Abstract
Background/Aims
Clinico-pathological manifestations of Ferroportin (Fpn) Disease (FD) are heterogeneous, with some patients presenting with iron overload predominantly in macrophages (“M” phenotype), others predominantly in hepatocytes (“H” phenotype). This appears to reflect functional heterogeneity of Fpn mutants, with loss-of-function generally resulting in the M type.
Methods
Two unrelated probands with “non-HFE” hemochromatosis were screened for Fpn mutations. Mutants were functionally characterized by immunofluorescence microscopy, evaluation of their ability to bind hepcidin and export iron, and by expressing them in zebrafish.
Results
Two novel Fpn mutations were identified: I152F in patient-1, presenting with typical M phenotype; and L233P in patient-2, presenting with ambiguous features (massive overload in both macrophages and hepatocytes). Molecular studies suggested loss of function in both cases. The I152F, normally localized on cell membrane and internalized by hepcidin, showed a unique “primary” deficit of iron export capability. The L233P did not appropriately traffic to cell surface. Loss of function was confirmed by expressing both mutants in vivo in zebrafish, resulting in iron limited erythropoiesis. Clinical manifestations were likely enhanced in both patients by nongenetic factors (HCV, alcohol).
Conclusions
The combination of careful review of clinico-pathological data with molecular studies can yield compelling explanations for phenotype heterogeneity in FD.
Keywords: iron overload, ferroportin, hemochromatosis, hepcidin, zebrafish
INTRODUCTION
Ferroportin (Fpn), a 571 amino acids transmembrane protein, plays a critical role in iron metabolism. Cells possess several different mechanisms for taking up iron [1], but Fpn is the only known cellular iron exporter. Fpn controls iron entry into the plasma through duodenal enterocytes (1–2 mg/day) or macrophages that recycle near 20–30 mg/day of iron from senescent erythrocytes [2]. Recently, Fpn has been recognized as the receptor for hepcidin, a master regulator of iron homeostasis [3]. Binding of hepcidin downregulates Fpn through induction of its internalization and degradation [4], the final results being a reduction of plasma iron concentration and an increase of intracellular ferritin. Missense mutations in the Fpn gene leads to Ferroportin Disease (FD), also known as type IV hereditary hemochromatosis (HH). This disorder is emerging as the second commonest inherited disorder of iron metabolism after “classic” HFE-related HH [5]. FD is relatively milder than other types of HH [6], although progression to liver cancer has been recently reported [7]. FD also differs from all other types of HH because of the dominant transmission, the pathophysiology (involving the hepcidin target rather than a defect of hepcidin itself), and the heterogeneous clinical presentation. Difference in disease presentation is thought to reflect the different functional behaviour of the various mutations. The most frequent category of mutations result in “loss-of-function”, mainly because of retention of the mutant Fpn into the endoplasmic reticulum leading to inappropriately low plasma membrane expression of Fpn. The decreased surface expression of Fpn leads to iron overload predominantly in macrophages (“M” phenotype). Patients with_this disorder present with hyperferritinemia, normal to low transferrin saturation (TS), and possibly transient iron restricted erythropoiesis, especially during phlebotomies. The second category of Fpn mutations includes “gain-of-function” mutations, mainly because of resistance to hepcidin-induced degradation. These mutants are associated with high TS and iron loading in hepatocytes (“H” phenotype). The presentation of this disorder is clinically indistinguishable from other types of HH. This classification may be an oversimplification that often does not fit with the observations in clinical practice. We describe here two novel mutations in the Fpn gene occurring in two unrelated patients, one of them presenting with clinico-pathological features of massive iron overload. The functional role of the two Fpn mutations was studied in mammalian cell culture and zebrafish.
MATERIALS AND METHODS
Case description
Both patients were referred to our regional Centre for Iron Overload Disorders in Verona for evaluation of “non-HFE” hemochromatosis. The study was approved by the local Ethical Committee (Azienda Ospedaliera di Verona). A written informed consent was obtained from either the patients or their relatives in accordance with the Declaration of Helsinki.
Case 1
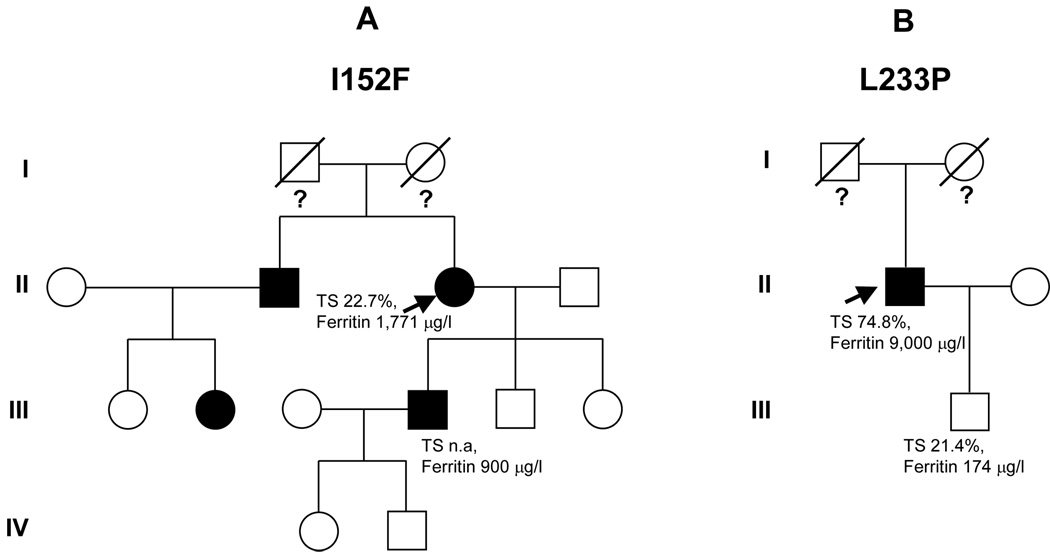
A 59 year-old female came to our observation in October 2004 because of hyperferritinemia (1,916 µg/l) and a slight increase of aminotransferases (AST 44 U/l, ALT 79 U/l; normal value <40), found during a family screening for hemochromatosis. A few months before, her 34 year-old son was incidentally found to have hyperferritinemia (900 µg/l, TS not available) (Figure 1A) during preliminary evaluation in becoming a blood donor. The patient was started on a phlebotomy program. The patient’s brother was also reported to have high ferritin levels. At the time of our first evaluation, the patient had already undergone 8 phlebotomies of about 400 ml each, her serum ferritin was 1,771 µg/L, and TS was 22.7%. A more comprehensive laboratory investigation revealed the presence of previously unrecognized diabetes mellitus (DM) and positive serology for hepatitis C virus (HCV)._HCV-RNA also resulted positive. Alcohol intake was negligible. She complained of arthralgias involving proximal and distal interphalangeal joints of both hands, where signs of slight inflammation were present. Other relevant data on physical examination were overweight (BMI 28 Kg/m2), and hepatomegaly (3 cm below the costal margin). Non-invasive quantification of liver iron content by means of MRI using the Gandon’s protocol [8] yielded a value of 150 µmol/g (normal value < 36 µmol/g). It was possible to perform a liver biopsy only after attaining normal ferritin levels by phlebotomies (total iron removed: 7 g). The histopathology showed portal and peri-portal lymphocytic infiltrates consistent with HCV-related chronic hepatitis, along with septal fibrosis and mild steatosis. On Pearl’s staining, no significant iron was found in hepatocytes, while rare deposits of hemosiderin were seen in macrophages (not shown).
Figure 1. Pedigree of two patients.
The probands are indicated by arrows. (A) patient no. 1; (B) patient no. 2. Iron parameters are reported when available.
Case 2
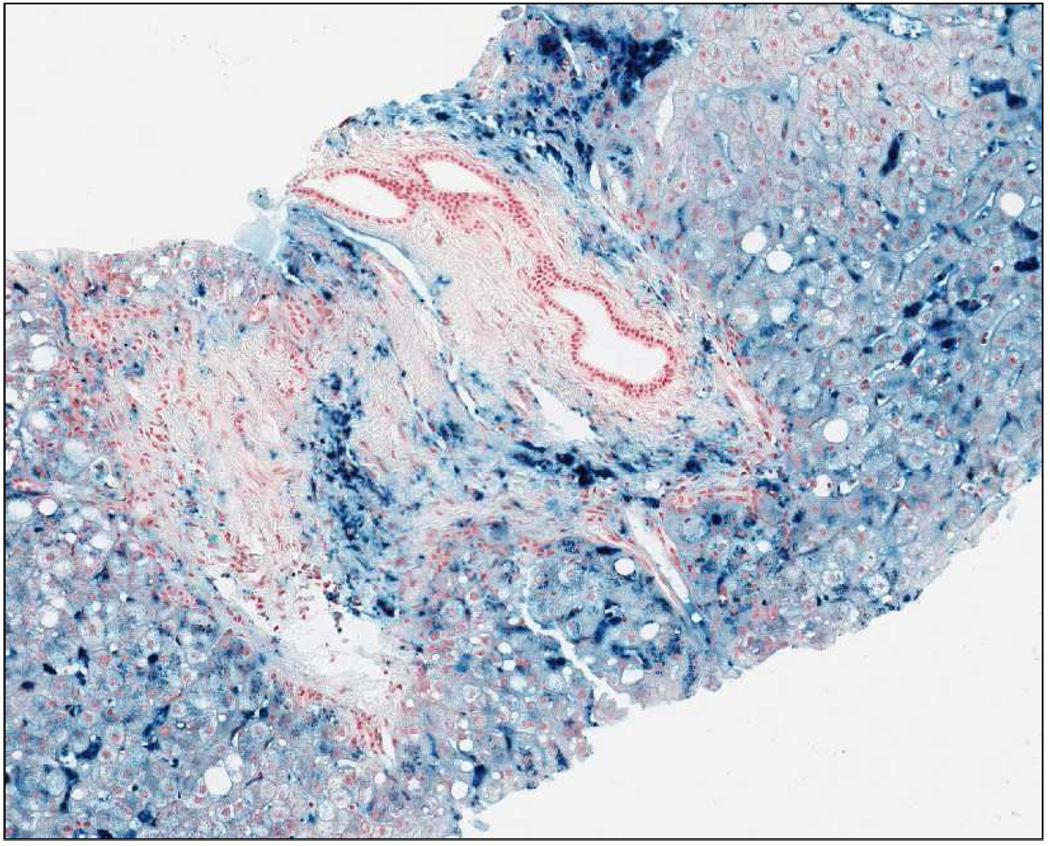
A 64 year-old man, came to our observation in September 2005. He first underwent laboratory investigations in 2000, at the age of 59 years, because of hepatomegaly (8 cm below the costal margin) found during a routine control examination by the family physician. On this occasion, he was found to have marked hyperferritinemia (9,000 µg/l) and increased TS (74,8%), along with slight elevation of aminotransferases (AST 44 U/L; ALT 96 U/L), and hyperglycaemia consistent with a previously unrecognized DM. He underwent a liver biopsy, the findings of which were consistent with HH. He was then started on an intensive phlebotomy program (one 500 ml-unit weekly). During the first month he developed a slight asymptomatic anemia (nadir of hemoglobin 12 g/dl). The phlebotomy program was unchanged, and hemoglobin levels rapidly rose and stabilized to 14.5–15 g/dl over the second month. At the time of our evaluation, he had nearly 300 phlebotomies (at least 75 g iron removed) throughout the previous four years. A precise retrospective estimation of iron deposits at diagnosis was not possible because of lack of data on serum ferritin during treatment. He declared a previous alcohol intake of about 1 liter of wine per week, and one drink of strong brandy per day until 2000. Until the age of 50 he had been an occasional blood donor (total donations: 10 units). There was no family history of iron overload or liver diseases (both parents were deceased for unrelated diseases). A biochemical screening of his year-old son revealed normal serum iron indices (TS 21,4%, ferritin 174 µg/l) (Figure 1B). A review of the original liver biopsy (Figure 2) showed massive iron overload with large coalescent deposits of hemosiderin especially in Kupffer cells, but also in hepatocytes (grade 4). There was also grade 1 steatosis, and portal fibrosis without overt cirrhosis. An abdominal MRI performed at diagnosis revealed the presence of iron deposits not only in the liver, but also in the spleen.
Figure 2. Liver histology of patient no. 2 at diagnosis.
Massive iron overload is evident, with large coalescent deposits of hemosiderin especially in Kupffer cells, but also in hepatocytes (grade 4). There was also grade 1 steatosis, and portal fibrosis without overt cirrhosis.
Genetic Analyses
Denaturing high-performance liquid chromatography (DHPLC) scanning of the hemochromatosis genes was performed on a Wave™ DNA fragment Analysis System (Transgenomic), as previously described [9,10].
Generation of Fpn Constructs
All Fpn mutations were generated in pFpn-EGFP-N1 by using the QuikChange site-directed mutagenesis kit (Stratagene), amplified in Escherichia coli, and sequence-verified before transfecting into mammalian cells.
Cells and Media
Mouse Fpn was expressed in a cytomegalovirus (CMV)-containing vector (pEGFP-N1, Clontech, Mountain View, CA) as described previously [11]. Human embryonic kidney (HEK) 293T cells were maintained in DMEM with 10% fetal bovine serum and transfected with pFpn-EGFP-N1 and pFpn(mutations)-EGFP-N1, by using Nucleofector technology (Amaxa, Gaithersburg, MD), according to the manufacturer’s directions.
Ferritin analysis
Cells expressing GFP only, wild-type (wt) Fpn-GFP, or mutant Fpn-GFP were incubated with 10 µM ferric ammonium citrate (FAC) for 24 hours. After FAC loading, cells were harvested, and ferritin content was determined by enzyme-linked immunosorbent assay (ELISA) as described previously [12].
Injection of Fpn constructs into zebrafish embryos
Maintenance of zebrafish stocks and embryo cultures were performed as previously described [13]. Mouse Fpn-GFP were purified using the EndoFree purification kit (Qiagen, Chatsworth, CA). Approximately 30 to 50 pg DNA was microinjected into zebrafish embryos at the 1-cell stage. Embryos were stained with o-dianisidine to detect hemoglobinized cells as described [13].
Urinary hepcidin assay
Urinary hepcidin was measured by SELDI-TOF-MS as previously described [14]. Relative concentrations of urinary hepcidin-25 were expressed as Mega Intensity units per millimole of creatinine (MIU/mM crea).
RESULTS
Genetic analyses
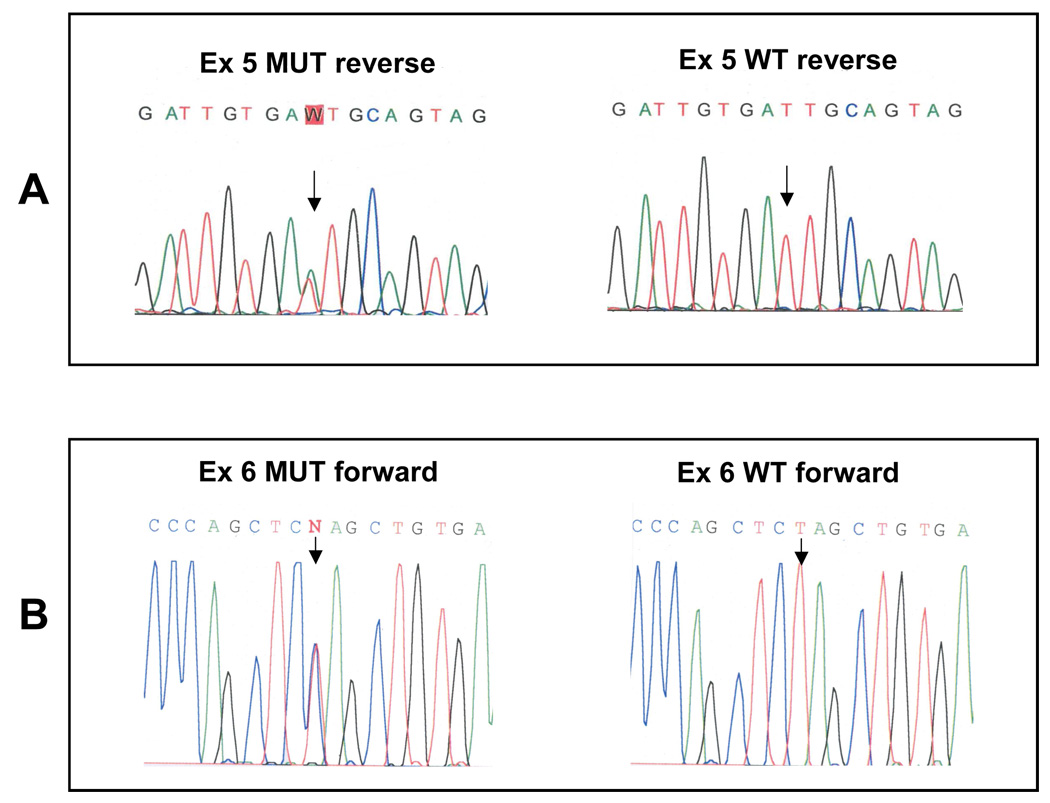
The molecular analyses of Fpn gene (SLC40A1) revealed the presence of two novel missense mutations (Figure 3). Patient 1 was found to carry an A758T change in exon 5 resulting in the substitution of isoleucine with phenylalanine at position 152 (I152F) (Figure 3A). Patient 2 was found to carry a T1012C change in exon 6, resulting in the substitution of leucine with proline at position 233 (L233P) (Figure 3B). Scanning of other HH-related genes showed the presence of heterozygosity for the H63D mutation in HFE gene in patient 1, and an intronic polymorphism of TfR2 (IVS15+7C>T) in patient 2. No variation was found in genes coding for hepcidin (HAMP) and hemojuvelin (HJV) in either patient.
Figure 3. Sequence analyses of the two novel mutations.
(A) the I152F mutation (arrowed); (B) the L233P mutation (arrowed); both compared with a wild-type sample.
Subcellular Localization and Response to Hepcidin of Mutant Fpn
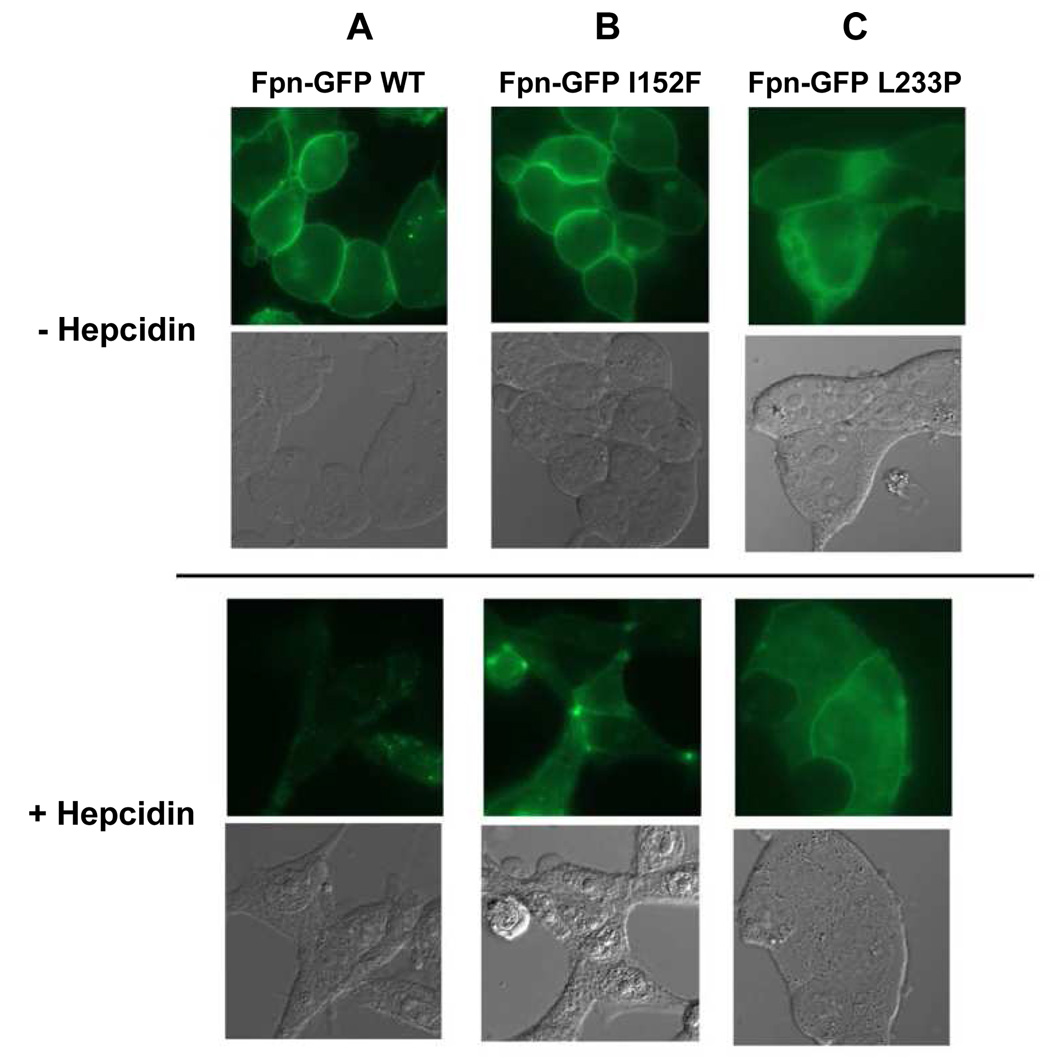
Cultured HEK293T cells were transfected with plasmids containing mutant Fpn-GFP under the control of the CMV promoter, and the cellular distribution of the expressed protein was examined by fluorescence microscopy. Wild-type Fpn was localized to the cell surface. After incubation with hepcidin for 24 hours, most of the wt Fpn was internalized (Figure 4, column A). The mutant Fpn-GFP I152F was properly localized on the cell membrane, but after incubation with hepcidin was internalized slower than wt Fpn (Figure 4, column B; Supplemental Figure 1). On the other hand, Fpn-GFP L233P showed a mixed localization in either the cytoplasm or the plasma membrane. Addition of hepcidin led to the internalization of cell surface Fpn-GFP L233P (Figure 4, column C).
Figure 4. Functional analyses of the new Fpn mutations and response to hepcidin.
HEK293T cells were transiently transfected with plasmids containing Fpn-GFP wt (A), Fpn-GFP I152F (B), or Fpn-GFP L233P (C), and localization was assessed by epifluorescence microscopy (top panel). Eighteen h after transfection, cells were incubated with 1 µg/ml hepcidin for 24 h and examined for Fpn-GFP localization (bottom panel).
Iron export capability
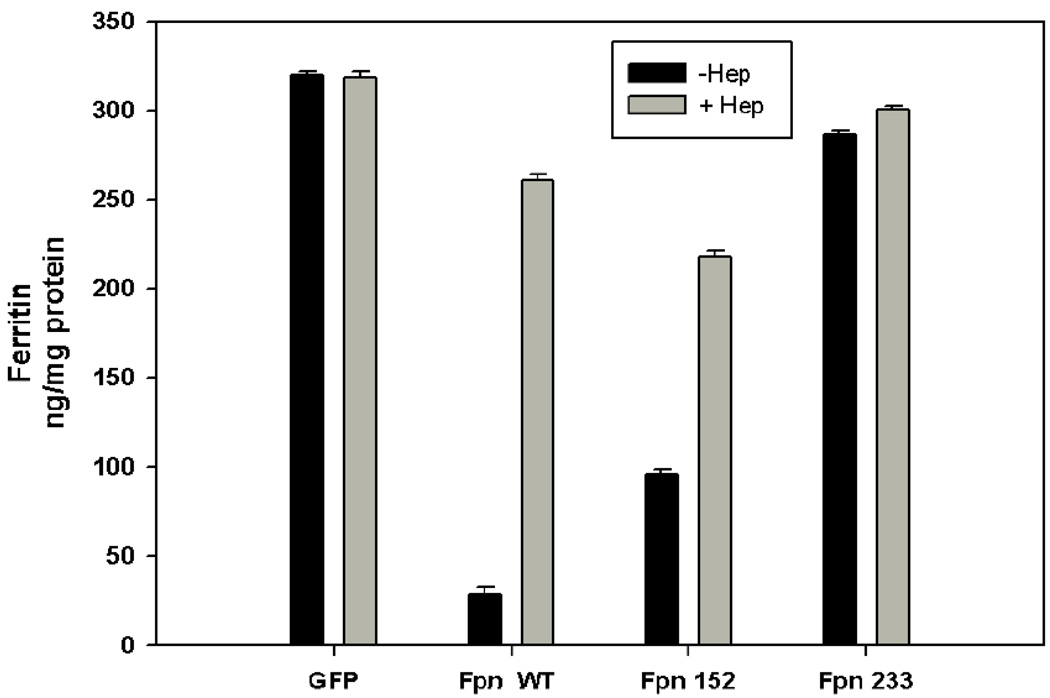
The ability of mutant Fpns to export iron was evaluated. Cells expressing wt or mutant Fpn were incubated in high iron (10 µM FAC) containing medium, which resulted in accumulation of the iron storage protein ferritin. As expected, expression of wt Fpn-GFP decreased substantially intracellular ferritin, (Figure 5). Expression of Fpn-GFP I152F resulted in a partial retention of ferritin as compared with wt Fpn-GFP. Since this mutant Fpn showed correct localization at the cell surface, the defect in iron export activity could be considered “primary”. Cells expressing Fpn-GFP L233P showed substantial retention of ferritin, suggesting a severe impairment of iron export capability that could be considered secondary to the inappropriate membrane localization of this mutant.
Figure 5. Fpn mutations affect intracellular ferritin levels.
HEK293T cells were transiently transfected with plasmids containing Fpn-GFP wt or mutant Fpn-GFP. Cells were cultured with ferric ammonium citrate (FAC) (10 µM). After FAC loading (24 h), cells were incubated with 1 µg/ml hepcidin (24 h), harvested, and ferritin levels were determined by ELISA. Error bars refers to three independent experiments.
Expression of Fpn mutations in Zebrafish
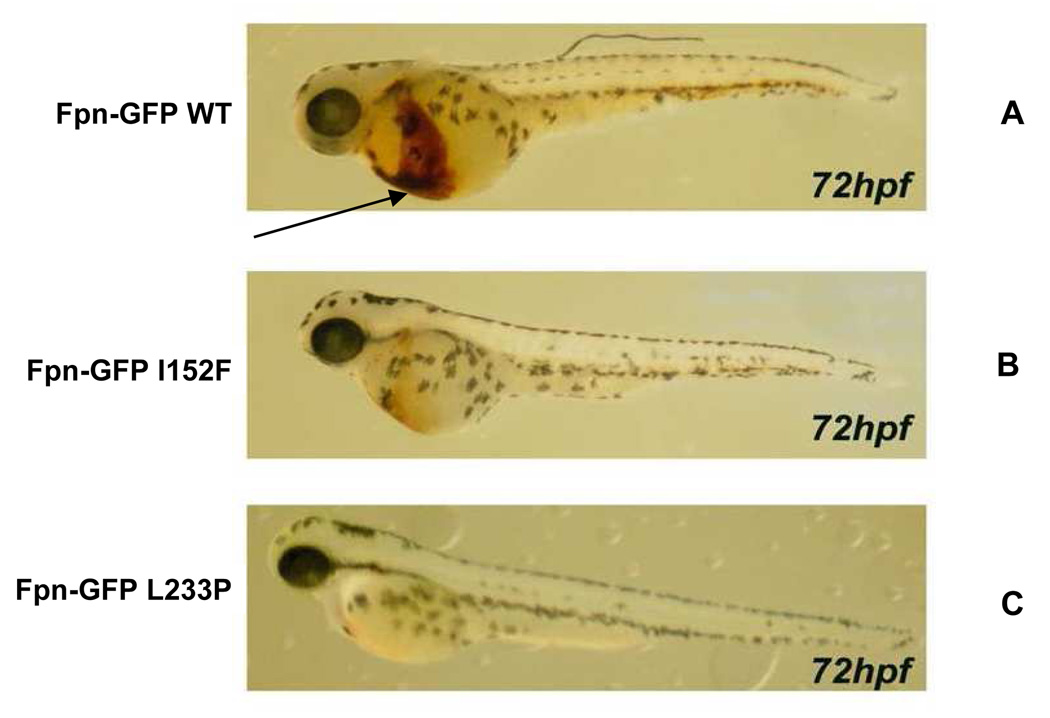
We investigated the effect of expression of Fpn mutants on erythroid development in zebrafish. Expression of wt Fpn had no effect on the development of erythrocytes (brown staining, denoted by arrow in Figure 6A). On the other hand, a severe reduction in hemoglobin production was observed in embryos expressing both the mutations, especially for the L233P (Figures 6B–C).
Figure 6. Expression of mutant Fpn in zebrafish affects hemoglobinization of erythrocytes.
Zebrafish embryos were injected with wt or mutant Fpn-GFP. At 72 hours post-fertilization the embryos were stained with o-dianisidine to detect hemoglobinized cells (brown color denoted by the arrow; A, wild-type Fpn). A severe reduction in hemoglobin production was observed in embryos expressing both the mutations, especially for the L233P (B–C). The figures are representative of 3 different experiments in which 100 embryos were injected with each construct. The survival rate was 85% for embryos injected with wild type constructs, 75% for embryos injected with I152F constructs, and 77% for embryos injected with L233P constructs.
Urinary hepcidin (u-hepcidin) by SELDI-TOF-MS
Urinary hepcidin level in both patients was below our reference range in control subjects: 0.14 MIU/mM creatinine in patient 1, and 0.19 MIU/mM creatinine in patient 2; reference range: 0.31–0.66 MIU/mM creatinine [14].
DISCUSSION
Functional studies of Fpn mutations have been often reported apart from antecedent clinical descriptions, making a proper interpretation of clinical/molecular correlations difficult. A further problem is represented by the relative paucity of liver biopsies reported [5]. In this study we performed a comprehensive functional description of two novel FPN mutations, and used this data to interpret the clinical phenotype.
Clinical phenotypes
The clinical presentation of the two index cases was different. Patient 1 presented with dominant hyperferritinemia and normal TS, consistent with a typical “M phenotype” associated to loss-of-function mutations. Patient 2, however, presented with ambiguous features because of a lack of a demonstrable positive family history, elevation of both ferritin and TS, and pathological findings of massive iron overload in both macrophages and hepatocytes.
Functional studies in cellular models
Transfection of mutant Fpn constructs into different cell types has been successfully used to assess their functional behaviour [11]. It permits an evaluation of their cellular localization, as well as their ability to bind hepcidin and to export iron. A proper membrane localization of Fpn is critical for iron export activity. Most “loss-of-function” mutations have been shown to result from defects of Fpn trafficking to the cell surface, probably because of a tendency to aggregate and/or misfold that leads to entrapment into the endoplasmic reticulum. At the opposite end of the spectrum are “gain-of-function” mutants that are properly localized on the membrane, but are resistant to hepcidin inactivation [11,15,16]. The new Fpn mutations described here appear to result in a loss-of-function, though with different mechanisms. Fpn I152F showed peculiar behaviour: it was properly localized on the cell surface and responded normally to hepcidin. Experiments in iron-loaded mammalian cells and in zebrafish expressing the mutant Fpn were consistent with an intrinsic defect of the iron export capability. Isoleucine at position 152 is part of a highly conserved region, and in alignments of both mouse and human Fpn proteins is placed at the border of the IV (out of 12) transmembrane domain by most prediction models [17]. Our observations suggest that this conserved amino acid may be critical for proper iron efflux. To our knowledge, this is the first report of a transport incompetent Fpn mutant that localizes properly to the cell membrane. A similar behaviour was previously reported for the N174I mutation [18], while in that case nearly half of the mutant protein showed intracellular localization.
The Fpn L233P mutant showed a typical defect in cell surface localization, which is sufficient per se to explain the inability to export iron. The leucine at position 233 is predicted to be part of an intracellular segment of the protein [17], which may result in retaining into the endoplasmic reticulum when mutated.
Expression of the mutations in Zebrafish
Zebrafish (Danio Rerio) represents an emerging tool as an animal model for human diseases because of the unique combination of easy manipulability and optical clarity of embryos, which allows real-time imaging of developing pathologies [19], particularly in the field of hematology [20]. Indeed, Fpn itself was originally identified by positional cloning in the weh zebrafish hypochromic anemia mutant [21]. Expression of Fpn mutants in zebrafish can identify those Fpn mutants that lead to macrophage iron retention [13]. The observed anemic phenotypes (Figure 6) unequivocally confirmed the assignation of the two novel mutations to the “loss-of-function” category, resulting in iron-restricted erythropoiesis because of inefficient iron release from macrophages.
Recapitulation of clinical and molecular data
While the apparent typical “M” phenotype observed in patient 1 is in agreement with molecular data, the relatively ambiguous phenotype observed in patient 2 is less obvious to interpret. The loss-of-function behaviour of the L233P suggests a type “M” phenotype with late secondary hepatocyte overload. A number of clinical considerations are consistent with this view: 1) the TS, though high, was lower than expected considering the extreme elevation of serum ferritin and the degree of iron overload at diagnosis, suggesting a late TS increase similar to that previously reported by others [5]; 2) the MRI at diagnosis showed substantial iron overload of the spleen (rich of macrophages), at variance with the iron-deprivation macrophage phenotype that occurs in HFE-related HH, as well as in “H” variants of FD [22]; 3) while histopathology examination showed a massive iron overload, there was a relative prevalence of iron accumulation in Kupffer cells, and absence of overt cirrhosis as one might expect to be present if primary hepatocyte overload had occurred over several years with ferritin levels consistently higher than 1,000 µg/l [23,24]. The relative sparing of lobular architecture in this patient is also in agreement with the general notion that primary Kupffer cell iron overload is better tolerated and less fibrogenic than its parenchymal counterpart [25].
Despite the “M” phenotype, both the patients tolerated phlebotomies, even if a marginal transient anemia was observed in patient 2 during the first month of weekly phlebotomies. Thus, phlebotomy is confirmed as an effective therapeutic tool in most FD patients, while the development of signs of iron-restricted erythropoiesis during treatment, when present, remains a clue suggesting the M form of FD.
We found low urinary hepcidin in both patients. Until now, only scanty data exist on hepcidin levels in FD. Papanikolau et al. [26] reported high levels in two subjects carrying the Val162del mutation, subsequently placed into the loss-of-function category [11]. This was attributed to a putative compensatory loop to hepcidin “insensitivity”. The hepcidin assay has been proposed as a useful tool for rapid differentiation of FD from other types of HH, all characterized by hepcidin deficiency [27]. Noteworthy, both our patients were under maintenance phlebotomy at time of sampling, and their hepcidin levels substantially overlapped with those of phlebotomized HFE-HH patients [14]. Two opposite forces are expected to regulate hepcidin in phlebotomized FD patients: inhibition by stimulated erythropoiesis [3], or induction related to the attempt to minimize iron overload and/or to overcome true “hepcidin resistance”. Our data suggest that the inhibitory erythropoietic signal tends to prevail, which is in agreement with observations in HFE-HH phlebotomized patients and in other conditions of activated erythropoiesis [28]. Thus, assay of hepcidin might be useful in diagnosing FD only in patients evaluated before starting phlebotomy and/or in certain mutants subtypes that show constitutive hepcidin resistance.
Finally, it is remarkable to note that clinical expressivity in both patients was likely enhanced by the interaction of nongenetic factors. Current views consider HH as the result of complex, nonlinear interactions between genetic and acquired factors. Clinical manifestations are determined not only by the underlying disease-related mutation(s), but also by coinheritance of modifier genes, and, particularly, by host-related factors leading to nongenetic hepcidin inhibition [22]. Among the latter, long known modifiers such as ethanol abuse and HCV have been recently demonstrated to inhibit hepcidin transcription/expression [29,30]. In light of this view, it appears that the relatively high clinical expressivity observed was mediated by the co-presence of HCV infection (patient 1), and alcohol intake (patient 2).
Conclusions
This study shows that the combination of functional studies with careful review of clinico-pathological data can yield compelling explanations for the heterogeneous phenotypes observed in FD.
Supplementary Material
Internalization of Fpn after a 24 h incubation with hepcidin. Quantifications represent the average Fpn internalization/cell from three data sets of >100 cells each. Internalization can be considered near-normal for I152F, while the substantial reduction observed for L233P was attributable to the primary defect in cell surface localization of this mutant.
ACKNOWLEDGMENTS
This work was supported by grants from Telethon Italy (no. GGP06213 to D.G. and no. GGP05141 to L.C.) and the Cariverona Foundation, Verona, Italy (to D.G) and NIH funding (DK 070947 to J.K.). The authors wish to thank Giovanna De Matteis and Giorgio Biasiotto for technical help in performing molecular analyses.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Hentze MW, Muckenthaler MU, Andrews NC. Balancing acts: molecular control of mammalian iron metabolism. Cell. 2004;117:285–297. doi: 10.1016/s0092-8674(04)00343-5. [DOI] [PubMed] [Google Scholar]
- 2.De Domenico I, McVey Ward D, Kaplan J. Regulation of iron acquisition and storage: consequences for iron-linked disorders. Nat Rev Mol Cell Biol. 2008;9:72–81. doi: 10.1038/nrm2295. [DOI] [PubMed] [Google Scholar]
- 3.Ganz T. Hepcidin and its role in regulating systemic iron metabolism. Hematology Am Soc Hematol Educ Program. 2006;507:29–35. doi: 10.1182/asheducation-2006.1.29. [DOI] [PubMed] [Google Scholar]
- 4.Nemeth E, Tuttle MS, Powelson J, Vaughn MB, Donovan A, Ward DM, et al. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science. 2004;306:2090–2093. doi: 10.1126/science.1104742. [DOI] [PubMed] [Google Scholar]
- 5.Pietrangelo A. The ferroportin disease. Blood Cells Mol Dis. 2004;32:131–138. doi: 10.1016/j.bcmd.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 6.Devalia V, Carter K, Walker AP, Perkins SJ, Worwood M, May A, et al. Autosomal dominant reticuloendothelial iron overload associated with a 3-base pair deletion in the ferroportin 1 gene (SLC11A3) Blood. 2002;100:695–697. doi: 10.1182/blood-2001-11-0132. [DOI] [PubMed] [Google Scholar]
- 7.Corradini E, Ferrara F, Pollicino T, Vegetti A, Abbati GL, Losi L, et al. Disease progression and liver cancer in the ferroportin disease. Gut. 2007;56:1030–1032. doi: 10.1136/gut.2007.122549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gandon Y, Olivié D, Guyader D, Aubé C, Oberti F, Sebille V, et al. Non-invasive assessment of hepatic iron stores by MRI. Lancet. 2004;363:357–362. doi: 10.1016/S0140-6736(04)15436-6. [DOI] [PubMed] [Google Scholar]
- 9.Cremonesi L, Forni GL, Soriani N, Lamagna M, Fermo I, Daraio F, et al. Genetic and clinical heterogeneity of ferroportin disease. Br J Haematol. 2005;131:663–670. doi: 10.1111/j.1365-2141.2005.05815.x. Erratum in: Br J Haematol 2006;132:806. [DOI] [PubMed] [Google Scholar]
- 10.Biasiotto G, Roetto A, Daraio F, Polotti A, Gerardi GM, Girelli D, et al. Identification of new mutations on hepcidin and hemojuvelin in patients with HFE C282Y allele. Blood Cells Mol Dis. 2004;33:338–343. doi: 10.1016/j.bcmd.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 11.De Domenico I, Ward DM, Nemeth E, Vaughn MB, Musci G, Ganz T, et al. The molecular basis of ferroportin-linked hemochromatosis. Proc Natl Acad Sci U S A. 2005;102:8955–8960. doi: 10.1073/pnas.0503804102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Domenico I, Vaughn MB, Li L, Bagley D, Musci G, Ward DM, et al. Ferroportin-mediated mobilization of ferritin iron precedes ferritin degradation by the proteasome. EMBO J. 2006;25:5396–5404. doi: 10.1038/sj.emboj.7601409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Domenico I, Vaughn MB, Yoon D, Kushner JP, Ward DM, Kaplan J. Zebrafish as a model for defining the functional impact of mammalian ferroportin mutations. Blood. 2007;110:3780–3783. doi: 10.1182/blood-2007-07-100248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bozzini C, Campostrini N, Trombini P, Nemeth E, Castagna A, Tenuti I, et al. Measurement of urinary hepcidin levels by SELDI-TOF-MS in HFE-hemochromatosis. Blood Cells Mol Dis. 2008;40:347–352. doi: 10.1016/j.bcmd.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 15.Drakesmith H, Schimanski LM, Ormerod E, Merryweather-Clarke AT, Viprakasit V, Edwards JP, et al. Resistance to hepcidin is conferred by hemochromatosis-associated mutations of ferroportin. Blood. 2005;106:1092–1097. doi: 10.1182/blood-2005-02-0561. [DOI] [PubMed] [Google Scholar]
- 16.Schimanski LM, Drakesmith H, Merryweather-Clarke AT, Viprakasit V, Edwards JP, Sweetland E, et al. In vitro functional analysis of human ferroportin (FPN) and hemochromatosis-associated FPN mutations. Blood. 2005;105:4096–4102. doi: 10.1182/blood-2004-11-4502. [DOI] [PubMed] [Google Scholar]
- 17.Liu XB, Yang F, Haile DJ. Functional consequences of ferroportin 1 mutations. Blood Cells Mol Dis. 2005;35:33–46. doi: 10.1016/j.bcmd.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 18.De Domenico I, Ward DM, Musci G, Kaplan J. Iron overload due to mutations in ferroportin. Haematologica. 2006;91:92–95. [PMC free article] [PubMed] [Google Scholar]
- 19.Lieschke GJ, Currie PD. Animal models of human disease: zebrafish swim into view. Nat Rev Genet. 2007;8:353–367. doi: 10.1038/nrg2091. [DOI] [PubMed] [Google Scholar]
- 20.Carradice D, Lieschke GJ. Zebrafish in hematology: sushi or science? Blood. 2008;111:3331–3342. doi: 10.1182/blood-2007-10-052761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Donovan A, Brownlie A, Zhou Y, Shepard J, Pratt SJ, Moynihan J, et al. Positional cloning of zebrafish ferroportin1 identifies a conserved vertebrate iron exporter. Nature. 2000;403:776–781. doi: 10.1038/35001596. [DOI] [PubMed] [Google Scholar]
- 22.Pietrangelo A. Hemochromatosis: an endocrine liver disease. Hepatology. 2007;46:1291–1301. doi: 10.1002/hep.21886. [DOI] [PubMed] [Google Scholar]
- 23.Beaton M, Guyader D, Deugnier Y, Moirand R, Chakrabarti S, Adams P. Noninvasive prediction of cirrhosis in C282Y-linked hemochromatosis. Hepatology. 2002;36:673–678. doi: 10.1053/jhep.2002.35343. [DOI] [PubMed] [Google Scholar]
- 24.Morrison ED, Brandhagen DJ, Phatak PD, Barton JC, Krawitt EL, El-Serag HB, et al. Serum ferritin level predicts advanced hepatic fibrosis among U.S. patients with phenotypic hemochromatosis. Ann Intern Med. 2003;138:627–633. doi: 10.7326/0003-4819-138-8-200304150-00008. Erratum in: Ann Intern Med 2003;139:235. [DOI] [PubMed] [Google Scholar]
- 25.Gualdi R, Casalgrandi G, Montosi G, Ventura E, Pietrangelo A. Excess iron into hepatocytes is required for activation of collagen type I gene during experimental siderosis. Gastroenterology. 1994;107:1118–1124. doi: 10.1016/0016-5085(94)90237-2. [DOI] [PubMed] [Google Scholar]
- 26.Papanikolaou G, Tzilianos M, Christakis JI, Bogdanos D, Tsimirika K, MacFarlane J, et al. Hepcidin in iron overload disorders. Blood. 2005;105:4103–4105. doi: 10.1182/blood-2004-12-4844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kemna EH, Tjalsma H, Podust VN, Swinkels DW. Mass spectrometry-based hepcidin measurements in serum and urine: analytical aspects and clinical implications. Clin Chem. 2007;53:620–628. doi: 10.1373/clinchem.2006.079186. [DOI] [PubMed] [Google Scholar]
- 28.Origa R, Galanello R, Ganz T, Giagu N, Maccioni L, Faa G, et al. Liver iron concentrations and urinary hepcidin in beta-thalassemia. Haematologica. 2007;92:583–588. doi: 10.3324/haematol.10842. [DOI] [PubMed] [Google Scholar]
- 29.Harrison-Findik DD, Schafer D, Klein E, Timchenko NA, Kulaksiz H, Clemens D, et al. Alcohol metabolism-mediated oxidative stress down-regulates hepcidin transcription and leads to increased duodenal iron transporter expression. J Biol Chem. 2006;281:22974–22982. doi: 10.1074/jbc.M602098200. [DOI] [PubMed] [Google Scholar]
- 30.Nishina S, Hino K, Korenaga M, Vecchi C, Pietrangelo A, Mizukami Y, et al. Hepatitis C virus–induced reactive oxygen species raise hepatic iron level in mice by reducing hepcidin transcription. Gastroenterology. 2008;134:226–238. doi: 10.1053/j.gastro.2007.10.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Internalization of Fpn after a 24 h incubation with hepcidin. Quantifications represent the average Fpn internalization/cell from three data sets of >100 cells each. Internalization can be considered near-normal for I152F, while the substantial reduction observed for L233P was attributable to the primary defect in cell surface localization of this mutant.