Abstract
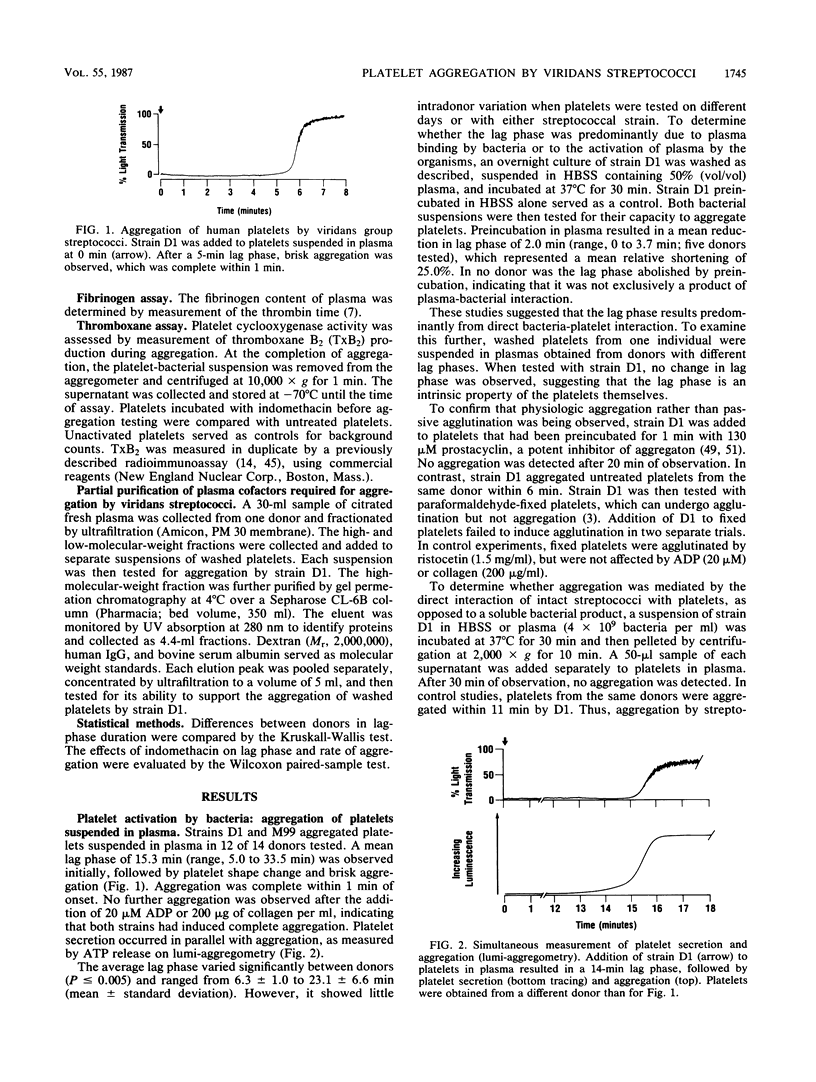
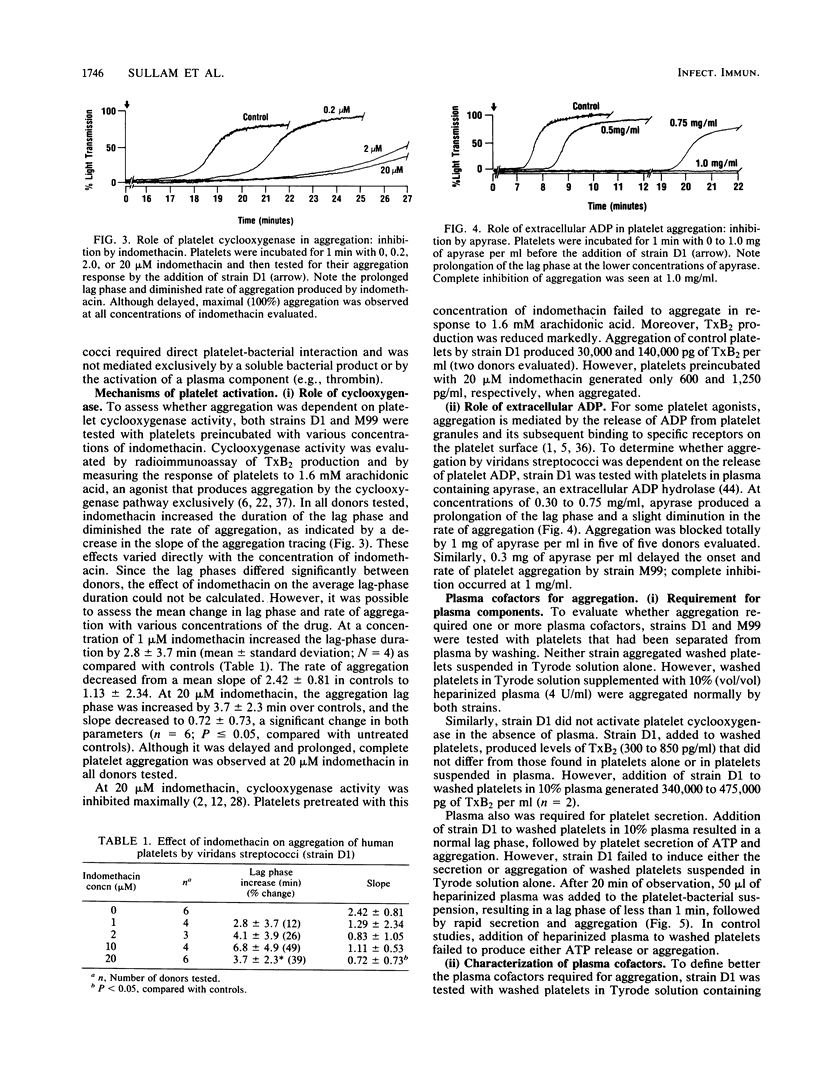
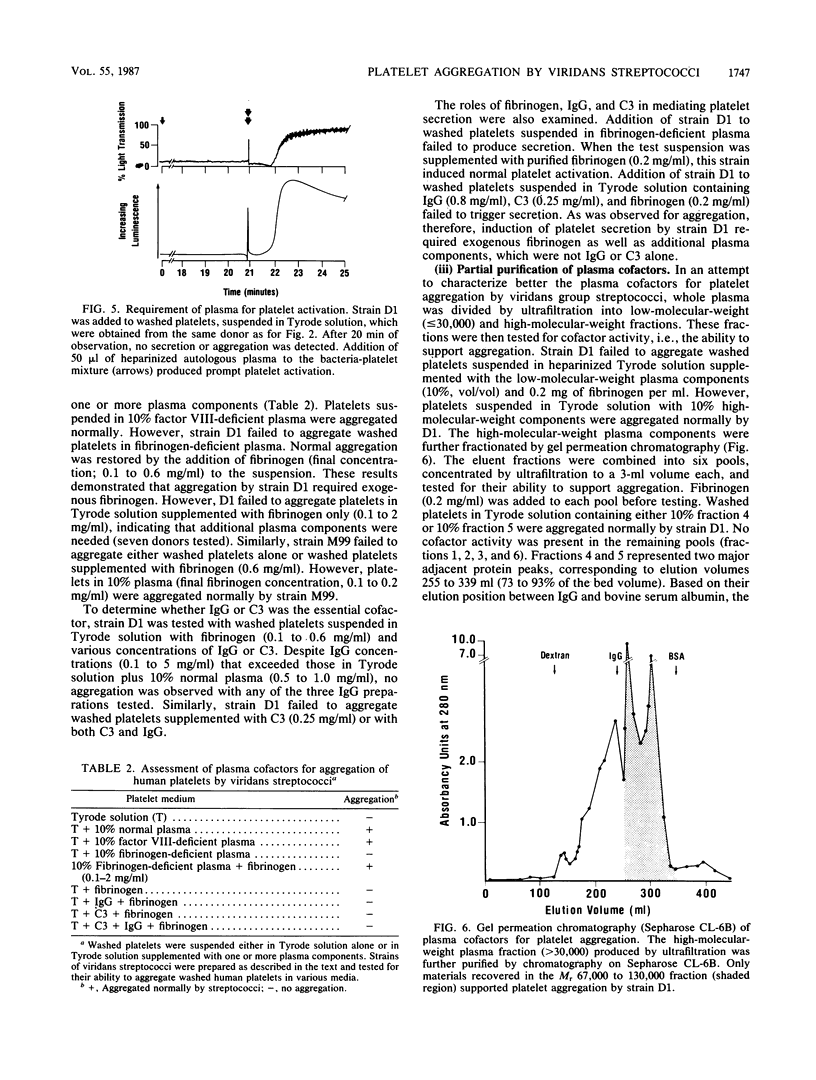
The direct aggregation of platelets is thought to be an important event in the pathogenesis of viridans streptococcal endocarditis, but the mechanisms for platelet activation are unknown. We evaluated the processes by which two endocarditis-producing strains of viridans group streptococci activated human platelets in vitro, as measured by platelet cyclooxygenase activity, secretion, and aggregation. Addition of either streptococcal strain to platelets suspended in whole plasma resulted in a mean lag phase of 15.3 min, followed by platelet secretion and brisk aggregation. The lag phase duration was dependent on the platelet donor and appeared to be a function of direct platelet-bacterial interaction. Aggregation was partially inhibited by 20 muM [corrected] indomethacin and blocked completely by 1 mg of apyrase, an extracellular ADP hydrolase, per ml. Neither strain aggregated washed platelets suspended in Tyrode solution alone. However, both strains produced maximal aggregation when the platelet suspension was supplemented with 10% (final concentration) normal plasma. Studies with factor-deficient plasmas demonstrated that exogenous fibrinogen was required for aggregation. One or more additional plasma components were needed, which eluted with a molecular weight of 67,000 to 130,000 on gel permeation chromatography. These cofactors have not been described for other platelet agonists, which suggests that viridans streptococci may aggregate human platelets by a novel mechanism.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler J. R., Handin R. I. Solubilization and characterization of a platelet membrane ADP-binding protein. J Biol Chem. 1979 May 25;254(10):3866–3872. [PubMed] [Google Scholar]
- Ali M., McDonald J. W. Reversible and irreversible inhibition of platelet cyclooxygenase and serotonin release by nonsteroidal antinflammatory drugs. Thromb Res. 1978 Dec;13(6):1057–1065. doi: 10.1016/0049-3848(78)90234-7. [DOI] [PubMed] [Google Scholar]
- Allain J. P., Cooper H. A., Wagner R. H., Brinkhous K. M. Platelets fixed with paraformaldehyde: a new reagent for assay of von Willebrand factor and platelet aggregating factor. J Lab Clin Med. 1975 Feb;85(2):318–328. [PubMed] [Google Scholar]
- Bang N. U., Heidenreich R. O., Trygstad C. W. Plasma protein requirements for human platelet aggregation. Ann N Y Acad Sci. 1972 Oct 27;201:280–299. doi: 10.1111/j.1749-6632.1972.tb16305.x. [DOI] [PubMed] [Google Scholar]
- Bennett J. S., Colman R. F., Colman R. W. Identification of adenine nucleotide binding proteins in human platelet membranes by affinity labeling with 5'-p-flurosulfonylbenzoyl adenosine. J Biol Chem. 1978 Oct 25;253(20):7346–7354. [PubMed] [Google Scholar]
- CLAUSS A. Gerinnungsphysiologische Schnellmethode zur Bestimmung des Fibrinogens. Acta Haematol. 1957 Apr;17(4):237–246. doi: 10.1159/000205234. [DOI] [PubMed] [Google Scholar]
- Charo I. F., Feinman R. D., Detwiler T. C. Interrelations of platelet aggregation and secretion. J Clin Invest. 1977 Oct;60(4):866–873. doi: 10.1172/JCI108841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clawson C. C., White J. G. Platelet interaction with bacteria. I. Reaction phases and effects of inhibitors. Am J Pathol. 1971 Nov;65(2):367–380. [PMC free article] [PubMed] [Google Scholar]
- Collins M. S., Dorsey J. H. Comparative in vivo activity between intravenous immune globulin prepared by reduction and alkylation or by low pH. J Infect Dis. 1985 Jun;151(6):1171–1173. doi: 10.1093/infdis/151.6.1171. [DOI] [PubMed] [Google Scholar]
- Cronberg S., Kubisz P. Demonstration of a plasmatic cofactor different from fibrinogen necessary for platelet release by ADP and adrenaline. Thromb Diath Haemorrh. 1970 Dec 31;24(3):409–418. [PubMed] [Google Scholar]
- Czuprynski C. J., Balish E. Interaction of rat platelets with Listeria monocytogenes. Infect Immun. 1981 Jul;33(1):103–108. doi: 10.1128/iai.33.1.103-108.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Caterina R., Giannessi D., Gazzetti P., Bernini W. Inhibition of platelet aggregation and thromboxane B2 production during aspirin treatment: dependence on the dose of the aggregating agent. Thromb Res. 1985 Jan 15;37(2):337–342. doi: 10.1016/0049-3848(85)90021-0. [DOI] [PubMed] [Google Scholar]
- Deykin D., Pritzker C. R., Scolnick E. M. Plasma co-factors in adenosine diphosphate-induced aggregation of human platelets. Nature. 1965 Oct 16;208(5007):296–298. doi: 10.1038/208296b0. [DOI] [PubMed] [Google Scholar]
- Dray F., Gerozissis K., Kouznetzova B., Mamas S., Pradelles P., Trugnan G. New approach to the RIA of prostaglandins and related compounds using iodinated tracers. Adv Prostaglandin Thromboxane Res. 1980;6:167–180. [PubMed] [Google Scholar]
- Durack D. T., Beeson P. B. Experimental bacterial endocarditis. II. Survival of a bacteria in endocardial vegetations. Br J Exp Pathol. 1972 Feb;53(1):50–53. [PMC free article] [PubMed] [Google Scholar]
- Durack D. T. Experimental bacterial endocarditis. IV. Structure and evolution of very early lesions. J Pathol. 1975 Feb;115(2):81–89. doi: 10.1002/path.1711150204. [DOI] [PubMed] [Google Scholar]
- Facklam R. R. Physiological differentiation of viridans streptococci. J Clin Microbiol. 1977 Feb;5(2):184–201. doi: 10.1128/jcm.5.2.184-201.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinman R. D., Lubowsky J., Charo I., Zabinski M. P. The lumi-aggregometer: a new instrument for simultaneous measurement of secretion and aggregation by platelets. J Lab Clin Med. 1977 Jul;90(1):125–129. [PubMed] [Google Scholar]
- Ferguson D. J., McColm A. A., Savage T. J., Ryan D. M., Acred P. A morphological study of experimental rabbit staphylococcal endocarditis and aortitis. I. Formation and effect of infected and uninfected vegetations on the aorta. Br J Exp Pathol. 1986 Oct;67(5):667–678. [PMC free article] [PubMed] [Google Scholar]
- Ganguly P., Fossett N. G. Inhibition of thrombin-induced platelet aggregation by a derivative of wheat germ agglutinin. Evidence for a physiologic receptor of thrombin in human platelets. Blood. 1981 Feb;57(2):343–352. [PubMed] [Google Scholar]
- Hammer C. H., Wirtz G. H., Renfer L., Gresham H. D., Tack B. F. Large scale isolation of functionally active components of the human complement system. J Biol Chem. 1981 Apr 25;256(8):3995–4006. [PubMed] [Google Scholar]
- Harfenist E. J., Guccione M. A., Packham M. A., Kinlough-Rathbone R. L., Mustard J. F. Arachidonate-induced fibrinogen binding to thrombin-degranulated rabbit platelets is independent of released ADP. Blood. 1982 May;59(5):956–962. [PubMed] [Google Scholar]
- Hawiger J., Steckley S., Hammond D., Cheng C., Timmons S., Glick A. D., Des Prez R. M. Staphylococci-induced human platelet injury mediated by protein A and immunoglobulin G Fc fragment receptor. J Clin Invest. 1979 Oct;64(4):931–937. doi: 10.1172/JCI109559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzberg M. C., Brintzenhofe K. L. ADP-like platelet aggregation activity generated by viridans streptococci incubated with exogenous ATP. Infect Immun. 1983 Apr;40(1):120–125. doi: 10.1128/iai.40.1.120-125.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzberg M. C., Brintzenhofe K. L., Clawson C. C. Aggregation of human platelets and adhesion of Streptococcus sanguis. Infect Immun. 1983 Mar;39(3):1457–1469. doi: 10.1128/iai.39.3.1457-1469.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzberg M. C., Brintzenhofe K. L., Clawson C. C. Cell-free released components of Streptococcus sanguis inhibit human platelet aggregation. Infect Immun. 1983 Oct;42(1):394–401. doi: 10.1128/iai.42.1.394-401.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hjelm H., Hjelm K., Sjöquist J. Protein A from Staphylococcus aureus. Its isolation by affinity chromatography and its use as an immunosorbent for isolation of immunoglobulins. FEBS Lett. 1972 Nov 15;28(1):73–76. doi: 10.1016/0014-5793(72)80680-x. [DOI] [PubMed] [Google Scholar]
- Honey A. C., Lad N., Tuffin D. P. Effect of indomethacin and dazoxiben on intravascular platelet aggregation in the anaesthetized rabbit. Thromb Haemost. 1986 Aug 20;56(1):80–85. [PubMed] [Google Scholar]
- Jamieson G. A., Okumura T., Hasitz M. Structure and function of platelet glycocalicin. Thromb Haemost. 1980 Feb 29;42(5):1673–1678. [PubMed] [Google Scholar]
- Kurpiewski G. E., Forrester L. J., Campbell B. J., Barrett J. T. Platelet aggregation by Streptococcus pyogenes. Infect Immun. 1983 Feb;39(2):704–708. doi: 10.1128/iai.39.2.704-708.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEVY H. B., SOBER H. A. A simple chromatographic method for preparation of gamma globulin. Proc Soc Exp Biol Med. 1960 Jan;103:250–252. doi: 10.3181/00379727-103-25476. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Malmgren R. ATP-secretion occurs as an initial response in collagen induced platelet activation. Thromb Res. 1986 Aug 15;43(4):445–453. doi: 10.1016/0049-3848(86)90089-7. [DOI] [PubMed] [Google Scholar]
- Mancini G., Carbonara A. O., Heremans J. F. Immunochemical quantitation of antigens by single radial immunodiffusion. Immunochemistry. 1965 Sep;2(3):235–254. doi: 10.1016/0019-2791(65)90004-2. [DOI] [PubMed] [Google Scholar]
- March S. C., Parikh I., Cuatrecasas P. A simplified method for cyanogen bromide activation of agarose for affinity chromatography. Anal Biochem. 1974 Jul;60(1):149–152. doi: 10.1016/0003-2697(74)90139-0. [DOI] [PubMed] [Google Scholar]
- Marguerie G. A., Edgington T. S., Plow E. F. Interaction of fibrinogen with its platelet receptor as part of a multistep reaction in ADP-induced platelet aggregation. J Biol Chem. 1980 Jan 10;255(1):154–161. [PubMed] [Google Scholar]
- Miller J. L., Kupinski J. M., Hustad K. O. Characterization of a platelet membrane protein of low molecular weight associated with platelet activation following binding by monoclonal antibody AG-1. Blood. 1986 Sep;68(3):743–751. [PubMed] [Google Scholar]
- Murer E. H. The role of platelet calcium. Semin Hematol. 1985 Oct;22(4):313–323. [PubMed] [Google Scholar]
- Mustard J. F., Packham M. A., Kinlough-Rathbone R. L., Perry D. W., Regoeczi E. Fibrinogen and ADP-induced platelet aggregation. Blood. 1978 Aug;52(2):453–466. [PubMed] [Google Scholar]
- Mustard J. F., Perry D. W., Ardlie N. G., Packham M. A. Preparation of suspensions of washed platelets from humans. Br J Haematol. 1972 Feb;22(2):193–204. doi: 10.1111/j.1365-2141.1972.tb08800.x. [DOI] [PubMed] [Google Scholar]
- Mustard J. F., Perry D. W., Kinlough-Rathbone R. L., Packham M. A. Factors responsible for ADP-induced release reaction of human platelets. Am J Physiol. 1975 Jun;228(6):1757–1765. doi: 10.1152/ajplegacy.1975.228.6.1757. [DOI] [PubMed] [Google Scholar]
- Phillips D. R., Agin P. P. Platelet plasma membrane glycoproteins. Identification of a proteolytic substrate for thrombin. Biochem Biophys Res Commun. 1977 Apr 25;75(4):940–947. doi: 10.1016/0006-291x(77)91473-5. [DOI] [PubMed] [Google Scholar]
- Rozenberg M. C., Holmsen H. Adenine nucleotide metabolism of blood platelets. IV. Platelet aggregation response to exogenous ATP and ADP. Biochim Biophys Acta. 1968 Apr 22;157(2):280–288. [PubMed] [Google Scholar]
- Sors H., Pradelles P., Dray F., Rigaud M., Maclouf J., Bernard P. Analytical methods for thromboxane B2 measurement and validation of radioimmunoassay by gas liquid chromatography-mass spectrometry. Prostaglandins. 1978 Aug;16(2):277–290. doi: 10.1016/0090-6980(78)90030-8. [DOI] [PubMed] [Google Scholar]
- Tangen O., Andrae M. L., Nilsson B. E. Nucleotide leakage from platelets in artificial media: prevention by albumin and other macromolecules and relation to ADP-induced platelet aggregation. Scand J Haematol. 1973;11(3):241–248. doi: 10.1111/j.1600-0609.1973.tb00124.x. [DOI] [PubMed] [Google Scholar]
- Tangen O., Berman H. J., Marfey P. Gel filtration. A new technique for separation of blood platelets from plasma. Thromb Diath Haemorrh. 1971 Jun 30;25(2):268–278. [PubMed] [Google Scholar]
- Terleckyj B., Willett N. P., Shockman G. D. Growth of several cariogenic strains of oral streptococci in a chemically defined medium. Infect Immun. 1975 Apr;11(4):649–655. doi: 10.1128/iai.11.4.649-655.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderwel M., Haslam R. J. Inhibition and subsequent enhancement of platelet responsiveness by prostacyclin in the rabbit. Relationship to platelet adenosine 3',5'-cyclic monophosphate. J Clin Invest. 1985 Jul;76(1):233–240. doi: 10.1172/JCI111952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh P. N. Albumin density gradient separation and washing of platelets and the study of platelet coagulant activities. Br J Haematol. 1972 Feb;22(2):205–217. doi: 10.1111/j.1365-2141.1972.tb08801.x. [DOI] [PubMed] [Google Scholar]
- Weiss H. J., Turitto V. T. Prostacyclin (prostaglandin I2, PGI2) inhibits platelet adhesion and thrombus formation on subendothelium. Blood. 1979 Feb;53(2):244–250. [PubMed] [Google Scholar]



