune effector cells (Olver et al., 2006). This relies more on a Type-1 cytokine response, including cytokines that stimulate the cellular immune response such as IFNγ, as opposed to a Type-2 response, which includes cytokines such as interleukin-4 (IL-4) that predominantly stimulate the humoral immune response (Kemp and Ronchese, 2001; Takashi et al., 1999). The Type-1 and Type-2 response, traditionally measured in helper T-cells (called TH1 and TH2 respectively), has been shown to also be relevant in cytotoxic T-cells (called TC1 and TC2, respectively) (Kemp and Ronchese, 2001). Low levels of intratumoral IFNγ expression have been related to poorer prognosis in ovarian cancer (Marth et al., 2004). In contrast, IL-4 in the tumor microenvironment has been linked to poorer CTL function and poorer tumor clearance (Olver et al., 2006), although not all findings are consistent (Mattes et al., 2003; Rodolfo et al., 1999).
Differences in cytokine profiles have been noted between peripheral blood lymphocytes and TIL. CTL cell lines generated from peripheral blood lymphocytes of ovarian cancer patients preferentially produced Type-1 cytokines such as IL-2 and IFNγ when cultured with autologous tumor cells (Goedegebuure et al., 1997). In contrast, TIL from ovarian tumors show a predominance of Type-2 cytokine gene expression (Rabinowich et al., 1996), although not all reports are consistent (Santin et al., 2004). Ovarian tumor cells have also been shown to inhibit IFNγ production by TIL (Kooi et al., 1993). Taken together, these findings show a general downregulation of the Type-1 response in the tumor microenvironment. However, little is known about host factors that may influence the polarity (relative Type-1 vs. Type 2 predominance) of the adaptive immune response in ovarian cancer.
Many of the immunologic activities relevant to cancer are known to be modulated by or reflective of psychological states such as depressed or anxious mood. Several meta-analytic studies and reviews have demonstrated consistent correlations between depressed mood, emotional distress and decrements in cellular immune parameters such as the T-cell response to mitogen stimulation and the cytotoxic activity of natural killer (NK) cells (Irwin, 1999, 2002; Irwin and Miller, 2007; Reiche et al., 2004; Segerstrom and Miller, 2004). Anxious mood has also been associated with impairments in the cellular immune response (Koga et al., 2001; Thornton et al., 2007), and interventions that decrease anxiety have shown increases in T-cell response to mitogens in women with breast cancer (Andersen et al., 2004) and long-term increases in CD3+CD8+ lymphocytes in men with HIV (Antoni et al., 2000a).
T-cells and NK cells express adrenergic and glucocorticoid receptors, enabling them to be responsive to distress-induced neuroendocrine hormones (Chrousos, 1992; Heilig et al., 1993). Glucocorticoids, catecholamines, and distressed mood have all been associated with a shift from a predominantly Type-1 response to a Type-2 cytokine response (Elenkov and Chrousos, 2000; Glaser et al., 2001). In cancer patients, elevated distress in early stage breast cancer patients at four months post-surgery has been associated with poorer T-cell response to mitogen stimulation in peripheral blood (Andersen et al., 1998; Thornton et al., 2007), whereas stress-reducing behavioral interventions were effective in increasing the T-cell lymphoproliferative response to mitogens in 2 trials in breast cancer patients (Andersen et al., 2004; McGregor et al., 2004).
Most psychoneuroimmunology studies in cancer patients have examined behavioral-immune relationships in the periphery. However because of tumor-induced immune downregulation, it is not known to what extent extrapolations from peripheral blood to the tumor microenvironment are justified. Thus the examination of behavioral-immune relationships in the vicinity of the tumor is extremely important in understanding the in vivo setting in cancer. Little is known about relationships between depressed or anxious mood and adaptive immunity in ovarian cancer patients, and to our knowledge this has not been previously examined in the tumor microenvironment.
With respect to innate immunity, we have previously reported that among epithelial ovarian cancer patients at surgery, depressed mood was related to lower NK cell activity in the tumor microenvironment (Lutgendorf et al., 2005). This finding has clinical relevance as NK cell activity among patients with advanced ovarian cancer has been related to disease progression both prospectively and at the time of recurrence (Garzetti et al., 1993). As many of the same adrenergic and glucocorticoid mechanisms that mediate relationships of psychosocial factors with NK cell activity also modulate T-cell production of cytokines (Elenkov, 2004; Elenkov and Chrousos, 1999; Elenkov et al., 1996) and T-cells have relevance for survival in ovarian cancer (Zhang et al., 2003), we investigated whether depressed and anxious mood were associated with further downregulation of adaptive immunity in ovarian cancer. The adaptive immune response was assessed by relative expression of Type-1 vs. Type 2 cytokines by both CD4+ and CD8+ T-cells in peripheral blood, ascites, and tumor. Cytokine expression stimulated by autologous tumor, by a polyclonal mitogen, or by no stimulation was examined. Stimulation by autologous tumor was performed to examine the T-cell response in the context of tumor-induced modulation of the immune response. In contrast, polyclonal mitogen stimulation allows for observation of the maximum cytokine response available to T-cells.
Based on previous associations of stress hormones and distress with lower levels of Type-1 cytokines and elevations in Type-2 cytokines, (Elenkov, 2004; Elenkov and Chrousos, 1999; Elenkov et al., 1996) we hypothesized that higher levels of depressed and anxious mood would be associated with lower levels of Type-1 cytokines as compared to Type-2 cytokines in T-cells in ovarian cancer patients. Based on our previous findings of behavioral immune links in NK cells isolated from peripheral blood and tumor but not in ascites, we predicted that these relationships would be seen in peripheral blood and in TIL but not in ascites.
2. Materials and methods
2.1. Participants
2.1.1. Inclusion and exclusion criteria
This study was approved by the University of Iowa Institutional Review Board. Inclusion as an ovarian cancer patient required confirmation by histological diagnosis of a primary invasive epithelial ovarian, primary papillary peritoneal, or fallopian tube malignant tumor. Patients found to have benign ovarian neoplasms with no inflammatory or other confounding conditions (e.g., endometriosis) were included as a comparison group. The benign comparison group was used to provide a standard of reference for immune measures in peripheral blood among women who were facing the stress of surgery for possible ovarian cancer in an immune system not compromised by the presence of tumor. Exclusion criteria included age under 18, previous cancer history, non-ovarian primary tumor, non-epithelial ovarian tumors or low malignant potential tumors, use of chronic systemic steroid medication in the last four months, or co-morbidities known to alter the immune response (e.g., multiple sclerosis, lupus).
2.1.2. Sample characteristics
One hundred thirty-two potentially eligible patients with a newly diagnosed pelvic or abdominal mass suspected as a possible ovarian malignancy were approached for study participation, and 114 agreed to participate (86.3%). Twenty-six patients were excluded for nonovarian or low malignant pathology, and 24 for complications including cancellation of surgery, surgical rescheduling that precluded study participation, neoadjuvant chemotherapy, inflammatory disease, or difficulty with venous access. Nine patients withdrew before surgery. Four patients (3 ovarian, 1 benign) had insufficient cells for an intracellular stain (ICS) and one assay was excluded for technical difficulties. Patients were included in the final sample if they had valid intracellular cytokine stain measurements available in any compartment. The final sample included 37 ovarian cancer patients and 14 patients with benign pelvic masses. Because not all patients had ascites or sufficient tumor available from pathology for analysis of TIL, the ICS was performed on cells from a subset of ovarian cancer patients. Sufficient T-cells for an ICS were isolated from ascites of 22 patients and from tumor tissue in 16 patients. No differences in Profile of Mood States (POMS) depression or anxiety scores were seen according to whether sufficient cells were able to be isolated from ascites (P = 0.74, P=0.32, respectively) or tumor tissue (P = 0.11 for both depression and anxiety). The majority of ovarian cancer patients (86.5%) had advanced stage disease (stages III and IV) with predominantly high grade tumors.
2.2. Procedure
Patients were recruited at their initial clinic visit, prior to surgery. Patients completed psychosocial questionnaires prior to surgery, and a 40 mL sample of peripheral venous blood was collected in heparinized vacutainer tubes (Becton Dickinson Co, Rutherford, NJ) on the morning of surgery before administration of pre-operative medication or general anesthesia. To control for potential circadian variation, peripheral blood samples were taken between approximately 6 a.m. and 12 p.m. Samples of ascites and tumor were obtained at the time of surgery and were immediately processed as described below.
2.3. Immunologic measures
PBMC were isolated from whole blood using a Ficoll gradient and centrifugation at 650×g at 4°C for 10 minutes. After washing three times with medium, the cells were resuspended in media and counted in 10% acetic acid. Separation of ascites and tumor into tumor- and lymphocyte-enriched fractions using enzymatic digestion and microbead separation was performed as previously described (Lutgendorf et al., 2005). T-cells were defined as CD3+ cells, and were further divided into CD3+CD4+ (helper T-cells) and CD3+CD8+ (cytotoxic T-cells [CTL]). Type-1 and Type-2 responses were defined by the expression of IFNγ and IL-4, respectively. Four different cell phenotypes were assessed: CD4+IFNγ+ Type-1 helper T-cells (TH1); CD4+IL-4+ Type-2 helper T-cells (TH2); CD8+IFNγ+ Type-1 cytotoxic T-cells (TC1); and CD8+IL-4+ Type-2 cytotoxic T-cells (TC2). Two-color flow cytometric analysis of cell surface markers and intracellular IFNγ or IL-4 expression was performed under 3 conditions: a) no stimulation; b) stimulation by the polyclonal mitogens phorbol myristate acetate and ionomycin (P/I); and c) stimulation by autologous tumor.
2.3.1. Intracellular cytokine stain
Lymphocyte suspensions from patients were prepared in CTL medium (RPMI 1640, 10% FBS, 1% sodium pyruvate, 1% HEPES, and 05% gentamycin [all from Gibco]) and plated with a resulting concentration of 1 × 106 cells/well. Phorbol myristate acetate (PMA: 10μL; Sigma) and ionomycin (I: 10μL; Sigma) or autologous tumor from ascites or tumor in a responder:stimulator ratio of 5:1, 10:1, or 50:1 were added to select wells. Other wells with cells remained unstimulated to demonstrate levels of cytokine production in the absence of stimulation. Cells were incubated at 37°C, with 5% CO2 and 95% humidity for 12 hours in the presence of 10μL/well of Golgistop (BD Pharmingen, San Diego, CA). Following incubation, plates were washed twice with human serum staining buffer (HSSB) (10% Human Serum AB [GemCell, Pennant Hills, NSW, Australia], 10% FBS, 0.02% sodium azide [NaN3, Fisher Scientific, Fair Lawn, NJ]), centrifuged at 1126×g at 4°C for 7 minutes, and supernatant removed. Fc blocking buffer (10% Fc-block [Miltenyi Biotec, Auburn, CA] in HSSB) at 70 μL/well was added to block non-specific binding to Fc receptors on the cells. After a 20-minute incubation at 4°C, the cells were washed once with HSSB. Anti-CD3-Cy5, anti-CD8-FITC, and isotype control IgG1μ-Cy5 and IgG1-FITC (BD Pharmingen) were added at 20μL/106cells. Cells were incubated at 4°C for 20 minutes, washed once with HSSB at 1126×g at 4°C for 7 minutes, aspirated and resuspended in Cytofix/Cytoperm (BD Pharmingen) at 100μL/well. Cells were incubated at 4°C for 20 minutes, and washed once with permwash buffer (10% PermWash [BD Pharmingen] in PBS) at 1697×g at 4°C for 10 minutes and then stained with either IFNγ-PE, IL-4-PE, or isotype control IgG1-PE (BD Pharmingen), at 20μL/106cells. After 30 minutes incubation at 4°C, cells were washed once with permwash buffer at 1697×g at 4°C for 10 minutes, aspirated, and stored in permwash buffer at 4°C until flow cytometry analysis (FACScan, Becton-Dickinson, Franklin Lakes, NJ). Data were analyzed using FlowJo Software, version 4.4 (Tree Star, Ashland, OR) and expressed as the percentage of CD3+ subsets identified as TH1, TH2, TC1, and TC2 with gating based on isotype controls to rule out nonspecific staining. Lymphocyte percentages were utilized as an outcome measure to allow for between subject differences in total number of immune cells in each compartment. In all analyses, CD8− cells were counted as CD4+. Previous experiments using anti-CD4 antibody confirmed that the percentage of cells in the CD4+ population was equal to those in the CD8− fraction using anti-CD8 antibody (data not shown).
2.4. Psychosocial assessments
2.4.1. Distressed Mood
Mood was assessed using the Profile of Mood States Short Form (POMS-SF) (Curran et al., 1995; Sacham, 1983). This scale lists 37 mood-related adjectives that subjects rate according to their mood over the past week. Ratings use a 5-point scale from “not at all” (0) to “extremely” (4). Six independent subscales have been validated by factor analysis: anxiety, depressed mood, anger, vigor, fatigue, and confusion. As both depressed and anxious mood are common in cancer patients prior to surgery (Andersen et al., 1989), the depression and anxiety subscales were analyzed and reported separately to provide specific data on these mood states.
2.4.2. Clinical and demographic information
Potential covariates such as hours of sleep in the week before surgery and the night before surgery, intake of alcohol (drinks/day), and cigarettes (yes/no) within the last 7 days prior to blood sampling were assessed to control for possible immune confounds. Use of hormone replacement therapy (yes/no) and beta blockers (yes/no), body mass index, and days between initial visit and surgery were extracted from medical records. The number of days between initial visit and surgery was tested as a possible covariate to control for time during which the patient might be dealing with the traumatic diagnosis of potential ovarian cancer. Demographic information was collected and clinical and histopathological information was obtained from medical records.
2.5. Statistical analyses
Statistical analyses were done in SAS version 9.1 (SAS Institute, Cary, N.C.) and SPSS version 15.0 (Statistical Package for the Social Sciences, Chicago, IL). All distributions were examined for outliers and non-normality, and natural logarithmic transformations were used to normalize immune data.
For comparison of benign and cancer patients on age and covariates, analyses of variance (ANOVAs) were used. The Chi Square test was used to compare categorical demographic variables between these two groups. Linear mixed model analyses (Snijders and Bosker, 1999) were performed to compare T-cell subpopulations in peripheral blood of cancer patients vs. benign patients. Among ovarian cancer patients, linear mixed model analyses were used to compare T-cell subpopulations according to type of stimulation and compartment. This statistical approach takes into account within subject correlations, and reduces Type I error by including multiple outcomes simultaneously. For these comparisons, fixed effects were stimulation, compartment, and cytokine All 2 and 3 factor interactions were included. Separate models were fitted for CD4+ cells and for CD8+ cells. Specific comparisons were tested using mean contrasts with Bonferroni adjusted P values. An adjusted P value of < 0.05 was considered significant.
Linear mixed models were fitted to test the relationships between depressed or anxious mood and TH1/TH2 or TC1/TC2 ratios among ovarian cancer patients in the three different compartments under the three different types of stimulation. Ratios were used to examine the overall response in a parsimonious manner. A linear mixed model was fit separately for each type of stimulation; the 3 compartments were included in each model to reduce number of comparisons. The fixed effects in the model were the mood variable, the compartment, and the interaction effect of mood and compartment, where the interaction effect tests for differences in slopes among the compartments. As a conservative measure, to control for the possibility that these associations might be influenced by the extent of a patient's illness and not by mood, cancer stage was used as a covariate in all analyses comparing psychological variables and immune variables. Because of small sample size, other covariates were tested one at a time along with disease stage in multivariate models to rule out these factors as potential confounds, and were kept in final models if they contributed significantly to the outcome variables.
3. Results
3.1. Patient characteristics
Clinical and demographic characteristics of patients are shown in Table 1. There were no significant differences between benign and ovarian cancer patients in age, ethnicity, education, alcohol use, hours of sleep during the last week or the last night, percentage of current smokers, body mass index, beta blockers or hormone replacement therapy (all P values > 0.18). There were no significant relationships between use of beta blockers or hormone replacement therapy and any of the immune outcome variables (all P values > 0.13). Ovarian cancer patients had predominantly Stage III (64.9%) and IV (21.6%) disease with predominantly serous histology (72.9%).
TABLE 1.
Patient Characteristics
| Measure |
Cancer Patients (n = 37) |
Benign Patients (n = 14) |
|---|---|---|
| Age (years) | ||
| Mean (Standard Deviation) | 58.81 (10.40) | 54.64 (11.08) |
| % of Patients | ||
| Education (n=35 ovarian, 12 benign) | ||
| Some high school | 11.5 | 16.7 |
| High school graduate | 37.1 | 16.7 |
| Trade school/some college | 40.0 | 41.6 |
| College graduate | 5.7 | 25.0 |
| Postgraduate | 5.7 | 0.0 |
| Ethnicity | ||
| Asian or Pacific Islander | 3 | 0 |
| Caucasian non Hispanic | 97 | 100 |
| Marital Status (n=36 ovarian, 14 benign) | ||
| Single | 11.1 | 7.1 |
| Divorced | 11.1 | 28.7 |
| Widowed | 5.6 | 7.1 |
| Married/living with partner | 72.2 | 57.1 |
| Stage (n=37) | ||
| I | 5.4 | |
| II | 8.1 | |
| III | 64.9 | |
| IV | 21.6 | |
| Grade | ||
| 1 | 8.1 | |
| 2 | 27.0 | |
| 3 | 54.1 | |
| 4 | 10.8 | |
| Tumor Histology | ||
| Serous | 72.9 | |
| Endometrioid | 8.1 | |
| Clear Cell | 2.8 | |
| Mucinous | 16.2 | |
| Residual Disease/Debulking | ||
| Suboptimal | 27.0 | |
| Optimal | 73.0 | |
3.2. Comparison of T-cell cytokine producing populations in benign and ovarian cancer patients
To provide a context for examining relationships of psychosocial factors with cytokine profiles in ovarian cancer patients, we first examined how adaptive immunity in ovarian cancer patients compared with that of women undergoing the stressor of surgery who turned out not to have ovarian cancer. No significant differences were found between benign and ovarian cancer patients in unstimulated or mitogen-stimulated production of Type-1 or Type-2 cytokines from CD4+ or CD8+ cells in peripheral blood (all P values > 0.31) (Supplement Figures S-1A and S-1B). Among benign patients, the unstimulated Type-1/Type-2 ratio in peripheral blood was approximately 1:2 for both CD4+ and CD8+ cells. Among ovarian cancer patients, the unstimulated TH1/TH2 ratio ranged from approximately 1:4 in peripheral blood to 1:8 in TIL (P = 0.008, TIL relative to benign peripheral blood). The unstimulated TC1/TC2 ratio in ovarian cancer patients was 1:6 in both peripheral blood and in TIL (P = 0.04, TIL relative to benign peripheral blood). This suggests a greater Type-1/Type -2 imbalance in TIL of ovarian cancer patients compared to peripheral blood of benign patients.
3.3. TH1 and TH2 cytokine producing populations among ovarian cancer patients
Next, we examined TH1 and TH2 subsets in ovarian cancer patients to determine: 1) relative proportions of TH1 vs. TH2 cells within each compartment and 2) differences between the 3 compartments in their TH1 and TH2 populations.
Unstimulated cells
A predominant TH2 response was seen in unstimulated cells, with significantly greater percentages of TH2 compared to TH1 cells in peripheral blood, ascites, and TIL (all P values < 0.0001). As seen in Figure 1A, percentages of TH1 cells were relatively low, and no significant differences in unstimulated TH1 populations were observed between the 3 compartments (P > 0.28). Significantly more TH2 cells were seen in unstimulated ascites and TIL as compared to peripheral blood (P = 0.02; P = 0.0003, respectively).
Figure 1.
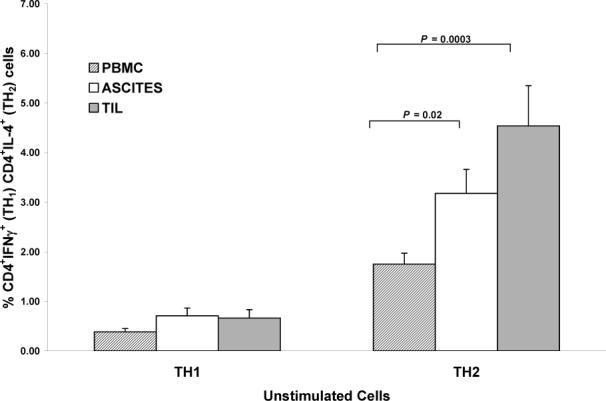
A) Comparisons of unstimulated TH1 and TH2 cells between compartments. For TH1 cells, all between compartment P values > 0.28. For TH2 cells, ascites vs. PBMC (P = 0.024) TIL vs. PBMC (P = 0.0003). Columns: mean, bars: SE. Y-axis in each panel expressed as percent positive cells in the CD3+ population.
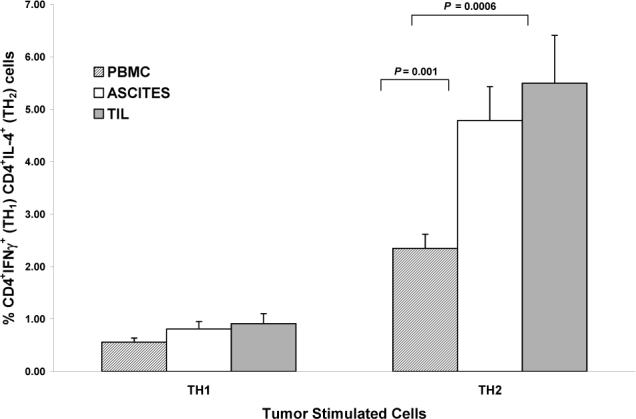
B) Comparisons of tumor-stimulated TH1 vs. TH2 cells between the 3 compartments. For TH1 cells all between compartment P values > 0.49. For TH2 cells, ascites vs. PBMC (P = 0.001), TIL vs. PBMC (P = 0.0006).
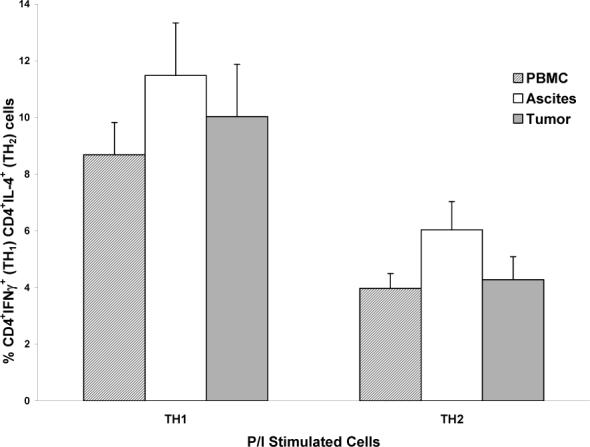
C) Comparisons of P/I-stimulated TH1 and TH2 cells between the 3 compartments. No significant differences seen between compartments for either TH1 or TH2 cells (all P values > 0.41).
Tumor-stimulated cells
Tumor stimulation induced a predominant TH2 response, with significantly greater percentages of tumor-stimulated TH2 compared to TH1 cells in all compartments (all P values < 0.0001). Tumor-stimulated TH1 populations did not significantly differ between the 3 compartments (P > 0.49). However, for tumor-stimulated TH2 cells, there were significantly greater percentages in ascites and TIL as compared to peripheral blood (P = 0.001; P = 0.0006; respectively). Tumor-stimulated TH2 percentages in TIL and ascites did not differ from each other (P > 0.90) (Figure 1B).
Polyclonal stimulation
Stimulation with P/I induced a strong TH1 response in all compartments, with significantly greater proportions of TH1 cells as compared to TH2 cells observed in all 3 compartments (peripheral blood: P = 0.0005; ascites: P = 0.049; TIL: P = 0.014). However, there were no significant differences between compartments in TH1 or TH2 cells with polyclonal stimulation (all P values > 0.41) (Figure 1C).
3.4 TC1 and TC2 cytokine producing populations in ovarian cancer
TC1 and TC2 subsets among ovarian cancer patients were examined as above, testing 1) relative proportions of TC1 vs. TC2 cells within each compartment; and 2) differences between the 3 compartments in their TC1 and TC2 populations.
Unstimulated cells
In unstimulated cells, a predominant TC2 response was seen, with significantly greater percentages of TC2 compared to TC1 cells in all 3 compartments (all P values < 0.0001). In unstimulated cells, greater percentages of both TC1 and TC2 cells were seen in ascites and TIL as compared to peripheral blood (TC1 cells: ascites vs. peripheral blood: P = 0.07; TIL vs. peripheral blood: P = 0.001; TC2 cells: ascites vs. peripheral blood: P = 0.005; TIL vs. peripheral blood: P < 0.0001) (Figure 2A).
Figure 2.
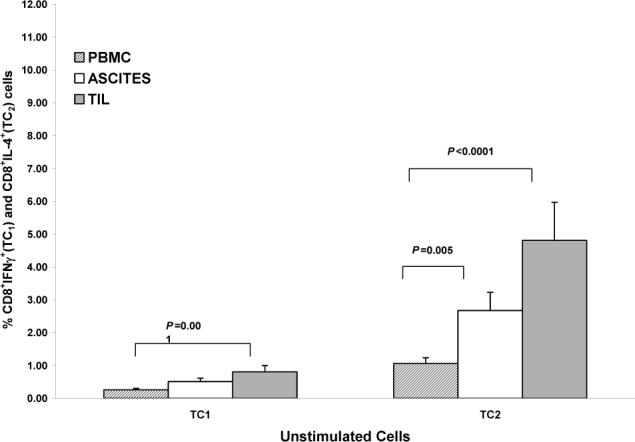
A) Comparisons of unstimulated TC1 and TC2 cells between compartments. For TC1 cells: ascites vs. PBMC (P = 0.07), TIL vs. PBMC (P = 0.001). For TC2 cells: ascites vs. PBMC (P = 0.005), TIL vs. PBMC (P < 0.0001). Columns: mean, bars: SE. Y-axis in each panel expressed as percent positive cells in the CD3+ population.
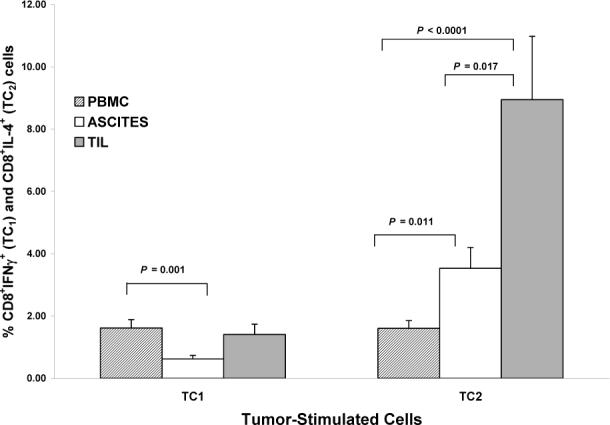
B) Comparisons of tumor-stimulated TC1 vs. TC2 cells between compartments. For TC1 cells: ascites vs. PBMC (P = 0.001), TIL vs. ascites (P = 0.06). For TC2 cells: ascites vs. PBMC (P = 0.011), TIL vs. PBMC (P < 0.0001), TIL vs. ascites (P = 0.017).
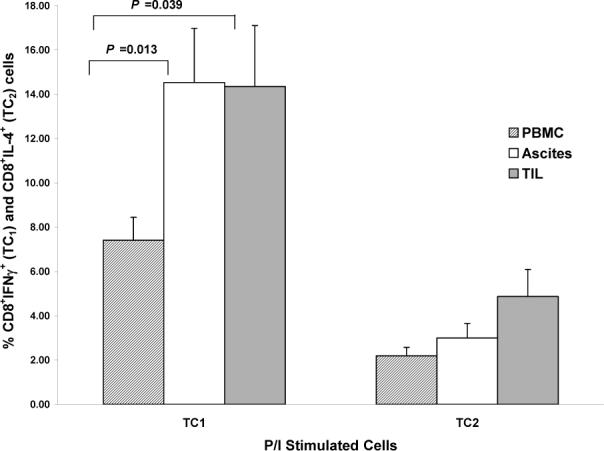
C) Comparisons of P/I-stimulated TC1 and TC2 cells between compartments. For TC1 cells: ascites vs. PBMC (P = 0.013), TIL vs. PBMC (P = 0.039).
Tumor-stimulated cells
Tumor stimulation induced a predominant TC2 response, with significantly greater percentages of tumor-stimulated TC2 compared to TC1 cells seen in ascites and TIL (P values < 0.0001) but not in peripheral blood (P > 0.95). Lower percentages of tumor-stimulated TC1 cells were seen in ascites compared to peripheral blood (P = 0.001) or TIL (P = 0.06). There were significantly greater percentages of tumor-stimulated TC2 cells in ascites and TIL compared to peripheral blood (P = 0.011; P < 0.0001; respectively), and greater percentages of TC2 cells in TIL as compared to ascites (P = 0.017) (Figure 2B).
Polyclonal stimulation
In contrast to tumor-stimulation, stimulation with P/I induced a robust TC1 response (TC1 vs. TC2 cells: peripheral blood and ascites, P < 0.0001; TIL P < 0.036). P/I-stimulated TC1 populations were higher in both ascites and TIL than in peripheral blood (ascites vs. peripheral blood: P = 0.013; TIL vs. peripheral blood: P = 0.039) (Figure 2C).
3.5. Mood and cytokine expressing populations in ovarian cancer patients
For each type of stimulation (tumor, polyclonal, or none) a separate model was fitted to examine whether depressed or anxious mood was associated with the T-cell cytokine response. Each model also tested whether these relationships differed among compartments. For depressed mood, no significant difference in slopes was observed between the 3 compartments for any type of stimulation (P = 0.99 for polyclonal stimulation; P = 0.24 for tumor stimulation; P = 0.91 for unstimulated). Thus all models were fitted using the assumption of common slope. All models adjusted for disease stage. Other potential covariates such as body mass index, cigarette smoking, alcohol use, hours of sleep in the past week or in the last night, and days since initial visit were considered as covariates for these equations. None of the covariates had a significant relationship with the TH1/TH2 ratio (all P values ≥ 0.12), and the significant relationship of depressed mood with the TH1/TH2 ratio was not altered by the inclusion of any covariate within the model, thus these covariates were not included in the final models.
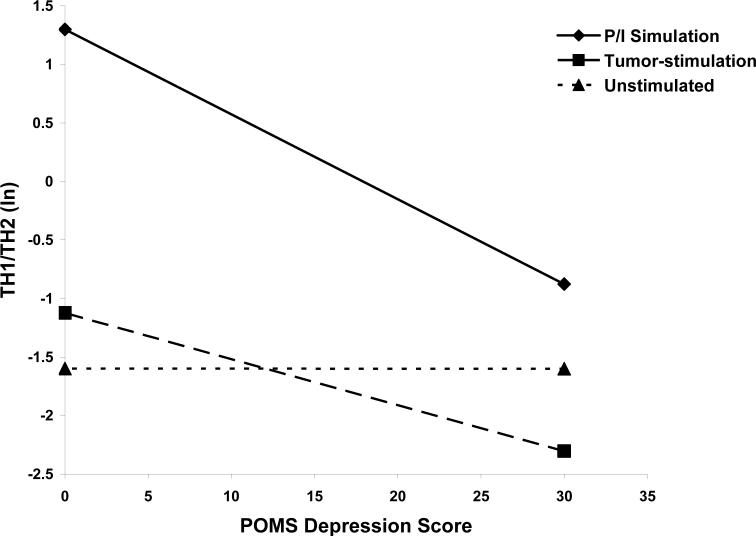
As seen in Figure 3, for polyclonally stimulated lymphocytes, higher levels of depressed mood were associated with a lower TH1/TH2 ratio (β= −0.07, P = 0.012), indicating that the general trend was for decreasing polyclonally-stimulated TH1/TH2 ratios as depressed mood increased. For every 5 unit increase in the POMS depression score, there was a 30.4% (S.E. = 9.2%) decrease in the TH1/TH2 ratio in polyclonally-stimulated cells. Depressed mood was also associated with lower TH1/TH2 ratios in tumor-stimulated cells, but this relationship was not significant (β= −0.04, P = 0.18). There was no relationship between depressed mood and TH1/TH2 ratios in unstimulated cells (β= 0.00005, P = 0.99).
Figure 3.
POMS depressed mood and TH1/TH2 ratio (natural log) in P/I-stimulated, tumor-stimulated, and unstimulated T-lymphocytes. Shown are fitted lines representing the slope of these relationships. (P < 0.012 for P/I-stimulated cells). Lines are fitted representing Stage III ovarian cancer and PBMC.
For anxious mood, slopes did not differ for polyclonal (P = 0.91) or no stimulation (P = 0.36) but there was a trend to differing slopes by compartment for tumor stimulation (P = 0.06). Thus, for tumor-stimulated cells, the model was fitted using a separate slope for each compartment. None of the covariates had a significant relationship with the TH1/TH2 ratio (all P values ≥ 0.12), and the significant relationship of anxious mood with the TH1/TH2 ratio was not altered by the inclusion of any covariate within the model1, thus these covariates were not included in the final models. Paralleling these findings, higher levels of anxious mood were related to significantly lower TH1/TH2 ratios in polyclonally-stimulated cells (β= −0.06, P = 0.038). Anxious mood was also associated with lower TH1/TH2 ratios in tumor-stimulated PBMC, but this relationship was not significant (β= −0.06, P = 0.11), and no relationships were observed for tumor-stimulated ascites (β= −0.02, P = 0.56) or TIL (β=0.06, P = 0.21), or for unstimulated cells (β= −0.015, P = 0.64)
For TC1/TC2 ratios, no differences in slopes were seen among the compartments (all P values > 0.24 for POMS depression and > 0.18 for POMS anxiety). Thus models were fitted using the assumption of common slope. All models adjusted for disease stage. For the depression model, greater sleep over the last night was related to significantly higher TC1/TC2 ratios in polyclonally-stimulated cells, (β=0.24, P=0.038). Other covariates were not significant in this model or in models with tumor-stimulated or non-stimulated cells, and sleep was not significant in models with other types of stimulation. Thus sleep was covaried along with disease stage in the depression-P/I model. Depressed mood was associated with lower TC1/TC2 ratios in polyclonally-stimulated cells, (β= −0.05, P = 0.035). For every 5 unit increase in the POMS depression score, there was a 27.1% (S.E. = 11.4%) decrease in the TC1/TC2 ratio in polyclonally stimulated cells. There were no associations between depressed mood and TC1/TC2 ratios in tumor-stimulated or unstimulated cells (all P values > 0.45). For anxiety no covariates were significant in any model (all P values > 0.13) and there were no associations between anxious mood and TC1/TC2 ratios in any model (all P values > 0.16).
4. Discussion
The present findings extend existing literature by demonstrating associations between higher levels of depressed mood and a shift from a Type-1 to a Type-2 pattern of cytokine expression in polyclonally-stimulated cells (both CD4+ and CD8+) among ovarian cancer patients. Similar patterns were observed in lymphocytes isolated from peripheral blood, ascites, and tumor. The presence of an inverse relationship between depressed mood and polyclonally-stimulated Type-1/Type-2 ratios in all compartments is noteworthy, suggesting that in spite of the down-regulation of signaling capacity that is likely to be present in lymphocytes isolated from ascites and from the tumor, further blunting of this response may occur with depressed mood. A similar pattern was observed between anxious mood and lower polyclonally-stimulated TH1/TH2 ratios. Depressed mood was also associated with lower TH1/TH2 ratios in tumor-stimulated lymphocytes in all compartments, and anxious mood was associated with lower TH1/TH2 ratios in tumor-stimulated peripheral blood cells. Although these associations had slopes similar to those observed with polyclonally-stimulated cells, these findings were not significant, likely due to decreased power in these models.
To better understand the context of these findings, data with respect to cytokine responses in ovarian cancer patients will first be discussed. We observed relative conservation of the unstimulated and polyclonally-induced TH1 and TC1 responses in peripheral blood of ovarian cancer patients compared to that of benign patients. However, this was coupled with profound elevations in the unstimulated TH2 and TC2 response in ovarian cancer patients, particularly in the tumor microenvironment, leading to a marked imbalance in both TH1/TH2 and TC1/TC2 ratios. Although ovarian cancer patients maintained the ability to mount Type-1 responses (both TH1 and TC1) to autologous tumor in peripheral blood, marked elevations in tumor-stimulated TH2 as compared to TH1 cells were observed in each compartment, most notably in ascites and TIL. A similar predominance of tumor-stimulated TC2 cells was seen in ascites and TIL.
In contrast to the predominant Type-2 responses to tumor stimulation, polyclonal stimulation induced strong TH1 and TC1 responses in lymphocytes from all compartments. The demonstration of a robust Type-1 response to polyclonal stimulation even in TIL suggests that these lymphocytes maintain the ability to mount a Type-1 response, but when in the presence of tumor, a Type-2 response predominates.
These findings are consistent with previous work demonstrating preferential induction of the Type-1 cytokines IL-2 and IFNγ by CTL cell lines isolated from PBMC of ovarian cancer patients when cultured with autologous tumor cells (Goedegebuure et al., 1997) and a predominant Type-2 cytokine response to autologous tumor stimulation in TIL (Goedegebuure et al., 1997; Rabinowich et al., 1996). Our findings are also consistent with the demonstrated inhibition of IFNγ production in T-lymphocyte cell lines derived from TIL by ovarian tumor cells (Kooi et al., 1993). IFNγ and CTL play a direct role in tumor clearance in vivo and IFNγ depletion can result in increased tumor load (Olver et al., 2006), suggesting a potential survival advantage for those patients who were able to mount a strong Type-1 response to autologous tumor, particularly in ascites or TIL. Tumor induced downregulation of T-cell signaling appears to involve features such as the loss or degradation of the T-cell receptor zeta (TCRζ) chain (Lockhart et al., 2001) which in turn has been associated with poorer production of IL-2 and IFNγ, loss of T-cell function, and poorer survival than patients (Zea et al., 1995).
The role of IL-4 in the tumor microenvironment has been somewhat controversial. IL-4 influences effector differentiation and function of CD4+ and CD8+ T-cells (Kelso, 1999). Although beneficial effects of IL-4 expression by tumors or T-cells on tumor clearance have been shown (Mattes et al., 2003; Rodolfo et al., 1999), other studies have noted that IL-4 producing TC2 cells were less effective than TC1 cells in promoting in vivo tumor rejection (Dobrzanski et al., 1999; Kemp and Ronchese, 2001). IL-4 also has been shown to inhibit the cytotoxic immune response and promote tumor growth and escape by several pathways, including increased expression of antiapoptotic genes in tumors (Conticello et al., 2004; Stassi et al., 2003), inhibition of IL-2 induced lymphocyte proliferation (Martinez et al., 1990; Tanaka et al., 1993), modification of antigen presentation by dendritic cells (King et al., 2001) and direct inhibition of tumor clearance (Olver et al., 2006). Thus, the higher levels of Type-2 cytokine expression induced by autologous tumor, particularly in ascites and TIL, would likely further support local downregulation of the immune response and tumor growth.
4.1. Associations between depressive and anxious mood and cytokine production
The observation of a relative shift in the polyclonally-stimulated TH1/TH2 ratio with depressed or anxious mood in all compartments is noteworthy, as is the association of depressed mood with a lower TC1/TC2 ratio. The fact that these observations were noted in polyclonally-stimulated cells but less so in tumor-stimulated cells may reflect the general impairment of lymphocyte activities by tumor cells (Santin et al., 2004), wherein the Type-1 response may already be so blunted that it cannot be further downregulated by depressed mood or anxiety. In contrast, as P/I predominantly induces a Type-1 response (Harada et al., 1996), relationships with mood may have been more visible in cells with polyclonal stimulation. Additionally, lower power may have contributed to the inability to see these relationships in tumor-stimulated cells.
Relationships between depressed and anxious mood and cytokine production are likely mediated by mechanisms that include adrenergic and glucocorticoid pathways (Chrousos, 1992; Heilig et al., 1993). Depressed mood and distress have been associated with higher tonic levels of glucocorticoids (Antoni, Cruess, S. et al., 2000; Chrousos, 1992) and catecholamines such as epinephrine and norepinephrine (Antoni, Cruess D. et al., 2000; Hughes et al., 2004; Mausbach et al., 2005). Glucocorticoids inhibit the production of Type-1 cytokines such as IFNγ and IL-12 by T-cells and antigen presenting cells while simultaneously upregulating Type-2 cytokines including IL-4, IL-10, and IL-13. This results not in a general immunosuppression, but in a specific shift in the Type-1/Type-2 balance (Elenkov, 2004). Similarly, adrenergic stimulation shifts the TH1/TH2 balance toward the Type-2 response (Elenkov, 2004; Elenkov and Chrousos, 1999; Elenkov et al., 1996). Norepinephrine also directly inhibits anti-tumor CTL activity via beta-adrenergic receptor signaling mechanisms (Kalinichenko et al., 1999). Synergistic effects of glucocorticoids and catecholamines may amplify these effects (Nakada et al., 1987). Beta-adrenergic receptors are present on all lymphocytes (VanTits and Graafsma, 1991). Although speculative at this point, it is possible that in tumor-stimulated lymphocytes, tumor-induced alterations in signaling pathways may mask or interfere with basic mechanisms by which ligation of beta-adrenergic receptors affect signaling pathways in lymphocytes. This is an important area for future research.
In models examining the relationship of depressed and anxious mood with the immune response in polyclonally-stimulated cells, similar patterns of association were observed in all 3 compartments. In our previous work with NK cell activity, we did not find a relationship between psychosocial factors and NK cell activity in ascites. Reasons for observing the relationship between depressive mood and T-cell cytokine production but not NK cell activity in ascites are not clear. It is possible that there are differences in cell signaling between the different types of lymphocytes in the ascites, or that the use of a polyclonal mitogen in some way compensated for signaling deficits that might have obscured a relationship in NK cells in ascites. This is also an area for future research.
4.2. Limitations
The findings of this study are limited by small sample size, particularly in tumor-stimulated TIL. For example, associations between depressed and anxious mood and TH1/TH2 cytokines in tumor-stimulated cells were similar in magnitude to those seen in polyclonally-stimulated cells, but likely due to power, this relationship was nonsignificant. Detection of differences between compartments in their secretion of IFNγ and IL-4 may also have been underestimated due to low sample size and Bonferroni correction. Confirmation and extension of these findings in a larger sample would be important.
As relationships between mood states and T-cell function are correlational, the direction of causality cannot be assumed from these findings. For example, inflammatory processes mediated by the immune response are known to produce vegetative depression and other “sickness behaviors” (Maier, 2003; Maier and Watkins, 1998); thus the depressive symptoms may be in part secondary to tumor-induced immunologic changes. Moreover, because associations with depressed and anxious mood were only significant for polyclonally-stimulated lymphocytes, extrapolation of these findings to processes that occur in vivo among ovarian cancer patients should be done with caution. Understanding of the clinical implications of these findings awaits longitudinal research.
Because the POMS is primarily a measure of mood over the last week and does not provide an index of the chronicity of depressed or anxious mood, which may have more clinical significance than short term changes in mood symptoms, findings of this study may not reflect relationships of chronic stress or mood disturbance with the adaptive immune response. Furthermore, assessment of depressed and anxious mood by a self-report measure may be vulnerable to social desirability bias.
It should be noted that it is possible that impairment in functioning associated with disease progression may be associated with both more severe depressed mood and with a poorer immune response. To address the issue of disease progression in the most objective way, we have controlled for disease stage in all regression models. It is difficult to further separate out effects of disease stage and impairment in a cross sectional model because these constructs are closely related. Thus it is not known whether functional impairment further contributed to the relationships observed.
4.3. Conclusion
Depressed and anxious mood were associated with a shift from a Type-1 to a Type-2 pattern of cytokine expression in polyclonally-stimulated lymphocytes among ovarian cancer patients. It is particularly noteworthy that these findings were observed in all compartments. Extrapolation to the in vivo immune response of ovarian cancer patients should be done with caution because findings reflect behavior of polyclonally-stimulated cells. Nevertheless, these findings suggest that depressed and anxious mood may contribute to the vulnerability of the adaptive immune response in ovarian cancer both in peripheral blood as well as in the tumor microenvironment.
Supplementary Material
S-1A. TH1, TH2, TC1 and TC2 cells in unstimulated PBMC of ovarian cancer and benign patients prior to surgery. S-1B) TH1 TH2, TC1 and TC2 cells in PMA-Ionomycin (P/I) stimulated PBMC of ovarian cancer and benign patients pre-surgery. Columns: mean, bars: SE. Y-axis in all figures expressed as percent positive cells in the CD3+ population.
Acknowledgements
This research was funded in part by grants #R21CA88293 and #RO1CA104825 to Susan Lutgendorf from the National Cancer Institute. We thank Andrew Misfeldt, Joshua Lukenbill, Hannah Chang, Daniel Pederson, and Elizabeth King for assistance with immunologic assays, Joel Sorosky, David Bender, and Michael Goodheart for assistance with patient recruitment, and Anna Hoffman for assistance in study administration.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The inclusion of BMI as a covariate which slightly lowered the significance of anxiety (from P= 0.038 to P= 0.06). However as BMI was not significantly related to the TH1/TH2 ratio, this was not included in the final model.
References
- American Cancer Society . Cancer facts and figures. Atlanta, GA: 2007. [Google Scholar]
- Andersen B, Anderson B, deProsse C. Controlled prospective longitudinal study of women with cancer: I. Psychological functioning and outcomes. J.Consult Clin.Psychol. 1989;57:683–691. doi: 10.1037//0022-006x.57.6.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen B, Farrar WB, Golden-Kreutz D, Kutz LA, MacCallum R, Courtney ME, Glaser R. Stress and immune responses after surgical treatment for regional breast cancer. J. Natl. Cancer Inst. 1998;90:30–36. doi: 10.1093/jnci/90.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen B, Farrar WB, Golden-Kreutz DM, Glaser R, Emery CF, Crespin TR, Shapiro CL, Carson WE. Psychological, behavioral, and immune changes after a psychological intervention: A clinical trial. J. Clin. Oncol. 2004;22:3570–3580. doi: 10.1200/JCO.2004.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoni M, Cruess D, Wagner S, Lutgendorf S, Kumar M, Ironson G, Klimas N, Fletcher M, Schneiderman N. Cognitive behavioral stress management reduces anxiety and 24-hour urinary catecholamine output among symptomatic HIV-infected gay men. J. Consult. Clin. Psychol. 2000;66:31–45. doi: 10.1037//0022-006x.68.1.31. [DOI] [PubMed] [Google Scholar]
- Antoni M, Cruess S, Cruess D, Kumar M, Lutgendorf S, Ironson G, Dettmer E, Williams J, Klimas N, Fletcher M, Scheiderman N. Cognitive-behavioral stress management reduces distress and 24-hour urinary free cortisol output among symptomatic HIV-infected gay men. Ann. Behav. Med. 2000;22:29–37. doi: 10.1007/BF02895165. [DOI] [PubMed] [Google Scholar]
- Badger A, Oh S, Moolten F. Differential effects of an immunosuppressive fraction from ascites fluid of patients with ovarian cancer on spontaneous and antibody dependent cytotoxicity. Cancer Res. 1981;41:1133–1139. [PubMed] [Google Scholar]
- Berek JS, Bast RC, Lichtenstein A. Lymphocyte cytotoxicity in the peritoneal cavity and blood of patients with ovarian cancer. Obstet. Gynecol. 1984;64:708–714. [PubMed] [Google Scholar]
- Chen C, Wu M, Chao K, Ho H, Sheu B, Huang S. T lymphocytes and cytokine production in ascitic fluid of ovarian malignancies. J. Formos. Med. Assoc. 1999;98:24–30. [PubMed] [Google Scholar]
- Chrousos G. The concepts of stress and stress system disorders. JAMA. 1992;267:1244–52. [PubMed] [Google Scholar]
- Conticello C, Pedini F, Zeuner A, Patti M, Zerilli M, Stassi G, Messina A, Peschle C, De Maria R. IL-4 protects tumor cells from anti-CD95 and chemotherapeutic agents via up-regulation of antiapoptotic proteins. J. Immunol . 2004;172:5467–5477. doi: 10.4049/jimmunol.172.9.5467. [DOI] [PubMed] [Google Scholar]
- Curran SL, Andrykowski MA, Studts JL. Short form of the profile of mood states (POMS-SF): Psychometric information. Psychol. Assess. 1995;7:80–83. [Google Scholar]
- Dobrzanski MJ, Reome JB, Dutton RW. Therapeutic effects of tumor-reactive type 1 and type 2 CD8+ T cell subpopulations in established pulmonary metastases. J. Immunol. 1999;162:6671–80. [PubMed] [Google Scholar]
- Elenkov IJ. Glucocorticoids and the Th1/Th2 balance. Ann. N.Y. Acad. Sci. 2004;1024:138–146. doi: 10.1196/annals.1321.010. [DOI] [PubMed] [Google Scholar]
- Elenkov IJ, Chrousos GP. Stress hormones, Th1/Th2 patterns, pro/anti-inflammatory cytokines and susceptibility to disease. Trends. Endocrinol. Metab. 1999;10:359–368. doi: 10.1016/s1043-2760(99)00188-5. [DOI] [PubMed] [Google Scholar]
- Elenkov IJ, Chrousos GP. Neuroendocrine regulation of IL-12 and TNFalpha/IL-10 balance: Clinical implications. Ann. N.Y. Acad. Sci. 2000;917:94–105. doi: 10.1111/j.1749-6632.2000.tb05374.x. [DOI] [PubMed] [Google Scholar]
- Elenkov IJ, Papanicolaou DA, Wilder RL, Chrousos GP. Modulatory effects of glucocorticoids and catecholamines on human interleukin-12 and interleukin-10 production: Clinical implications. Proc. Assoc. Am. Physicians. 1996;108:374–81. [PubMed] [Google Scholar]
- Garzetti G, Cignitti M, Ciavattini A, Fabris N, Romanini C. Natural killer cell activity and progression-free survival in ovarian cancer. Gynecol. Obstet. Invest. 1993;35:118–120. doi: 10.1159/000292678. [DOI] [PubMed] [Google Scholar]
- Glaser R, MacCallum R, Laskowski B, Malarkey W, Sheridan J, Kiecolt-Glaser J. Evidence for a shift in the Th-1 to Th-2 cytokine response associated with chronic stress and aging. J. Gerontol. A. Biol. Sci. Med. Sci. 2001;56:M477–482. doi: 10.1093/gerona/56.8.m477. [DOI] [PubMed] [Google Scholar]
- Goedegebuure P, Douville CC, Doherty JM, Linehan DC, Lee KY, Ganguly EK, Eberlein TJ. Simultaneous production of T helper-1-like cytokines and cytolytic activity by tumor-specific T cells in ovarian and breast cancer. Cell. Immunol. 1997;175:150–156. doi: 10.1006/cimm.1996.1055. [DOI] [PubMed] [Google Scholar]
- Harada Y, Watanabe S, Yssel H, Arai K. Factors affecting the cytokine production of human T cells stimulated by different modes of activation. J. Allergy Clin. Immunol. 1996;98:S161–173. doi: 10.1016/s0091-6749(96)70063-5. [DOI] [PubMed] [Google Scholar]
- Heilig M, Irwin M, Grewal I, Sercarz E. Sympathetic regulation of T-helper cell function. Brain Behav. Immun. 1993;7:154–163. doi: 10.1006/brbi.1993.1017. [DOI] [PubMed] [Google Scholar]
- Hughes JW, Watkins L, Blumenthal JA, Kuhn C, Sherwood A. Depression and anxiety symptoms are related to increased 24-hour urinary norepinephrine excretion among healthy middle-aged women. J. Psychosom. Res. 2004;57:353–358. doi: 10.1016/j.jpsychores.2004.02.016. [DOI] [PubMed] [Google Scholar]
- Ioannides C, Freedman R, Platsoucas C, Rashed S, Kim Y. Cytotoxic T cell clones isolated from ovarian tumor-infiltrating lymphocytes recognize multiple antigenic epitopes on autologous tumor cells. J. Immunol. 1991;146:1700–1707. [PubMed] [Google Scholar]
- Irwin M. Immune correlates of depression. Adv. Exp. Med. Bio. 1999;461:1. doi: 10.1007/978-0-585-37970-8_1. [DOI] [PubMed] [Google Scholar]
- Irwin M. Psychoneuroimmunology of depression: clinical implications. Brain Behav. Immun. 2002;16:1–16. doi: 10.1006/brbi.2001.0654. [DOI] [PubMed] [Google Scholar]
- Irwin MR, Miller AH. Depressive disorders and immunity: 20 years of progress and discovery. Brain Behav. Immun. 2007;21:374–383. doi: 10.1016/j.bbi.2007.01.010. [DOI] [PubMed] [Google Scholar]
- Kalinichenko VV, Mokyr MB, Graf LHJ, Cohen RL, Chambers DA. Norepinephrine-mediated inhibition of antitumor cytotoxic T lymphocyte generation involves a beta-adrenergic receptor mechanism and decreased TNF-alpha gene expression. J. Immunol. 1999;163:2492–2499. [PubMed] [Google Scholar]
- Kelso A. Educating T cells: early events in the differentiation and commitment of cytokine-producing CD4+ and CD8+ T cells. Springer Seminars in Immunopathol. 1999;21:231–48. doi: 10.1007/BF00812255. [DOI] [PubMed] [Google Scholar]
- Kemp RA, Ronchese F. Tumor-specific Tc1, but not Tc2, cells deliver protective antitumor immunity. J. Immunol. 2001;167:6497–6502. doi: 10.4049/jimmunol.167.11.6497. [DOI] [PubMed] [Google Scholar]
- King C, Mueller Hoenger R, Malo Cleary M, Murali-Krishna K, Ahmed R, King E, Sarvetnick N. Interleukin-4 acts at the locus of the antigen-presenting dendritic cell to counter-regulate cytotoxic CD8+ T-cell responses. Nature Med. 2001;7:206–214. doi: 10.1038/84659. [DOI] [PubMed] [Google Scholar]
- Koga C, Itoh K, Aoki M, Suefuji Y, Yoshida M, Asosina S, Esaki K, Kameyama T. Anxiety and pain suppresses the natural killer cell activity in oral surgery outpatients. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2001;91:654–658. doi: 10.1067/moe.2001.115465. [DOI] [PubMed] [Google Scholar]
- Kooi S, Freedman R, Rodriguez-Villanueva J, Platsoucas C. Cytokine production by T-cell lines derived from tumor-infiltrating lymphocytes from patients with ovarian carcinoma: Tumor-specific immune responses and inhibition of antigen-independent cytokine production by ovarian tumor cells. Lymphokine Cytokine Res. 1993;12:429–437. [PubMed] [Google Scholar]
- Lai P, Rabinowich H, Crowley-Nowick P, Bell M, Mantovani G, Whiteside T. Alteration in expression and function of signal-transducing proteins in tumor-associated T and natural killer cells in patients with ovarian carcinoma. Clin. Cancer Res. 1996;2:161–173. [PubMed] [Google Scholar]
- Lockhart DC, Chan AK, Mak S, Joo HG, Daust HA, Carritte A, Douville CC, Goedegebuure PS, Eberlein TJ. Loss of T-cell receptor-CD3zeta and T-cell function in tumor-infiltrating lymphocytes but not in tumor-associated lymphocytes in ovarian carcinoma. Surg. 2001;129:749–756. doi: 10.1067/msy.2001.114554. [DOI] [PubMed] [Google Scholar]
- Lutgendorf S, Sood AK, Anderson B, McGinn S, Maiseri H, Dao M, Sorosky J, DeGeest K, Ritchie J, Lubaroff DM. Social support, psychological distress, and natural killer cell activity in ovarian cancer. J. Clin. Oncol. 2005;23:7106–13. doi: 10.1200/JCO.2005.10.015. [DOI] [PubMed] [Google Scholar]
- Maier S. Bi-directional immune-brain communication: Implications for understanding stress, pain, and cognition. Brain Behav. Immun. 2003;17:69–85. doi: 10.1016/s0889-1591(03)00032-1. [DOI] [PubMed] [Google Scholar]
- Maier S, Watkins L. Cytokines for psychologists: Implications of bidirectional immune to brain communication for understanding behavior, mood, and cognition. Psychol. Rev. 1998;105:83–107. doi: 10.1037/0033-295x.105.1.83. [DOI] [PubMed] [Google Scholar]
- Marth C, Fiegl H, Zeimet AG, Muller-Holzner E, Deibl M, Doppler W, Daxenbichler G. Interferon-gamma expression is an independent prognostic factor in ovarian cancer. Am. J. Obstet. Gynecol. 2004;191:1598–1605. doi: 10.1016/j.ajog.2004.05.007. [DOI] [PubMed] [Google Scholar]
- Martinez OM, Gibbons RS, Garovoy MR, Aronson FR. IL-4 inhibits IL-2 receptor expression and IL-2-dependent proliferation of human T cells. J Immunol. 1990;144:2211–15. [PubMed] [Google Scholar]
- Mattes J, Hulett M, Xie W, Hogan S, Rothenberg ME, Foster P, Parish C. Immunotherapy of cytotoxic T cell-resistant tumors by T helper 2 cells: an eotaxin and STAT6-dependent process. J. Exp. Med. 2003;197:387–393. doi: 10.1084/jem.20021683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mausbach BT, Dimsdale JE, Ziegler MG, Mills PJ, Ancoli-Israel S, Patterson TL, Grant I. Depressive symptoms predict norepinephrine response to a psychological stressor task in Alzheimer's caregivers. Psychosom. Med. 2005;67:638–42. doi: 10.1097/01.psy.0000173312.90148.97. [DOI] [PubMed] [Google Scholar]
- McGregor B, Antoni M, Boyers A, Alferi S, Blomberg B, Carver C. Cognitive-behavioral stress management increases benefit finding and immune function among women with early-stage breast cancer. J. Psychosom. Res. 2004;56:1–8. doi: 10.1016/S0022-3999(03)00036-9. [DOI] [PubMed] [Google Scholar]
- Nakada MT, Stadel JM, Poksay KS, Crooke ST. Glucocorticoid regulation of beta-adrenergic receptors in 3T3-L1 preadipocytes. Mol. Pharmacol. 1987;31:377–84. [PubMed] [Google Scholar]
- Olver S, Groves P, Buttigieg K, Morris E, Janas M, Kelso A, Kienzle N. Tumor-derived interleukin-4 reduces tumor clearance and deviates the cytokine and granzyme profile of tumor-induced CD8+ T cells. Cancer Res. 2006;66:571–580. doi: 10.1158/0008-5472.CAN-05-1362. [DOI] [PubMed] [Google Scholar]
- Rabinowich H, Suminami Y, Reichert TE, Crowley-Nowick P, Bell M, Edwards R, Whiteside TL. Expression of cytokine genes or proteins and signaling molecules in lymphocytes associated with human ovarian carcinoma. Int. J. Cancer. 1996;68:276–284. doi: 10.1002/(SICI)1097-0215(19961104)68:3<276::AID-IJC2>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Raspollini MR, Castiglione F, Rossi Degl'innocenti D, Amunni G, Villanucci A, Garbini F, Baroni G, Taddei GL. Tumour-infiltrating gamma/delta T-lymphocytes are correlated with a brief disease-free interval in advanced ovarian serous carcinoma. Ann. Oncol. 2005;16:590–596. doi: 10.1093/annonc/mdi112. [DOI] [PubMed] [Google Scholar]
- Reiche EM, Nunes SO, Morimoto HK. Stress, depression, the immune system, and cancer. Lancet Oncol. 2004;5:617–625. doi: 10.1016/S1470-2045(04)01597-9. [DOI] [PubMed] [Google Scholar]
- Rodolfo M, Zilocchi C, Accornero P, et al. IL-4-transduced tumor cell vaccine induces immunoregulatiory type 2 CD8 T lymphocytes that cure lung metastases upon adoptive transfer. J. Immunol. 1999;163:1923–1928. [PubMed] [Google Scholar]
- Sacham S. A shortened version of the Profile of Mood States. J. Pers. Assess. 1983;47:305–306. doi: 10.1207/s15327752jpa4703_14. [DOI] [PubMed] [Google Scholar]
- Santin AD, Bellone S, Palmieri M, Bossini B, Cane S, Bignotti E, Roman JJ, Cannon MJ, Pecorelli S. Restoration of tumor specific human leukocyte antigens class I-restricted cytotoxicity by dendritic cell stimulation of tumor infiltrating lymphocytes in patients with advanced ovarian cancer. Int. J. Gynecol. Cancer. 2004;14:64–75. doi: 10.1111/j.1048-891x.2004.014175.x. [DOI] [PubMed] [Google Scholar]
- Segerstrom SC, Miller GE. Psychological stress and the human immune system: a meta-analytic study of 30 years of inquiry. Psychol. Bull. 2004;130:601–630. doi: 10.1037/0033-2909.130.4.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snijders TA, Bosker RJ. Multilevel analysis: An introduction to basic and advanced multilevel modeling. Sage, London: 1999. [Google Scholar]
- Stassi G, Todaro M, Zerilli M, Ricci-Vitiani L, Di Liberto D, Patti M, Florena A, Di Gaudio F, Di Gesu G, De Maria R. Thyroid cancer resistance to chemotherapeutic drugs via autocrine production of interleukin-4 and interleukin-10. Cancer Res. 2003;63:6784–6790. [PubMed] [Google Scholar]
- Takashi N, Iwakabe K, Sekimoto M, et al. Distinct role of antigen-specific T helper Type 1 (Th1) and Th2 cells in tumor eradication in vivo. J. Exp. Med. 1999;190:617–24. doi: 10.1084/jem.190.5.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton LM, Andersen BL, Crespin TR, Carson WE. Individual trajectories in stress covary with immunity during recovery from cancer diagnosis and treatments. Brain Behav. Immun. 2007;21:185–194. doi: 10.1016/j.bbi.2006.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanTits L, Graafsma S. Stress influences CD4+ lymphocyte counts. Immunol. Lett. 1991;30:141–142. doi: 10.1016/0165-2478(91)90103-h. [DOI] [PubMed] [Google Scholar]
- Yamada A, Kawano K, Harashima N, Niiya F, Nagai K, Kobayashi T, Mine T, Ushijima K, Nishida T, Itoh K. Study of HLA class 1 restriction and the directed antigens of cytotoxic T lymphocytes at the tumor sites of ovarian cancer. Cancer Immunol. Immunother. 1999;48:147. doi: 10.1007/s002620050559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zea A, Curti B, Longo D, Alvord W, Strobl S, Mizoguchi H, Creekmore S, O'Shea J, Powers G, Urba W. Alterations in T cell receptor and signal transduction molecules in melanoma patients. Clin. Cancer Res. 1995;1:1327–1335. [PubMed] [Google Scholar]
- Zhang L, Conejo-Garcia JR, Katsaros D, Gimotty PA, Massobrio M, Regnani G, Makrigiannakis A, Gray H, Schlienger K, Liebman MN, Rubin SC, Coukos G. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N. Eng. J. Med. 2003;348:203–213. doi: 10.1056/NEJMoa020177. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.