Abstract
Changing the direction of the line of sight is essential for the visual exploration of our environment. When the head does not move, re-orientation of the visual axis is accomplished with high velocity, conjugate movements of the eyes known as saccades. Our understanding of the neural mechanisms that control saccadic eye movements has advanced rapidly as specific hypotheses have been developed, evaluated and sometimes rejected on the basis of new observations. Constraints on new hypotheses and new tests of existing models have often arisen from the careful assessment of behavioral observations. The definition of the set of features (or rules) of saccadic eye movements was critical in the development of hypotheses of their neural control.
When the head is free to move, changes in the direction of the line of sight can involve simultaneous saccadic eye movements and movements of the head. When the head moves in conjunction with the eyes to accomplish these shifts in gaze direction, the rules that helped define head-restrained saccadic eye movements are altered. For example, the slope relationship between duration and amplitude for saccadic eye movements is reversed (the slope is negative) during gaze shifts of similar amplitude initiated with the eyes in different orbital positions. Modifications to the hypotheses developed in head-restrained subjects may be needed to account for these new observations. This review briefly recounts features of head-restrained saccadic eye movements, and then describes some of the characteristics of coordinated eye-head movements that have led to development of new hypotheses describing the mechanisms of gaze shift control.
Scope of this Review
The goal of this review is to present and discuss the development of ideas about the mechanisms that control visual orienting movements. An appreciation of the rules that characterize the coordination of the eyes and head is a critical step in constraining and possibly rejecting alternative hypotheses describing their control. Development of specific models of gaze control, and designing neurophysiological experiments that further define the implementation of these mechanisms has been and continues to be dependent upon a clear understanding of how the eyes and head move together in order to redirect the line of sight. This review will focus on eye-head coordination and the kinematics (temporal progression) of gaze shifts, the alterations of saccadic eye movements that occur when coupled with movements of the head, and how these observations have influenced formal descriptions of this system. Visual orienting behaviors will be emphasized and data from non-human primates will be discussed in detail. In addition, movements to targets displaced along the horizontal meridian will be the primary focus; there has been significantly less work on vertical and oblique gaze shifts (although see (Tomlinson and Bahra 1986a; Tweed et al. 1995; Goossens and vanOpstal 1997; Freedman 2005; Freedman and Cecala 2008)). The neural elements and description of neurophysiological observations will be touched on only briefly (see (Fuchs et al. 1985; Sparks and Hartwich-Young 1989; Keller 1991; Sparks 1991; Freedman and Sparks 1997a; Scudder et al. 2002)). Although the ultimate goal of much of the discussed research is the revelation of the neural mechanisms that underlay the observed behavior, correlations between aspects of gaze, eye and head movements can confound interpretations of neural recording studies. Unambiguous conclusions can be drawn only when movement parameters are dissociable, and this review will focus on identifying conditions of these dissociations.
Context
The foveal region of the primate retina subtends about 5° of visual angle. As a result of this concentration of photoreceptors (cones), at any one time, high acuity visual information is available from only ∼0.03% of the surrounding environment. With each shift in the direction of gaze, new images fall on the highly specialized region of the retina permitting sequential extraction of high resolution visual information from a much larger portion of the world. Volitional shifts of the line of sight can be accomplished with high velocity, saccadic eye movements. These conjugate movements of the eyes are defined by a set of stereotypical relationships between movement amplitude, duration and peak velocity, and generally occur at a rate of 3−4 per second. Saccades can greatly increase the range of the visual environment that can be surveyed. However, eye movements are neuromechanically restricted such that when the eyes begin in a central orbital position, the largest movements do not typically exceed ±40 − 45° (in humans and rhesus monkeys; the oculomotor range in many other species is significantly smaller). To extend these limits without moving the body, animals with mobile heads can redirect the line of sight by simultaneously moving the head and eyes. While the control of eye-head movements is of interest because it provides insight into the generation and control of coordinated multi-joint movements, gaze shifts are also behaviorally relevant and critically important for proper functioning of the visual system.
When the head does not participate
Behavioral observations
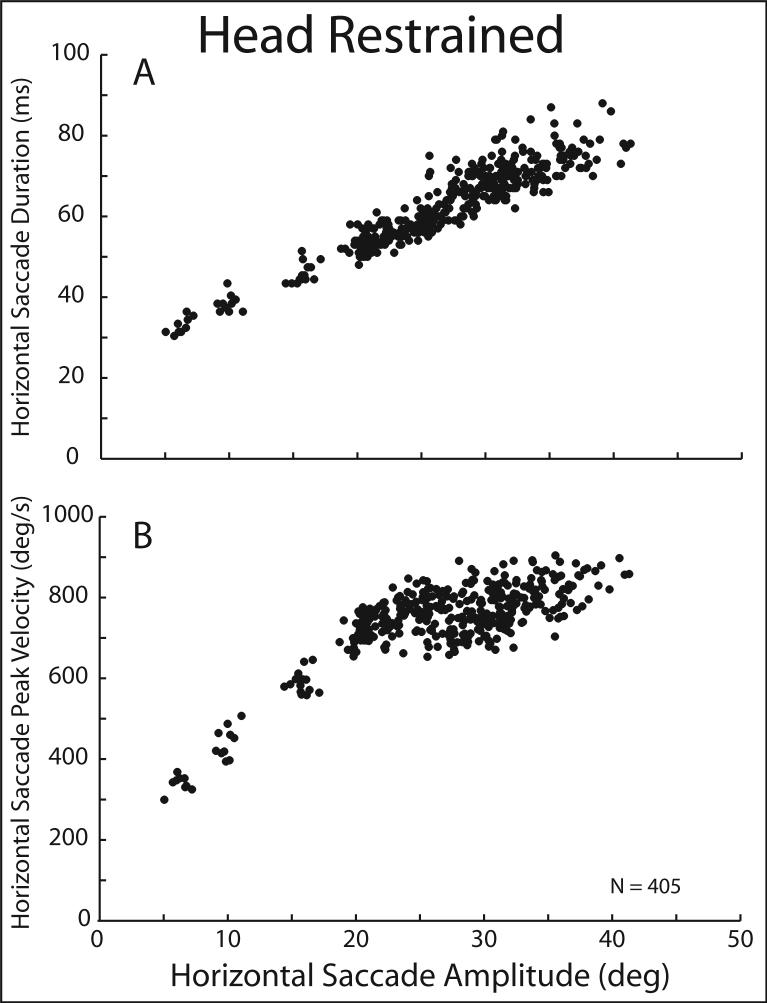
In a number of species (including humans, non-human primates, cats, rabbits, et al.) that have mobile eyes and heads, changing the direction of the line of sight does not necessarily require simultaneous movements of the head. The oculomotor range of humans and rhesus monkeys is approximately ±40° (along the horizontal meridian – it is slightly smaller along the vertical axis) and movements to targets within this central 80° region could be accomplished without head movements. As described below, the actual size of the region of visual space over which saccades are made without contributions from simultaneous head movements is much smaller than 80°. Nonetheless, saccades can be made in the absence of head movements (in fact as you read this, you are making a series of small saccades to the right followed by a larger saccade to the left, but unless you are sitting very close to your computer screen or to the manuscript, it is unlikely that head movements are helping you redirect your line of sight; see (Lee 1999) for discussion of eye-head coordination during reading). Experimentally, saccadic eye movements were described under conditions that prevented movements of the head. Several measures of saccades made under these restricted conditions are illustrated in Figure 1. As shown in panel A, during saccades made along the horizontal meridian and within the amplitude limits displayed (from 5−45°), the duration of a saccade increases linearly with increases in movement amplitude. In this example the slope of this relationship was 1.4 ms/deg. Similar values have been reported by others, although with human subjects the slopes of these relationships tend to be between 1.5−3 ms/deg (Bahill et al. 1975; Baloh et al. 1975; Bahill et al. 1981). Over the same range of saccade amplitudes, peak velocity increases monotonically as amplitude increases (Fig. 1B), but the relationship is non-linear and approaches a limit that in rhesus monkeys is ∼900−1000°/s. The linear duration-amplitude and non-linear peak velocity- amplitude relationships are the defining characteristics of saccadic eye movements.
Figure 1.
Saccade duration (A) and peak velocity (B) are plotted as functions of saccade amplitude for a set of 405 movements made when the head was prevented from moving. Data are from a rhesus monkey; movements initiated with the eyes centered in the orbits.
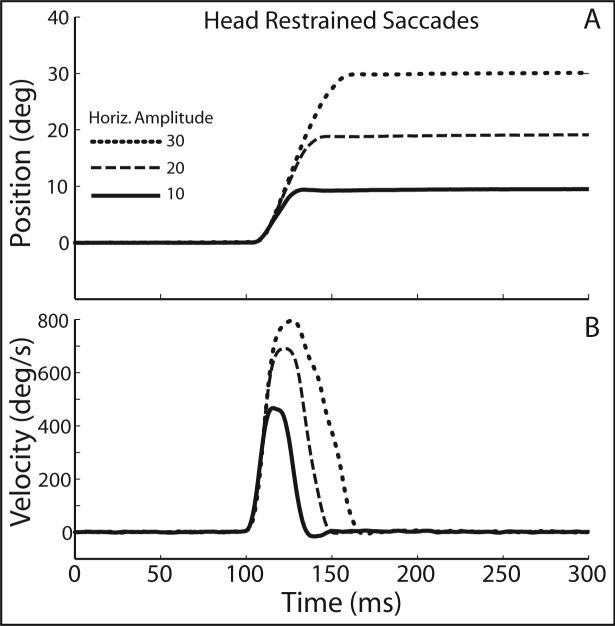
In addition to the metrical relationships shown above, saccadic eye movements made when the head is restrained evolve as a function of time in a stereotyped pattern that is amplitude dependent. Figure 2 illustrates this pattern by plotting eye position (A) and velocity (B) as functions of time for 3 head restrained saccades. Movements were directed along the horizontal meridian and had amplitudes of 9° (black), 18° (dark gray), and 30° (light gray). Position and velocity traces are aligned on movement initiation. As movement amplitudes increased, peak velocity and duration increased. In addition, as shown in Fig. 2B, saccadic eye movements generally accelerate along the same trajectories regardless of amplitude. Velocity profiles are typically symmetrical, especially during movements between ∼5−25°. During larger saccades the deceleration phase is often longer than the acceleration phase, skewing the velocity profile (vanGisbergen et al. 1984; vanOpstal and vanGisbergen 1987). Nonetheless, for a given amplitude (and direction) saccade velocity profiles are stereotyped. These relationships make it possible to predict (with reasonable accuracy) the duration and peak velocity of a saccade as well as the shape of the velocity profile given only information about movement amplitude and direction, or in fact, given only information about the locations of the visual targets since the vector of a saccade is correlated with target displacement.
Figure 2.
Three example saccades made when the head was restrained. Position (A) and velocity (B) are plotted as functions of time. Movements are aligned at saccade onset.
Models of the saccadic system
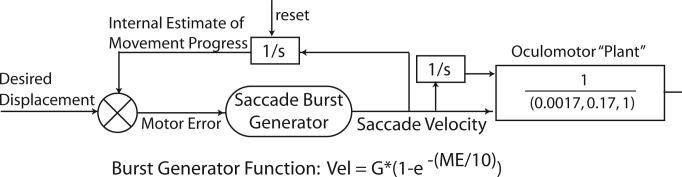
Using information about the saccadic system, the activity of motor neurons (Robinson 1970) and the biomechanics of the eye, its musculature and connective tissues (Robinson 1964; Robinson et al. 1969), David A. Robinson developed a control systems-based model of the saccadic system (Robinson 1973b; Robinson 1973a; vanGisbergen et al. 1981). This model was based on the assumption that eye position in the orbit was controlled via feedback. Based on experimental observations of saccades of different speeds this assumption was challenged. It was suggested that eye displacement (the change in position) was the controlled parameter (Jürgens et al. 1981a; Becker and Jürgens 1990). This modified hypothesis has survived repeated tests of its predictions and remains one of the clearest examples of the benefits and proper use of modeling in understanding brain function. The Becker and Jürgens model (schematized in Fig. 3) uses a second order linear filter that approximates the elastic and viscous properties of the orbits, muscles and connective tissue (collectively known as the oculomotor ‘plant’). As shown, in Fig. 3, there are two inputs to this filter, one that is proportional to saccade velocity (the output of the saccade burst generator), and a second that is proportional to eye position relative to the head (derived by mathematically integrating the velocity command (the integrator is indicated using Laplace transform notation: 1/s)). The velocity command is produced by a high gain, non-linear element known as the burst generator (the exponential function that converts motor error (ME) into a signal proportional to saccade velocity as given in Fig. 3). The input to this hypothesized element is the current motor error – the difference between the current position of the eyes and the position that is intended or desired at the end of the saccade. This error signal is calculated by comparing an input of desired position with an internal estimate of the current position – an estimate based on the integral of the output of the burst generator. This local feedback concept was introduced to avoid the difficulties that arise from using visual feedback to assess current eye position. In particular, the long retinal processing delays (40−50 ms) can be longer than the duration of many saccades. As a result of this long delay, feedback control using visual information would be unstable. Using an internal estimate of the current position permits the increased accuracy of feedback control without the instability of long delay times. In a slight modification of this original model, the input is changed to a signal of the desired displacement of the eyes (a signal independent of the positions of the eyes in the orbits) and the current displacement estimate is calculated through a resettable feedback integrator (reset after completion of each saccade) that is separate from the feed-forward integrator. Several reviews of these ideas describe the neural mechanisms that make up many of the analytical elements hypothesized in the model (Jürgens et al. 1981a; Fuchs et al. 1985; Hepp et al. 1989; Keller 1991; Moschovakis 1994; Leigh and Zee 1999; Scudder et al. 2002; Sparks 2002).
Figure 3.
Schematic diagram showing the major features of the saccade control circuit. Desired displacement of the eye serves as the reference input to a comparator. An internal estimate of current eye displacement is subtracted from the reference signal to produce a signal of current motor error. This is the input to the saccade burst generator – an exponential function that produces a signal proportional to saccade velocity (burst generator function is displayed: “Vel” is the velocity command as a function of time; G is the asymptotic value of the exponential function or “gain”; ME is the motor error input). The saccade velocity command is the direct input to the “oculomotor plant” (muscles, connective tissue and orbital mechanical properties approximated by a second order linear filter). The second input to the plant is the integral of the velocity command (1/s = Laplace notation for integration). In addition a second resettable integrator resides in the feedback pathway and contributes to the generation of the internal estimate of current displacement.
When the head contributes
A number of issues arise when gaze shifts are made under conditions that permit movements of the head. Among these are: Under what conditions does the head contribute to the gaze shift? Are the eye and head components of gaze shifts tightly linked or are they dissociable? What factors determine how large the head contribution will be? Do the rules that define head-restrained saccades remain unaltered when the head moves? If not, what are the defining characteristics of head-unrestrained gaze shifts? When the head does make a contribution, it is moving at the same time and in the same direction as the eyes, so what role does the vestibuloocular reflex (VOR) play during these movements? How do these two competing subsystems (the gaze-stabilizing VOR and the gaze-destabilizing gaze shift command) coexist? Although many of these issues have been investigated, there is not necessarily consensus on the answers that have been proposed. As discussed below, the study of the neural control of coordinated movements continues to be a rich and vigorous pursuit.
Temporal coupling of eyes and head
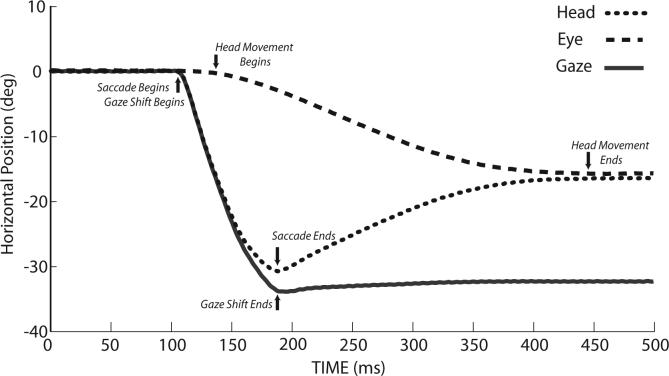
In some of the earliest work on eye-head coordination, Emilio Bizzi and his colleagues recorded horizontal eye and head movements as well as neck muscle EMG in trained rhesus monkeys (Bizzi et al. 1971; Bizzi et al. 1972a; Bizzi et al. 1972b; Morasso et al. 1973). They described a typical gaze shift beginning when the eyes and head were stationary. A similar movement is shown in Fig. 4, in order to highlight several of the key points made by Bizzi and colleagues; this figure also provides an opportunity to define important aspects of gaze shifts. For example, gaze shifts are defined as the change in the direction of the line of sight relative to a fixed, external referent (i.e. the line of sight in space). Head movements are similarly defined as head positions in space. Eye movements or saccades, are defined as the change in eye positions relative to the head. In Fig. 4 gaze (black), eye (dark gray) and head (light gray) positions are plotted as functions of time and superimposed for comparison. As indicated, the gaze shift begins when the saccadic eye movement rapidly changes the positions of the eyes relative to the head. The gaze shift ends when the line of sight is directed toward the visual target, and the rapid eye movement component of the gaze shift ends at approximately the same time. At this point the eyes are deviated in the orbits by ∼30° and the head has moved < 2°. Although the line of sight is now directed at the visual target and the saccadic eye movement has ended, the head does not stop moving for an additional 250ms and 15° of rotation. During this continuing movement of the head toward the target, the eyes move in the opposite direction at a velocity that is approximately the same as that of the head. As a result the direction of the line of sight changes very little. Two distinct measures of the head movement can be defined. The first is the total movement of the head from start to finish (in this example 17°). The second is a measure of the amount that the head movement contributed to the accomplishment of the gaze shift (often referred to as the head contribution), and in this example the head contributed ∼2° to the overall change in gaze.
Figure 4.
Single trial example of a gaze shift (and the eye and head components of the movements) made when the head is free to move. Gaze is the direction of the line of sight relative to an external/spatial reference, Head is the position of the head relative to an external/spatial reference, and Eye is the position of the eye relative to the head. Eye position + Head position at each time sample equals Gaze position. Experimentally, gaze and head positions are measured directly using scleral and head-mounted coils sitting inside alternating magnetic fields (Fuchs and Robinson 1966; Judge et al. 1980) and eye position is calculated by subtracting head from gaze positions. Arrows indicate features of eye-head movements, discussed more fully in related text.
The movement described above is fairly typical of gaze shifts of this amplitude made to visual targets that appear in unexpected locations and/or at unpredictable times (Bizzi et al. 1972a; Zangemeister and Stark 1982). The saccadic eye movement begins 25−40 ms before the head movement begins, and it ends just after the onset of the head movement. Despite the delay in the beginning of the head movement, measurement of eye and neck muscle activity using electromyography (EMG) indicates that an increase in agonist (and decrease in antagonist) neck muscle EMG precedes the increase in eye muscle EMG by 20 ms (Bizzi et al. 1972a; Zangemeister and Stark 1981). These data suggest that the neural signals used to “initiate triggered eye-head coordination are delivered synchronously to all neck muscles and shortly thereafter to the eyes (Bizzi et al. 1972a)”.
In addition to synchronous activation of neck and eye muscles seen during head unrestrained gaze shifts, other data suggest a tight coupling of the timing of the eye and head components of gaze shifts. For example, in rabbits (Collewijn 1977), cats (Vidal et al. 1982), and monkeys (Lestienne et al. 1984) with restrained heads, there is a clear coupling of neck muscle EMG and eye position. During saccadic eye movements, vestibular nystagmus and pursuit, when the eyes move away from the center of the orbits, neck muscle activity on the side ipsilateral to the direction of the eye movement increases, and contralateral neck muscle activity decreases. Lestienne et al. (1984) point out the tight coupling between saccadic eye movements and attempted head movements (assessed using neck muscle EMG in the head restrained subject) and suggest that eye-head coupling although perhaps not compulsory in primates may be an underlying mechanism for eye-head coordination. In the head unrestrained cat, the coupling between eyes and head during gaze shifts is even more pronounced (Guitton et al. 1990). These authors argue that the covariance of eye and head movement velocities, covariance of eye and head latencies, and the generally linear phase plane relationships (plots of head acceleration as a function of eye velocity are linear during the rapid portion of gaze shifts in the cat) are indicative of a common motor command that drives both the head and eyes (Guitton et al. 1990; Galiana and Guitton 1992).
Although movements of the eyes and head can occur synchronously, suggesting a tight coupling and perhaps indicating a common motor command, it is possible to dissociate the initiation of eye and head movements. For instance, the relative timing of the eyes and head depends upon several factors including the spatial and/or temporal predictability of the target (Bizzi et al. 1972a; Zangemeister and Stark 1982), gaze shift amplitude (Barnes 1979; Guitton and Volle 1987; Freedman and Sparks 1997b), and a propensity to move the head (Fuller 1992; Stahl 1999). Task requirements are also likely factors. For example, in some reports using cats as subjects the saccadic eye movement tended to precede head movement onset by ∼30ms (Blakemore and Donaghy 1980), whereas in other studies the timing of eyes and head was reversed: head movements preceded saccade onset by ∼40ms (Guitton et al. 1984). This variability in the relative timing of the start of eye and head movements suggests that the triggering of the eyes and head are separable. The EMG data also indicate that the underlying neural control signals occur at different times (Bizzi et al. 1972a; Morasso et al. 1973).
Additional support for the idea that the triggering of eye and head components of gaze shifts are not tightly coupled comes from comparison of the relative timing of eye and head movement onset during gaze shifts of different amplitudes. The relationship between gaze shift amplitude and relative eye-head onset times was implicit in data from Zangemeister and Stark (1982). One type of movements had small amplitudes and the head lagged saccade onset (Zangemeister and Stark Type 1), whereas, Other movements were much larger and the head movement began before the onset of the saccade (Type 3). In non-human primates, as gaze shift amplitude increases, the time from saccade onset to head movement onset declines monotonically, until head movement onset is simultaneous with the onset of saccades, or even precedes saccade onset (Freedman and Sparks 1997b). In human subjects, head movements that preceded the onset of saccades were also observed, particularly when the target location was predictable (Moschner and Zangmeister 1993).
Finally, it is possible to artificially delay the onset of saccadic eye movements by electrically stimulating in the pontine omnipause region. When stimulation in this region occurs just prior to the onset of a gaze shift, the beginning of the saccadic eye movement is delayed until after the end of the stimulation train. In contrast, head movement initiation occurs at approximately the same time relative to the movement initiation cue (Gandhi and Sparks 2007). These data are consistent with the hypothesis that the activity in omnipause neurons prevents the onset of saccadic eye movements, but does not appear to affect the triggering of movements of the head.
To summarize, from observations of visual orienting behavior it is clear that movements of the eyes and head can begin at approximately the same times. However, recording the activity of neck and eye muscles reveals that even when movement onsets are synchronous, the command to move the head precedes the command to move the eyes. Furthermore, inspection of the behavior over a broad range of gaze shift amplitudes, task requirements, and target predictability indicates that the relative timing of eye and head movements is variable: the head can lag the onset of eye movements during small amplitude gaze shifts, but during large amplitude movements, or movements to target locations that are predictable head movements can begin well before saccades. And, electrical stimulation in the omnipause neuron region can delay saccade onset without altering the initiation of head movements; evidence that the triggering mechanisms for the eyes and head are not shared. Taken together these data suggest a separation (at least with respect to movement initiation) of head and eye command signals within the brainstem structures that control coordinated eye-head movements.
Amplitude coupling of eyes and head
Although the initiation of eye and head movements during gaze shifts can be asynchronous it remains plausible that the control of the amplitude of these movements is not separable. Reconsider the example gaze shift illustrated in Fig. 4. During this trial an initial fixation light was illuminated directly in front of the subject. After fixation of this target it was turned off and a second visual stimulus was presented at a location 35° to the left. A gaze shift to redirect the line of sight to this location was initiated and the eyes began to rotate relative to the head. When the line of sight was relocated, the eyes were deviated by 32° in the orbits. The head also moved to the left during this trial and contributed 2−3° to the redirection of the line of sight. As pointed out above, the head continued to rotate after the gaze shift was complete and during this interval the eyes rotated in the opposite direction at approximately the same velocity, stabilizing the line of sight at the new location. An important question about this movement is whether this pattern is always observed when 35° gaze shifts are made, suggesting an obligatory link between the visual inputs and the movements of the eyes and head, or whether a 35° gaze shift can be accomplished using a variety of eye and head movement patterns. Are the relative roles of the eyes and head determined by the amplitude of the gaze shift? Or are there other factors that influence the coordination of these two mobile elements?
There is a systematic relationship between the amplitude of the eye movement, the contribution of the head and the size of the gaze shift. During movements along the horizontal meridian that begin with the eyes and head directed at the initial fixation target, saccade amplitude increases linearly with a slope near unity when small gaze shifts are made. As gaze shift amplitudes increase, however, the slope of the relationship between eye and gaze amplitude declines, and over a range of large amplitude gaze shifts the amplitude of the saccade component remains relatively constant. Observations of this type have been made in a variety of species including cats (Guitton et al. 1984; Guitton et al. 1990), non-human primates (Tomlinson and Bahra 1986a; Phillips et al. 1995; Freedman and Sparks 1997b), and human subjects (Guitton and Volle 1987; Stahl 1999). Figure 5 illustrates an example from non-human primates in which gaze shifts that ranged in amplitude from 5° to 80° were made along the horizontal meridian. As shown, during movements between 5° and ∼20°, eye movement amplitude (Fig. 5A) was nearly equal to gaze shift amplitude (dashed line in 5A has slope = 1). Although the head moved during some of these trials (Fig. 5B) it contributed < 5° to the overall change in the direction of the line of sight (Fig. 5C). As gaze shift amplitude increased beyond 20°, saccade amplitude continued to increase linearly, but the slope of the eye amplitude – gaze amplitude relationship was less than 1 (solid gray line in Fig. 5A). For these larger movements, gaze shift amplitude increased as a result of the increasing contribution of the head (Fig. 5C). The transition from gaze shifts made almost exclusively by the eyes (with little or no head contribution) to gaze shifts that have similar saccadic contributions and steadily increasing head contributions occurred between 20−25° in this example.
Figure 5.
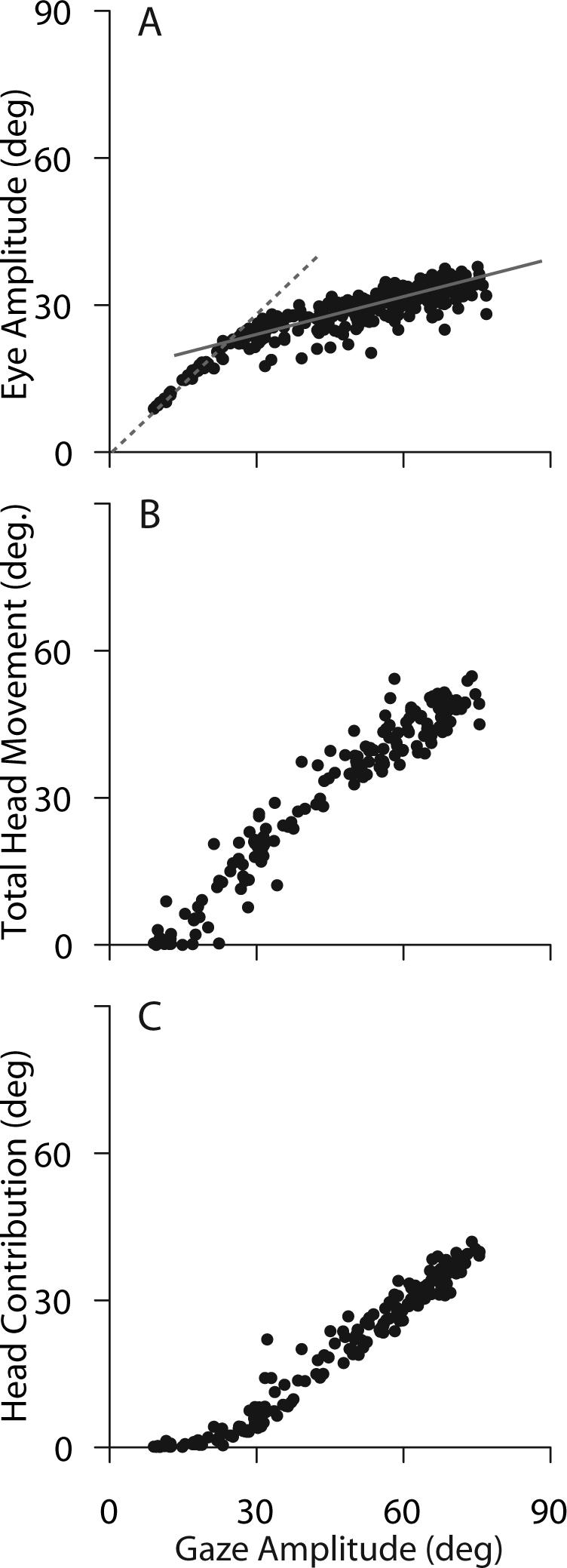
Relative contributions of the eyes and head to gaze shifts of different amplitudes directed along the horizontal meridian. Movements initiated with the eyes centered in the orbits. Eye movement amplitude (A), total head movement amplitude (B), and head contribution (C) are plotted for the same set of trials. Total head movement amplitude is the change in head position from the beginning to end of head movements; head contribution is the change in position of the head that occurs during the gaze shift and actually contributes to the overall change in the direction of the line of sight. Dashed and solid lines in A are described in the text.
The amplitude of the gaze shift at which the above transition occurs and the saturating function that describes the relationship between eye and gaze shift amplitude was perhaps expected if we consider the mechanical limitations on movements of the eyes. As stated above, when the eyes are initially in a central position in the orbits, they are mechanically constrained; in rhesus monkeys the eyes can rotate only ±45°. Under these conditions gaze shifts larger than 45° require a contribution of the head, and as gaze shift amplitude increases beyond 45° the head contribution must increase linearly with a slope near 1. Interestingly, this mechanical limit is rarely (if ever) reached during gaze shifts made under experimental conditions. As shown in Fig. 5, saccade amplitudes did not exceed a functional limit (∼35°) that was significantly smaller than the physical limitations of the orbits (Guitton et al. 1984; Guitton and Volle 1987; Phillips et al. 1995; Freedman and Sparks 1997b).
The observations above led to the uncovering of one of the critical factors governing eye-head coordination: the positions of the eyes in the orbits at the beginning of the gaze shift (Tomlinson and Bahra 1986a; Delreux et al. 1991; Becker and Jürgens 1992; Volle and Guitton 1993; Fuller 1996; Freedman and Sparks 1997b; Stahl 1999; Freedman and Sparks 2000). The effects of initial eye position on the relative amplitudes of the eyes and head during gaze shifts is illustrated in Fig. 6. In panel B, horizontal eye movement amplitude (gray) and head contribution (black) are plotted as functions of gaze shift amplitude for a set of movements made when the eyes and head were initially aligned. Although from a different subject, these data are similar to those shown in Fig.5; however, in Fig. 6 gaze shift amplitudes did not exceed 50°. Nonetheless, similar relationships between eye, head and gaze shift amplitudes were observed. During small gaze shifts the head did not contribute and saccadic eye movements made up the entire gaze shift. However as gaze shift amplitudes increased beyond ∼25°, the rate of increase in eye movement amplitude as a function of gaze amplitude began to decline and head contribution began to increase. As gaze shift amplitude continued to increase, eye movement amplitude increased at a much slower rate. Further increases in gaze amplitude were accomplished by increases in the contribution of the head. However, when the eyes began deviated in the orbits, the relative amplitudes of the eyes and head were altered. In panel A, for example, the eyes began deviated away from the direction of the gaze shift (eyes began deviated 15° to the left of center relative to the head). The oculomotor range that is now available for the eyes to rotate has been increased by ∼15°before the neuro-mechanical limit is approached. Under these conditions, as shown in panel A, gaze shifts >30° were accomplished without contributions of the head. In addition, the rate of increase in the contribution of the head to increases in gaze amplitude was reduced (Freedman and Sparks 1997b). In contrast, when the eyes began deviated in the orbits in the direction of the ensuing gaze shift (panel C), the head began to contribute to gaze shifts that were smaller than 20°. Also, the slope of the linear relationship between head contribution and gaze amplitude was steeper during movements made under these conditions. As a result, during gaze shifts having similar amplitudes (in this example consider gaze shifts of 45° - dashed line) saccade amplitudes were 40° when the eyes begin deviated away from the direction of movement (6A), 35° when the eyes and head were centered (6B), and ∼28° when the eyes began deviated in the direction of movement (6C). Greater differences in saccade amplitude during 45° gaze shifts would be seen if the eyes were deviated by more than 15° at the onset of movements. Similarly, as a result of initiating 45° movements with the eyes in different positions, head contributions varied from 5−20°.
Figure 6.
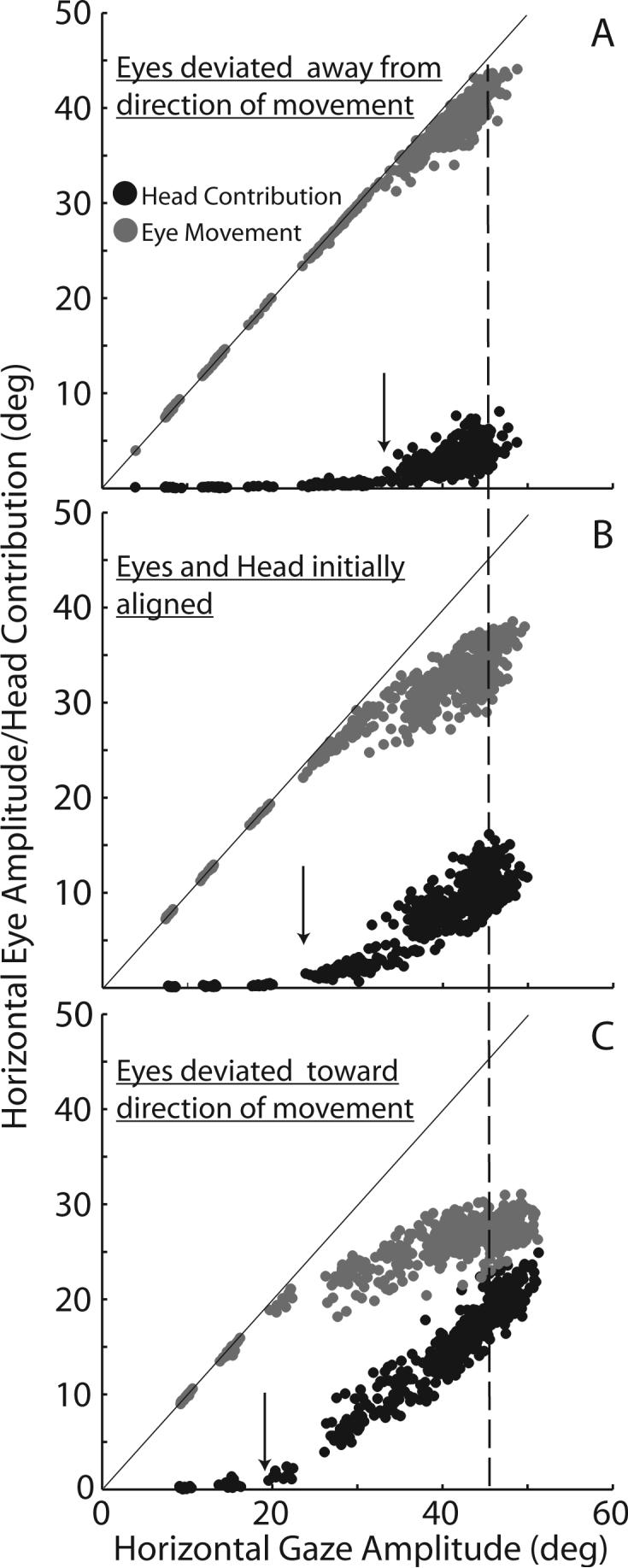
Eye movement amplitude (gray) and head contribution (black) during horizontal gaze shifts of different amplitudes initiated with the eyes in 3 different orbital positions: deviated away from the direction of movement (A) centered in the orbits (B: similar to data in Fig. 5), and deviated in the direction of the ensuing gaze shift (C). Lines in each panel have unity slopes and y-intercepts = 0. Arrows indicate gaze amplitudes at which the head begins to contribute to accomplishing the gaze shift. Dashed vertical line highlights gaze shifts of 45° to facilitate comparison of relative eye and head contributions.
The effects of initial eye position on the relative contributions of the eyes and head are very important in understanding eye-head coordination. As shown, gaze shifts of a particular amplitude are not made up of a particular eye movement coupled with a particular head movement. Instead, similar amplitude gaze shifts can be constructed of eye and head movements having a variety of amplitudes, accomplishing the gaze shift without a head contribution on one trial and on another the head might contribute more than 50% of the overall change in gaze direction. This might lead to the erroneous conclusion that there is great variability in the control of eye and head movements. But this variability can be accounted for by considering the positions of the eyes at the onset of the gaze shift. Given information about initial eye position, and the amplitude of the intended movement, the amplitudes of the eye and head components of gaze can be accurately predicted (Stahl 1999; Freedman and Sparks 2000; Freedman 2001; Freedman 2005). As discussed in a later section, this observation will constrain hypotheses concerning the control of gaze shifts.
Kinematics and Head-Eye Interactions
Gaze shifts are often accomplished with simultaneous eye and head movements. One possible mechanism for coordinating the eyes and head would be to initiate a saccade of a particular amplitude and execute it in exactly the same way regardless of whether or not a head movement contributes to the gaze shift. Given the amplitude of the required gaze shift and the positions of the eyes, the head might or might not contribute, but the saccadic eye movement would have properties that did not depend upon the movement of the head. The relationships between amplitude, peak velocity and duration of saccades would remain unaltered, and the velocity profiles of saccades would continue to be (nearly) symmetrical. However, from some of the earliest studies of eye-head coordination, there is clear evidence that this is not the case. For instance, Bizzi and colleagues (Bizzi et al. 1972a) report that during “predictive” movements, the early head movement had a significant impact on the time course of saccades: peak velocities were lower and durations longer than comparable saccades made under “triggered” conditions (i.e. when the occurrence of the movement initiation cue was unpredictable). In addition to differences in task requirements, one of the critical distinctions between the movements they observed during “triggered” and “predictive” gaze shifts was that during “predictive movements the head contribution was systematically larger than during ‘triggered’ movements to the same targets”.
As part of their systematic description of eye-head coordination in the rhesus monkey Phillips and colleagues (Phillips et al. 1995) describe the relationship between peak eye velocity and gaze shift amplitude (their Fig. 10). As gaze amplitude increases from ∼5° to 25°, peak eye velocity increases and begins to saturate – as it does when the head is restrained. However, there is significantly increased variability in peak saccade velocity during gaze shifts when the head is allowed to move. For example, Phillips reports that during 30° gaze shifts, peak saccade velocity could range from 800°/s to as little as 300°/s. Compare this range to that observed during head restrained saccades (Fig. 1). In addition, it was reported that rather than increasing monotonically with saccade amplitude, peak saccade velocity increased initially but then began to decline as gaze shift amplitudes continued to increase (Phillips et al. 1995). Tomlinson and Bahra (1986) reported a similar reduction in peak saccadic velocity compared to head restrained saccades having similar amplitudes.
Figure 10.
Effects of head movements on eye velocity during gaze shifts of similar amplitudes. During 35° gaze shifts directed along the horizontal meridian, eye solid lines) and head (dotted lines) from two movements are plotted as function of time. In one example the head contribution was small (gray traces) and in the other the head contribution was much larger (black traces). Examples in panel A are from a rhesus monkey, in panel B similar movements are made by a human subject. Vertical lines in each panel highlight the large changes in saccade velocity at a point in time at which the head had barely begun to move.
As discussed above, gaze shifts of any particular amplitude can be composed of a variety of combinations of eye and head movements. The relationship between saccade velocity and gaze shift amplitude is much less variable when the initial positions of the eyes are considered (Freedman and Sparks 1997b). In Fig. 7, peak saccade velocity is plotted as a function of gaze shift amplitude during horizontal gaze shifts beginning with the head and eyes aligned. As gaze shift amplitude increased from 5−20° saccade velocity increased monotonically, as it does during head restrained saccades. With the eyes and head aligned, the head contributes very little to gaze shifts over this range of amplitudes. As a result, there is little if any difference between these gaze shifts made with the head unrestrained (but accomplished without a head contribution), and saccades having similar amplitudes made when the head is restrained. As gaze amplitude increased and head contribution increased, saccade velocity declined. This decline continued as gaze shift amplitudes increased from 30°−50°, until peak saccade velocity was < 300°/s. Saccade velocity remained at this reduced level for gaze shifts between 50° and 80° (Freedman and Sparks 1997b). For comparison, Fig. 7B plots the peak saccade velocity data from panel A as a function of the amplitude of the saccadic eye movement. As shown the relationship between saccade peak velocity and amplitude is very different when the head and eyes move together. The monotonic, soft-saturation of peak velocity plotted as a function of saccade amplitude observed when the head is prevented from moving, is replaced by an initial increase in velocity followed by a marked decline as head contribution increases.
Figure 7.
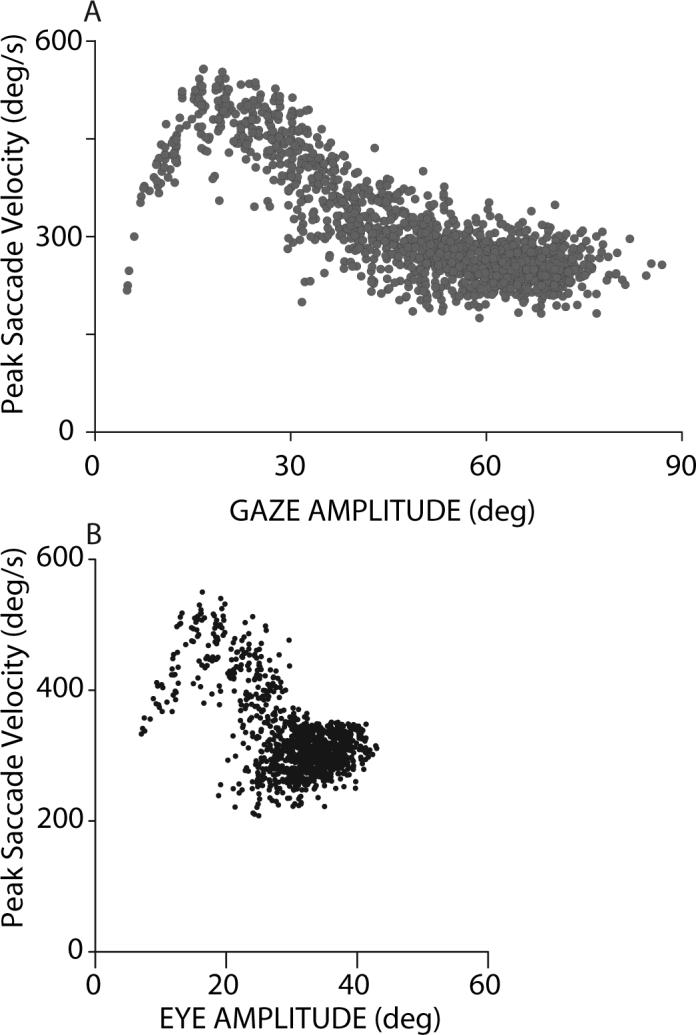
Peak saccade velocity (A) plotted as a function of gaze shift amplitude observed during head unrestrained horizontal gaze shifts initiated with the eyes centered in the orbits. For comparison, peak saccade velocity is also plotted as a function of eye movement (saccade) amplitude (B), during a similar set of horizontal gaze shifts initiated when the eyes were centered in the orbits.
As shown earlier, as gaze shift amplitudes increase, the contribution of the head also increases (Figs. 5 and 6 ). The reduction in gaze shift velocity and saccade velocity might result either from a gaze shift command that specifies a reduced velocity, or as a result of an interaction between the eyes and head. It is fairly simple to separate these alternatives by considering gaze shifts having similar amplitudes that are initiated with the eyes in different orbital positions. Under these conditions, a gaze displacement command remains constant, but the contribution of the head will vary depending upon the initial eye position. Figure 8A illustrates the relationship between peak gaze velocity and head contribution during horizontal gaze shifts with amplitudes of 35°. As shown, during gaze shifts of similar amplitude, when the head contribution was small, peak gaze velocity was >800°/s, however, gaze velocity declined to ∼400°/s when the head contribution increased. A similar effect of head contribution on average saccade velocity during saccades of constant amplitude (Fig. 8B) was reported by Freedman and Sparks (1997a), and was consistent with the suggestion that the head and eye movement commands were interacting to reduce saccade velocity as a function of the associated head movement (Tomlinson and Bahra 1986b; Freedman 2001).
Figure 8.
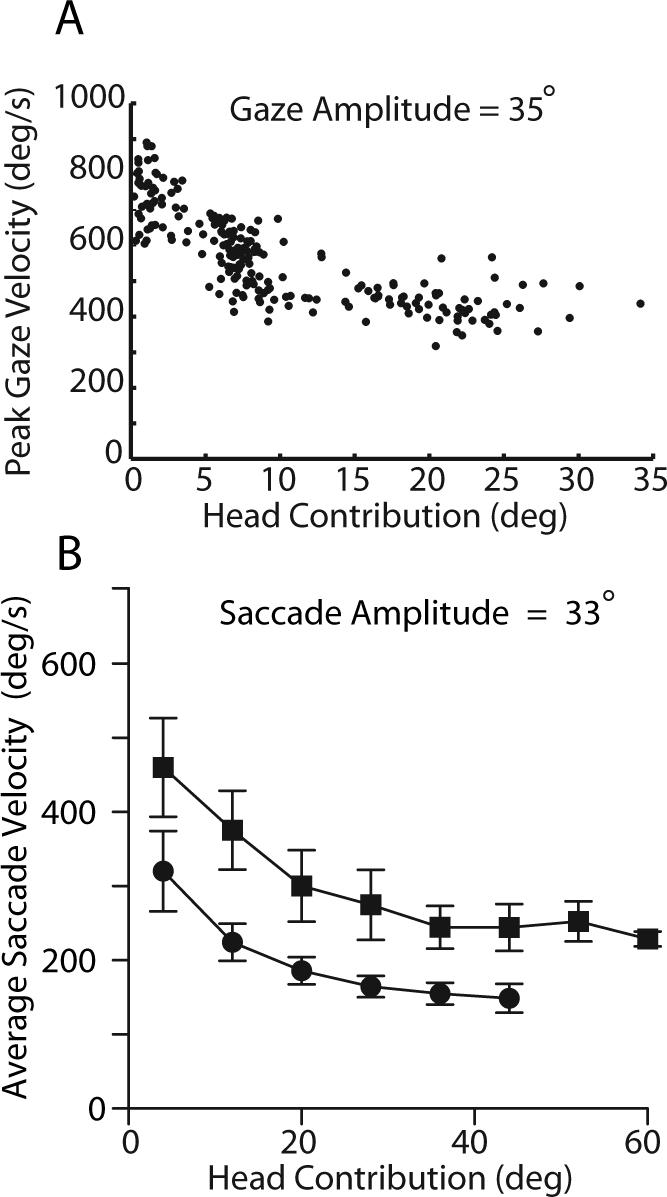
The effects of increasing head contribution on the velocity of constant amplitude gaze shifts. During head unrestrained gaze shifts having amplitudes of 35° and directed along the horizontal meridian peak gaze velocity is plotted as a function of head contribution to the gaze shift (A). During horizontal gaze shifts having different amplitudes, movements with similar eye movement amplitudes (33°) were selected and average saccade velocity is plotted as a function of head contribution (B). Data from two rhesus monkeys represented respectively by squares and circles. Error bars are 1 SD.
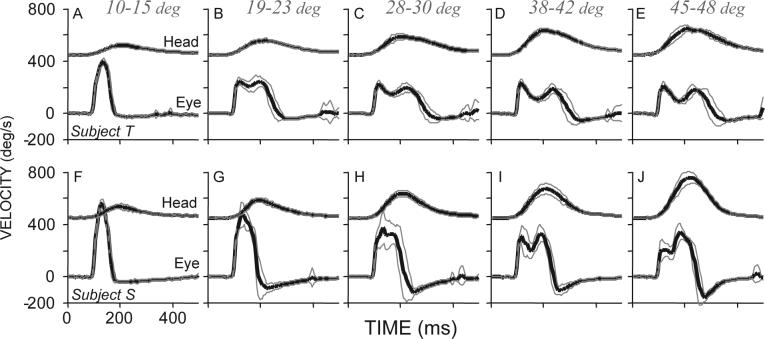
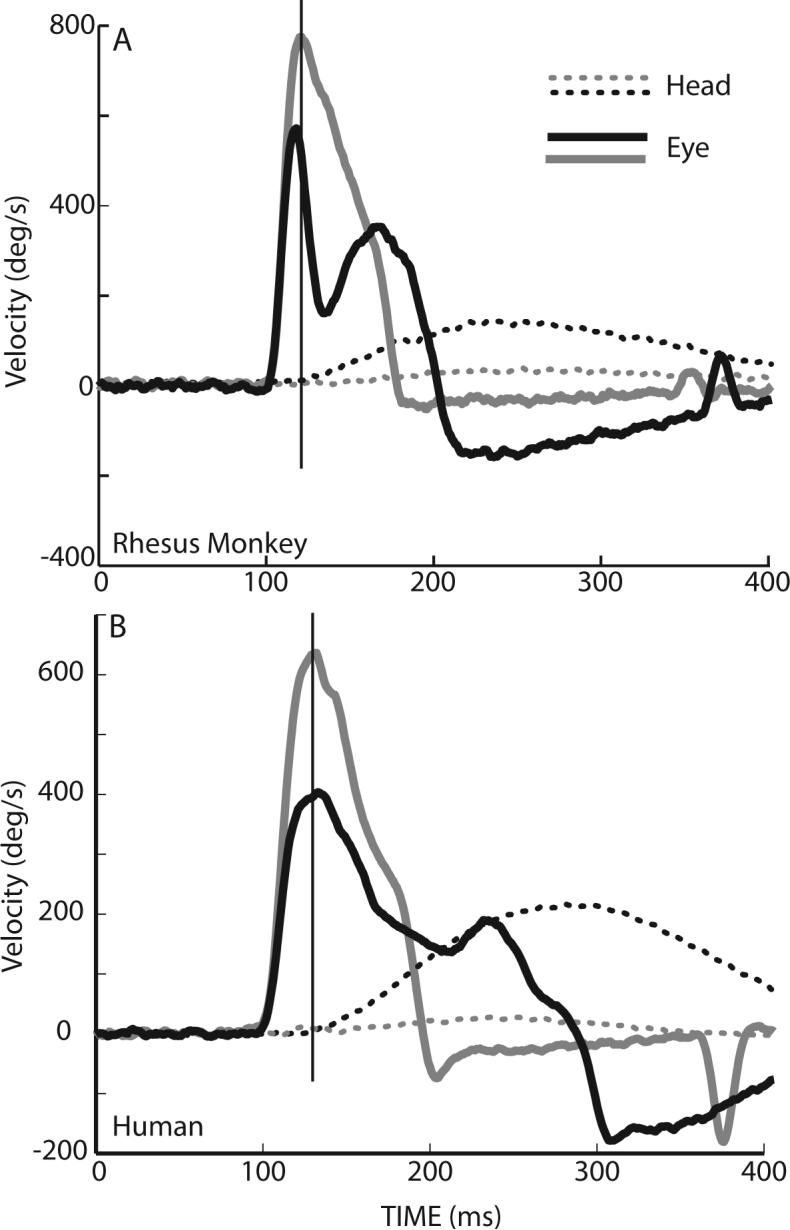
In addition to the decline in saccade velocity that occurs as the velocity of the associated head movements increases, velocity profiles of saccades depend upon the associated movements of the head. In Fig. 9, eye and head velocities are plotted as functions of time during saccades having similar amplitudes (38°) and directions (along the horizontal meridian). Head movement amplitudes varied from ∼12° to >45°. Note in this figure as head velocities are displaced 450°/s along the ordinate; each panel shows the mean (±SD) of between 12 and 20 trials. When 38° saccades were coupled with small head movements (A and F) saccade velocity profiles had single peaks, and movements had short durations and high peak velocities. However, as head movement amplitudes increased, peak saccade velocity declined, duration increased and velocity profiles often had two peaks. This type of double peaked saccade velocity profile has been seen repeatedly in data from both rhesus monkeys (Tomlinson and Bahra 1986a; Cullen and Guitton 1997b; Cullen and Guitton 1997a; Freedman and Sparks 1997b; Roy and Cullen 1998; Freedman and Sparks 2000; Freedman 2005) and humans (see Fig. 10B; control data from (Cecala and Freedman 2008)), during horizontal (Freedman and Sparks 2000) and vertical gaze shifts (Freedman 2005), and during large amplitude gaze shifts evoked by electrical stimulation of the superior colliculus (Freedman et al. 1996). The occurrence of saccade or gaze velocity profiles with two peaks depends not on the amplitude of gaze shifts, but on the velocity of the associated head movement, and on the relative timing of the eye and head components of gaze. In fact, large reductions in saccade velocity often occur before the onset of head movements as illustrated in Fig. 10. Figure 10 illustrates 2 pairs of gaze shifts one pair made by a rhesus monkey (A) and the other by a human (B) subject. In both cases, eye (solid lines) and head (dotted lines) velocities are plotted as functions of time. Gaze shift amplitudes were ∼35° in all cases, but the amplitudes of the associated head movements were different; the eyes began either aligned with the head (gray traces) or deviated in the direction of the ensuing gaze shift (black traces). Note that the occurrence of reduced saccade velocity, and secondary velocity peaks are correlated with the amplitude of the associated head movement and not on the size of the gaze shift which in these examples were identical and cannot account for the differences in velocity profile. The examples in Fig. 10, highlight an additional point about the reduction in saccade velocity that occurs as a function of increasing velocity of the head. The vestibulo-ocular reflex (VOR) cannot account for these results. This statement is based on the observation illustrated in Fig. 10, that the difference in saccade velocity seen when the two movements are compared, occurs at a point in time when there is no difference in the ongoing head movements (vertical lines in A and B). At this point in time movements of the head were identical; head movement sensors will be carrying the same signals during these two movements. As a result, the differences in eye velocity profiles correlated with differences in head movement velocity cannot be accounted for with a mechanism that relies on sensing the movement of the head. Rather the data suggest the possibility of an interaction between head movement commands and the velocity of associated saccades (Tomlinson and Bahra 1986b; Freedman 2001; Freedman and Quessy 2004). The role of the VOR during gaze shifts, in particular the gain of the reflex and the methods that have been used to assess the nature of the interaction between gaze shift commands and stabilizing reflexes will be addressed in the next section.
Figure 9.
Mean (black) ± SD (gray) eye and head velocities plotted as functions of time. Movements were directed along the horizontal meridian and saccade amplitudes were (38°). Head movement amplitudes (given above each column) varied. Data from two rhesus monkey subjects are shown (modified from Freedman and Sparks 2000). Head velocities are displaced 450°/s along the ordinate for display. Movements are aligned on saccade onset.
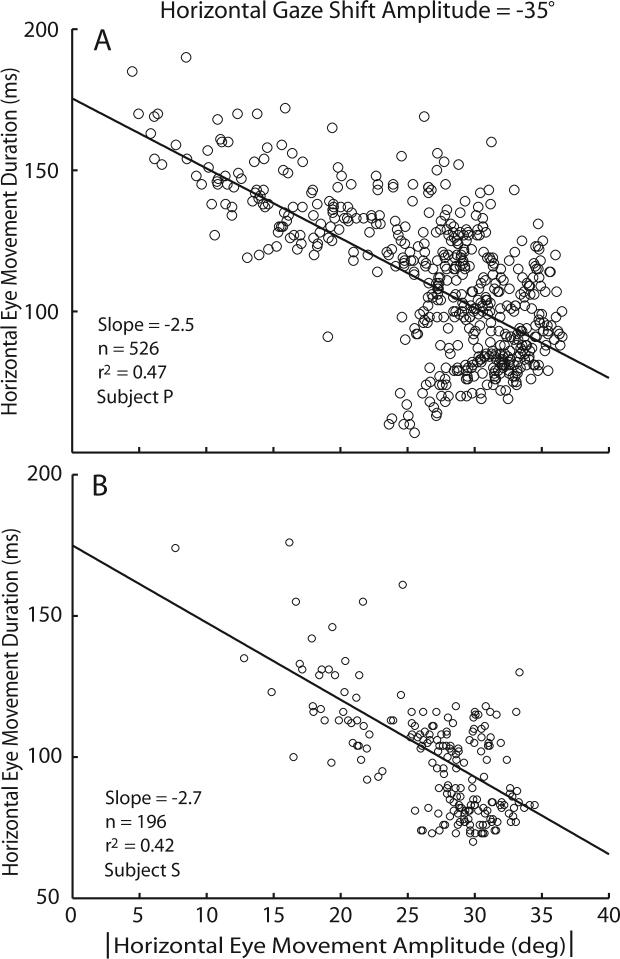
Several key observations have been outlined above, and each of them must be accounted for as hypotheses of gaze control are developed. Two of these observations, when considered together lead directly to what might appear to be an unexpected result. First, during constant amplitude gaze shifts when the eyes begin in different orbital positions, saccade amplitude and head movement amplitude are inversely related. Second, as head movement amplitude increases the velocity of the associated gaze shift and saccadic eye movements decline (note that because head amplitude and velocity are linearly related, increases in amplitude are inextricably linked with increases in head movement velocity). A direct consequence of these rules of eye-head coordination is that during gaze shifts having constant amplitude and direction, the linear correlation between duration and amplitude that is one of the defining characteristics of saccadic eye movements, can be reversed (Freedman 2008; Freedman and Cecala 2008). An illustration of this point is provided in Fig. 11. In this figure, 35° gaze shifts along the horizontal meridian were made both with the eyes and head aligned, and with the eyes and head not aligned. Because of the varying starting positions of the eyes in the orbits, during these gaze shifts of similar amplitude, the amplitude of the saccadic eye movement varied from 5−35°. When the saccade amplitude was small, the head contribution was necessarily large (all gaze shifts had similar amplitudes). As shown, during small saccades made under these conditions saccade durations were long (>150ms). In contrast, during larger saccades the duration of horizontal saccades was <100ms. For this set of movements, the duration-amplitude relationship for saccadic eye movements (one of the defining characteristics of saccades) is reversed; the slope of the duration-amplitude relationship is negative. Panel B illustrates similar data from a second subject. This is another important example of the dramatic changes in saccadic eye movements that result when gaze shifts are accomplished with coordinated movements of the eyes and head.
Figure 11.
Reversal of the duration-amplitude relationship observed during constant amplitude gaze shifts directed along the horizontal meridian. Saccade duration is plotted as a function of saccade amplitude for two rhesus monkey subjects (A and B). In each case, gaze shifts were to a target displaced 35° from the fixation target. Movements were initiated with the eyes in a variety of orbital positions. In both case the slopes of the lines of best fit are negative. (based on (Freedman and Cecala 2008)).
Vestibulo-Ocular Reflex
Controlling the coordination of various mobile elements (whether we consider reaching, walking or changing the direction of the line of sight), must address the inherent conflict between stabilizing reflexes and de-stabilizing motor commands. The vestibulo-ocular reflex (VOR) and cervico-ocular reflex (COR) act to stabilize the retinal image in response to head and body movements. In particular, the VOR uses signals proportional to head velocity derived from the semi-circular canals in order to drive the eyes at a speed equal to that of the head but in the opposite direction. If this compensation were perfect, the retinal image would remain unchanged despite perturbations of the head. Gaze shift commands necessarily de-stabilize (albeit temporarily) the retinal image, moving the retina so that new images fall on the fovea. If the VOR and/or COR were operational (at a gain close to 1) during gaze shifts made up of coordinated movements of the eyes and head, the expected eye velocity (that observed during saccades of similar amplitudes when the head did not move) would be reduced by approximately the velocity of the concomitant head movement. This potential reduction in saccade velocity would result from the addition of two competing signals: the saccadic command that is part of the gaze shift driving the eyes to a new location, and the reflex signals attempting to stabilize the retina in space in response to a movement of the head.
An additive mechanism like this, that came to be known as the linear summation hypothesis (Laurutis and Robinson 1986; Guitton and Volle 1987), was proposed by Bizzi and colleagues (Bizzi et al. 1971; Bizzi et al. 1972a; Bizzi et al. 1972b; Dichgans et al. 1973; Morasso et al. 1973; Dichgans et al. 1974) as a mechanism for coordinating the eyes and head during gaze shifts. Thus the planning of the gaze shift would be independent of head movement planning; any head movement would be eliminated by the VOR-induced reduction in saccade velocity during execution. This greatly simplifies the coordination of the eyes and head. However, several problems arise from this approach. The first is that the head contribution to gaze shifts will always be canceled by the VOR. This would prevent gaze shifts to targets beyond the neuromechanical limits of ocular motility; with the eyes initially centered in the orbits it would not be possible to make a single gaze shift larger than ∼45°. The linear addition hypothesis also assumes that the VOR operates near unity gain during gaze shifts. Numerous experiments testing this assumption have since been carried out.
Tests of the hypothesis that the VOR remains active throughout gaze shifts have taken a variety of forms. Despite the technical differences, conceptually these experiments were similar in that each attempts to perturb the movement of the head during gaze shifts and then assess the ocular response to the perturbation. In some cases the perturbation took the form of an unexpected halting of the head movement via an electronic braking mechanism (for examples see (Guitton et al. 1984; Guitton and Volle 1987)), others imposed brief torque pulses using an electronic clutch (Tomlinson and Bahra 1986b; Cullen et al. 2004), and in one case head perturbations were produced using a light tap with a rubber hammer on a yoke encircling the subject's head and clamped in the teeth (Laurutis and Robinson 1986). In all cases a modification of the head movement was used to assess the gain of the VOR. The results, however, have varied. For instance, several studies concluded that the VOR was turned off during gaze shifts larger than ∼30° in cats (Roucoux et al. 1980), in human subjects (Jürgens et al. 1981b) and in rhesus monkeys (Tomlinson and Bahra 1986b). In contrast during smaller gaze shifts (< 30°), it was concluded that the VOR remained active (Blakemore and Donaghy 1980; Roucoux et al. 1980; Guitton et al. 1984; Tomlinson and Bahra 1986b). This result is consistent with the idea that during small gaze shifts the head contributes very little and so an active VOR would be useful in correcting for unwanted head motion. However, during larger gaze shifts when the head might be expected to be an important contributor to the active change in gaze direction, an active VOR would be counterproductive. In fact it was suggested that the VOR gain might be modulated as a function of gaze motor error. Thus, VOR gain would be low at the onset of larger gaze shifts but return to unity gain near the end of the gaze shift (Pelisson et al. 1988). A test of this hypothesis that kept gaze amplitudes constant and varied the contribution of the head would be informative, but has not yet been carried out.
The majority of tests of VOR gain used to arrive at these conclusions, however, were not sufficiently sensitive to determine the time course of changes in VOR gain during gaze shifts. Using a novel approach Tabak and colleagues (Tabak et al. 1996) were able to measure VOR gain throughout gaze shifts of different amplitudes. Using human subjects, these authors designed a helmet with a torque-motor-driven fly wheel. Acceleration of the fly wheel produced a reactive torque on the helmet and on the head of the subject. Using high frequency oscillations it was possible to assess VOR gain continuously throughout gaze shifts; brief torque pulses could also be used to perturb the head. Their results suggested that VOR gain was modulated – reduced sometimes by as much as 50% - but never fully suppressed, even at the beginning of large amplitude gaze shifts.
In a slightly different approach, the time course of VOR gain changes was investigated by applying short duration (∼5 ms) torque pulses at different points throughout gaze shifts (Freedman et al. 1998; Cullen et al. 2004). In the example shown in Fig. 12 (from Freedman et al. 1998), mean eye velocity is plotted as a function of time for 3 sets of trials. The first (black) were control gaze shifts of 70°. In other trials that were randomly interspersed with the controls, are trials in which a 5ms torque pulse was applied to the head driving the head either in the direction of the ongoing movement (dark gray), or driving the head in the direction opposite to the ongoing movement (light gray). Torque pulses were applied 5ms before gaze shift onset. The effects of the torque pulse on head velocity during perturbations in each direction are compared to head velocity during control trials (inset). As illustrated when the head was unexpectedly rotated in the direction opposite to the ongoing head movement, eye velocities increased compared to control movements. Similarly when head perturbations drove the head in the direction of the gaze shift, eye velocities were reduced. The latency of the effect of head perturbation on eye velocities was ∼10ms, consistent with VOR latencies. When VOR gain was calculated by comparing the changes in head and eye velocities during perturbations, it was found to remain elevated (near unity gain) throughout gaze shifts. Cullen and colleagues (2004), in a more comprehensive study using a similar technique, describe the time course of VOR gain from 3 subjects during 60° gaze shifts. During perturbations in the direction opposite ongoing movements, one of their subjects showed very little modulation of the VOR gain. In this subject only a 10% reduction in gain was observed when the torque pulse was applied 100ms after movement onset. Data from this subject were similar to that described by Freedman et al. (1998). However, in a second subject, during perturbations delivered 50ms after movement onset Cullen found a nearly 80% reduction in VOR gain, and the reduction declined (VOR gain increased) as movements progressed. Data from this subject were similar to earlier work (for example Pelisson et al. 1988) in which the VOR gain appeared to be modulated as a function of gaze motor error. A third subject in the Cullen experiment also showed a modulation of VOR gain but VOR gain was reduced only by ∼35−40% (data consistent with the study of Tabak et al. 1996). Data during head perturbations in the direction of the ongoing movement were more consistent, but revealed much less attenuation (∼20%) of VOR gain (Cullen et al. 2004).
Figure 12.
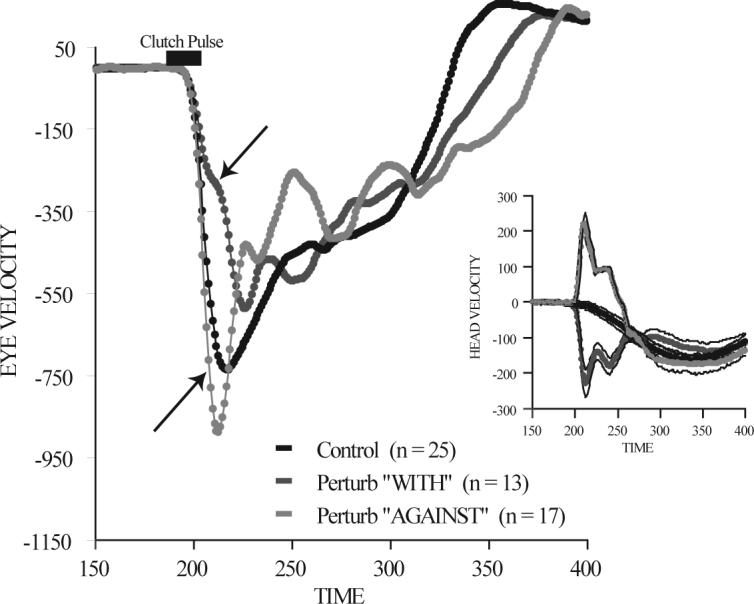
Head perturbation during an ongoing 70° gaze shift. Mean eye velocity is plotted as a function of time during three sets of trials. Control trials (black), trials in which a brief torque pulse was applied to the head driving the head in the direction of the ongoing gaze shift (dark gray), and trials during which the torque pulse drove the head in the direction opposite the ongoing gaze shift (light gray). The inset shows the effects of the torque pulse on the head. Movements were to the left. (modified from Freedman et al. 1998).
As may be clear from the variety of conclusions drawn from the experiments outlined above, there is no consensus on the role of the VOR during visual orienting behaviors. Yet, understanding how the conflict between stabilizing reflexes and destabilizing motor commands is resolved is a critical question for furthering the study of eye-head coordination and for a more general understanding of the neural control of coordinated actions. One possible solution to this general problem and also a potential resolution of the confusing results of tests of the VOR during gaze shifts is an idea that was clearly delineated by von Holst and Mittlestaedt in 1950 (translated into English by P. Dodwell (von Holst and Mittelstaedt 1971)), and simultaneously by Sperry (Sperry 1950). In the von Holst and Mittlestaedt exposition of reafference (the inflow of sensory information caused by a centrally generated movement) these authors suggest that rather than operating on the sensory inflow directly, that “reflexes” operate on the difference between expected and actual reafference. Expected reafference is an internal prediction of the reafference caused by the motor commands that produce the movement in question and is derived from a copy of the motor command. If sensory inflow exactly matches the expected inflow no corrective action is required. If on the other hand the expectation differs from the actual inflow, corrective motor commands will be issued. This mechanism has been clearly established in the electric fish electrosensory system (Bell 1981; Bell 1982; Bell 1984; Bell et al. 1997a; Bell et al. 1997b).
Efforts to demonstrate a functioning “reafference comparator” mechanism that may modulate the gain of the VOR during gaze shifts in primates have been more difficult. Nonetheless, if one assumes that the expected reafferent signal can be altered by experience (see Bell 1982), this hypothesis suggests that the variability in results of head perturbation experiments could stem from the predictability (or lack thereof) of the perturbation. If the timing, direction and/or amplitude of perturbations are predictable and the sensory inflow caused by head perturbations becomes part of the expected reafferent signal, the VOR will appear to be inactive during gaze shifts (Roucoux et al. 1980; Jürgens et al. 1981b; Fuller et al. 1983; Tomlinson and Bahra 1986b; Pelisson et al. 1988; Cullen et al. 2004); there will be reduced ocular compensation for the head perturbation. In contrast, if perturbations are unpredictable, the expected sensory inflow will not account for the activity produced by the perturbation and the VOR will appear to be active with near unity gain (Blakemore and Donaghy 1980; Guitton et al. 1984; Laurutis and Robinson 1986; Freedman et al. 1998; Cullen et al. 2004). This suggests an experimental approach to addressing this issue using adaptation to the head perturbation as a method of probing the buildup of the expected reafferent signal. If correct, the gain of the VOR should appear to be high when the head is perturbed unexpectedly, but should decline steadily with repeated and predictable head perturbations as the expected reafferent signal is modified to include the effects of the perturbation. The data from the electric fish as well as the nature of the adaptation process required by this hypothesis make the cerebellum a good candidate for implementing this type of mechanism. The advantage of such a mechanism is clear: stabilizing reflexes will only operate on unexpected sensory inflow correcting movements that are not progressing as planned. Reflexes in this type of scheme will also leave unaltered the “destabilizing” motor commands as long as they are being executed as predicted.
Modeling the Gaze Displacement Control System
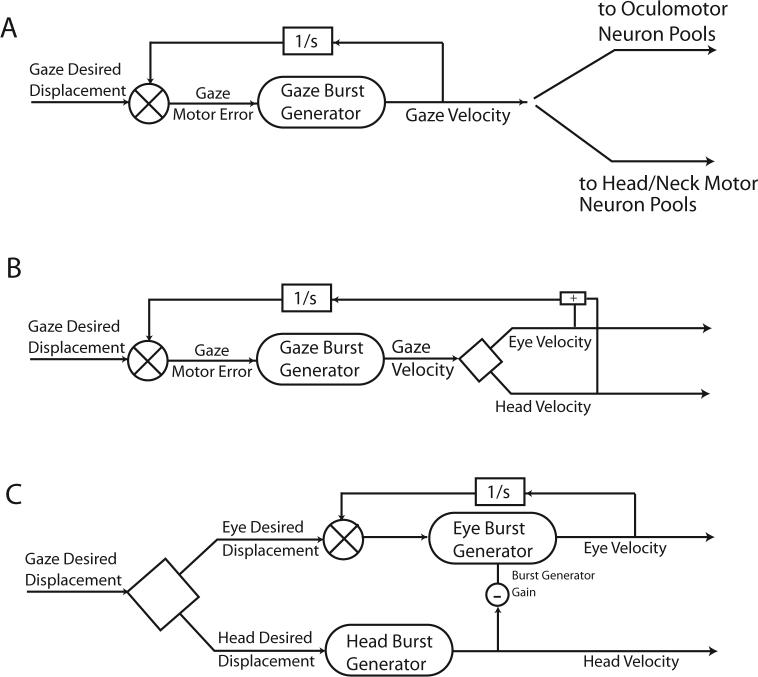
Figure 3 presented a schematic diagram of a saccade control system (Jürgens et al. 1981a; Moschovakis 1994). This hypothesis was designed to capture the main features of saccadic eye movements made when the head is prevented from moving. Extensions of this model to include coordinated movements of both the eyes and head have taken several different forms. In one of the early hypotheses of gaze control the strategy was to maintain the basic structure of the head-restrained model but proposed that gaze displacement (rather than eye displacement relative to the head) might be the controlled variable. Thus, in this scheme a signal of desired gaze displacement was compared to an internal representation of current gaze displacement to provide a gaze motor error signal. This served as input to the non-linear gaze burst generator that produced a signal proportional to gaze velocity. It was suggested that the gaze velocity command could drive both the head and the eyes (Guitton et al. 1990; Galiana and Guitton 1992). Figure 13A illustrates a schematic diagram showing the basic structure of this type of gaze displacement model. As illustrated the gaze velocity command provided a common drive to both the eyes and head. This hypothesis accounted for the tight coupling observed in both the relative timing and relative amplitudes of eye and head movements seen in some subjects. However, as described above, eye and head movements are not coupled in an obligatory fashion. In fact, the apparent coupling only arises when gaze shifts are made under a narrow subset of conditions: when the eyes and head always begin in the same relative positions. During more natural movements, or when the initial positions of the eyes relative to the head are experimentally varied, there is little temporal coupling of eye and head movements, and the amplitudes of eye and head movements vary inversely. Furthermore, as head velocities increase, saccade and gaze shift velocities decline. It is difficult to reconcile the gaze control strategy outlined in Fig. 13A with the variety of eye-head movements observed during gaze shifts and the degree of dissociation seen in the metrics and kinematics of gaze, eye and head movements.
Figure 13.
Schematic diagrams of three classes of gaze control model. In A, desired gaze displacement is compared with an internal estimate of current gaze displacement to produce a gaze motor error signal. A single gaze burst generator produces a signal proportional to gaze velocity, and this common signal drives both the eye and head movements. The scheme in panel B is similar, but proposes that the gaze velocity signal is decomposed into separate eye and head velocity commands inside the dynamic feedback control loop. In C, the decomposition of gaze signals occurs upstream of the control loop. Here signals of desired eye and desired head displacement are used by separate burst generator elements to produce head and eye related velocity commands. A signal proportional to head movement velocity is proposed to reduce the gain of the saccadic burst generator. This head-eye interaction allows hypotheses of this type to capture the changes in saccade velocity that occur as head movement velocities increase.
An alternative scheme (Fig. 13B), accounts for some of the eye-head dissociation data by assuming that a gaze velocity command is separated into eye and head velocity signals inside the feedback control loop. The reliance on a common gaze velocity command remains problematic for models of this type. As head velocity increases, both gaze and eye velocities decline. But in this scheme there is no apparent mechanism that can account for reductions in eye and gaze shift velocities that are correlated with increases in the velocity of the head.
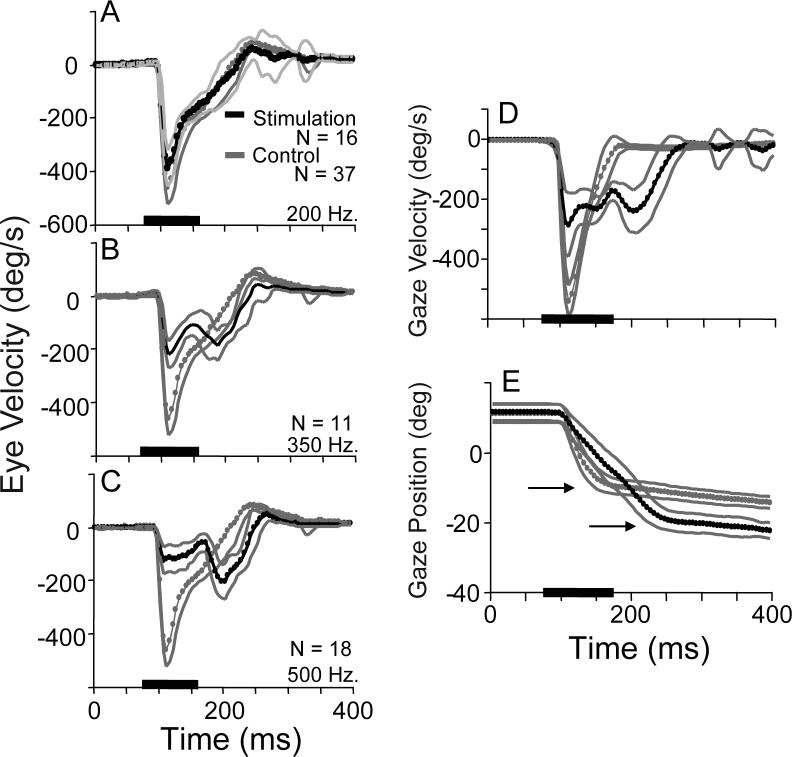
A third class of alternative assumes that a desired gaze displacement signal is separated into eye and head commands before the dynamic control elements (Tomlinson and Bahra 1986b; Tomlinson 1990; Freedman 2001). An interaction between head and eye signals (a copy of a signal proportional to head velocity reduces the gain of the saccadic burst generator) leads to the observed dissociations of eye and head metrics and kinematics. Note that the head-related signal that interacts with the saccadic burst generator is a copy of the head motor command, and not derived from sensory input from the neck proprioceptive or vestibular sensors. This hypothesis makes two critical and unique predictions. If a signal can be added along the head command pathway (after it has been separated from the eye movement command path), an unexpected reduction in saccade velocity should occur via the proposed inhibition of the burst generator gain. In addition, because gaze shift amplitude is not controlled via feedback (the feedback controller operates on desired eye movement amplitude), prolonging the saccade by adding to the head movement command should lead to hypermetric gaze shifts. Using electrical stimulation of the nucleus reticularis gigantocellularis (NRG), Freedman and Quessy (Freedman and Quessy 2004) tested these predictions. Note that during fixation of a central target, NRG stimulation evokes horizontal head rotations that are not accompanied by saccadic eye movements (Cowie and Robinson 1994; Quessy and Freedman 2004), and neurons in NRG receive direct input from the superior colliculus and project to neck motor neurons (Peterson and Richmond 1988; Isa and Sasaki 2002). Figure 14 (A-C) illustrates the effects of NRG stimulation on an ongoing movement made to the location of a briefly flashed visual target. In each panel, mean (-SD) eye velocity during control movements is shown. On stimulation trials, NRG stimulation was applied 25ms before movement onset; mean eye velocity (±SD) is plotted in black. When stimulation frequency was 200Hz (A) there was a minor reduction in the velocity of eye movements caused by NRG stimulation. However, when stimulation frequency was increased to 350Hz, eye velocity at the beginning of movements was reduced, and then declined until around the time at which the stimulation train ended when there was a reacceleration of the eyes. Eye movements clearly had two velocity peaks and prolonged durations compared to control movements. During 500Hz stimulation of the same NRG site, initial eye velocity was further reduced, reaching only ∼125°/s. This reduced velocity was maintained until near the end of the stimulation train when a large increase in eye velocity was observed. The velocity profiles observed during NRG stimulation were quite similar to the variety of velocity profiles observed during gaze shifts associated with head movements of increasing amplitudes (see Fig. 9). These results are consistent with the effects of the hypothesized reduction of the saccadic burst generator gain as a function of a head velocity command.
Figure 14.
Experimental tests of two critical predictions of the model proposed by Freedman (2001). Using electrical stimulation of the nucleus reticularis gigantocellularis (NRG) to artificially increase the head movement command, the model makes two types of predictions. The first is that compared to controls during which there is no NRG stimulation, saccade velocities should be reduced during NRG stimulation. In panels A-C mean eye velocity during control trials (gray) is superimposed on mean eye velocity profiles during NRG stimulation (black). Stimulation frequency was 200 Hz (A), 350Hz (B), and 500 Hz (C). A second critical prediction is that gaze shifts should be hypermetric when NRG stimulation increases the head contribution. Mean gaze velocity during control (gray) and NRG stimulation trials (black) are plotted as function of time (D), and for the same sets of trials mean control (gray) and NRG stimulation (black) position are also shown (E). Arrows in E highlight the endpoints of control and NRG stimulation trials. (modified from Freedman and Quessy,2004).
A second critical prediction of the Freedman (2001) hypothesis arises because the model assumes that gaze amplitude is not controlled directly. As a result injecting an additional head velocity signal (via NRG stimulation) should lead to hypermetric gaze shifts. In Fig. 14D, gaze velocity during control movements (gray) and during NRG stimulation trials (black) are plotted as functions of time. As shown, NRG stimulation reduces gaze shift velocity compared to control movements, and increases movement duration. In panel 14E, gaze positions are plotted as functions of time for the same sets of movements. As indicated, the amplitude of gaze shifts during NRG stimulation was much larger than during control gaze shifts to the same flashed target. In fact, NRG stimulation resulted in gaze shifts that could be 150% as large as control movements. To the extent that NRG stimulation provides a way in which to alter the head movement command signal, these experiments tested two critical and unique predictions of the hypothesis outlined above. Results were not inconsistent with the hypothesized predictions. Note too that this hypothesis can account for the somewhat surprising reversal in the duration-amplitude relationship described in Fig. 11 (Freedman 2008; Freedman and Cecala 2008).
Concluding Remarks
The ways in which the nervous system mediates behavior, translates sensory information into actions, and carries out the computations required for orienting and navigating through our environment are critical components in our investigation of brain function. Despite the failure to reject the hypothesis above (Fig. 13C) based on tests of two critical predictions, much remains to be accomplished if we are to elucidate the neural mechanisms that result in coordinated movements of the eyes and head. While modeling has proven to be a useful tool for generating explicit predictions, models are, after all, only particular instantiations of a specific hypothesis. They are only as useful as they are falsifiable.
A clear delineation of characteristics of visual orienting behaviors will constrain current and future hypotheses (i.e. modeling efforts), but in order to evaluate these models there must be consensus on the data that each is required to simulate. In the sections above I have tried to present several features of gaze shifts that I deem to be essential in this regard: the ability to dissociate the timing and amplitude of eye and head movements; the effects of initial eye position on the relative contributions of the eyes and head; the changes in saccade kinematics that are correlated with increasing head movement velocities; the reversal of the duration-amplitude relationship for saccades during gaze shifts of similar amplitudes; the apparent failure of the VOR or other sensory signals to account for the observed changes in saccade kinematics. This is not (nor is it meant to be) a complete list of all gaze shift rules that must be considered. But it is a place to begin. It remains to determine the neural mechanisms that may support these behaviors, clarify the role of the VOR during gaze shifts, divulge the contributions of the cerebellum and other brainstem structures, and unravel the nature of the interactions between the several subsystems (gaze changing, pursuit etc.) controlling visual orienting behaviors when the head is free to move.
Acknowledgements
The author thanks the members of his lab at the University of Rochester: Dr. J. Quinet, Dr. S. Quessy, Dr. M. Walton, A. Cecala, and G. Parker. In addition the comments and suggestions of Prof. Patrick Haggard and two anonymous reviewers led to important improvements in this manuscript. The author is also indebted to Dr. David Sparks for his continuing intellectual contributions, encouragement and friendship. Supported in part by NIH Grants EY13239 and EY01319.
References
- Bahill AT, Brockenbrough A, Troost BT. Variability and development of a normative data base for saccadic eye movements. Res. Vis. Ophthal. 1981;21:116–126. [PubMed] [Google Scholar]
- Bahill AT, Clark MR, Stark L. The main sequence, a tool for studying human eye movements. Mathematical Biosciences. 1975;24:191–204. [Google Scholar]
- Baloh RW, Sills AW, Kumley WE, Honrubia V. Quantitative measurement of saccade amplitude, duration, and velocity. Neurology. 1975;25:1065–1070. doi: 10.1212/wnl.25.11.1065. [DOI] [PubMed] [Google Scholar]
- Barnes GR. Vestibulo-ocular function during co-ordinated head and eye movements to acquire visual targets. J. Physiol. 1979;287:127–147. doi: 10.1113/jphysiol.1979.sp012650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker W, Jürgens R. Human oblique saccades: Quantitative analysis of the relation between horizontal and vertical components. Vision Res. 1990;30:893–920. doi: 10.1016/0042-6989(90)90057-r. [DOI] [PubMed] [Google Scholar]
- Becker W, Jürgens R. Gaze saccades to visual targets: do head movements change the metrics. In: Berthoz A, Graf W, Vidal P-P, editors. The head-neck sensory motor system. Oxford Univ. Press; New York: 1992. pp. 427–433. [Google Scholar]
- Bell C, Bodznick D, Montgomery J, Bastian J. The Generation and Subtraction of Sensory Expectations within Cerebellum-Like Structures. Brain, Behaviour and Evolution. 1997a;50:17–31. doi: 10.1159/000113352. [DOI] [PubMed] [Google Scholar]
- Bell CC. An efference copy which is modified by reafferent input. Science. 1981;214:449–453. doi: 10.1126/science.7291985. [DOI] [PubMed] [Google Scholar]
- Bell CC. Properties of a modifiable efference copy in an electric fish. J. Neurophysiology. 1982;47:1043–1056. doi: 10.1152/jn.1982.47.6.1043. [DOI] [PubMed] [Google Scholar]
- Bell CC. Effects of motor commands on sensory inflow, with examples from electric fish. In: Bolis L, Keynes RD, Maddrell SHP, editors. Comparative Physiology of Sensory Systems. Cambridge Univ. Press; 1984. pp. 637–646. [Google Scholar]
- Bell CC, Han VZ, Sugawara Y, Grant K. Synaptic Plasticity in a Cerebellum-Like Structure Depends on Temporal Order. Nature. 1997b;387:278–281. doi: 10.1038/387278a0. [DOI] [PubMed] [Google Scholar]
- Bizzi E, Kalil RE, Morasso P. Two modes of active eye-head coordination in monkeys. Brain Reserch. 1972a;40:45–48. doi: 10.1016/0006-8993(72)90104-7. [DOI] [PubMed] [Google Scholar]
- Bizzi E, Kalil RE, Morasso P, Tagliasco V. Central programming and peripheral feedback during eye-head coordination in monkeys. Bibl. Ophthal. 1972b;82:220–232. [PubMed] [Google Scholar]
- Bizzi E, Kalil RE, Tagliasco V. Eye-head coordination in monkeys: evidence for centrally patterned organization. Science. 1971;173:452–454. doi: 10.1126/science.173.3995.452. [DOI] [PubMed] [Google Scholar]
- Blakemore C, Donaghy M. Co-Ordination of Head and eyes in the Gaze Changing Behaviour of Cats. J. Physiol. 1980;300:317–335. doi: 10.1113/jphysiol.1980.sp013164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cecala AL, Freedman EG. Amplitude changes in response to target displacements during human eye-head movements. Vision Res. 2008;48:149–166. doi: 10.1016/j.visres.2007.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collewijn H. Eye- and head movements in freely moving rabbits. J. Physiol. 1977;266:471–498. doi: 10.1113/jphysiol.1977.sp011778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowie RJ, Robinson DL. Subcortical Contributions to Head Movements in Macaques I. Contrasting Effects of Electrical Stimulation of a Medial Pontomedullary Region and the Superior Colliculus. Journal of Neurophysiology. 1994;71:2648–2664. doi: 10.1152/jn.1994.72.6.2648. [DOI] [PubMed] [Google Scholar]
- Cullen KE, Guitton D. Analysis of Primate IBN Spike Trains Uding system Identification Techniques. I. Relationship to Eye Movement Dynamics During Head-Fixed Saccades. J. Neurophysiol. 1997a;78:3259–3282. doi: 10.1152/jn.1997.78.6.3259. [DOI] [PubMed] [Google Scholar]
- Cullen KE, Guitton D. Analysis of primate IBN spike trains using system identification techniques. II. Relationshp to gaze, eye and head movement dynamics during head-free gaze shifts. Journal of Neurophysiology. 1997b;78:3283–3306. doi: 10.1152/jn.1997.78.6.3283. [DOI] [PubMed] [Google Scholar]
- Cullen KE, Huterer M, Braidwood DA, Sylvestre PA. Time course of vestibuloocular reflex suppression during gaze shifts. J Neurophysiol. 2004;92:3408–3422. doi: 10.1152/jn.01156.2003. [DOI] [PubMed] [Google Scholar]
- Delreux V, Abeele SV, Lefevre P, Roucoux A. Influences of Eye Position on the Control of Head Movement Amplitude. In: Paillard J, editor. Brain and Space. Oxford U. Press; Oxford: 1991. pp. 38–48. [Google Scholar]
- Dichgans J, Bizzi E, Morasso P, Tagliasco V. Mechanisms underlying recovery of eye-head coordination follo wing bilateral labyrinthectomy in monkeys. Exp. Brain Res. 1973;18:548–562. doi: 10.1007/BF00234137. [DOI] [PubMed] [Google Scholar]
- Dichgans J, Bizzi E, Morasso P, Tagliasco V. The Role of Vestibular and Neck Afferents During Eye-Head Coordination in the Monkey. Brain Research. 1974;71:225–232. doi: 10.1016/0006-8993(74)90964-0. [DOI] [PubMed] [Google Scholar]
- Freedman EG. Interactions between eye and head control signals can account for movement kinematics. Biological Cybernetics. 2001;84:453–462. doi: 10.1007/PL00007989. [DOI] [PubMed] [Google Scholar]
- Freedman EG. Head-eye interactions during vertical gaze shifts made by rhesus monkeys. Exp Brain Res. 2005:1–14. doi: 10.1007/s00221-005-0051-9. [DOI] [PubMed] [Google Scholar]
- Freedman EG. Coupling Between Horizontal and Vertical Components of Saccadic Eye Movements during Constant Amplitude and Direction Gaze Shifts in the Rhesus Monkey. J. Neurophysiol. 2008 doi: 10.1152/jn.90669.2008. In review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman EG, Cecala AL. Oblique Gaze Shifts: Head Movements Reveal New Aspects of Component Coupling. In: Leigh RJ, Kennard C, editors. Using Eye Movements as an Experimental Probe of Brain Function. Elsevier; Amsterdam: 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman EG, Ling L, Fuchs AF. Perturbing the head: the gain of reflex interactions during orienting eye-head movements. Abstract, Society for Neurosci. 1998;24:1412. [Google Scholar]
- Freedman EG, Quessy S. Electrical stimulation of rhesus monkey nucleus reticularis gigantocellularis II. Effects on metrics and kinematics of ongoing gaze shifts to visual targets. Exp Brain Res. 2004;156:357–376. doi: 10.1007/s00221-004-1840-2. [DOI] [PubMed] [Google Scholar]
- Freedman EG, Sparks DL. Activity of cells in the deeper layers of the superior colliculus of the rhesus monkey: evidence for a gaze displacement command. J. Neurophysiology. 1997a;78:1669–1690. doi: 10.1152/jn.1997.78.3.1669. [DOI] [PubMed] [Google Scholar]
- Freedman EG, Sparks DL. Eye-head coordination during head-unrestrained gaze shifts in rhesus monkeys. J. Neurophysiol. 1997b;77:2328–2348. doi: 10.1152/jn.1997.77.5.2328. [DOI] [PubMed] [Google Scholar]
- Freedman EG, Sparks DL. Coordination of the eyes and head: movement kinematics. Exp. Brain Research. 2000;131:22–32. doi: 10.1007/s002219900296. [DOI] [PubMed] [Google Scholar]
- Freedman EG, Stanford TR, Sparks DL. Combined eye-head gaze shifts produced by electrical stimulation of the superior colliculus in rhesus monkeys. J. Neurophysiol. 1996;76:927–951. doi: 10.1152/jn.1996.76.2.927. [DOI] [PubMed] [Google Scholar]
- Fuchs AF, Kaneko CRS, Scudder CA. Brainstem control of saccadic eye movements. Ann. Rev. Neurosci. 1985;8:307–337. doi: 10.1146/annurev.ne.08.030185.001515. [DOI] [PubMed] [Google Scholar]
- Fuchs AF, Robinson DA. A method for measuring horizontal and vertical eye movement chronically in the monkey. J. Applied Physio. 1966;21:1068–1070. doi: 10.1152/jappl.1966.21.3.1068. [DOI] [PubMed] [Google Scholar]
- Fuller JH. Head movement propensity. Exp. Brain res. 1992;92:152–165. doi: 10.1007/BF00230391. [DOI] [PubMed] [Google Scholar]
- Fuller JH. Eye position and target amplitude effects on human visual saccadic latencies. Exp. Brain Res. 1996;109:457–466. doi: 10.1007/BF00229630. [DOI] [PubMed] [Google Scholar]
- Fuller JH, Maldonado H, Schlag J. Vestibular-oculomotor interaction in cat eye-head movements. Brain Research. 1983;271:241–250. doi: 10.1016/0006-8993(83)90286-x. [DOI] [PubMed] [Google Scholar]
- Galiana HL, Guitton D. Central organization and modeling of eye-head coordination during orienting gaze shifts. In: Cohen B, Tomko D, Guedry F, editors. Sensing and Controlling Motion. Annals of New York Academy of Sciences; New York: 1992. pp. 452–471. [DOI] [PubMed] [Google Scholar]
- Gandhi NJ, Sparks DL. Dissociation of eye and head components of gaze shifts by stimulation of the omnipause neuron region. J Neurophysiol. 2007;98:360–373. doi: 10.1152/jn.00252.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goossens HHLM, vanOpstal AJ. Human eye-head coordination in two dimensions under different sensorimotor conditions. Exp. Brain Res. 1997;114:542–560. doi: 10.1007/pl00005663. [DOI] [PubMed] [Google Scholar]
- Guitton D, Douglas RM, Volle M. Eye-head coordination in cats. J. Neurophysiology. 1984;52:1030–1050. doi: 10.1152/jn.1984.52.6.1030. [DOI] [PubMed] [Google Scholar]
- Guitton D, Munoz DP, Gallana HL. Gaze control in the cat: Studies and modeling of the coupling between orienting eye and head movements in different behavioral tasks. J. Neurophysiology. 1990;64:509–531. doi: 10.1152/jn.1990.64.2.509. [DOI] [PubMed] [Google Scholar]
- Guitton D, Volle M. Gaze control in humans: Eye-head coordination during orienting movements to targets within and beyond the oculomotor range. J Neurophysiol. 1987;58:427–439. doi: 10.1152/jn.1987.58.3.427. [DOI] [PubMed] [Google Scholar]
- Hepp K, Henn V, Vilis T, Cohen B. Brainstem regions related to saccade generation. Rev Oculomot Res. 1989;3:105–212. [PubMed] [Google Scholar]
- Isa T, Sasaki S. Brainstem control of head movements during orienting; organization of the premotor circuits. Prog Neurobiol. 2002;66:205–241. doi: 10.1016/s0301-0082(02)00006-0. [DOI] [PubMed] [Google Scholar]
- Judge SJ, Richmond BJ, Chu FC. Implantation of magnetic search coils for measurement of eye position: an improved method. Vision Res. 1980;20:535–538. doi: 10.1016/0042-6989(80)90128-5. [DOI] [PubMed] [Google Scholar]
- Jürgens R, Becker W, Kornhuber HH. Natural and drug-induced variations of velocity and duration of human saccadic eye movements: Evidence for a control of the neural pulse generator by local feedback. Biological Cybernetics. 1981a;39:87–96. doi: 10.1007/BF00336734. [DOI] [PubMed] [Google Scholar]
- Jürgens R, Becker W, Reiger P. Different effects involved in the interactions of saccades and the vestibulo-ocular reflex. In: Cohen B, editor. Vestibular and Oculomotor Physiology. Vol. 374. NY Acad. Sci.; New York: 1981b. pp. 744–754. [DOI] [PubMed] [Google Scholar]
- Keller EL. The Brainstem. In: Carpenter RHS, editor. Eye Movements. Vol. 8. CRC Press; Boca Raton: 1991. pp. 200–223. [Google Scholar]
- Laurutis V, Robinson D. The vestibulo-ocular reflex during human saccadic eye movements. J. Physiol. 1986;373:209–233. doi: 10.1113/jphysiol.1986.sp016043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C. Eye and Head Coordination in Reading: Roles of Head Movement and Cognitive Control. Vision Research. 1999;39:3761–3768. doi: 10.1016/s0042-6989(99)00111-x. [DOI] [PubMed] [Google Scholar]
- Leigh RJ, Zee DS. The neurology of eye movements. Oxford University Press; Oxford: 1999. [Google Scholar]
- Lestienne F, Vidal PP, Berthoz A. Gaze Changing Behaviour in Head Restrained Monkey. Exp. Brain Res. 1984;53:349–356. doi: 10.1007/BF00238165. [DOI] [PubMed] [Google Scholar]
- Morasso P, Bizzi E, Dichgans J. Adjustment of saccade characteristics during head movements. Exp. Brain Res. 1973;16:492–500. doi: 10.1007/BF00234475. [DOI] [PubMed] [Google Scholar]
- Moschner C, Zangmeister WH. Preview control of gaze saccades: efficacy of prediction modulates eye-head interaction during human gaze saccades. Neurol Res. 1993;15:417–432. doi: 10.1080/01616412.1993.11740176. [DOI] [PubMed] [Google Scholar]
- Moschovakis AK. Neural network simulations of the primate oculomotor system I. The vertical saccadic burst generator. Biol. Cybern. 1994;70:291–302. doi: 10.1007/BF00197610. [DOI] [PubMed] [Google Scholar]
- Pelisson D, Prablanc C, Urquizar C. Vestibuloocular reflex inhibition and gaze saccade control characteristics during eye-head orientation in humans. J Neurophysiol. 1988;59:997–1013. doi: 10.1152/jn.1988.59.3.997. [DOI] [PubMed] [Google Scholar]
- Peterson BW, Richmond FJ. Control of head movement. Oxford University Press; Oxford: 1988. [Google Scholar]
- Phillips JO, Ling L, Fuchs AF, Seibold C, Plorde JJ. Rapid horizontal gaze movement in the monkey. J. Neurophysiol. 1995;73:1632–1652. doi: 10.1152/jn.1995.73.4.1632. [DOI] [PubMed] [Google Scholar]
- Quessy S, Freedman EG. Electrical stimulation of rhesus monkey nucleus reticularis gigantocellularis I. Characteristics of evoked head movements. Exp Brain Res. 2004;156:342–356. doi: 10.1007/s00221-003-1787-8. [DOI] [PubMed] [Google Scholar]
- Robinson DA. The mechanics of human saccadic eye movement. J. Physiol. 1964;174:245–264. doi: 10.1113/jphysiol.1964.sp007485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson DA. Oculomotor unit behavior in the monkey. J. Physiol. 1970;33:393–404. doi: 10.1152/jn.1970.33.3.393. [DOI] [PubMed] [Google Scholar]
- Robinson DA. Models of the saccadic eye movement control system. Kybernetik. 1973a;14:71–83. doi: 10.1007/BF00288906. [DOI] [PubMed] [Google Scholar]
- Robinson DA. Oculomotor control system. Invest. Ophthalmol. 1973b;12:164–166. [PubMed] [Google Scholar]
- Robinson DA, O'meara DM, Scott AB, Collins CC. Mechanical components of human eye movements. J. Applied Physiology. 1969;26:548–553. doi: 10.1152/jappl.1969.26.5.548. [DOI] [PubMed] [Google Scholar]
- Roucoux A, Guitton D, Crommelinck M. Stimulation of the superior colliculus in the alert cat. Exp. Brain Res. 1980;39:75–85. doi: 10.1007/BF00237071. [DOI] [PubMed] [Google Scholar]
- Roy JE, Cullen KE. A neural correlate of vestibulo-ocular reflex suppression during voluntary eye-head gaze shifts. Nature Neurosci. 1998;1:404–410. doi: 10.1038/1619. [DOI] [PubMed] [Google Scholar]
- Scudder CA, Kaneko CRS, Fuchs AF. The brainstem burst generator for saccadic eye movement: A modern synthesis. Exp. Brain Res. 2002;142:439–462. doi: 10.1007/s00221-001-0912-9. [DOI] [PubMed] [Google Scholar]
- Sparks DL. The Neural Control of Orienting Eye and Head Movements. In: Humphrey DR, Freund H-J, editors. Motor Control: Concepts and Issues. John Wiley & Sons, ltd.; 1991. pp. 263–275. [Google Scholar]
- Sparks DL. The brainstem control of saccadic eye movements. Nat Rev Neurosci. 2002;3:952–964. doi: 10.1038/nrn986. [DOI] [PubMed] [Google Scholar]
- Sparks DL, Hartwich-Young R. The deep layers of the superior colliculus. Rev Oculomot Res. 1989;3:213–255. [PubMed] [Google Scholar]
- Sperry R. Neural basis of the spontaneous optokinetic response produced by visual inversion. J. Comp. Physiol. Psychol. 1950;43:482–489. doi: 10.1037/h0055479. [DOI] [PubMed] [Google Scholar]
- Stahl J. Amplitude of human head movements associated with horizontal saccades. Exp. Brain Res. 1999;126:41–54. doi: 10.1007/s002210050715. [DOI] [PubMed] [Google Scholar]
- Tabak S, Smeets JBJ, Collewijn H. Modulation of the human vestibuloocular reflex during saccades: probing by high frequency oscillation and torque pulses to the head. J Neurophysiol. 1996;76:3249–3263. doi: 10.1152/jn.1996.76.5.3249. [DOI] [PubMed] [Google Scholar]
- Tomlinson RD. Combined eye-head gaze shifts in primate III. contributions to the accuracy of gaze saccades. J. Neurophysiology. 1990;64:1873–1981. doi: 10.1152/jn.1990.64.6.1873. [DOI] [PubMed] [Google Scholar]
- Tomlinson RD, Bahra PS. Combined eye-head gaze shifts in the primate I. metrics. J. Neurophysiology. 1986a;56:1542–1557. doi: 10.1152/jn.1986.56.6.1542. [DOI] [PubMed] [Google Scholar]
- Tomlinson RD, Bahra PS. Combined eye-head gaze shifts in the primate II. Interactions between saccades and the vestibuloocular reflex. J. Neurophysiology. 1986b;56:1558–1570. doi: 10.1152/jn.1986.56.6.1558. [DOI] [PubMed] [Google Scholar]
- Tweed D, Glenn B, Vilis T. Eye-head coordination during large gaze shifts. J. Neurophysiol. 1995;73:766–799. doi: 10.1152/jn.1995.73.2.766. [DOI] [PubMed] [Google Scholar]
- vanGisbergen JAM, Robinson DA, Gielen SA. A quantitative analysis of generation of saccadic eye movements by burst neurons. J. Neurophysiology. 1981;45:417–442. doi: 10.1152/jn.1981.45.3.417. [DOI] [PubMed] [Google Scholar]
- vanGisbergen JAM, vanOpstal J, Ottes FP. Parametrization of saccadic velocity profiles in man. In: Gale AG, Johnson F, editors. Theoretical and Applied aspects of Eye Movement Research. Elsevier Science; New York: 1984. pp. 87–94. [Google Scholar]
- vanOpstal AJ, vanGisbergen JAM. Skewness of saccadic velocity profiles a unifying parameter for normal and slow saccades. Vision Res. 1987;27:731–745. doi: 10.1016/0042-6989(87)90071-x. [DOI] [PubMed] [Google Scholar]
- Vidal PP, Roucoux A, Berthoz A. Horizontal eye position-related activity in neck muscles of t he alert cat. Exp. Brain Research. 1982;46:448–453. doi: 10.1007/BF00238639. [DOI] [PubMed] [Google Scholar]
- Volle M, Guitton D. Human gaze shifts in which the head and eyes are not initially aligned. Exp. Brain Res. 1993;94:463–470. doi: 10.1007/BF00230204. [DOI] [PubMed] [Google Scholar]
- von Holst E, Mittelstaedt H. The Principle of reafferance: Interactions between the central nervous system and the peripheral organs (translated by P.C. Dodwell and reprinted from Die Naturwissenschaften (1950)). In: Dodwell PC, editor. Perceptual Processing: Stimulus equivalence and pattern recognition. Appleton-Century-Crofts; New York: 1971. pp. 41–71. [Google Scholar]
- Zangemeister WH, Stark L. Active head rotations and eye-head coordination. Ann N.Y. Acad. Sci. 1981;374:540–559. doi: 10.1111/j.1749-6632.1981.tb30899.x. [DOI] [PubMed] [Google Scholar]
- Zangemeister WH, Stark L. Types of gaze movement: variable interactions of eye and head movements. Exp. Neurol. 1982;77:563–577. doi: 10.1016/0014-4886(82)90228-x. [DOI] [PubMed] [Google Scholar]