Abstract
The hormone leptin has widespread actions in the CNS. Indeed, leptin markedly influences hippocampal excitatory synaptic transmission and synaptic plasticity. However, the effects of leptin on fast inhibitory synaptic transmission in the hippocampus have not been evaluated. Here we show that leptin modulates GABAA receptor-mediated synaptic transmission onto hippocampal CA1 pyramidal cells. Leptin promotes a rapid and reversible increase in the amplitude of evoked GABAA receptor-mediated IPSCs; an effect that was paralleled by increases in the frequency and amplitude of miniature IPSCs, but with no change in paired pulse ratio or CV, suggesting a postsynaptic expression mechanism. Following washout of leptin, a persistent depression (I-LTD) of evoked IPSCs was observed. Whole cell dialysis or bath application of inhibitors of PI 3-kinase or Akt prevented leptin-induced enhancement of IPSCs indicating involvement of a postsynaptic PI 3-kinase/Akt-dependent pathway. In contrast, blockade of PI 3-kinase or Akt activity failed to alter the ability of leptin to induce I-LTD, suggesting that this process is independent of PI 3-kinase/Akt. In conclusion these data indicate that the hormone leptin bi-directionally modulates GABAA receptor-mediated synaptic transmission in the hippocampus. These findings have important implications for the role of this hormone in regulating hippocampal pyramidal neuron excitability.
Keywords: Leptin, inhibitory synaptic transmission, PI 3-kinase, Akt, I-LTD
Introduction
Leptin is a circulating hormone that can enter the brain via transport across the blood brain barrier. Leptin receptors are widely expressed in many regions of the brain including the hypothalamus, hippocampus, cerebellum, amygdala and brain stem (Hakansson et al, 1998; Elmquist et al, 1998; Shanley et al, 2002a). It is well established that leptin plays a pivotal role in regulating energy homeostasis, thermoregulation and reproductive function via its actions on specific hypothalamic neurons (Spiegelman & Flier 2001; Mantzoros, 2000). However, it is becoming apparent that leptin has widespread actions in the brain. Indeed, several lines of evidence indicate that this hormone rapidly modulates excitatory synaptic transmission in the CNS. Indeed in the hippocampus, leptin enhances NMDA receptor-mediated synaptic transmission, but depresses AMPA receptor-mediated function (Shanley et al, 2001). Similarly in cerebellar granule cells, leptin selectively facilitates NMDA, but not AMPA-receptor-mediated responses (Irving et al, 2006). Moreover several lines of evidence indicate that leptin markedly influences the efficacy of hippocampal excitatory synapses (Harvey, 2007; Shanley et al, 2001; Li et al, 2002; Wayner et al, 2004; Durakoglugil et al, 2005). In addition, recent studies have shown that leptin promotes rapid remodeling of hippocampal dendrites which in turn leads to a rapid increase in synaptic density (O’Malley et al, 2007). However the acute effects of leptin on fast inhibitory synaptic transmission in the hippocampus are not known. In hypothalamic neurons, the effects of leptin on GABAergic synaptic transmission have been investigated. Indeed, acute application of leptin results in attenuation of the frequency of fast inhibitory synaptic transmission onto POMC (Cowley et al, 2001; Munzberg et al, 2007), but not NPY neurons (Glaum et al, 1997) suggesting that leptin acts presynaptically to reduce GABA release onto POMC neurons. Moreover in accordance with these findings, ob/ob mice with chronic deficiencies in leptin display marked increases in GABAergic inhibitory tone onto POMC neurons (Pinto et al, 2004). However little is known about the locus, time course or precise cellular mechanisms underlying the effects of leptin on hypothalamic inhibitory synaptic transmission.
Fast inhibitory synaptic transmission in the mammalian brain is mediated predominantly by the ligand-gated A-type γ-aminobutyric acid (GABAA) receptor (MacDonald & Olsen, 1994). GABAA receptors are thought to exist as hetero-pentameric complexes, assembled from various subunits including α (1-6), β (1-3), γ (1-3), δ, ε and θ (Barnard et al., 1998; MacDonald and Olsen, 1994; McKernan and Whiting, 1996). GABAA receptors are known to be the site of action of various psychoactive drugs such as benzodiazepines and barbiturates and may be subject to physiological regulation by certain neurosteroids (Sieghart and Ernst, 2005). GABAA receptor function can also be modulated by alterations of channel gating and conductance properties or by changes to the density of postsynaptic cell surface receptors. Recent studies have shown that the hormone insulin increases GABAA receptor-mediated synaptic transmission in the hippocampus via Akt (also known as protein kinase B)-dependent delivery of GABAA receptor subunits to the plasma membrane (Wang et al, 2003; Vetiska et al, 2007).
It is well established that activity-dependent changes in the efficacy of excitatory synapses are crucial for neuronal development and learning and memory (Bliss and Collingridge, 1993). Growing evidence indicates that the strength of inhibitory GABAA synapses is also regulated in an activity-dependent manner (Gaiarsa, 2004). Indeed, in hippocampal pyramidal cells GABAA receptor-mediated synaptic responses can be transiently reduced following postsynaptic membrane depolarization (Pitler and Alger, 1992). High frequency stimulation combined with presynaptic and postsynaptic activity also evokes long term modification of GABAergic synapses onto CA1 pyramidal cells (Lu et al, 2000; Shew et al, 2000; Chevaleyre & Castillo, 2003). The LTD of inhibitory synapses (I-LTD) induced following high frequency stimulation is due to a persistent reduction in GABA release that is mediated by endocannabinoids (Chevaleyre & Castillo, 2003). Moreover this form of plasticity is thought to underlie changes in pyramidal cell excitability associated with LTP at excitatory synapses.
Although considerable attention has focused on the effects of leptin on excitatory synaptic input onto CA1 pyramidal cells, the effects of this hormone on inhibitory synaptic transmission at this synapse are unknown. In this study we provide the first compelling evidence that leptin evokes an initial increase, followed by a persistent reduction in the efficacy of GABAA receptor-mediated synaptic transmission onto hippocampal pyramidal cells. These findings have important implications for the regulation of hippocampal neuron excitability by leptin.
Materials and Methods
Hippocampal slice preparation
Young Sprague Dawley rats of either sex (13-19 days old) were killed by cervical dislocation in accordance with Schedule 1 of the U.K. Government Animals (Scientific Procedures) Act, 1986. After decapitation the brain was removed and placed in ice-cold artificial cerebrospinal fluid (aCSF) consisting of (mM): NaCl 124; KCl 3; NaHCO3 26; NaH2PO4 1.25; MgSO4 1; CaCl2 2; D-glucose 10 (bubbled with 95% O2/ 5% CO2; pH 7.4). Transverse hippocampal slices (400 μm) were cut using a Vibratome or DSK tissue slicer (Intracel, Royston, UK) and were maintained in oxygenated aCSF at room temperature for an hour before use.
Electrophysiological recordings
Whole-cell patch-clamp recordings were made at ∼33°C from hippocampal CA1 pyramidal neurons visually identified with an Olympus BX51 (Olympus, Southall, UK) microscope using differential interference contrast optics. Miniature inhibitory synaptic current (IPSC) recordings were obtained at a holding potential of -60mV using borosilicate glass pipettes (4-6 MΩ) containing (in mM): 130 CsCl, 10 HEPES, 10 EGTA, 2 MgCl2, 2 Mg-ATP, 1 CaCl2, pH 7.2, with CsOH. Hippocampal slices were bathed in ACSF containing 2 mM kynurenate acid to block AMPA and NMDA receptor mediated currents, and 0.5 μM tetrodotoxin (TTX) to block action potential driven synaptic transmission. Miniature IPSC data were recorded onto a digital audiotape (DAT) using a Bio-Logic (Claix, France) DTR 1200 recorder and analyzed off-line using the Strathclyde Electrophysiology Software, WinEDR/WinWCP (courtesy of Dr. J. Dempster, University of Strathclyde, Glasgow, UK). Individual mIPSCs were detected using a -4pA amplitude threshold detection algorithm and were visually inspected for validity. To minimize the contribution of dendritic currents, analysis was restricted to events with a rise time ≤ 1ms. The mIPSC frequency was determined over 10s bins for 2 min with the WinEDR programme (J. Dempster, University of Strathclyde) using a detection method based on the rise time of events (35-40 pA/ms) as well as visual scrutiny.
Evoked IPSCs (eIPSCs) were obtained using pipettes comprising (mM): 130 KGlu, 5 NaCl, 10 HEPES, 10 EGTA, 2 MgCl2, 2 Mg-ATP, 1 CaCl2, pH 7.2 KOH. Inhibitory postsynaptic potentials (IPSCs) were evoked by single shock electrical stimulation at a frequency of 0.0333 Hz using a monopolar stimulating electrode placed on Schaffer collateral-commissural fibers, and cells were voltage-clamped at -50 mV to -60mV. Whole-cell recordings were made using an Axopatch 200B patch clamp amplifier (Axon Instruments, USA), and data were filtered at 5 kHz and digitized at 10 kHz. Electrical signals were recorded and analysed on- and off-line using LTP software (Courtesy of Dr Bill Anderson, University of Bristol, UK). Whole-cell access resistances were in the range 7-15 MΩ prior to electrical compensation by 65-80%. Access resistance was continuously monitored and experiments abandoned if changes >20% were encountered.
To evaluate the effects of leptin on GABAA receptor-mediated currents, whole cell recordings were made from hippocampal CA1 pyramidal neurons voltage clamped at -70 mV using pipettes comprising (mM): CsCl 140, CaCl2 1, MgCl2 2, HEPES 10 and EGTA 11 (pH 7.2). Tetrodotoxin (0.5 μM) was also added to the ACSF to inhibit action potential firing. The GABAA receptor agonist muscimol (1 μM) was bath applied which resulted in a peak inward current within 3-5 min of application. Once a steady state muscimol current was achieved leptin (50 nM) was applied in the continued presence of muscimol.
Materials
Human recombinant leptin (R & D Systems; 95-98% purity) was prepared as a stock solution in normal aCSF and was diluted in normal aCSF containing 0.2% bovine serum albumin. Wortmannin and the Akt inhibitor (IV) were obtained from Calbiochem (La Jolla, CA, USA), kynurenate and muscimol were obtained from Sigma-Aldrich (St Louis, MO, USA) whereas TTX and picrotoxin were obtained from Tocris Cookson (Avonmouth, UK), respectively.
Analyses
Individual mIPSCs were detected using a -4 pA amplitude threshold detection algorithm and visually inspected for validity. Accepted events were analyzed with respect to peak amplitude, 10-90% rise time, charge transfer, and time for events to decay by 50% (T50) and 90% (T90). All results are reported as the arithmetic mean ± SEM. The large sample approximation of the Kolmogorov-Smirnoff (KS) test (SPSS software; SPSS, Chicago, IL) was used to compare the distribution of the mIPSCs parameters. Statistical significance of mean data was assessed with the unpaired Student’s t test or repeated measures ANOVA post hoc followed by the Newman-Keuls test as appropriate, using the SigmaStat (SPSS) software package.
In all experiments, the peak amplitude and time to decay of eIPSCs were monitored continuously. For studies comparing the actions of leptin and all other agents on eIPSCs, the mean amplitude (average of 5 min recording) of eIPSCs obtained during the 5 min period immediately prior to leptin and/or agent addition was compared to that after 25-30 min exposure to leptin.
The coefficient of variation (CV) was calculated as described previously (Kullmann, 1994). Briefly, the mean and SD were calculated for the IPSC amplitudes recorded during successive 5 min epochs (SDIPSC and meanIPSC) in control conditions and following hormone/drug treatment. The SD of the background noise was also calculated for each 5 min epoch using a period immediately before electrical stimulation (SDNoise). The CV for each epoch was calculated as (SDIPSC - SDNoise)/meanIPSC.
Results
Leptin rapidly facilitates GABAA receptor-mediated IPSCs
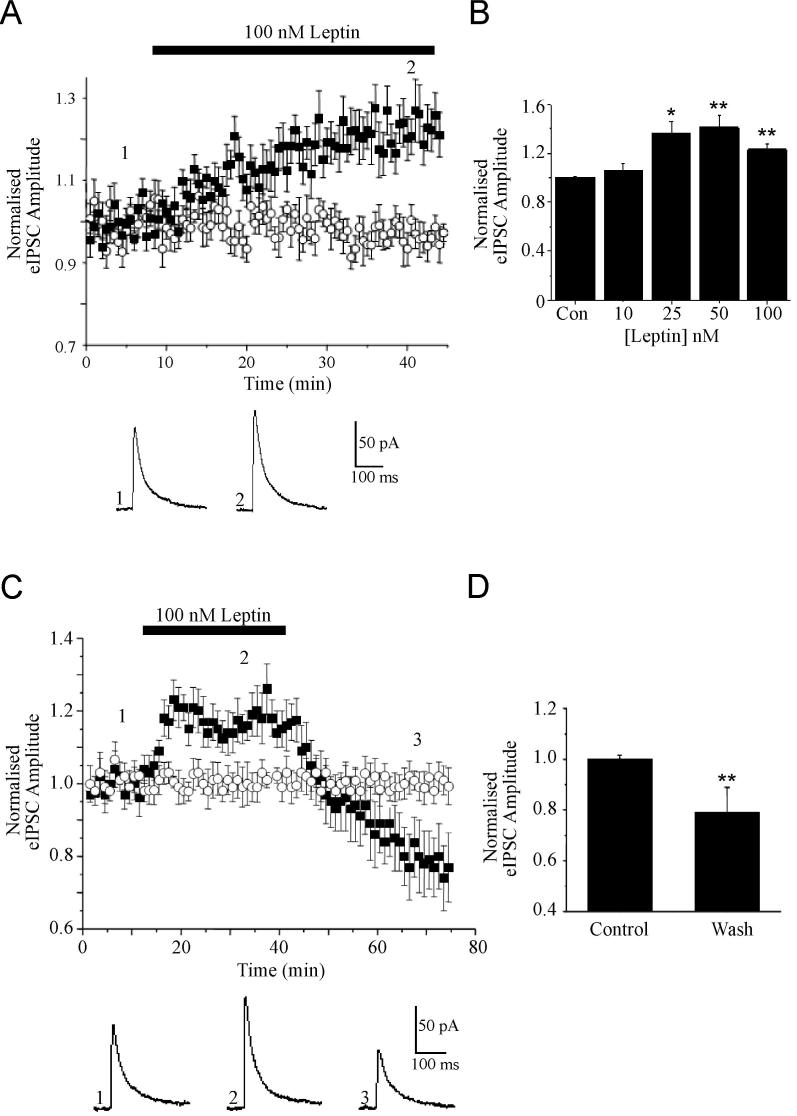
Monosynaptic IPSCs evoked by electrical stimulation of GABAergic axons in stratum radiatum in hippocampal slices (from 13-19 day old rats) were recorded from CA1 pyramidal neurons voltage clamped at -50 mV. eIPSCs were pharmacologically isolated by the addition of kynurenic acid (2 mM) to the aCSF. Under these conditions, the evoked currents were outward and were completely blocked by the GABAA receptor antagonist, picrotoxin (50 μM; n=4). To test whether leptin receptor activation can regulate fast inhibitory GABAergic synaptic transmission in the hippocampus, the effects of bath application of leptin (10-100 nM) were evaluated. Application of 10 nM leptin had no significant effect on eIPSC amplitude (mean amplitude of 107 ± 5.1% of baseline, n=3; P>0.05), whereas at higher concentrations leptin increased eIPSC amplitude (Fig 1B). Thus, at 100nM, leptin produced a rapid (within 3-5 min) facilitation of eIPSC amplitude (to 127 ± 3.9% of baseline, n=12; P<0.01; Fig 1A,B), that was sustained in the presence of leptin. No significant change in the holding current or input resistance was observed during leptin application. Following washout of leptin, the amplitude of eIPSCs returned to pre-leptin levels (99.9 ± 5.4% of control; n=9; P>0.05) within 5-10 mins indicating that this is a reversible process. However, on prolonged washout of leptin (up to 30 min) the amplitude of eIPSCs were reduced further to 78.3 ± 10.3% of control (n=6; P<0.01); an action that persisted for the duration of recordings (Fig 1C,D).
Figure 1. The effects of leptin on evoked GABAA receptor-mediated synaptic currents.
A, Plot of the pooled data illustrating the amplitude of normalized evoked IPSCs against time. Application of leptin (100 nM) for the time indicated by the bar increased eIPSC amplitude that was sustained in the presence of leptin (filled squares). Evoked IPSC amplitude did not vary significantly in interleaved control experiments (open circles). Below the plot are representative examples of synaptic currents obtained prior to (1) and during exposure to leptin (2). B, Histogram of the pooled data showing the relative increase in eIPSC amplitude induced by varying concentrations leptin relative to control. C, Plot of the pooled data of the normalized eIPSC amplitude against time illustrating the effects of leptin washout (filled squares). In interleaved control experiments eIPSC amplitude did not vary significantly (open circles). Below the plot are examples of synaptic currents obtained before (1) and during (2) leptin application and after washout for 30 min (3). D, Histogram of the pooled data showing the relative reductions in eIPSC amplitude induced following washout of leptin for 10 min and 30 min. In this and subsequent figures, *, ** and *** represent P<0.05, P<0.01 and P<0.001, respectively.
Leptin enhances eIPSCs via a postsynaptic mechanism
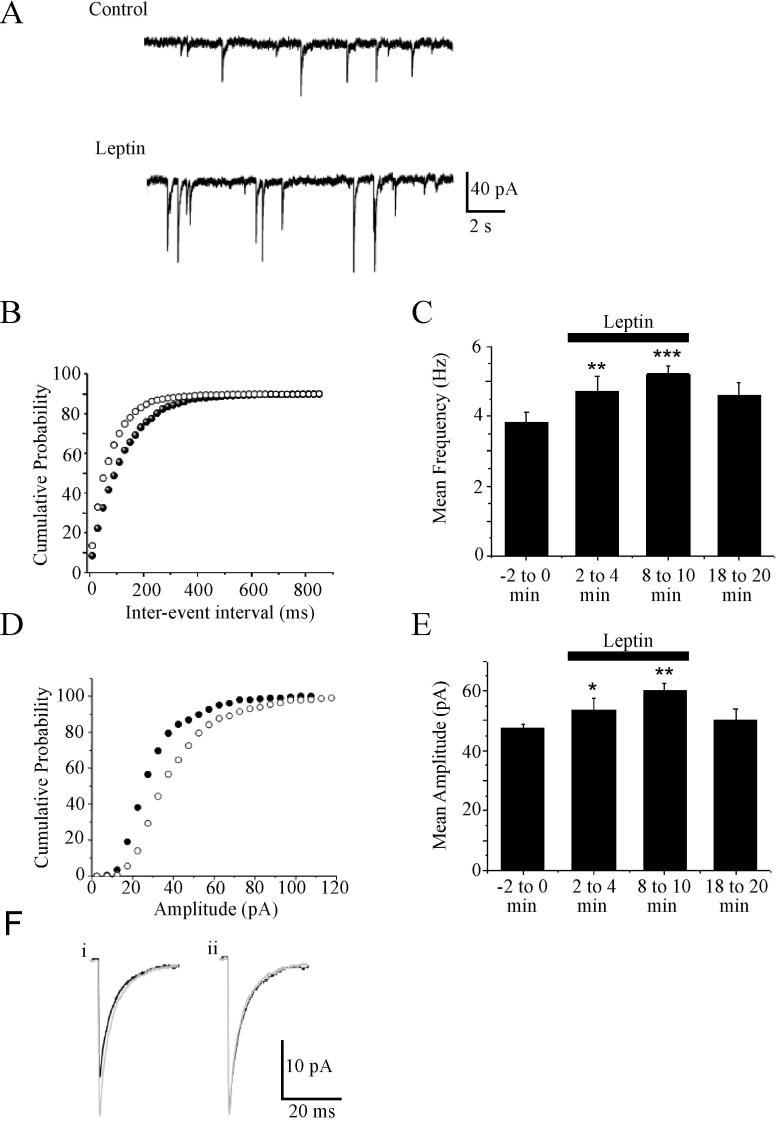
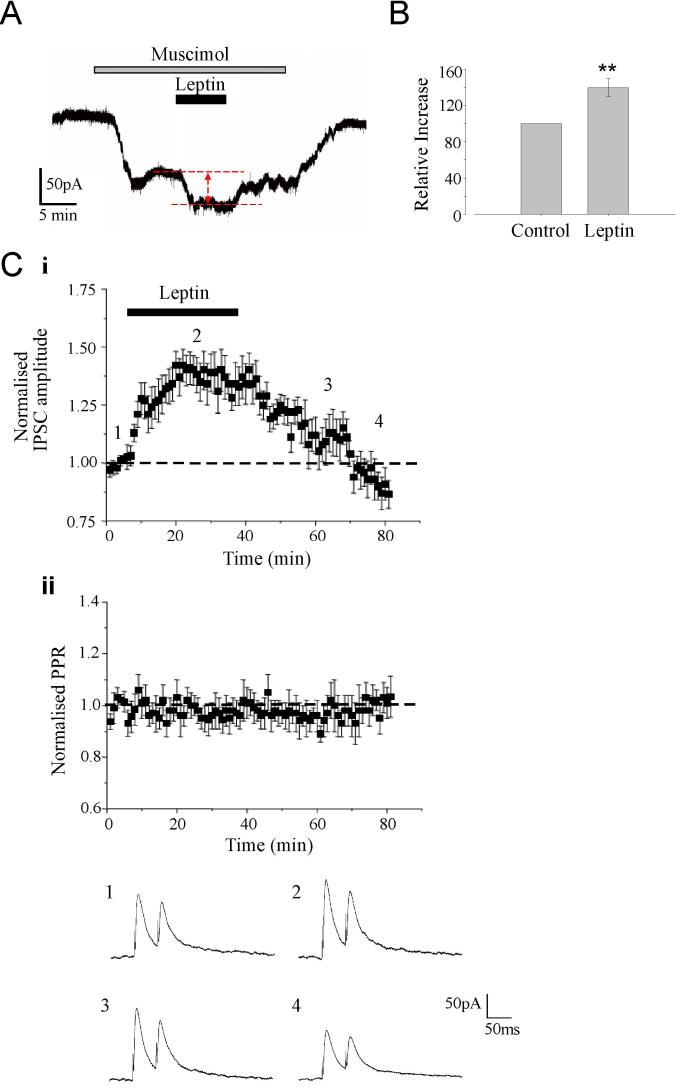
In order to determine the locus of the facilitatory effect of leptin on GABAergic synaptic transmission, the effects of leptin on action potential-independent miniature IPSCs recorded in CA1 pyramidal cells were also assessed. Under control conditions (in the absence of leptin), mIPSCs had a mean frequency of 3.91 ± 0.27 Hz (n=43) and amplitude of -47.5 ± 1.2 pA (n=43), values that are comparable to those reported previously in CA1 pyramidal neurons. Application of leptin (50nM or 100 nM) resulted in an increase in both the frequency and amplitude of these miniature events (Fig 2A-F). Thus after 5-10 min exposure to leptin the frequency and amplitude of mIPSCs were increased to 5.21 ± 0.23 Hz (n=19; P<0.001) and -60.2 ± 2.4 pA (n=19; P<0.01), respectively. At these concentrations, leptin failed to alter the rise time (10-90% of peak; 2.9 ± 0.2 ms in control conditions versus 3.1 ± 0.1ms after leptin; P>0.05) or the decay time constants (τ of 29.4 ± 2ms versus 27.9 ± 3ms; P>0.05) of mIPSCs (Fig 2G). It is often presumed that a change in the frequency of miniature events indicates a direct regulation of neurotransmitter release by a receptor located at the presynaptic terminal, whilst changes in the amplitude and/or kinetics is usually associated with a postsynaptic regulatory mechanism. However, there is also evidence that changes in both the frequency and amplitude of miniature events can indicate the insertion of new receptors into the postsynaptic membrane. Indeed, recent evidence suggests that synaptic GABAA receptors may be highly mobile, being temporarily tethered by synaptic anchoring proteins, but able to dynamically interchange with the extrasynaptic receptor pool (Thomas et al., 2005). Thus, as both the frequency and amplitude are increased by leptin, it was feasible that a combination of both pre- and postsynaptic mechanisms or alternatively that trafficking of postsynaptic receptors contribute to this process. Thus in order to differentiate between these possibilities, we also calculated the paired pulse ratio (PPR), by delivering two pulses at an interval of 50 ms. The coefficient of variation (CV) was also determined before, during and after washout of leptin, as this parameter is widely used to assess changes in the probability of transmitter release (Malinow & Tsien, 1990; Bekkers et al, 1990; McAllister & Stevens, 2000). In this series of experiments, neither the PPR (control PPR of 1.48 ± 0.18; during leptin 1.46 ± 0.13; washout 1.49 ± 0.17; n=5; P>0.05) nor the CV (control CV of 0.16 ± 0.03; during leptin 0.14 ± 0.04; washout 0.15 ± 0.04; n=12; P>0.05) changed significantly either during or after application of leptin (Fig 3C). In addition leptin enhanced inward currents evoked by bath application of GABAA receptor agonist, muscimol (1 μM) to 40 ± 10 % of control (n=5; P<0.01; Fig 3A,B). Thus these data indicate that the leptin-induced facilitation of eIPSCs and the persistent depression of eIPSCs after leptin washout are not expressed presynaptically.
Figure 2. Leptin increases the frequency and amplitude of mIPSCs.
A, Representative traces of mIPSCs recorded from hippocampal CA1 neurons under control conditions and following exposure to leptin (100 nM). Leptin increased the frequency and amplitude of these events. B, Cumulative probability plot illustrating the distribution of the inter-event interval in control (filled circle) and leptin-treated neurons (open circle). C, Histogram of the pooled data of mean mIPSC frequency before (-2 to 0min), during (2 to 4min, 8 to 10 min) and following (18 to 20 min) leptin (100 nM) addition. Leptin increased the frequency of mIPSCs relative to control. D, Cumulative probability plot illustrating the distribution of the amplitude in control conditions (filled circle) and following leptin application (open circle). E Histogram of the pooled data illustrating the mean mIPSC amplitude before (-2 to 0min), during (2 to 4min, 8 to10 min) and following (18 to 20 min) leptin addition. F, Representative averaged traces of mIPSCs in control conditions (black line) and following leptin addition (grey line). i, Leptin increased the mIPSC amplitude relative to control. In ii, the mIPSCs obtained in (i) has been scaled to match the size of mIPSCs obtained following leptin exposure. The effects of leptin are not associated with any change in the kinetic properties of mIPSCs.
Figure 3. The effects of leptin on eIPSCs involve postsynaptic mechanisms.
A. Representative trace of the inward current evoked by the GABAA receptor agonist, muscimol (1 μM). Application of leptin (50nM) for the time indicated resulted in an increase in the muscimol current that reversed on leptin washout. B. Histogram of pooled data of the mean muscimol current amplitude in control conditions and in presence of leptin. C. The effects of leptin were not accompanied by any marked change in PPR. (i) Plot of the pooled data illustrating the normalized eIPSC amplitude against time. (ii) Plot of the mean PPR against time for the experiments depicted in (i). Below the plots are representative pairs of eIPSCs evoked with a 50 ms inter-stimulus interval obtained at the times indicated in (i).
A PI 3-kinase-dependent pathway underlies leptin-induced facilitation of eIPSCs
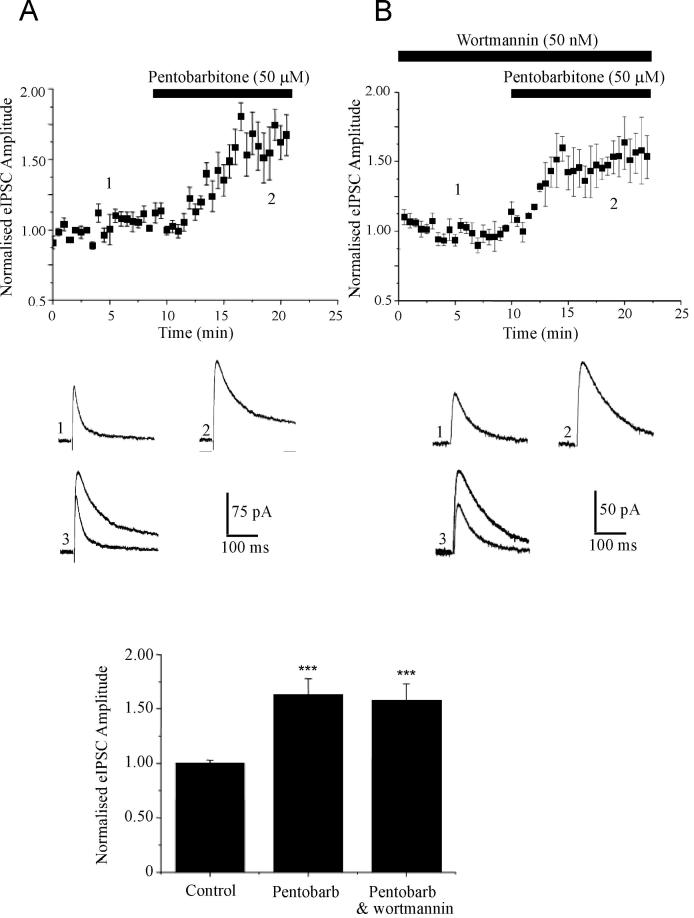
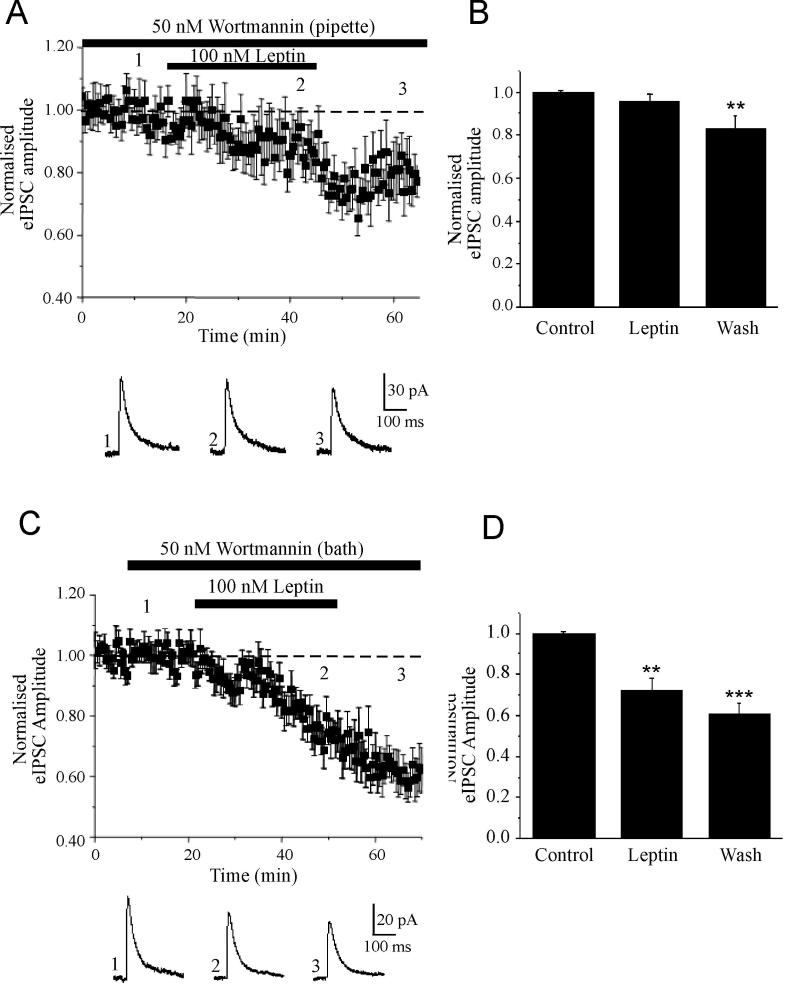
As our data indicate that the facilitation of eIPSCs by leptin has a postsynaptic locus of expression, we evaluated whether whole cell dialysis with specific inhibitors of leptin receptor-driven signaling pathways attenuated the effects of leptin. We have shown previously that PI 3-kinase is a key component of the signaling cascades activated by hippocampal leptin receptors (Shanley et al, 2001; Shanley et al, 2002). Moreover insulin, which activates similar signaling pathways to leptin (van der Heide et al, 2006) facilitates GABAA receptor-mediated synaptic currents via a PI 3-kinase-dependent process (Wang et al, 2003). Thus, the role of this pathway was assessed using a specific inhibitor of this enzyme, namely wortmannin (Powis et al, 1994). Whole cell dialysis with wortmannin (50 nM) had no significant effect on the amplitude of eIPSCs per se (P>0.05). Moreover, wortmannin did not have non-specific effects on GABAA receptors as the ability of the anaesthetic barbiturate, pentobarbitone to directly facilitate the amplitude of eIPSCs and alter the decay kinetics of eIPSCs was not affected (n=4; Fig 4A). Thus following exposure to wortmannin, application of pentobarbitone (50 μM) increased the amplitude of eIPSCs to 157 ± 16% (n=4; P<0.001; Fig 4B); an effect that was not significantly different to that obtained in control slices (163 ± 15%; n=4; P<0.001). However, following at least 20 min dialysis with wortmannin the ability of leptin (100 nM) to facilitate eIPSCs was completely occluded such that the mean amplitude of eIPSCs was 96.8 ± 3.3 % of baseline after 15-20 min exposure to leptin (n=7; P>0.05; Fig 5A,B). Whole cell dialysis with LY294002 (10 μM), a structurally distinct inhibitor of PI 3-kinase completely prevented facilitation of eIPSCs by leptin such that the mean eIPSC amplitude was 98 ± 5.2% of baseline after leptin treatment (n=3; P>0.05). Moreover, postsynaptic dialysis of either wortmannin (n=4) or LY294002 (n=4) completely blocked the ability of leptin to increase the amplitude or frequency of mIPSCs. Thus these data suggest that the leptin-induced facilitation of eIPSCs is likely to involve a postsynaptic PI 3-kinase-dependent process.
Figure 4. Wortmannin does not alter the properties of GABAA receptors.
A,B, Plot of the pooled data illustrating the effects of pentobarbitone on eIPSC amplitude in control (A) and wortmannin treated (B) slices. Below the plot are representative synaptic current traces obtained in control conditions (A1) or in the presence of wortmannin (B1), and following addition of pentobarbitone (A2) or in the combined presence of wortmannin and pentobarbitone (B2). In (A3 and B3) the superimposed synaptic currents obtained in (1) and (2) illustrate that pentobarbitone significantly alters the decay kinetics of eIPSCs. B, Histogram of the pooled data of normalised eIPSC amplitude in control conditions, following application of pentobarbitone and in the combined presence of wortmannin and pentobarbitone.
Figure 5. Leptin facilitates fast inhibitory transmission via a PI 3-kinase-driven process.
A,B, Leptin-induced facilitation of eIPSCs is PI 3-kinase-dependent. A, Plot of the pooled data of amplitude of normalized eIPSCs against time during whole cell dialysis with wortmannin (50 nM). Below the plot are representative synaptic currents obtained from an individual experiment in the presence of wortmannin (1), in the combined presence of wortmannin and leptin (2), and following washout of leptin (3). B, Histogram of the pooled data illustrating the relative changes in eIPSC amplitude in control conditions, in the presence of leptin and following its washout, for neurones dialysed with wortmannin. C, D, Inhibition of pre and postsynaptic PI 3-kinase facilitates leptin induced I-LTD. C, Plot of the pooled data illustrating the amplitude of normalized eIPSCs against time from slices bathed in wortmannin (50 nM). Below the plot are representative synaptic current traces obtained in the presence of wortmannin (1), in the combined presence of wortmannin and leptin (2), and following washout of leptin (3). D, Histogram of the pooled data of normalised eIPSC amplitude in control conditions, in the presence of leptin and following its washout, in slices incubated with wortmannin
In contrast, however postsynaptic inhibition of PI 3-kinase activity following dialysis with wortmannin (n=5) or LY294002 (n=3) had no effect on the ability of leptin to induce a long lasting depression of eIPSCs (I-LTD). Thus in neurons dialysed with wortmannin the amplitude of eIPSCs was reduced to 83 ± 6.1% of control (n=5; P<0.01) after at least 15 min washout of leptin (Fig 5A,B). Moreover, these data also indicate that leptin-induced I-LTD is mediated by a distinct signaling mechanism.
Leptin-induced I-LTD is independent of postsynaptic PI 3-kinase activity
Our studies indicate that leptin-induced LTD of eIPSCs is likely to be mediated by a PI 3-kinase-independent process. However, as leptin receptors are expressed both pre and postsynaptically in the hippocampus (Shanley et al, 2002), it is feasible that leptin-induced LTD of eIPSCs involves a presynaptic process that is also regulated by PI 3-kinase activity. To assess this possibility, we determined if bath application of wortmannin, to inhibit both presynaptic and postsynaptic PI 3-kinase activity, influenced the actions of leptin. In control slices, bath application of either wortmannin (50 nM; n=6) or LY294002 (10 μM; n=3) had no effect on the amplitude or decay kinetics of eIPSCs. In accordance with the experiments involving whole cell dialysis with the PI 3-kinase inhibitors, the initial leptin-induced facilitation of eIPSCs was prevented by bath application of LY294002 (n=3) or wortmannin (n=9; Fig 5C,D). Moreover, the ability of leptin to depress eIPCSs was unaffected following PI 3-kinase inhibition. Thus in wortmannin-treated slices eIPSCs were depressed to 72.4 ± 5.5% of control (n=9; P<0.01; Fig 5C,D) following 15 min exposure to leptin; an effect that was significantly different to the effects of wortmannin alone in paired control slices (P<0.01). Moreover, the synaptic depression was enhanced further (to 60.3 ± 5.4%; n=9; P<0.001) following leptin washout. Thus these data suggest not only that leptin-induced I-LTD involves a distinct mechanism to the facilitation of fast inhibitory synaptic transmission, but also that leptin-induced I-LTD is mediated by a PI-3 kinase-independent process.
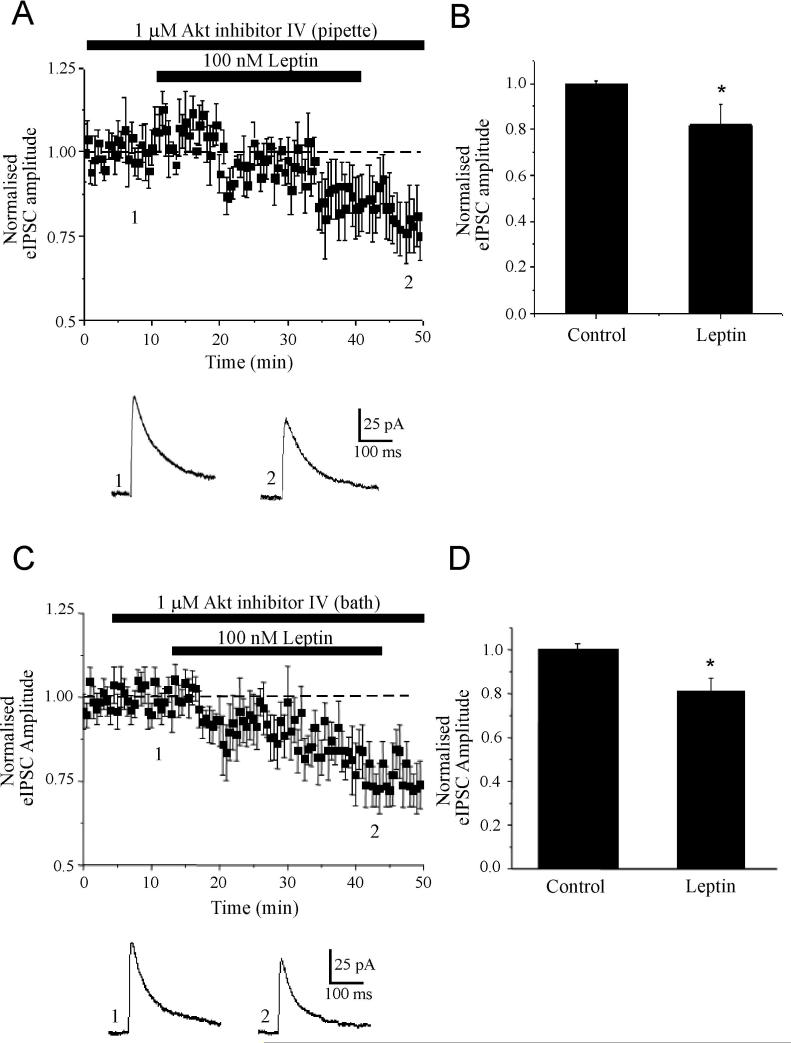
Role of Akt in the bi-directional modulation of GABAergic synaptic transmission by leptin
It is well known that PI 3-kinase possesses serine kinase activity, and both the regulatory and catalytic domains of the enzyme can interact with a number of signalling molecules, including AGC protein kinases, TEC tyrosine kinases and rho GTPases. There is also evidence that one potential target of PI 3-kinase, namely Akt (also known as protein kinase B), can rapidly increase GABAA receptor-mediated synaptic currents in the hippocampus, via a postsynaptic mechanism (Wang et al, 2003). Moreover leptin can stimulate the phosphorylation of Akt in hypothalamic neurons (Mirshamsi et al, 2004). Thus, the role of Akt in leptin-induced facilitation of eIPSCs was assessed via postsynaptic dialysis of CA1 pyramidal neurons with a specific inhibitor of this enzyme, Akt inhibitor IV. In control experiments, dialysis of neurons with Akt inhibitor IV (1 μM) for at least 30 min had no effect on the amplitude or decay kinetics of eIPSCs per se (n=5). However, the ability of leptin to facilitate eIPSCs was markedly attenuated following inhibition of Akt, such that in the presence of leptin (100 nM) the amplitude of eIPSC was 93 ± 7.8% of baseline (n=6; P>0.05; Fig 6A,B). In contrast, the ability of leptin to depress fast inhibitory synaptic transmission was unaffected by Akt inhibition as the eIPSC amplitude was depressed to 82 ± 8.9% of control on leptin washout (n=6; P<0.05; Fig 6B); an effect that was not significantly different to the I-LTD induced by leptin in control conditions (P>0.05). Moreover, postsynaptic dialysis with Akt inhibitor IV blocked the ability of leptin to increase the amplitude and frequency of mIPSCs (n=3). Thus these data indicate that PI 3-kinase-dependent activation of Akt underlies leptin-induced postsynaptic facilitation of GABAA receptor-mediated synaptic transmission.
Figure 6. Akt is involved in the facilitation but not the long lasting depression of eIPSCs by leptin.
A,B, Leptin-induced facilitation of eIPSCs involves an Akt-dependent process. A, Whole cell recordings were obtained using pipettes filled with Akt inhibitor IV (1 μM). Below the plot are representative synaptic currents obtained from an individual experiment in the presence of the Akt inhibitor alone (1) and in the combined presence of the Akt inhibitor and leptin (2). B, Histogram of the pooled data illustrating the relative changes in eIPSC amplitude in control conditions and in the presence of leptin, following dialysis with the Akt inhibitor. C, D, Combined inhibition of pre and postsynaptic Akt facilitates leptin induced I-LTD. C, Plot of the pooled data of amplitude of normalized eIPSCs against time obtained from slices bathed in the Akt inhibitor IV (1 μM). Below the plot are representative synaptic current traces obtained in the presence of the Akt inhibitor (1) and in the combined presence of the Akt inhibitor and leptin (2). D, Histogram of the pooled data of normalised eIPSC amplitude in control conditions and in the presence of leptin in slices incubated with the Akt inhibitor.
In accordance with these findings, the ability of leptin to facilitate eIPSCs was also prevented in slices perfused with the Akt inhibitor (n=7). In contrast, however, the synaptic depression induced by leptin was unaffected by bath application of the Akt inhibitor IV (1 μM; Fig 6C,D). Thus, the peak amplitude of eIPSCs was reduced to 81 ± 6.0 % of control (n=7; P<0.05) in the combined presence of leptin (100 nM) and the Akt inhibitor; an effect that was significantly different to the effects of the Akt inhibitor alone in paired control slices (P<0.05). Thus, together these data indicate that leptin-induced I-LTD is most likely to be mediated by a process that is independent of the PI 3-kinase/Akt signaling pathway.
Discussion
The hormone leptin was originally identified by its ability to regulate energy homeostasis via its actions in the hypothalamus. However, there is growing evidence that leptin has widespread actions in the brain. In the hippocampus and cerebellum, leptin selectively facilitates NMDA receptor-mediated responses (Shanley et al, 2001; Irving et al, 2006). Leptin is also implicated in activity-dependent synaptic plasticity as leptin promotes the conversion of hippocampal STP into LTP (Shanley et al, 2001; Harvey et al, 2005), whereas under conditions of enhanced excitability leptin has the ability to induce a novel form of NMDA receptor-dependent LTD (Durakoglugil et al, 2005). Moreover, genetically-obese rodents with defective leptin receptors display deficits in hippocampal synaptic plasticity and in spatial memory tasks (Li et al, 2002). Here we present the first compelling evidence that GABAA receptor synapses onto hippocampal CA1 pyramidal cells are also regulated by leptin. The predominant effect of leptin was an initial rapid enhancement of GABAA receptor-mediated IPSCs that was readily reversed on washout of leptin. However on prolonged washout of leptin, a long lasting depression of IPSCs (I-LTD) was also observed that persisted for the duration of recordings.
The facilitation of inhibitory synaptic transmission by leptin was paralleled by an increase in both the frequency and amplitude of action-potential-independent mIPSCs, suggesting that this may be attributable to a combination of presynaptic increase in GABA release and altered postsynaptic GABAA receptor function. It is well established that GABA released from a single vesicle almost completely saturates postsynaptic GABAA receptors at central synapses (Mody et al, 1994). As a consequence, enhanced quantal release of GABA would have little impact on the amplitude of fast inhibitory synaptic transmission. Indeed, the facilitation of inhibitory currents by leptin was not accompanied by any change in the CV or PPR, indicating that leptin is unlikely to enhance synaptic transmission by changing GABA release probability. Furthermore leptin enhanced postsynaptic currents induced by application of muscimol. Thus, leptin is likely to enhance fast inhibitory synaptic transmission via a postsynaptic mechanism. Indeed, whole cell dialysis with inhibitors of PI 3-kinase or Akt, respectively completely prevented the facilitation of eIPSCs induced by leptin indicating that this process involves a postsynaptic PI 3-kinase/Akt-dependent process. Similarly, in cortical neurons, a PI 3-kinase-dependent signaling cascade mediates the enhancement of GABAA receptor currents by muscarinic receptors (Ma et al, 2003), whereas blockade of PI 3-kinase activity attenuates the ability of muscarinic receptors to depress GABAA receptor currents (Salgado et al, 2007). It is well documented that PI 3-kinase plays a pivotal role in receptor/protein trafficking processes. Moreover, as changes in both the frequency and amplitude of miniature synaptic events can indicate the postsynaptic insertion of new functional receptors, it is feasible that leptin promotes the insertion of GABAA receptors into the postsynaptic membrane. In support of this possibility, the metabolic hormone insulin, which signals via similar signaling pathways to leptin, enhances GABAergic synaptic transmission by increasing the cell surface expression of GABAA receptors in hippocampal neurons (Wan et al, 1997). In addition, activation of a PI 3-kinase/Akt-driven process is pivotal for phosphorylation of GABAA receptor β subunits and subsequent regulation of GABAA receptor cell surface expression (Wang et al, 2003; Vetiska et al, 2006).
Our previous studies have shown that leptin (1-10 nM) enhances NMDA receptor function which in turn promotes facilitation of hippocampal LTP (Shanley et al, 2001). However, in contrast to its effects on NMDA responses (Shanley et al, 2001) application of low nanomolar concentrations of leptin (10 nM) failed to influence GABAA receptor-mediated IPSCs in this study. Thus there are clear potency differences in the ability of leptin to modulate NMDA versus GABAA receptor-mediated events in hippocampal neurons. Thus it is likely that the predominant effect of leptin is to enhance NMDA receptor function and thereby facilitate the induction of hippocampal LTP. However under conditions where leptin levels are elevated, leptin would also facilitate fast inhibitory synaptic transmission onto CA1 synapses and subsequently reduce the likelihood of LTP induction at excitatory synapses (Wigstrom and Gustafsson, 1985). This in turn would be likely to counteract the facilitatory effects of leptin on hippocampal LTP.
Activation of PI 3-kinase-dependent signalling is implicated in the regulation and trafficking of other ion channel by leptin. Indeed, leptin enhances NMDA receptor function and hippocampal synaptic plasticity via a PI 3-kinase-dependent mechanism (Shanley et al, 2001; Harvey, 2005), whereas the ability of leptin to activate and translocate BK channels to hippocampal synapses involves a PI 3-kinase-dependent mechanism (O’Malley et al, 2005). Our previous studies have shown that BK channel activation underlies the regulation of hippocampal pyramidal neuron excitability by leptin (Shanley et al, 2002a), and this process is likely to underlie the anti-epileptogenic properties of leptin (Shanley et al, 2000b; Xu et al, 2008). In contrast the present data suggest that leptin regulates hippocampal neuron excitability by modulating GABAA receptor mediated synaptic transmission onto pyramidal neurons. This suggests that leptin depresses the excitability of hippocampal pyramidal neurons by either direct activation of pyramidal neurons BK channels, or indirectly by enhancing the inhibitory drive onto pyramidal neurons. It is interesting however, that both modes for regulating pyramidal neuron excitability involve leptin-driven PI 3-kinase-dependent mechanisms. It is not entirely clear how such distinct cellular targets, namely BK channels and GABAA receptors, are both triggered by the activation of PI 3-kinase. As PI 3-kinase can activate a range of downstream effector molecules, the likeliest scenario is that distinct signaling pathways are activated downstream of PI 3-kinase, which in turn couple leptin to different cellular outputs. Indeed, the present data indicate that activation of Akt is required for modulation of GABAA receptor-mediated synaptic transmission, whereas our previous studies indicate that BK channel stimulation requires a PtdIns(3,4,5)P3-dependent alteration in actin dynamics (O’Malley et al, 2005). As PI 3-kinase-dependent stimulation of Akt and PI 3-kinase-driven changes in the actin cytoskeleton both require an increase in PtdIns(3,4,5)P3 levels, it is not clear how specificity in the activation of downstream effectors is achieved. It is known that PI 3-kinase is a heterodimeric enzyme that consists of a catalytic subunit and an adaptor subunit. As different families of the adaptor subunits are expressed in the brain, it is feasible that distinct PI 3-kinase isoforms differentially regulate the spatial and temporal generation of PtdIns(3,4,5)P3 (Hirsch et al, 2007). Thus, it is possible that leptin by activating different PI 3-kinase isoforms results in spatially segregated accumulation of PtdIns(3,4,5)P3. Consistent with this, the subcellular localization of PtdIns(3,4,5)P3 is pivotal for directional cell migration in leukocytes (Hirsch et al, 2007) and in semaphorin 3A-mediated growth cone collapse in neurons (Chadborn et al, 2006).
In addition to facilitating fast inhibitory synaptic transmission, leptin evoked a persistent depression of fast inhibitory synaptic transmission (I-LTD) onto hippocampal CA1 synapses; an effect not associated with any change in PPR or CV suggesting a postsynaptic locus of expresion. This is the first demonstration of a long-lasting change in the efficacy of fast inhibitory synaptic transmission in response to acute application of leptin. Previous studies have explored the effects of leptin on GABAergic synaptic transmission in hypothalamic neurons. Indeed, acute application of leptin reduced the frequency of fast inhibitory synaptic transmission onto POMC neurons (Cowley et al, 2001; Munzberg et al, 2007), but not NPY neurons (Glaum et al, 1997) suggesting that leptin acts presynaptically to reduce GABA release onto POMC neurons. Consistent with these findings chronic deficiencies in leptin (ob/ob mice) result in marked increases in GABAergic inhibitory tone onto POMC neurons (Pinto et al, 2004). However, the cellular mechanisms responsible for these hypothalamic effects of leptin remain to be determined.
The present data indicate that leptin-induced I-LTD is likely to be distinct from the rapid facilitation of IPSCs as disinhibition was still observed under conditions that completely blocked the initial rapid facilitation induced by leptin. Thus, leptin-induced I-LTD was unaffected by blockade of both pre- and postsynaptic PI 3-kinase/Akt activity, suggesting that the persistent disinhibition evoked by leptin is likely to be mediated by a PI 3-kinase/Akt-independent signaling pathway. It is known that alterations in GABAergic tone can directly influence neuronal excitability and also indirectly regulate the induction of excitatory synaptic plasticity. Indeed, inhibitory synapses can modulate the induction of activity-dependent LTP at excitatory glutamatergic synapses, by regulating the degree of postsynaptic depolarization, as LTP induction is facilitated in the presence of GABAA receptor antagonists (Wigstrom and Gustafsson, 1985). LTP is also associated with altered neuronal excitability as it increases the ability of the EPSP to fire an action potential; a phenomenon known as E-S coupling component of LTP (Bliss & Lynch, 1988). Furthermore, persistent attenuation of GABAA-receptor mediated inhibitory drive has been shown to mediate the E-S coupling component of hippocampal LTP (Abraham et al, 1987, Chavez-Noriega et al, 1989; Lu et al, 2000; Chevaleyre & Castillo, 2003). Thus, the ability of leptin to induce a persistent reduction of GABAergic synaptic transmission onto CA1 pyramidal neurons is likely to facilitate the induction of LTP at this synapse.
Evidence is growing that the anti-obesity hormone leptin has widespread actions in the CNS. Indeed, several studies have implicated leptin in the regulation of excitatory synaptic plasticity (Shanley et al, 2001; Wayner et al 2004; Durakoglugil et al, 2005; Li et al, 2002; O’Malley et al, 2007). The capacity of leptin to promote long term modification of the inhibitory drive onto hippocampal CA1 pyramidal neurons is likely to affect the output of target neurons and in turn alter the excitability of neuronal networks. Thus, leptin-driven alterations in fast inhibitory synaptic transmission may play an important role during neuronal development when neuronal networks are stabilizing, and the maturation of synapses and neuronal networks is greatly influenced by neurotrophic factors. In support of this possibility, several studies have implicated leptin in neuronal development processes as leptin-deficiency results in abnormal CNS development (Ahima et al, 1999; Bouret and Simerly, 2004). Alternatively, leptin-induced changes in the inhibitory drive may be important for a number of CNS-driven diseases. For instance, it is well known that obesity and obesity-related disorders such as type II diabetes are associated with insulin resistance. However, individuals with these disorders also have high circulating peripheral levels of leptin, but display CNS-resistance to leptin. Obesity and type II diabetes are not only associated with a range of memory impairments, but are also causally linked to an increased incidence of Alzheimer’s disease (Craft, 2007). Thus it is likely that dysregulation and/or deficiencies in the leptin system play a role in the cognitive deficits associated with neurodegenerative disorders such as Alzheimer’s disease. In support of this possibility, recent studies have detected reductions in the circulating levels of leptin in Alzheimer’s disease patients (Power et al, 2001), whereas leptin significantly decreases the levels of amyloid β (Fewlass et al, 2004) and improves memory performance (Farr et al, 2005) in mouse models of Alzheimer’s disease. Thus the ability of leptin to bi-directionally modulate fast inhibitory synaptic transmission in the hippocampus may be pivotal for the role of this hormone in normal brain function but also in the cognitive deficits associated with leptin resistance.
Acknowledgements
This work is supported by the BBSRC (grant number: 94/C18771). JH is supported by a Wellcome University Award.
References
- Abraham WC, Gustafsson B, Wigstrom H. Long-term potentiation involves enhanced synaptic excitation relative to synaptic inhibition in guinea-pig hippocampus. J Physiol. 1987;394:367–80. doi: 10.1113/jphysiol.1987.sp016875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahima RS, Bjorbaek C, Osei S, Flier JS. Regulation of neuronal and glial proteins by leptin: implications for brain development. Endocrinology. 1999;140:2755–62. doi: 10.1210/endo.140.6.6774. [DOI] [PubMed] [Google Scholar]
- Bekkers JM, Richerson GB, Stevens CF. Origin of variability in quantal size in cultured hippocampal neurons and hippocampal slices. Proc. Natl. Acad. Sci. U S A. 1990;87:5359–62. doi: 10.1073/pnas.87.14.5359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss TVP, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–9. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- Bliss TVP, Lynch MA. Long-term potentiation of synaptic transmission in the hippocampus: properties and mechanisms. In: Landfield p.W., Deadwyler SA., editors. Long-Term Potentiation:From Biophysics to Behaviour. Alan R. Liss; New York: 1988. pp. 3–72. [Google Scholar]
- Bouret SG, Simerly RB. Minireview: Leptin and development of hypothalamic feeding circuits. Endocrinology. 2004;145:2621–6. doi: 10.1210/en.2004-0231. [DOI] [PubMed] [Google Scholar]
- Chadborn NH, Ahmed AI, Holt MR, Prinjha R, Dunn GA, Jones GE, Eickholt BJ. PTEN couples Sema3A signalling to growth cone collapse. J. Cell Sci. 2006;119:951–7. doi: 10.1242/jcs.02801. [DOI] [PubMed] [Google Scholar]
- Chavez-Noriega LE, Bliss TV, Halliwell JV. The EPSP-spike (E-S) component of long-term potentiation in the rat hippocampal slice is modulated by GABAergic but not cholinergic mechanisms. Neurosci Lett. 1989;104:58–64. doi: 10.1016/0304-3940(89)90329-7. [DOI] [PubMed] [Google Scholar]
- Chevaleyre V, Castillo PE. Heterosynaptic LTD of hippocampal GABAergic synapses: a novel role of endocannabinoids in regulating excitability. Neuron. 2003;38:461–72. doi: 10.1016/s0896-6273(03)00235-6. [DOI] [PubMed] [Google Scholar]
- Craft S. Insulin resistance and Alzheimer’s disease pathogenesis: potential mechanisms and implications for treatment. Curr Alzheimer Res. 2007;4:147–52. doi: 10.2174/156720507780362137. [DOI] [PubMed] [Google Scholar]
- Durakoglugil M, Irving AJ, Harvey J. Leptin induces a novel form of NMDA receptor-dependent long-term depression. J. Neurochem. 2005;95:396–405. doi: 10.1111/j.1471-4159.2005.03375.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmquist JK, Bjorbaek C, Ahima RS, Flier JS, Saper CB. Distributions of leptin receptor mRNA isoforms in the rat brain. J. Comp. Neurol. 1998;395:535–47. [PubMed] [Google Scholar]
- Farr SA, Banks WA, Morley JE. Effects of leptin on memory processing. Peptides. 2005;27:1420–5. doi: 10.1016/j.peptides.2005.10.006. [DOI] [PubMed] [Google Scholar]
- Fewlass DC, Noboa K, Pi-Sunyer FX, Johnston JM, Yan SD, Tezapsidis K. Obesity-related leptin regulates Alzheimer’s Abeta. FASEB J. 2004;18:1870–8. doi: 10.1096/fj.04-2572com. [DOI] [PubMed] [Google Scholar]
- Gaiarsa JL. Plasticity of GABAergic synapses in the neonatal rat hippocampus. J. Cell Mol. Med. 2004;8:31–7. doi: 10.1111/j.1582-4934.2004.tb00257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaum SR, Hara M, Bindokas VP, Lee CC, Polonsky KS, Bell GI, Miller RJ. Leptin, the obese gene product, rapidly modulates synaptic transmission in the hypothalamus. Mol. Pharmacol. 1996;50:230–5. [PubMed] [Google Scholar]
- Hakansson ML, Brown H, Ghilardi N, Skoda RC, Meister B. Leptin receptor immunoreactivity in chemically defined target neurons of the hypothalamus. J. Neurosci. 1998;18:559–72. doi: 10.1523/JNEUROSCI.18-01-00559.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey J, Solovyova N, Irving A. Leptin and its role in hippocampal synaptic plasticity. Prog. Lipid Res. 2006;45:369–78. doi: 10.1016/j.plipres.2006.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch E, Costa C, Ciraolo E. Phosphoinositide 3-kinases as a common platform for multi-hormone signaling. J. Endocrinol. 2007;194:243–56. doi: 10.1677/JOE-07-0097. [DOI] [PubMed] [Google Scholar]
- Irving AJ, Wallace L, Durakoglugil D, Harvey J. Leptin enhances NR2B-mediated N-methyl-D-aspartate responses via a mitogen-activated protein kinase-dependent process in cerebellar granule cells. Neurosci. 2006;138:1137–48. doi: 10.1016/j.neuroscience.2005.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kairiss EW, Abraham WC, Bilkey DK, Goddard GV. Field potential evidence for long-term potentiation of feed-forward inhibition in the rat dentate gyrus. Brain Res. 1987;401:87–94. doi: 10.1016/0006-8993(87)91167-x. [DOI] [PubMed] [Google Scholar]
- Kullmann DM. Amplitude fluctuations of dual component EPSCs in hippocampal pyramidal cells: implications for long-term potentiation. Neuron. 1994;12:1111–20. doi: 10.1016/0896-6273(94)90318-2. [DOI] [PubMed] [Google Scholar]
- Li XL, Aou S, Oomura Y, Hori N, Fukunaga K, Hori T. Impairment of long-term potentiation and spatial memory in leptin receptor-deficient rodents. Neurosci. 2002;113:607–15. doi: 10.1016/s0306-4522(02)00162-8. [DOI] [PubMed] [Google Scholar]
- Lu YM, Mansuy IM, Kandel ER, Roder J. Calcineurin-mediated LTD of GABAergic inhibition underlies the increased excitability of CA1 neurons associated with LTP. Neuron. 2000;26:197–205. doi: 10.1016/s0896-6273(00)81150-2. [DOI] [PubMed] [Google Scholar]
- Ma X-H, Zhong P, Gu Z, Feng J, Yan Z. Muscarinic potentiation of GABAA receptor currents is gated by insulin signaling in the prefrontal cortex. J. Neurosci. 2003;23:1159–68. doi: 10.1523/JNEUROSCI.23-04-01159.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdonald RL, Olsen RW. GABAA receptor channels. Annu. Rev. Neurosci. 1994;17:569–602. doi: 10.1146/annurev.ne.17.030194.003033. [DOI] [PubMed] [Google Scholar]
- McAllister AK, Stevens CF. Nonsaturation of AMPA and NMDA receptors at hippocampal synapses. Proc Natl Acad Sci U S A. 2000;97:6173–8. doi: 10.1073/pnas.100126497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKernan RM, Whiting PJ. Which GABAA-receptor subtypes really occur in the brain? Trends Neurosci. 1996;19:139–43. doi: 10.1016/s0166-2236(96)80023-3. [DOI] [PubMed] [Google Scholar]
- Malinow R, Tsien RW. Presynaptic enhancement shown by whole-cell recordings of long-term potentiation in hippocampal slices. Nature. 1990;346:177–80. doi: 10.1038/346177a0. [DOI] [PubMed] [Google Scholar]
- Mantzoros CS. Role of leptin in reproduction. Ann N Y Acad Sci. 2000;900:174–83. doi: 10.1111/j.1749-6632.2000.tb06228.x. [DOI] [PubMed] [Google Scholar]
- Mirshamsi S, Laidlaw HA, Ning K, Anderson E, Burgess LA, Gray A, Sutherland C, Ashford ML. Leptin and insulin stimulation of signalling pathways in arcuate nucleus neurones: PI3K dependent actin reorganization and KATP channel activation. BMC Neurosci. 2004;5:54. doi: 10.1186/1471-2202-5-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mody I, De Koninck Y, Otis TS, Soltesz I. Bridging the cleft at GABA synapses in the brain. Trends Neurosci. 1994;17:517–25. doi: 10.1016/0166-2236(94)90155-4. [DOI] [PubMed] [Google Scholar]
- Niswender KD, Schwartz MW. Insulin and leptin revisited: adiposity signals with overlapping physiological and intracellular signaling capabilities. Front. Neuroendocrinol. 2003;24:1–10. doi: 10.1016/s0091-3022(02)00105-x. [DOI] [PubMed] [Google Scholar]
- O’Malley D, Irving AJ, Harvey J. Leptin-induced dynamic alterations in the actin cytoskeleton mediate the activation and synaptic clustering of BK channels. FASEB J. 2005;19:1917–19. doi: 10.1096/fj.05-4166fje. [DOI] [PubMed] [Google Scholar]
- O’Malley D, Macdonald N, Mizielinska S, Connolly CN, Irving AJ, Harvey J. Leptin promotes rapid dynamic changes in hippocampal dendritic morphology. Mol. Cell. Neurosci. 2007;35:559–72. doi: 10.1016/j.mcn.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto S, Roseberry AG, Liu H, Diano S, Shanabrough M, Cai X, Friedman JM, Horvath TL. Rapid rewiring of arcuate nucleus feeding circuits by leptin. Science. 2004;304:110–5. doi: 10.1126/science.1089459. [DOI] [PubMed] [Google Scholar]
- Pitler TA, Alger BE. Postsynaptic spike firing reduces synaptic GABAA responses in hippocampal pyramidal cells. J Neurosci. 1992;12:4122–32. doi: 10.1523/JNEUROSCI.12-10-04122.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power DA, Noel J, Collins R, O’Neill D. Circulating leptin levels and weight loss in Alzheimer’s disease patients. Dement Geriatr Cogn Disord. 2001;12:167–70. doi: 10.1159/000051252. [DOI] [PubMed] [Google Scholar]
- Powis G, Bonjouklian R, Berggren MM, Gallegos A, Abraham R, Ashendel C, Zalkow L, Matter WF, Dodge J, Grindey G, et al. Wortmannin, a potent and selective inhibitor of phosphatidylinositol-3-kinase. Cancer Res. 1994;54:2419–23. [PubMed] [Google Scholar]
- Salgado H, Bellay T, Nichols JA, Bose M, Martinolich L, Perrotti L, Atzori M. Muscarinic M2 and M1 receptors reduce GABA release by Ca2+ channel modulation through activation of PI3K/Ca2+ -independent and PLC/Ca2+ -dependent PKC. J. Neurophysiol. 2007;98:952–965. doi: 10.1152/jn.00060.2007. [DOI] [PubMed] [Google Scholar]
- Shanley LJ, Irving AJ, Harvey J. Leptin enhances NMDA receptor function and modulates hippocampal synaptic plasticity. J. Neurosci. 2001;21:RC186. doi: 10.1523/JNEUROSCI.21-24-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanley LJ, Irving AJ, Rae MG, Ashford ML, Harvey J. Leptin inhibits rat hippocampal neurons via activation of large conductance calcium-activated K+ channels. Nature Neurosci. 2002a;5:299–300. doi: 10.1038/nn824. [DOI] [PubMed] [Google Scholar]
- Shanley LJ, O’Malley D, Irving AJ, Ashford ML, Harvey J. Leptin inhibits epileptiform-like activity in rat hippocampal neurones via PI 3-kinase-driven activation of BK channels. J. Physiol. 2002b;545:933–44. doi: 10.1113/jphysiol.2002.029488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shew T, Yip S, Sastry BR. Mechanisms involved in tetanus-induced potentiation of fast IPSCs in rat hippocampal CA1 neurons. J Neurophysiol. 2000;83:3388–401. doi: 10.1152/jn.2000.83.6.3388. [DOI] [PubMed] [Google Scholar]
- Sieghart W, Ernst M. Heterogeneity of GABAA receptors: revived interest in the development of subtype-selective drugs. Curr. Med. Chem.-Central Nervous System Agents. 2005;5:217–242. [Google Scholar]
- Spiegelman BM, Flier JS. Obesity and the regulation of energy balance. Cell. 2001;104:531–43. doi: 10.1016/s0092-8674(01)00240-9. [DOI] [PubMed] [Google Scholar]
- Thomas P, Mortensen M, Hosie AM, Smart TG. Dynamic mobility of functional GABAA receptors at inhibitory synapses. Nat. Neurosci. 2005;8:889–897. doi: 10.1038/nn1483. [DOI] [PubMed] [Google Scholar]
- Van der Heide LP, Ramakers GM, Smidt MP. Insulin signalling in the central nervous system: learning to survive. Prog. Neurobiol. 2006;79:205–21. doi: 10.1016/j.pneurobio.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Vetiska SM, Ahmadian G, Ju W, Liu L, Wymann MP, Wang YT. GABAA receptor-associated phosphoinositide 3-kinase is required for insulin-induced recruitment of postsynaptic GABAA receptors. Neuropharmacol. 2007;52:146–55. doi: 10.1016/j.neuropharm.2006.06.023. [DOI] [PubMed] [Google Scholar]
- Wan Q, Xiong ZG, Man HY, Ackerley CA, Braunton J, Lu WY, Becker LE, MacDonald JF, Wang YT. Recruitment of functional GABAA receptors to postsynaptic domains by insulin. Nature. 1997;388:686–90. doi: 10.1038/41792. [DOI] [PubMed] [Google Scholar]
- Wang Q, Liu L, Pei L, Ju W, Ahmadian G, Lu J, Wang Y, Liu F, Wang YT. Control of synaptic strength, a novel function of Akt. Neuron. 2003;38:915–28. doi: 10.1016/s0896-6273(03)00356-8. [DOI] [PubMed] [Google Scholar]
- Wayner MJ, Armstrong DL, Phelix CF, Oomura Y. Orexin-A (Hypocretin-1) and leptin enhance LTP in the dentate gyrus of rats in vivo. Peptides. 2004;25:991–6. doi: 10.1016/j.peptides.2004.03.018. [DOI] [PubMed] [Google Scholar]
- Wigström H, Gustafsson B. Facilitation of hippocampal long-lasting potentiation by GABA antagonists. Acta. Physiol. Scand. 1985;125:159–72. doi: 10.1111/j.1748-1716.1985.tb07703.x. [DOI] [PubMed] [Google Scholar]
- Xu L, Rensing N, Yang XF, Zhang HX, Thio LL, Rothman SM, Weisenfeld AE, Wong M, Yamada KA. Leptin inhibits 4-aminopyridine- and pentylenetetrazole-induced seizures and AMPAR-mediated synaptic transmission in rodents. J. Clin. Invest. 2008;118:272–80. doi: 10.1172/JCI33009. [DOI] [PMC free article] [PubMed] [Google Scholar]