Abstract
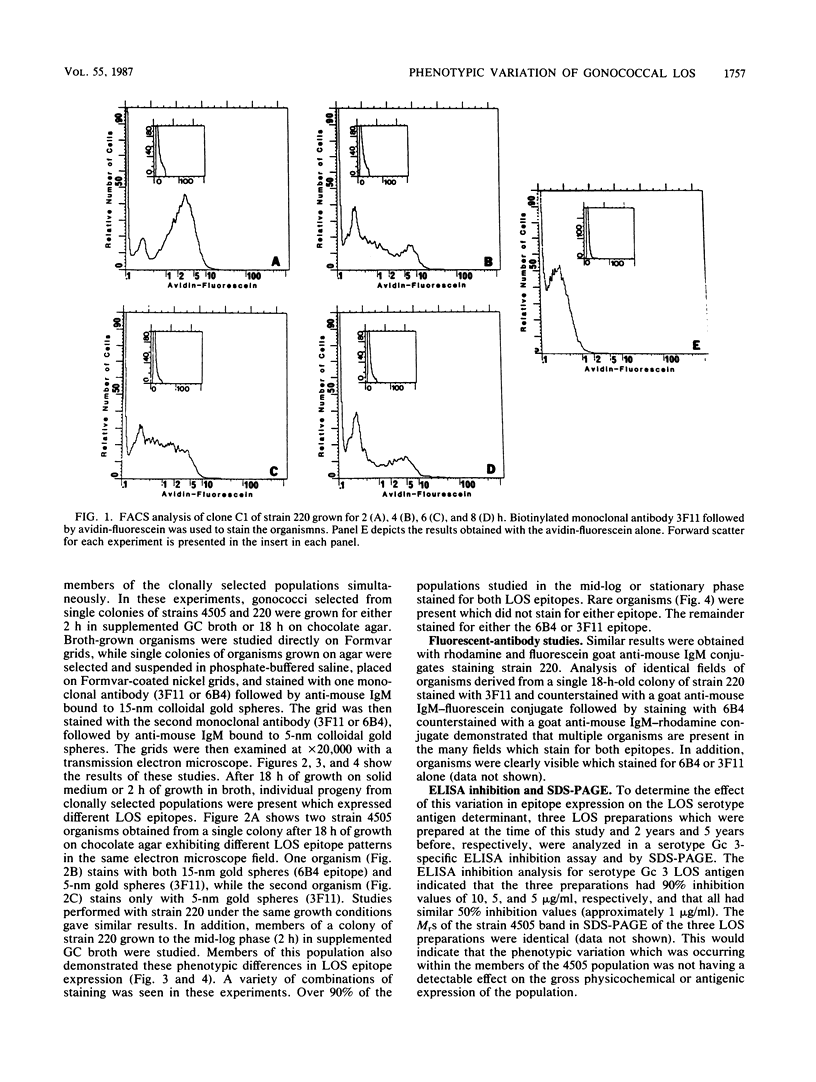
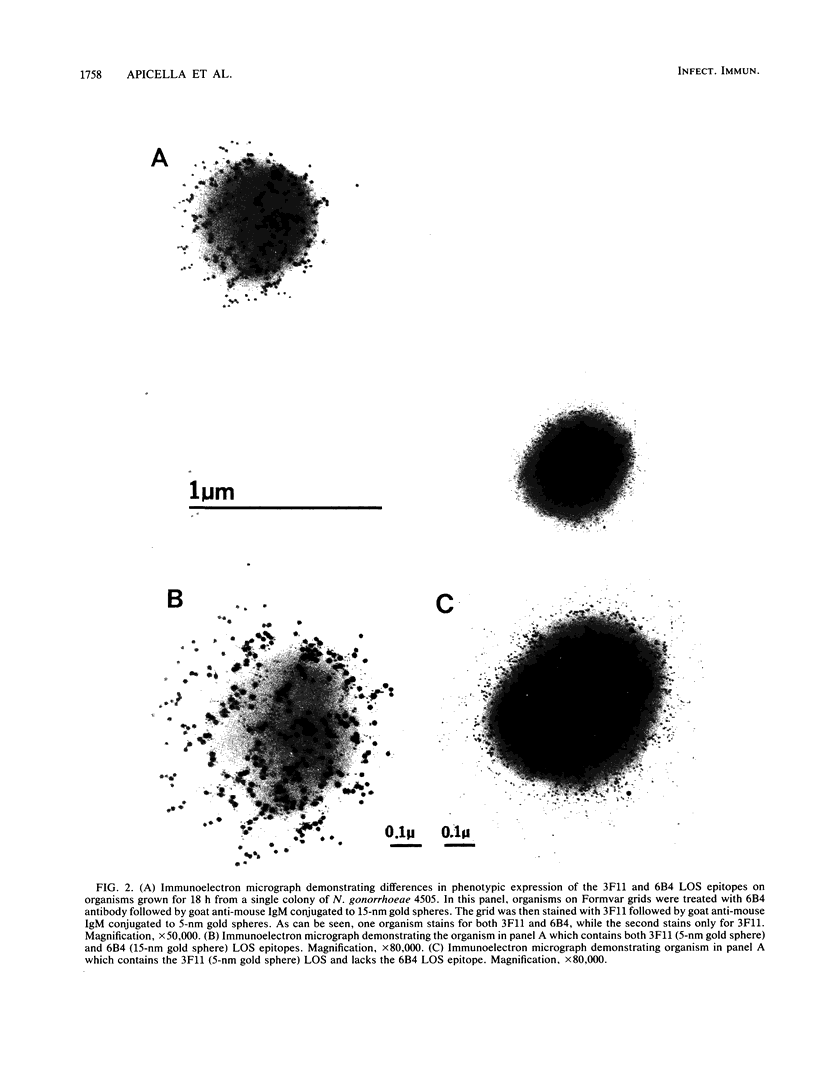
Gonococcal lipooligosaccharides (LOSs) are a series of antigenically complex heteropolymers. To investigate whether all members of clonally selected populations of Neisseria gonorrhoeae express antigenically similar LOS, we studied gonococcal strains 4505 and 220 with monoclonal antibodies 6B4 and 3F11 which have specificity for different oligosaccharide epitopes on the same or comigrating LOS unit(s) on sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Fluorescent-antibody and immunoelectron microscopy studies indicated that all members of the clonally selected populations were not homogenous for the epitopes these antibodies recognized. Fluorescence-activated cell sorting studies of 3F11-coated strain 220 indicated that the density of epitope expression was a function of time of growth. The population could be separated into two broad groups corresponding to organisms staining strongly or weakly for the 3F11 epitope, and the epitope density decreased during the late-log and stationary phases of growth. Sequentially staining organisms on Formvar grids with 6B4 and 3F11, followed by staining with either 5- or 15-nm colloidal gold spheres conjugated to goat anti-mouse immunoglobulin M demonstrated the following populations of cells among organisms derived from a single clone: organisms which stained for both 6B4 and 3F11 epitopes and organisms which stained for either 6B4 epitopes alone or 3F11 epitopes alone. Immunofluorescence microscopy studies with rhodamine and fluorescein goat anti-mouse immunoglobulin M conjugates sequentially staining organisms on Formvar grids with 3F11 and 6B4 also demonstrated these three populations. Analysis of LOS preparations made over the last 5 years indicated no change in serotype antigen concentration or in sodium dodecyl sulfate-polyacrylamide gel electrophoresis migration pattern. These studies indicate that while clonally selected strains of Neisseria gonorrhoeae undergo phenotypic variation at the epitope level, the impact of this variation on the total LOS of the population has little overall effect on its antigenic or physicochemical properties.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Apicella M. A., Bennett K. M., Hermerath C. A., Roberts D. E. Monoclonal antibody analysis of lipopolysaccharide from Neisseria gonorrhoeae and Neisseria meningitidis. Infect Immun. 1981 Dec;34(3):751–756. doi: 10.1128/iai.34.3.751-756.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apicella M. A., Gagliardi N. C. Antigenic heterogeneity of the non-serogroup antigen structure of Neisseria gonorrhoeae lipopolysaccharides. Infect Immun. 1979 Dec;26(3):870–874. doi: 10.1128/iai.26.3.870-874.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apicella M. A. Serogrouping of Neisseria gonorrhoeae: identification of four immunologically distinct acidic polysaccharides. J Infect Dis. 1976 Oct;134(4):377–383. doi: 10.1093/infdis/134.4.377. [DOI] [PubMed] [Google Scholar]
- Apicella M. A., Westerink M. A., Morse S. A., Schneider H., Rice P. A., Griffiss J. M. Bactericidal antibody response of normal human serum to the lipooligosaccharide of Neisseria gonorrhoeae. J Infect Dis. 1986 Mar;153(3):520–526. doi: 10.1093/infdis/153.3.520. [DOI] [PubMed] [Google Scholar]
- Bergström S., Robbins K., Koomey J. M., Swanson J. Piliation control mechanisms in Neisseria gonorrhoeae. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3890–3894. doi: 10.1073/pnas.83.11.3890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gmeiner J., Lüderitz O., Westphal O. Biochemical studies on lipopolysaccharides of Salmonella R mutants. 6. Investigations on the structure of the lipid A component. Eur J Biochem. 1969 Jan;7(3):370–379. doi: 10.1111/j.1432-1033.1969.tb19618.x. [DOI] [PubMed] [Google Scholar]
- Gregg C. R., Johnson A. P., Taylor-Robinson D., Melly M. A., McGee Z. A. Host species-specific damage to oviduct mucosa by Neisseria gonorrhoeae lipopolysaccharide. Infect Immun. 1981 Dec;34(3):1056–1058. doi: 10.1128/iai.34.3.1056-1058.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hase S., Reitschel E. T. The chemical structure of the lipid A component of lipopolysaccharides from Chromobacterium violaceum NCTC 9694. Eur J Biochem. 1977 May 2;75(1):23–34. doi: 10.1111/j.1432-1033.1977.tb11500.x. [DOI] [PubMed] [Google Scholar]
- Hitchcock P. J., Brown T. M. Morphological heterogeneity among Salmonella lipopolysaccharide chemotypes in silver-stained polyacrylamide gels. J Bacteriol. 1983 Apr;154(1):269–277. doi: 10.1128/jb.154.1.269-277.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hämmerling G., Lehmann V., Lüderitz O. Structural studies on the heptose region of Salmonella lipopolysaccharides. Eur J Biochem. 1973 Oct 18;38(3):453–458. doi: 10.1111/j.1432-1033.1973.tb03079.x. [DOI] [PubMed] [Google Scholar]
- Kadam S. K., Rehemtulla A., Sanderson K. E. Cloning of rfaG, B, I, and J genes for glycosyltransferase enzymes for synthesis of the lipopolysaccharide core of Salmonella typhimurium. J Bacteriol. 1985 Jan;161(1):277–284. doi: 10.1128/jb.161.1.277-284.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennett R. H. Cell fusion. Methods Enzymol. 1979;58:345–359. doi: 10.1016/s0076-6879(79)58149-x. [DOI] [PubMed] [Google Scholar]
- Knecht D. A., Dimond R. L. Visualization of antigenic proteins on Western blots. Anal Biochem. 1984 Jan;136(1):180–184. doi: 10.1016/0003-2697(84)90321-x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lehmann V., Lüderitz O., Westphal O. The linkage of pyrophosphorylethanolamine to heptose in the core of Salmonella minnesota lipopolysaccharides. Eur J Biochem. 1971 Aug 16;21(3):339–347. doi: 10.1111/j.1432-1033.1971.tb01474.x. [DOI] [PubMed] [Google Scholar]
- Mandrell R., Schneider H., Apicella M., Zollinger W., Rice P. A., Griffiss J. M. Antigenic and physical diversity of Neisseria gonorrhoeae lipooligosaccharides. Infect Immun. 1986 Oct;54(1):63–69. doi: 10.1128/iai.54.1.63-69.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning P. A., Heuzenroeder M. W., Yeadon J., Leavesley D. I., Reeves P. R., Rowley D. Molecular cloning and expression in Escherichia coli K-12 of the O antigens of the Inaba and Ogawa serotypes of the Vibrio cholerae O1 lipopolysaccharides and their potential for vaccine development. Infect Immun. 1986 Aug;53(2):272–277. doi: 10.1128/iai.53.2.272-277.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse S. A., Apicella M. A. Isolation of a lipopolysaccharide mutant of Neisseria gonorrhoeae: an analysis of the antigenic and biologic difference. J Infect Dis. 1982 Feb;145(2):206–216. doi: 10.1093/infdis/145.2.206. [DOI] [PubMed] [Google Scholar]
- Morse S. A., Mintz C. S., Sarafian S. K., Bartenstein L., Bertram M., Apicella M. A. Effect of dilution rate on lipopolysaccharide and serum resistance of Neisseria gonorrhoeae grown in continuous culture. Infect Immun. 1983 Jul;41(1):74–82. doi: 10.1128/iai.41.1.74-82.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikaido H. Biosynthesis of cell wall lipopolysaccharide in gram-negative enteric bacteria. Adv Enzymol Relat Areas Mol Biol. 1968;31:77–124. doi: 10.1002/9780470122761.ch3. [DOI] [PubMed] [Google Scholar]
- Nikaido H. Biosynthesis of cell wall polysaccharide in mutant strains of Salmonella. 3. Transfer of L-rhamnose and D-galactose. Biochemistry. 1965 Aug;4(8):1550–1561. doi: 10.1021/bi00884a014. [DOI] [PubMed] [Google Scholar]
- Rick P. D., Fung L. W., Ho C., Osborn M. J. Lipid A mutants of Salmonella typhimurium. Purification and characterization of a lipid A precursor produced by a mutant in 3-deoxy-D-mannooctulosonate-8-phosphate synthetase. J Biol Chem. 1977 Jul 25;252(14):4904–4912. [PubMed] [Google Scholar]
- Robinson E. N., Jr, McGee Z. A., Kaplan J., Hammond M. E., Larson J. K., Buchanan T. M., Schoolnik G. K. Ultrastructural localization of specific gonococcal macromolecules with antibody-gold sphere immunological probes. Infect Immun. 1984 Nov;46(2):361–366. doi: 10.1128/iai.46.2.361-366.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal E., Hagblom P., Seifert H. S., So M. Antigenic variation of gonococcal pilus involves assembly of separated silent gene segments. Proc Natl Acad Sci U S A. 1986 Apr;83(7):2177–2181. doi: 10.1073/pnas.83.7.2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparling P. F., Cannon J. G., So M. Phase and antigenic variation of pili and outer membrane protein II of Neisseria gonorrhoeae. J Infect Dis. 1986 Feb;153(2):196–201. doi: 10.1093/infdis/153.2.196. [DOI] [PubMed] [Google Scholar]
- Steen H. B., Boye E., Skarstad K., Bloom B., Godal T., Mustafa S. Applications of flow cytometry on bacteria: cell cycle kinetics, drug effects, and quantitation of antibody binding. Cytometry. 1982 Jan;2(4):249–257. doi: 10.1002/cyto.990020409. [DOI] [PubMed] [Google Scholar]
- Yuasa R., Levinthal M., Nikaido H. Biosynthesis of cell wall lipopolysaccharide in mutants of Salmonella. V. A mutant of Salmonella typhimurium defective in the synthesis of cytidine diphosphoabequose. J Bacteriol. 1969 Oct;100(1):433–444. doi: 10.1128/jb.100.1.433-444.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]