Summary
Objective
Prader–Willi syndrome (PWS) is a genetic syndrome characterized by relative hypoinsulinaemia and normal or increased insulin sensitivity despite profound obesity. We hypothesized that this increased insulin sensitivity is mediated by increased levels of total and high molecular weight adiponectin and associated with changes in levels of satiety hormones.
Design, patients and measurements
We measured total adiponectin and its isoforms [high molecular weight (HMW), middle molecular weight (MMW) and low molecular weight (LMW) adiponectin] and satiety hormones in 14 children with PWS [median age 11.35 years, body mass index (BMI) Z-score 2.15] and 14 BMI-matched controls (median age 11.97 years, BMI Z-score 2.34).
Results
Despite comparable BMI Z-scores and leptin levels, the PWS children exhibited lower fasting insulin and HOMA-IR (homeostasis model assessment of insulin resistance) scores compared to obese controls. For any given BMI Z-score, the PWS children showed higher concentrations of fasting total and HMW adiponectin and higher HMW/total adiponectin ratios. The HMW/total adioponectin ratio was preserved in children with PWS at high degrees of obesity. In PWS children, fasting plasma total adiponectin, HMW adiponectin and HMW/total adiponectin ratio correlated negatively with age (P < 0.05), HOMA-IR (P < 0.01), BMI Z-score (P < 0.05), insulin (P < 0.01) and leptin (P < 0.05). In addition to higher fasting ghrelin concentrations, the PWS children showed significantly higher fasting levels of total peptide YY (PYY) and gastric inhibitory polypeptide (GIP) compared to obese controls.
Conclusions
Relative to controls of similar age and BMI Z-score, the PWS children had significantly higher levels of total and HMW adiponectin, and increased ratios of HMW/total adiponectin. These findings may explain in part the heightened insulin sensitivity of PWS children relative to BMI-matched controls.
Introduction
Prader–Willi syndrome (PWS) is a genetic syndrome of human obesity involving disruption of the normal neuroendocrine control of energy balance. It is characterized in infancy by failure to thrive, decreased arousal, poor suck, and hypotonia. By age 1–5 years, the child with PWS develops insatiable hunger, progressive obesity and short stature. Motor and cognitive development are also delayed, in association with behavioural difficulties and sleep disturbances.
Despite their profound obesity, a state of relative hypoinsulinaemia occurs in PWS individuals. For example, fasting insulin concentrations are lower in PWS compared to body mass index (BMI)-matched children,1–3 and insulin sensitivity is increased.3 Compared to BMI-matched controls, children with PWS have a decreased insulin response to mixed meals and to oral and intravenous glucose loading. Additionally, the first- and second-phase insulin secretion responses are significantly lower in PWS adults compared to obese controls during an intravenous glucose tolerance test (IVGTT). Finally, normal or increased sensitivity to exogenous insulin has been observed in PWS individuals.4–6 These findings suggest that subjects with PWS may be protected from obesity-associated insulin resistance.3,7–9
Type 2 diabetes and other states of insulin resistance are associated with low levels of adiponectin, a protein that is exclusively expressed and secreted by adipose tissue.10–12 Adiponectin is the most abundant adipose-derived protein and circulates in plasma in a variety of multimeric forms. It combines through its collagen domain to create three major oligomeric structures: a low molecular weight (LMW) trimer, a middle molecular weight (MMW) hexamer and a high molecular weight (HMW) 12- to 18-mer.13–15
The higher-order multimeric complexes are thought to mediate the biological activities of adiponectin.14,15 Several lines of evidence suggest that the HMW adiponectin form has a dominant role in modulation of insulin sensitivity and protection against diabetes. First, certain rare human adiponectin mutations of the collagen domain (G84R and G90S) result in very low circulating HMW adiponectin levels and are closely associated with type 2 diabetes.16 Second, the improvement in insulin sensitivity associated with thiazolidinedione treatment correlates more strongly with increases in the ratio of HMW adiponectin levels to total adiponectin levels than to increases in total adiponectin levels alone.17 Third, in obese adults, the ratio of plasma HMW adiponectin to total adiponectin correlates more significantly with glucose and insulin levels than does total adiponectin levels.18,19 Thus, the distribution of circulating adiponecin isoforms plays a central role in adiponectin action and insulin sensitivity.
A few reports have implicated high total adiponectin levels in the altered insulin sensitivity found in adults and children with PWS.20–23 However, to our knowledge no study has compared the different adiponectin isoforms (HMW, MMW and LMW) in PWS children and BMI-matched controls. We hypothesized that the increased insulin sensitivity found in children with PWS is mediated by increased levels of total and HMW adiponectin. To test this hypothesis, we compared the isoform species of adiponectin in children with PWS with those of children of similar age and BMI Z-score and examined the relationships among fasting plasma adiponectin, glucose, insulin and homeostasis model assessment of insulin resistance (HOMA-IR) score in both groups. Because adiponectin acts centrally in the brain to regulate energy expenditure, food intake and body weight,24 and because changes in insulin sensitivity are associated with changes in food intake and satiety, we hypothesized that differences in adiponectin expression in PWS and BMI-matched controls might be associated with changes in satiety factors such as leptin, ghrelin, total peptide YY (PYY), neuropeptide Y (NPY), pancreatic polypeptide (PP) and gastric inhibitory polypeptide (GIP).
Subjects and methods
Subjects
Fourteen children with PWS [median age 11.35 years, body mass index (BMI) Z-score 2.15] and 14 BMI-matched control children without PWS (median age 11.97 years, BMI Z-score 2.34) were studied. BMI Z-scores were calculated using EpiInfo Version 3.3.2 (Centers for Disease Control and Prevention, CDC, Atlanta, GA). Subjects with chronic secondary illness, such as diabetes mellitus, liver or kidney disease, or active malignancy or those taking investigational drugs were excluded. Nine PWS subjects were taking a stable dose of GH at the time of study. Referring physicians made the initial decisions whether or not to treat with GH; the average GH dose of each subject was 0.02 mg/kg/day.
All PWS subjects had free T4 and TSH levels in the normal range (either endogenous or on replacement). One PWS subject was on thyroid replacement to treat central hypothyroidism. Four subjects with PWS had uniparental disomy; all others had a deletion in chromosome 15.25 Control subjects were recruited from the insulin resistance and obesity clinics at Duke University Medical Center. Table 1 shows the subjects’ clinical characteristics. This study was approved by the Institutional Review Board of Duke University Medical Center. A parent or legal guardian of each child gave consent and children ≥ 12 years gave assent before entry into the study.
Table 1.
Baseline characteristics of the study participants
| Prader–Willi syndrome (PWS) | BMI-matched control (C) | P-value* | |
|---|---|---|---|
| Age (years) | 11.35 (7.13–14.61) | 11.97 (10.56–14.17) | 0.505 |
| Males/females | 5/9 | 6/8 | |
| BMI Z-score | 2.15 (1.465–2.60) | 2.335 (1.78–2.65) | 0.698 |
| Average baseline | Average baseline | ||
| Glucose (mmol/l) | 4.87 (4.54–5.63) | 5.27 (4.94–5.52) | 0.448 |
| Leptin (nM) | 1.97 (0.81–2.67) | 2.01 (0.976–3.41) | 0.800 |
| Insulin (pmol/l) | 93.69 (70.64–204.02) | 161.13 (119.26–241.01) | 0.029 |
| HOMA-IR | 3.199 (2.494–5.676) | 5.109 (4.130–8.611) | 0.029 |
| Total adiponectin (ng/ml) | 8769.000 (6363.500–15 045.500) | 5798.000 (3216.500–7352.500) | 0.012 |
| Adiponectin HMW: total ratio | 0.706 (0.480–0.840) | 0.403 (0.273–0.505) | 0.033 |
| Adiponectin HMW (ng/ml) | 5370.500 (2763.000–11 867.500) | 2523.250 (819.000–3147.000) | 0.013 |
| Adiponectin LMW/MMW (ng/ml) | 2557.500 (2065.500–3503.000) | 2746.500 (1501.500–3191.000) | 0.765 |
| Ghrelin (pM) | 434.66 (339.13–479.32) | 248.55 (213.05–263.34) | < 0.001 |
| IGF-I SDS | 2.36 (−0.81 to 3.50) | 0.15 (−0.60 to 0.84) | 0.11 |
| GIP (pM) | 7.86 (5.88–9.65) | 5.60 (4.81–6.05) | 0.031 |
| PYY (pM) | 31.72 (29.32–35.03) | 26.23 (24.13–29.50) | 0.016 |
| NPY (pM) | 49.987 (36.062–62.088) | 49.858 (43.007–52.470) | 0.909 |
| Pancreatic polypeptide (pM) | 4.93 (3.32–6.44) | 5.02 (4.49–6.04) | 0.872 |
Wilcoxon sign rank test for comparison of PWS vs. OC. All data reported as medians (interquartile range).
Average baseline = (BL1 + BL2)/2 for each individual subject and then averaged overall. No significant differences detected between BL1 and BL2 values.
Experimental design
All subjects were admitted to the General Clinical Research Center at Duke University Medical Center. After an observed 10-h overnight fast, each subject had an initial fasting blood sample for determination of circulating levels of serum ghrelin and plasma glucose, insulin, total adiponectin, HMW, MMW and LMW adiponectin isoforms and IGF-I, leptin, PYY, NPY, GIP and PP. Each subject had a fasting blood sample taken on two separate days; one sample was obtained fasting prior to a high carbohydrate meal tolerance test and a second sample was obtained fasting prior to a high fat meal tolerance test as part of a larger yet unpublished study. The average of these two fasting baseline measurements is the value that is reported in this analysis. The median length of the washout period between these two test meals was 35 days. There were no significant differences between these two baseline measures and comparable results were obtained when only a single fasting measure was used.
Blood sample analysis
Following an overnight fast, blood samples were collected between 0800 and 1000 h into red-top Vacutainer tubes (for serum) or purple Vacutainer tubes (for plasma). Aprotinin (500 KIU/ml of blood, Roche, Indianapolis, IN) was added to each vacutainer. Serum samples were allowed to clot on ice for 30 min and each sample was centrifuged and the serum or plasma was removed and stored at −70 °C until assay. All analytes were measured in duplicate.
Hormonal assays: adiponectin analysis
Fasting plasma total adiponectin concentrations and the HMW isoform of adiponectin were measured by enzyme immunoassay (EIA) (Multimeric adiponectin EIA, Alpco Diagnostics, Salem, NH).26 This assay measures total adiponectin and the combined MMW + LMW isoforms (calculated by the difference between total and HMW adiponectin fractions). The intra-assay coefficient of variation (CV) was 5.4% for total adiponectin and 5.0% for HMW adiponectin while the interassay CV was 5.0% for total adiponectin and 5.7% for HMW adiponectin. The sensitivity was 0.075 ng/ml for both total and HMW adiponectin.
Additional hormone assays
Fasting serum samples were assayed for immunoreactive total ghrelin concentration using a commercial radioimmunoassay (RIA; Linco, St Charles, MO). The intra- and interassay CVs were < 10% and < 18%, respectively. The lower and upper limits of detection for this assay were 100 and 6000 pg/ml, respectively. The assay measures both active (octanoylated) and inactive ghrelin, and shows no significant cross-reactivity.
Plasma leptin concentrations were determined using a commercially available RIA (Linco) with a detection limit of 0.5 ng/ml (upper limit 100 ng/ml), and intra-assay and interassay CVs of each < 10%.
Plasma insulin concentrations were measured by RIA (Linco). The intra- and interassay CVs were each < 10%. The normal range of fasting plasma insulin is 2–100 μU/ml. HOMA was calculated using the formula: fasting glucose (mg/dl) × fasting insulin (μIU/ml) divided by 405.27 Plasma IGF-I was measured by immunoradiometric assay (IRMA; Diagnostic Services Laboratory); the sensitivity as 2.0 ng/ml and intra- and interassay CVs were 3.9–7.0% and 3.8–7.4%, respectively.
Plasma NPY and PP were measured by RIA (Alpco Diagnostics). For the NPY assay, the intra- and interassay CVs were 2.6–3.9% and 10.5–12.7%, respectively, with a sensitivity of 6 pM. For the PP assay, the intra- and interassay CVs were 1.8–2.6% and 2.0–3.5%, respectively, with a sensitivity of 3 pM. Plasma GIP was measured by enzyme-linked immunosorbent assay (ELISA; Linco); the sensitivity was 8.2 pg/ml and intra- and interassay CVs were 3.0–8.8% and 1.8–6.1%., respectively. Plasma total PYY was measured by RIA (Linco); the sensitivity was 10 pg/ml and intra- and interassay CVs were 2.9–9.4% and 5.5–8.5%, respectively.
Statistical analysis
Because of the non-normal distribution of many variables, all values are summarized using the median and interquartile range. Between-group comparisons were performed using the nonparametric Wilcoxon rank-sum test. Spearman rank correlation coefficients were used to measure monotonic associations between variables. Simple linear regression was used to characterize the relationships between adiponectin isoforms and BMI Z-scores within and between the groups. Statistical analyses were performed using SAS 9.1 for Windows (SAS Institute, Cary, NC) and SigmaStat (SPSS, Inc., Chicago, IL). Two-sided P-values at the standard 0.05 level were used to determine statistical significance.
Results
Baseline characteristics of the study participants are illustrated in Table 1. PWS and BMI-matched control subjects were of similar age, gender and BMI Z-score and had comparable fasting plasma glucose and leptin concentrations.
Fasting insulin, HOMA-IR and adiponectin
Despite comparable BMI Z-score and leptin levels, fasting insulin levels were significantly lower in PWS children (P = 0.029) compared with BMI-matched controls. Similarly, the HOMA-IR was significantly lower in PWS children (P = 0.029) compared to BMI-matched controls. Total plasma adiponectin levels were significantly higher in the PWS subjects compared to the BMI-matched controls (P = 0.012), consistent with higher insulin sensitivity in PWS subjects (Table 1).
Adiponectin isoforms
Analysis of the isoforms of adiponectin revealed significantly higher levels of HMW adiponectin in PWS children compared to BMI-matched controls (P = 0.013). The HMW/total adiponectin ratio was also significantly increased in PWS children compared to BMI-matched controls (P = 0.033); No significant differences were seen in the LMW + MMW molecular isoforms of adiponectin (Table 1).
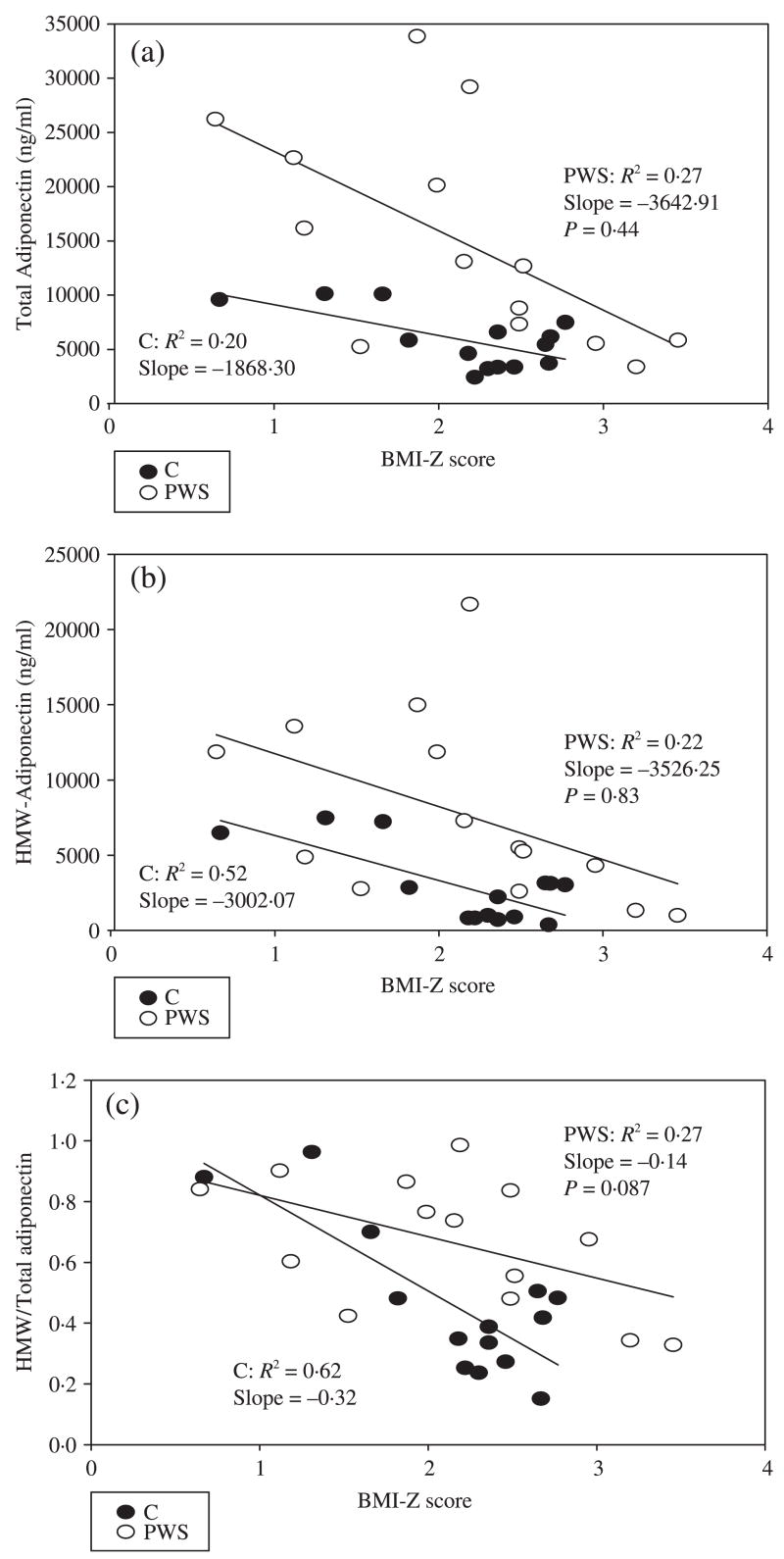
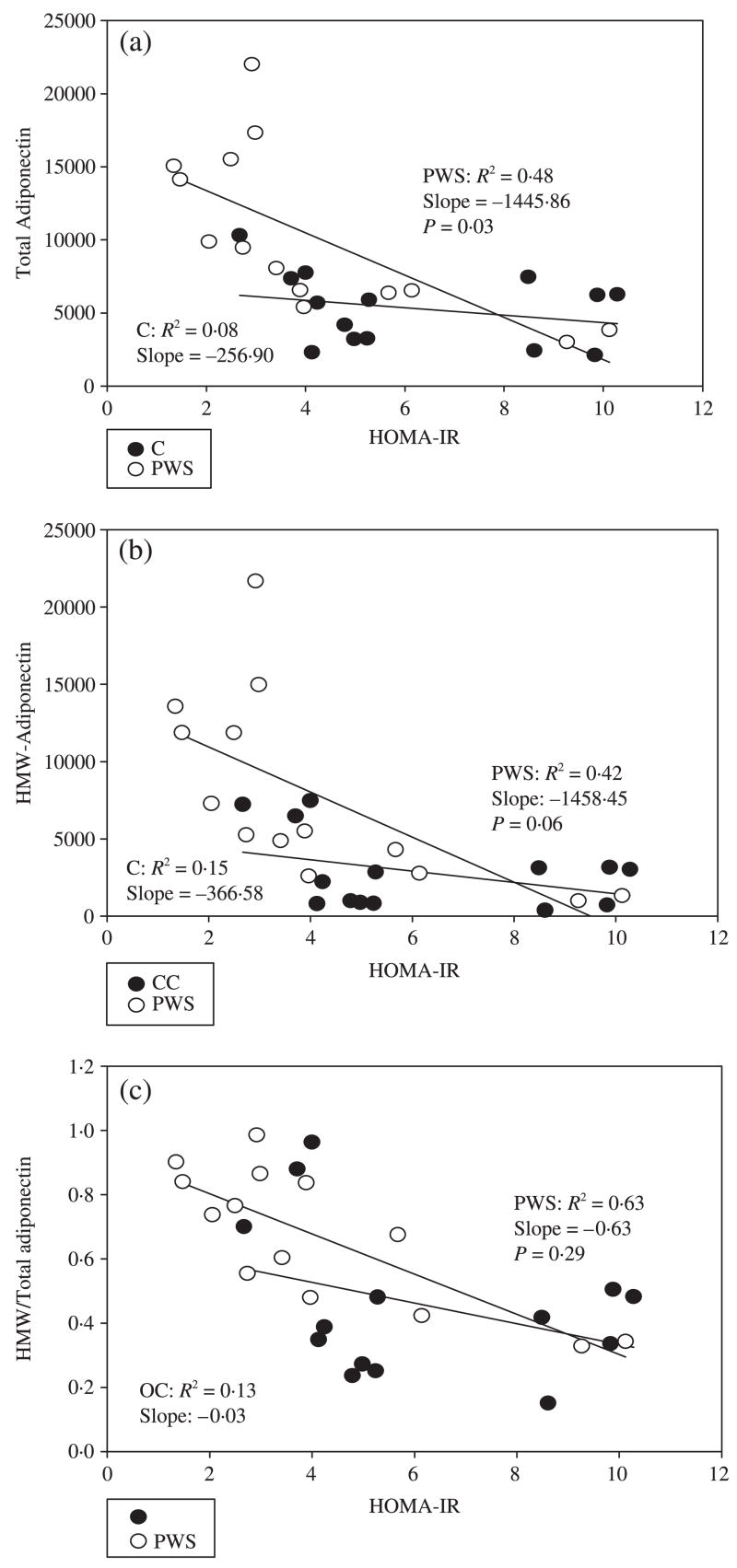
Total adiponectin, HMW adiponectin and the ratio of HMW/total adiponectin decreased with increasing BMI Z-score in children with PWS as well as in controls (Fig. 1a–c). However, total and HMW adiponectin and the ratio of HMW/total adiponectin were higher in children with PWS than in controls at any given BMI Z-score. Of note, the ratio of HMW/total adiponectin appeared to decline more precipitously with increasing BMI Z-score in obese controls (OC) than in children with PWS, although the differences in the slopes of the two regression lines were not statistically significant [slopes of regression curves −3002.1 (PWS), −3526.3 (OC); P-value for difference in slopes = 0.087); thus, a relative excess of HMW adiponectin may be preserved even as BMI Z-score increases in children with PWS. In BMI-matched controls, HOMA-IR correlates weakly with, and is of similar magnitude to, total adiponectin (r2 = 0.08), HMW adiponectin (r2 = 0.15) and HMW/total adiponectin ratio (r2 = 0.13). In PWS children, all three measures [total (r2 = 0.48), HMW (r2 = 0.42) and HMW/total adiponectin ratio (r2 = 0.63)] correlated more strongly with HOMA-IR (Fig. 2a–c).
Fig. 1.
Relationship between (a) total adiponectin, (b) high molecular weight (HMW) adiponectin and (c) HMW/total adiponectin ratio and BMI Z-score in children with Prader–Willi syndrome (PWS) and BMI-matched controls (C).
Fig. 2.
Relationship between (a) total adiponectin, (b) high molecular weight (HMW) adiponectin and (c) HMW/total adiponectin ratio and HOMA-IR in children with Prader–Willi syndrome (PWS) and BMI-matched controls (C).
Ghrelin, satiety peptides and IGF-I
Consistent with previous findings, fasting serum ghrelin levels were higher in PWS children compared to BMI-matched controls (P < 0.001). To determine whether PWS is associated with dysregulation of other gut satiety peptides, we compared the fasting levels of total PYY and GIP in PWS with those in BMI-matched controls. Fasting total PYY (P = 0.016) and GIP (P = 0.031) were significantly higher in PWS children than in BMI-matched controls. Thus, we found that PWS is accompanied by high levels of total PYY and GIP, as well as ghrelin. No significant differences were seen in fasting IGF-I SDS, NPY or PP concentrations in PWS children compared with BMI-matched controls. It should be noted, however, that those PWS subjects on GH therapy had higher IGF-I SDS values compared to those PWS subjects not on treatment (P = 0.003).
Correlations of adiponectin to auxological and metabolic parameters
We analysed the correlations of total adiponectin, HMW adiponectin, HMW/total adiponectin ratio and LMW + MMW adiponectin to age, HOMA, BMI, BMI Z-score, and fasting ghrelin, glucose, insulin, leptin, PYY, NPY, PP, IGF-I and GIP in both BMI-matched control and PWS children (Table 2). In PWS children, fasting plasma total adiponectin, HMW adiponectin and HMW/total adiponectin ratio correlated negatively with age (P < 0.05), HOMA-IR (P < 0.01), BMI and BMI Z-score (P < 0.05), insulin (P < 0.01) and leptin (P < 0.05). Notably, the LMW + MMW adiponectin isoforms did not correlate significantly with any parameters. In BMI-matched control children, fewer and less powerful significant correlations were detected. The absence of significant negative correlations between adiponectin and BMI, insulin and HOMA-IR in BMI-matched controls might be explained in part by the relatively small sample size.
Table 2.
Correlations between adiponectin fractions and other variables in PWS children and BMI-matched controls
| Total adiponectin | HMW adiponectin | HMW/total adiponectin | LMW + MMW adiponectin | |
|---|---|---|---|---|
| PWS children | ||||
| Age | −0.578* | −0.604* | −0.640* | −0.169 |
| HOMA | −0.798** | −0.802** | −0.736** | −0.226 |
| BMI | −0.656* | −0.663** | −0.641* | −0.236 |
| BMI Z-score | −0.620* | −0.603* | −0.557* | −0.321 |
| Ghrelin | 0.495 | 0.495 | 0.481 | 0.095 |
| Glucose | 0.235 | 0.200 | 0.088 | −0.277 |
| Insulin | −0.780** | −0.793** | −0.719** | −0.187 |
| Leptin | −0.565* | −0.591* | −0.609* | −0.081 |
| PYY | −0.112 | −0.143 | −0.187 | −0.024 |
| NPY | 0.305 | 0.385 | 0.530* | −0.152 |
| PP | 0.178 | 0.222 | 0.310 | −0.051 |
| GIP | −0.484 | −0.478 | −0.401 | 0.005 |
| IGF-I | 0.345 | 0.424 | 0.569* | −0.156 |
| Control children | ||||
| Age | −0.587* | −0.552* | −0.244 | −0.565* |
| HOMA | −0.314 | −0.363 | −0.305 | 0.240 |
| BMI | −0.471 | −0.546* | −0.447 | 0.145 |
| BMI Z-score | −0.244 | −0.352 | −0.411 | 0.515 |
| Ghrelin | 0.490 | 0.578* | 0.407 | −0.226 |
| Glucose | −0.224 | −0.262 | −0.022 | −0.251 |
| Insulin | −0.270 | −0.310 | −0.314 | 0.218 |
| Leptin | −0.341 | −0.393 | −0.336 | 0.248 |
| PYY | 0.332 | 0.248 | 0.007 | 0.468 |
| NPY | 0.560* | 0.569* | 0.473 | 0.213 |
| PP | 0.160 | 0.0286 | −0.108 | 0.222 |
| GIP | 0.380 | 0.240 | 0.099 | 0.327 |
| IGF-I | 0.521 | 0.398 | 0.253 | 0.319 |
Spearman rank correlations reported.
P < 0.05;
P < 0.01.
There were negative correlations between total adiponectin, HMW adiponectin and LMW + MMW adiponectin isoforms and age (P < 0.05), and a negative correlation between HMW adiponectin and BMI (P < 0.05). HMW adiponectin correlated positively with plasma ghrelin (P < 0.05), but total adiponectin did not. There was a positive correlation between total adiponectin and HMW adiponectin and NPY (P < 0.05). Other than a negative correlation with age, no significant correlations were found between the LMW + MMW adiponectin isoforms and any other parameters. In the PWS group only, HMW/total adiponectin ratio correlated positively with IGF-I.
Discussion
Previous studies,20–23,28 with one exception,29 have reported an increase in total adiponectin in adults and children with PWS relative to obese controls. However, other investigators have not characterized the distribution of adiponectin isoforms in PWS. This is important because the isoform distribution plays a crucial role in adiponectin action. Our study reveals novel findings of elevations in the HMW isoform of adiponectin in children with PWS. Additionally, we found an increased ratio of HMW/total adiponectin in the PWS children, but no changes in the LMW + MMW fraction of adiponectin compared to BMI-matched controls. For any given BMI Z-score, the PWS children had higher levels of total and HMW adiponectin and HMW/total adiponectin ratios compared to controls.
The preferential increase in the HMW form of adiponectin in children with PWS is of considerable interest because this isoform is thought to correlate better with insulin sensitivity than total adiponectin. Thus, the relative excess of higher total and HMW adiponectin in children with PWS may explain the increased insulin sensitivity in these children. Plasma adiponectin levels are inversely correlated with degree of adiposity and correlated positively with insulin sensitivity in both healthy subjects and subjects with diabetes. Furthermore, a lower concentration of plasma adiponectin has been found in obese adolescents with high visceral adipose tissue (VAT) compared to those with lower VAT.30 Negative correlations between concentrations of adiponectin and glucose, insulin and triglyceride levels, BMI, percentage body fat and HOMA-IR have been demonstrated in non-PWS subjects.31 These relationships between adiponectin and insulin, BMI and HOMA-IR were also found in the PWS children studied here.
In theory, high levels of adiponectin may be a consequence, rather than a cause, of increased insulin sensitivity and hypoinsulinaemia because insulin reduces adiponectin concentrations both in vivo and in vitro.32,33 It is therefore possible that higher adiponectin concentrations in PWS reflect their lower insulin levels relative to BMI-matched controls. Of note, we did find significant negative correlations between adiponectin and insulin, and between adiponectin and HOMA-IR in the PWS children. Given the cross-sectional nature of this study, we cannot determine the causal direction of these relationships.
The cause of the relative hyperadiponectinaemia in PWS is not fully understood at present. Elevated levels of adiponectin in PWS subjects may be the result of the proportionally lower levels of visceral fat in these PWS patients compared with BMI-matched controls,8 as lower levels of adiponectin expression have been found in omental or visceral fat vs. subcutaneous fat.34–36 It is also possible that post-transcriptional processing of adiponectin may be different between visceral fat and subcutaneous fat, leading to higher levels of HMW adiponectin in PWS. Finally, because adiponectin might cross the blood–brain barrier and bind to adiponectin receptors 1 and 2 localized in the central nervous system,24 it is possible that PWS involves central dysregulation of adiponectin through unknown mechanisms.
A number of lines of evidence suggest that hyperadiponectinaemia of PWS is not explained simply by GH deficiency or therapy. First, plasma IGF-I levels were not significantly different between our PWS and BMI-matched controls and there was no correlation between fasting total or HMW adiponectin and IGF-I in the PWS children. In addition, a previous study detected no change in adiponectin levels in response to GH treatment of adult subjects with PWS.22 Furthermore, a recent report concludes that 12 months of GH treatment of 34 GH-deficient children did not change adiponectin levels.37 These findings suggest that GH treatment of a subset of PWS subjects does not explain the differences in adiponectin isoforms that we detected in this study. By contrast, one recent report found an increase in total adiponectin levels in children with PWS during initiation of GH treatment.23 We cannot therefore exclude the possibility that GH deficiency or therapy might have had effects on adiponectin that were not reflected in differences in plasma IGF-I levels. One limitation of our study is the inability to perform a sub-analysis of PWS subjects on GH vs. nontreated. A limited sample size and differences in age, BMI Z-score, gender and pubertal status among the GH- and non-GH-treated PWS subjects made it impossible to analyse the effects of GH therapy on adiponectin levels or the distribution of adiponectin isoforms. Differences between GH-treated and nontreated PWS subjects in adiponectin levels need to be studied further in larger, prospective studies.
Because adiponectin acts centrally in the brain to regulate energy expenditure, food intake and body weight, and because differences in insulin sensitivity can lead to changes in food intake and satiety, we also examined the relationship between adiponectin and a number of hormones that regulate satiety and appetite.
Ghrelin is an orexigenic hormone produced primarily in the stomach. Markedly elevated ghrelin concentrations have been reported in previous studies of both children and adults with PWS.38–41 Two other papers found no difference in ghrelin levels among PWS vs. control subjects; one paper examined ghrelin levels in a small number of PWS (n = 9) young infants (17–60 months) compared to healthy controls of equivalent age, sex and BMI (n = 8) and found no difference between groups,42 while another found no difference in ghrelin levels in children with PWS compared to controls.43 Because the human ghrelin gene on chromosome 3p26–25 is not located within the known deleted gene sequence that causes PWS, the cause of the elevation in ghrelin concentrations in PWS is unclear.
The increased concentrations of ghrelin found in children with PWS are postulated to cause stimulation of food intake through the hypothalamic NPY/agouti-related protein (AGRP) signalling pathways. However, the regulation of other gut satiety peptides such as PYY, GIP, PP and circulating NPY in PWS has been less well characterized. In this study, we found higher fasting levels of total PYY (0.014) and GIP (P = 0.031), as well as ghrelin. Plasma NPY and PP in children with PWS were comparable to those in BMI-matched controls. These findings contrast with those of other investigators. Zipf et al. reported lower concentrations of both fasting GIP and PP in PWS children compared to lean or obese adults.44 In that study, the mean age of the PWS children was significantly less than that of the obese or lean adult controls; thus, the confounding effect of age might have given different results from ours. Butler et al. reported that PYY levels were lower in 12 infants and children with PWS compared with values for normal children reported in the literature. However, as control samples were not run concurrently with the measured PWS samples in the same assay, these findings might not be valid.45 On the contrary, Goldstone et al. reported that fasting PYY in PWS adults was not significantly different from that of obese or hypothalamic obese controls.46 Finally, Gimenez-Palop et al. reported similar fasting PYY concentrations in subjects with PWS compared to obese controls, but lower levels in PWS compared to lean controls.41 Given the variability of results for levels of satiety factors in PWS, further studies are warranted. Because PYY suppresses food intake in humans and rodents,47 we speculate that the elevated total PYY levels in children with PWS that we found in our study may represent a compensatory response to the high ghrelin levels. Alternatively, PWS children might oversecrete ghrelin, PYY and GIP in response to disordered autonomic or vagal efferent input to the GI tract.
In summary, we find that despite similar degrees of obesity, the PWS children had significantly higher levels of total and HMW adiponectin and increased ratios of HMW to total adiponectin relative to controls of similar age and BMI Z-score. The findings of high total and HMW adiponectin and low insulin levels in children with PWS support a link between altered processing or secretion of adiponectin forms and the increased insulin sensitivity demonstrated here and in previous reports of children and adults with PWS. Our findings provide a possible mechanism that might explain the relative paucity of metabolic complications in obese PWS children and adults compared to those of equal adiposity.7–9,48 The study of PWS, a unique model of obesity, may provide further insight into the mechanisms that control the relative distribution of the different molecular weight forms of circulating adiponectin. This might, in turn, lead to novel treatment strategies for the amelioration of obesity-related insulin resistance, type 2 diabetes and the metabolic syndrome.
Acknowledgments
We thank Juanita Cuffee (study coordinator) for help with recruitment of study subjects and Diane Stadler for her critical review of this manuscript. We thank all the families and children who participated in these studies. This study was conducted through the Duke University Medical Center, General Clinical Research Center (MO1-RR-30, National Center for Research Resources, Clinical Research Centers Program, NIH) and was supported by 1K23-RR-021979 (to A.M.H) and NIDDK R01 DK071161 (to J.Q.P) and additional funding from the Lawson Wilkins Pediatric Endocrine Society and the Sarah W. Stedman Nutrition and Metabolism Center.
References
- 1.Eiholzer U, Stutz K, Weinmann C, Torresani T, Molinari L, Prader A. Low insulin, IGF-I and IGFBP-3 levels in children with Prader–Labhart–Willi syndrome. European Journal of Pediatrics. 1998;157:890–893. doi: 10.1007/s004310050961. [DOI] [PubMed] [Google Scholar]
- 2.Lindgren AC, Hagenas L, Ritzen EM. Growth hormone treatment of children with Prader–Willi syndrome: effects on glucose and insulin homeostasis. Swedish National Growth Hormone Advisory Group. Hormone Research. 1999;51:157–161. doi: 10.1159/000023350. [DOI] [PubMed] [Google Scholar]
- 3.Schuster DP, Osei K, Zipf WB. Characterization of alterations in glucose and insulin metabolism in Prader–Willi subjects. Metabolism. 1996;45:1514–1520. doi: 10.1016/s0026-0495(96)90181-x. [DOI] [PubMed] [Google Scholar]
- 4.Landwirth J, Schwartz AH, Grunt JA. Prader–Willi syndrome. American Journal of Diseases of Children. 1968;116:211–217. doi: 10.1001/archpedi.1968.02100020213017. [DOI] [PubMed] [Google Scholar]
- 5.Bray GA, Dahms WT, Swerdloff RS, Fiser RH, Atkinson RL, Carrel RE. The Prader–Willi syndrome: a study of 40 patients and a review of the literature. Medicine (Baltimore) 1983;62:59–80. [PubMed] [Google Scholar]
- 6.Sarren C, Ruvalcaba RH, Kelley VC. Some aspects of carbohydrate metabolism in Prader–Willi syndrome. Journal of Mental Deficiency Research. 1975;19:113–119. doi: 10.1111/j.1365-2788.1975.tb01264.x. [DOI] [PubMed] [Google Scholar]
- 7.Krochik AG, Ozuna B, Torrado M, Chertkoff L, Mazza C. Characterization of alterations in carbohydrate metabolism in children with Prader–Willi syndrome. Journal of Pediatric Endocrinology and Metabolism. 2006;19:911–918. doi: 10.1515/jpem.2006.19.7.911. [DOI] [PubMed] [Google Scholar]
- 8.Goldstone AP, Thomas EL, Brynes AE, Bell JD, Frost G, Saeed N, Hajnal JV, Howard JK, Holland A, Bloom SR. Visceral adipose tissue and metabolic complications of obesity are reduced in Prader–Willi syndrome female adults: evidence for novel influences on body fat distribution. Journal of Clinical Endocrinology and Metabolism. 2001;86:4330–4338. doi: 10.1210/jcem.86.9.7814. [DOI] [PubMed] [Google Scholar]
- 9.Mogul HR, Lee PD, Whitman B, Zipf W, Frey M, Myers SE, Cahan M, Pinyerd B. Preservation of insulin sensitivity and paucity of metabolic syndrome symptoms in Prader–Willi Syndrome adults: preliminary data from the US multicenter study. Obesity Research. 2004;12:171. [Google Scholar]
- 10.Scherer PE, Williams S, Fogliano M, Baldini G, Lodish HF. A novel serum protein similar to C1q, produced exclusively in adipocytes. Journal of Biological Chemistry. 1995;270:26746–26749. doi: 10.1074/jbc.270.45.26746. [DOI] [PubMed] [Google Scholar]
- 11.Berg AH, Scherer PE. Adipose tissue, inflammation, and cardiovascular disease. Circulation Research. 2005;96:939–949. doi: 10.1161/01.RES.0000163635.62927.34. [DOI] [PubMed] [Google Scholar]
- 12.Kern PA, Di Gregorio GB, Lu T, Rassouli N, Ranganathan G. Adiponectin expression from human adipose tissue: relation to obesity, insulin resistance, and tumor necrosis factor-alpha expression. Diabetes. 2003;52:1779–1785. doi: 10.2337/diabetes.52.7.1779. [DOI] [PubMed] [Google Scholar]
- 13.Berg AH, Combs TP, Scherer PE. ACRP30/adiponectin: an adipokine regulating glucose and lipid metabolism. Trends in Endocrinological Metabolism. 2002;13:84–89. doi: 10.1016/s1043-2760(01)00524-0. [DOI] [PubMed] [Google Scholar]
- 14.Pajvani UB, Du X, Combs TP, Berg AH, Rajala MW, Schulthess T, Engel J, Brownlee M, Scherer PE. Structure–function studies of the adipocyte-secreted hormone Acrp30/adiponectin. Implications for metabolic regulation and bioactivity. Journal of Biological Chemistry. 2003;278:9073–9085. doi: 10.1074/jbc.M207198200. [DOI] [PubMed] [Google Scholar]
- 15.Tsao TS, Tomas E, Murrey HE, Hug C, Lee DH, Ruderman NB, Heuser JE, Lodish HF. Role of disulfide bonds in Acrp30/adiponectin structure and signaling specificity. Different oligomers activate different signal transduction pathways. Journal of Biological Chemistry. 2003;278:50810–50817. doi: 10.1074/jbc.M309469200. [DOI] [PubMed] [Google Scholar]
- 16.Waki H, Yamauchi T, Kamon J, Ito Y, Uchida S, Kita S, Hara K, Hada Y, Vasseur F, Froguel P, Kimura S, Nagai R, Kadowaki T. Impaired multimerization of human adiponectin mutants associated with diabetes. Molecular structure and multimer formation of adiponectin. Journal of Biological Chemistry. 2003;278:40352–40363. doi: 10.1074/jbc.M300365200. [DOI] [PubMed] [Google Scholar]
- 17.Pajvani UB, Hawkins M, Combs TP, Rajala MW, Doebber T, Berger JP, Wagner JA, Wu M, Knopps A, Xiang AH, Utzschneider KM, Kahn SE, Olefsky JM, Buchanan TA, Scherer PE. Complex distribution, not absolute amount of adiponectin, correlates with thiazolidinedione-mediated improvement in insulin sensitivity. Journal of Biological Chemistry. 2004;279:12152–12162. doi: 10.1074/jbc.M311113200. [DOI] [PubMed] [Google Scholar]
- 18.Lara-Castro C, Luo N, Wallace P, Klein RL, Garvey WT. Adiponectin multimeric complexes and the metabolic syndrome trait cluster. Diabetes. 2006;55:249–259. [PubMed] [Google Scholar]
- 19.Hara K, Horikoshi M, Yamauchi T, Yago H, Miyazaki O, Ebinuma H, Imai Y, Nagai R, Kadowaki T. Measurement of the high-molecular weight form of adiponectin in plasma is useful for the prediction of insulin resistance and metabolic syndrome. Diabetes Care. 2006;29:1357–1362. doi: 10.2337/dc05-1801. [DOI] [PubMed] [Google Scholar]
- 20.Caixas A, Gimenez-Palop O, Gimenez-Perez G, Potau N, Berlanga E, González-Glemente JM, Arroyo J, Laferrère B, Mauricio D. Postprandial adiponectin levels are unlikely to contribute to the pathogenesis of obesity in Prader–Willi syndrome. Hormone Research. 2006;65:39–45. doi: 10.1159/000090513. [DOI] [PubMed] [Google Scholar]
- 21.Kennedy L, Bittel DC, Kibiryeva N, Kalra SP, Torto R, Butler MG. Circulating adiponectin levels, body composition and obesity-related variables in Prader–Willi syndrome: comparison with obese subjects. International Journal of Obesity (London) 2006;30:382–387. doi: 10.1038/sj.ijo.0803115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoybye C, Bruun JM, Richelsen B, Flyvbjerg A, Frystyk J. Serum adiponectin levels in adults with Prader–Willi syndrome are independent of anthropometrical parameters and do not change with GH treatment. European Journal of Endocrinology. 2004;151:457–461. doi: 10.1530/eje.0.1510457. [DOI] [PubMed] [Google Scholar]
- 23.Festen DA, van Toorenenbergen A, Duivenvoorden HJ, Hokken-Koelega AC. Adiponectin levels in pre-pubertal children with Prader–Willi syndrome before and during growth hormone therapy. Journal of Clinical Endocrinology and Metabolism. 2007;92:1549–1554. doi: 10.1210/jc.2006-2241. [DOI] [PubMed] [Google Scholar]
- 24.Qi Y, Takahashi N, Hileman SM, Patel HR, Berg AH, Pajvani UB, Scherer PE, Ahima RS. Adiponectin acts in the brain to decrease body weight. Nature Medicine. 2004;10:524–529. doi: 10.1038/nm1029. [DOI] [PubMed] [Google Scholar]
- 25.Nicholls RD, Saitoh S, Horsthemke B. Imprinting in Prader–Willi and Angelman syndromes. Trends in Genetics. 1998;14:194–200. doi: 10.1016/s0168-9525(98)01432-2. [DOI] [PubMed] [Google Scholar]
- 26.Ebinuma H, Miyazaki O, Yago H, Hara K, Yamauchi T, Kadowaki T. A novel ELISA system for selective measurement of human adiponectin multimers by using proteases. Clinica Chimica Acta. 2006;372:47–53. doi: 10.1016/j.cca.2006.03.014. [DOI] [PubMed] [Google Scholar]
- 27.Conwell LS, Trost SG, Brown WJ, Batch JA. Indexes of insulin resistance and secretion in obese children and adolescents: a validation study. Diabetes Care. 2004;27:314–319. doi: 10.2337/diacare.27.2.314. [DOI] [PubMed] [Google Scholar]
- 28.Pagano C, Marin O, Calcagno A, Schiappelli P, Pilon C, Milan G, Bertelli M, Fanin E, Andrighetto G, Federspil G, Vettor R. Increased serum resistin in adults with Prader–Willi syndrome is related to obesity and not to insulin resistance. Journal of Clinical Endocrinology and Metabolism. 2005;90:4335–4340. doi: 10.1210/jc.2005-0293. [DOI] [PubMed] [Google Scholar]
- 29.Unanue N, Bazaes R, Iniguez G, Cortes F, Avila A, Mericq V. Adrenarche in Prader–Willi syndrome appears not related to insulin sensitivity and serum adiponectin. Hormone Research. 2007;67:152–158. doi: 10.1159/000096742. [DOI] [PubMed] [Google Scholar]
- 30.Bacha F, Saad R, Gungor N, Arslanian SA. Adiponectin in youth: relationship to visceral adiposity, insulin sensitivity, and beta-cell function. Diabetes Care. 2004;27:547–552. doi: 10.2337/diacare.27.2.547. [DOI] [PubMed] [Google Scholar]
- 31.Diez JJ, Iglesias P. The role of the novel adipocyte-derived hormone adiponectin in human disease. European Journal of Endocrinology. 2003;148:293–300. doi: 10.1530/eje.0.1480293. [DOI] [PubMed] [Google Scholar]
- 32.Lihn AS, Ostergard T, Nyholm B, Pedersen SB, Richelsen B, Schmitz O. Adiponectin expression in adipose tissue is reduced in first-degree relatives of type 2 diabetic patients. American Journal of Physiology. Endocrinology and Metabolism. 2003;284:E443–E448. doi: 10.1152/ajpendo.00358.2002. [DOI] [PubMed] [Google Scholar]
- 33.Fasshauer M, Klein J, Neumann S, Eszlinger M, Paschke R. Hormonal regulation of adiponectin gene expression in 3T3-L1 adipocytes. Biochemical and Biophysical Research Communications. 2002;290:1084–1089. doi: 10.1006/bbrc.2001.6307. [DOI] [PubMed] [Google Scholar]
- 34.Fisher FM, McTernan PG, Valsamakis G, Chetty R, Harte AL, Anwar AJ, Starcynski J, Crocker J, Barnett AH, McTernan CL, Kumar S. Differences in adiponectin protein expression: effect of fat depots and type 2 diabetic status. Hormone and Metabolic Research. 2002;34:650–654. doi: 10.1055/s-2002-38246. [DOI] [PubMed] [Google Scholar]
- 35.Wajchenberg BL, Giannella-Neto D, da Silva ME, Santos RF. Depot-specific hormonal characteristics of subcutaneous and visceral adipose tissue and their relation to the metabolic syndrome. Hormone and Metabolic Research. 2002;34:616–621. doi: 10.1055/s-2002-38256. [DOI] [PubMed] [Google Scholar]
- 36.Lihn AS, Bruun JM, He G, Pedersen SB, Jensen PF, Richelsen B. Lower expression of adiponectin mRNA in visceral adipose tissue in lean and obese subjects. Molecular and Cellular Endocrinology. 2004;219:9–15. doi: 10.1016/j.mce.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 37.Ciresi A, Amato MC, Criscimanna A, Mattina A, Vetro C, Galluzzo A, D’Acquisto G, Giordano C. Metabolic parameters and adipokine profile during GH replacement therapy in children with GH deficiency. European Journal of Endocrinology. 2007;156:353–360. doi: 10.1530/eje.1.02343. [DOI] [PubMed] [Google Scholar]
- 38.Cummings DE, Clement K, Purnell JQ, Vaisse C, Foster KE, Frayo RS, Schwartz MW, Basderant A, Weigle DS. Elevated plasma ghrelin levels in Prader Willi syndrome. Nature Medicine. 2002;8:643–644. doi: 10.1038/nm0702-643. [DOI] [PubMed] [Google Scholar]
- 39.DeIParigi A, Tschop M, Heiman ML, Salbe AD, Vozarova B, Sell SM, Bunt JC, Tataranni PA. High circulating ghrelin: a potential cause for hyperphagia and obesity in Prader–Willi syndrome. Journal of Clinical Endocrinology and Metabolism. 2002;87:5461–5464. doi: 10.1210/jc.2002-020871. [DOI] [PubMed] [Google Scholar]
- 40.Haqq AM, Farooqi IS, O’Rahilly S, Stadler DD, Rosenfield RG, Pratt KL, LaFranchi S, Purnell JC. Serum ghrelin levels are inversely correlated with body mass index, age, and insulin concentrations in normal children and are markedly increased in Prader–Willi syndrome. Journal of Clinical Endocrinology and Metabolism. 2003;88:174–178. doi: 10.1210/jc.2002-021052. [DOI] [PubMed] [Google Scholar]
- 41.Gimenez-Palop O, Gimenez-Perez G, Mauricio D, González–Glemente JM, Potau N, Berlanga E, Trallero R, Laferrère B, Caixas A. A lesser postprandial suppression of plasma ghrelin in Prader–Willi syndrome is associated with low fasting and a blunted postprandial PYY response. Clinical Endocrinology. 2007;66:198–204. doi: 10.1111/j.1365-2265.2006.02707.x. [DOI] [PubMed] [Google Scholar]
- 42.Erdie-Lalena CR, Holm VA, Kelly PC, Frayo RS, Cummings DE. Ghrelin levels in young children with Prader–Willi syndrome. Journal of Pediatrics. 2006;149:199–204. doi: 10.1016/j.jpeds.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 43.Butler MG, Bittel DC. Plasma obestatin and ghrelin levels in subjects with Prader–Willi syndrome. American Journal of Medical Genetics A. 2007;143:415–421. doi: 10.1002/ajmg.a.31687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zipf WB, O’Dorisio TM, Cataland S, Sotos J. Blunted pancreatic polypeptide responses in children with obesity of Prader–Willi syndrome. Journal of Clinical Endocrinology and Metabolism. 1981;52:1264–1266. doi: 10.1210/jcem-52-6-1264. [DOI] [PubMed] [Google Scholar]
- 45.Butler MG, Bittel DC, Talebizadeh Z. Plasma peptide YY and ghrelin levels in infants and children with Prader–Willi syndrome. Journal of Pediatric Endocrinology and Metabolism. 2004;17:1177–1184. doi: 10.1515/jpem.2004.17.9.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Goldstone AP, Patterson M, Kalingag N, Ghatei MA, Brynes AE, Bloom SR, Grossman AB, Korbonits M. Fasting and postprandial hyperghrelinemia in Prader–Willi syndrome is partially explained by hypoinsulinemia, and is not due to peptide YY3-36 deficiency or seen in hypothalamic obesity due to craniopharyngioma. Journal of Clinical Endocrinology and Metabolism. 2005;90:2681–2690. doi: 10.1210/jc.2003-032209. [DOI] [PubMed] [Google Scholar]
- 47.Batterham RL, Cowley MA, Small CJ, Herzog H, Cohen MA, Dakin CL, Wren AM, Brynes AE, Low MJ, Ghatei MA, Cone RD, Bloom SR. Gut hormone PYY (3–36) physiologically inhibits food intake. Nature. 2002;418:650–654. doi: 10.1038/nature00887. [DOI] [PubMed] [Google Scholar]
- 48.Zipf WB. Glucose homeostasis in Prader–Willi syndrome and potential implications of growth hormone therapy. Acta Paediatrica Supplement. 1999;88:115–117. doi: 10.1111/j.1651-2227.1999.tb14418.x. [DOI] [PubMed] [Google Scholar]