Abstract
Growing evidence implicates a key role for extracellular nucleotides in cellular regulation, including of ion channels and renal function, but the mechanisms for such actions are inadequately defined. We investigated purinergic regulation of the epithelial Na+ channel (ENaC) in mammalian collecting duct. We find that ATP decreases ENaC activity in both mouse and rat collecting duct principal cells. ATP and other nucleotides, including UTP, decrease ENaC activity via apical P2Y2 receptors. ENaC in collecting ducts isolated from mice lacking this receptor have blunted responses to ATP. P2Y2 couples to ENaC via PLC; direct activation of PLC mimics ATP action. Tonic regulation of ENaC in the collecting duct occurs via locally released ATP; scavenging endogenous ATP and inhibiting P2 receptors, in the absence of other stimuli, rapidly increases ENaC activity. Moreover, ENaC has greater resting activity in collecting ducts from P2Y2-/- mice. Loss of collecting duct P2Y2 receptors in the knock-out mouse is the primary defect leading to increased ENaC activity based on the ability of direct PLC stimulation to decrease ENaC activity in collecting ducts from P2Y2-/- mice in a manner similar to ATP in collecting ducts from wild-type mice. These findings demonstrate that locally released ATP acts in an autocrine/paracrine manner to tonically regulate ENaC in mammalian collecting duct. Loss of this intrinsic regulation leads to ENaC hyperactivity and contributes to hypertension that occurs in P2Y2 receptor-/- mice. P2Y2 receptor activation by nucleotides thus provides physiologically important regulation of ENaC and electrolyte handling in mammalian kidney.
Systemic Na+ balance influences blood pressure. Consequently, body Na+ content is under tight negative-feedback control by the renin-angiotensin-aldosterone system. Discretionary Na+ reabsorption in the aldosterone-sensitive distal renal nephron, including the collecting duct, fine-tunes plasma Na+ levels. Here, the activity of the luminal epithelial Na+ channel (ENaC)2 is limiting for Na+ transport (1-3). ENaC is an end-effector of the renin-angiotensin-aldosterone system with aldosterone increasing ENaC activity. The importance of ENaC and its proper regulation to control of blood pressure is highlighted by several diseases associated with gain and loss of ENaC function (3, 4). For instance, gain of ENaC function results in inappropriate Na+ conservation and hypertension (e.g. Liddle syndrome). Conversely, loss of ENaC function results in renal salt wasting associated with hypotension (e.g. pseudohypoaldosteronism type 1).
Although extrinsic regulation of ENaC in the distal nephron by hormones originating outside the kidney is considered pivotal to blood pressure control, complementary regulation of ENaC by autocrine/paracrine factors originating from intrarenal sources is just now becoming appreciated. ATP has been identified as a candidate signaling molecule possibly mediating intrinsic control of distal nephron Na+ reabsorption (5-14).
ATP and other nucleotide signaling factors are released by epithelial cells, including those in the collecting duct, in a highly controlled and regulated manner. Both constitutive release and release in response to physiologic stimuli, including shear and mechanical stress resulting from flow, have been documented (7-9, 12, 13, 15). This finding and the fact that the spatial and temporal efficacy of nucleotides as signaling molecules are tightly controlled by extracellular enzymes (e.g. ectonucleotidases), make them well suited to convey information in a paracrine manner (5, 6, 8, 16).
ATP targets two major types of receptors: ionotropic P2X and metabotropic P2Y purinergic receptors (6, 17-19). The former are ligand-gated nonselective cation channels and the latter G protein-coupled receptors. Each mammalian P2 receptor family contains several receptor subtypes (P2Y1,2,4,6,11-14 and P2X1-7) that have distinct function and expression profiles, as well as distinct biophysical and pharmacological profiles (6, 17-20).
Solid experimental evidence documents the expression and function of P2Y2,4,&6 and perhaps P2Y1&11, as well as, P2X3-6, and possibly P2X1 in the mammalian collecting duct (5, 6, 8, 21). Activation of luminal P2X receptors (likely P2X1&4) in the collecting duct promotes Ca2+ entry and mobilization in mice and rats (22, 23). Activation of apical P2Y receptors (perhaps P2Y2) also promotes Ca2+ signaling, which, in some cases results in activation of Cl- channels, in mouse and rat collecting duct cells (11, 12, 14, 24). Similarly, activation of P2Y receptors (possibly P2Y2) decreases the activity of luminal K+ channels in the mouse collecting duct (25).
Previous work provides strong support for a role for P2 receptors in the regulation of Na+ reabsorption in the mammalian collecting duct (10, 11). The prevailing consensus is that luminal P2Y receptors decrease amiloride-sensitive, electrogenic Na+ transport across this nephron segment. Because amiloride is a blocker of ENaC and activity of this channel is limiting for electrogenic Na+ reabsorption in the collecting duct, these findings point to ENaC as possibly being sensitive to P2Y signaling. Although the P2Y2 receptor has been proposed to be important in regulating amiloride-sensitive Na+ transport, firm evidence for this idea has not been available, in part, because of the limitation of available selective pharmacological agonists/antagonists for P2Y receptor subtypes. Moreover, the associated intracellular mechanism for regulation of Na+ transport is as-yet poorly defined. Similarly, ENaC in the mammalian collecting duct has yet to be definitively shown to be a target for ATP via P2Y signaling, and controversy surrounds which membrane contains the relevant P2 receptor in the regulation of Na+ reabsorption.
To address these issues, we combined pharmacologic and molecular genetic approaches in which we studied P2Y2 receptor knock-out mice to directly test ATP regulation of ENaC with patch clamp electrophysiology using single channel analysis of ENaC in the isolated split-open collecting duct. We find that the P2Y2 agonists ATP and UTP rapidly and dynamically decrease ENaC open probability in principal cells in the rat and mouse collecting duct. Luminal P2Y2 receptors signaling through PLC are the primary arbiters of this action. Importantly, our results also provide compelling support for the idea that ENaC activity in the collecting duct is under intrinsic regulation by endogenous ATP tonically released from collecting duct epithelial cells. Loss of this regulation results in ENaC being hyperactive, thus likely contributing to the hypertensive phenotype of P2Y2-/- mice (30).
EXPERIMENTAL PROCEDURES
Materials—All chemicals and materials were purchased from Calbiochem, BioMol (Plymouth Meeting, PA), or Sigma unless noted otherwise and were of reagent grade. The PI(4,5)P2 reporter, GFP-PLC-δ-PH, is a chimera consisting of the PI(4,5)P2-binding pleckstrin homology (PH) domain from PLC-δ1 conjugated to GFP. The cDNA encoding this reporter was a gift from T. Meyer (26). The immortalized mouse collecting duct principal cell line, mpkCCDc14, used in some experiments was a gift from A. Vandewalle (27). These cells, which contain functional mineralocorticoid receptors and ENaC, readily polarize, forming monolayers with high trans-epithelial resistance and avid aldosterone-sensitive Na+ reabsorption. In brief, these cells were cultured in defined medium on permeable supports (Costar Transwells, 0.4 μm pore, 24-mm diameter) as described previously (27-29). Cells were maintained in culture with fetal bovine serum and corticosteroids.
Collecting Duct Isolation—The activity of ENaC in the apical plasma membrane of principal cells in isolated, split-open cortical collecting ducts from wild-type (C57BL/6) and P2Y2 receptor -/- (backcrossed and inbred onto a C57BL/6 background) mice was assessed directly with patch clamp electrophysiology. P2Y2 receptor -/- mice have been described previously (30). Likewise, isolation of collecting ducts suitable for electrophysiology has been described (29, 31, 32). In brief, mice were sacrificed by cervical dislocation and the kidneys immediately removed. Kidneys were cut into thin slices (<1 mm) with slices placed into ice-cold physiologic saline solution (pH 7.4). Collecting ducts were mechanically isolated from these slices by microdissection using watchmaker forceps under a stereomicroscope. Isolated cortical collecting ducts were allowed to settle onto 5 × 5-mm cover glass coated with poly-l-lysine. Cover glass containing collecting ducts were placed within a perfusion chamber mounted on an inverted Nikon TE2000 microscope and superfused for 2 min with a physiologic saline solution buffered with HEPES (pH 7.4) prior to experimentation. Collecting ducts were split-open with a sharpened micropipette controlled with a micromanipulator to gain access to the apical membrane. Collecting ducts were used within 1-2 h of isolation. Animal use and welfare adhered to the NIH Guide for the Care and Use of Laboratory Animals following a protocol reviewed and approved by the Institutional Laboratory Animal Care and Use Committee at the University of Texas Health Science Center at San Antonio.
In most cases, mice were maintained, for at least 1 week, on a nominally Na+-free diet ([Na+] < 0.01%, Harlan TEKLAD TD.90228) prior to experimentation. This dietary regimen increases ENaC activity (29, 31, 32). For other experiments, such as the comparison between resting ENaC activity in wild-type and P2Y2-/- mice and experiments testing regulation by endogenous ATP, mice were maintained with standard chow (TD.7912) containing 0.32% [Na+]. Standard chow was used to set ENaC activity to resting levels in the absence of perturbed Na+ balance allowing comparison of ENaC activity in wild-type and knock-out mice under this control condition.
Electrophysiology—Cell-attached patches were made under voltage-clamp conditions (-Vp = -60 mV) on the apical plasma membranes of principal cells in isolated, split-open collecting ducts from wild-type and P2Y2-/- mice using standard procedures (28, 29, 33). Current recordings were made in a paired manner with a still bath where experimental reagents added sequentially were placed near the patch electrode (final concentration calculated using bath volume). Recording pipettes had resistances of 10-15 megaohm. Typical bath and pipette solutions were (in mm): 150 NaCl, 5 mm KCl, 1 CaCl2, 2 MgCl2, 5 glucose, and 10 HEPES (pH 7.4); and 140 LiCl, 2 MgCl2, and 10 HEPES (pH 7.4), respectively. Gap-free single channel current data from gigaohm seals were acquired (and subsequently analyzed) with an EPC-9 (HEKA Instruments Inc.) or Axopatch 200B (Axon Instruments) patch clamp amplifier interfaced via a Digidata 1322A (Axon Instruments) to a PC running the pClamp 9.2 suite of software (Axon Instruments). Currents were low-pass filtered at 100 Hz with an eight-pole Bessel filter (Warner Instruments). Unitary current (i) and the number of ENaC in a patch, N, were determined from all-point amplitude histograms. Channel activity defined as NPo was calculated using the following equation: NPo = Σ(t1 + 2t2 +...ntn), where tn is the fractional open time spent at each of the observed current levels. Po was calculated by normalizing NPo for the number of channels observed within a given patch. Only patches containing five channels or fewer were used to estimate Po.
Total Internal Reflection Fluorescence (TIRF) Microscopy—Plasmid cDNA encoding the PI(4,5)P2 reporter was introduced into mpkCCDc14 principal cells within a confluent monolayer with a biolistic particle delivery system (Biolistic PDS-1000/He Particle Delivery System; Bio-Rad). Use of this system has been described previously (29, 34, 35). In brief, mpkCCDc14 cells were grown to confluence on permeable supports. After forming high-resistance monolayers that avidly transport Na+, cells were washed twice with physiologic saline, aspirated, and quickly bombarded (at the apical membrane) under vacuum with microcarriers coated with reporter cDNA. Medium was immediately returned to the cells, which were then placed within a tissue culture incubator for 2-3 days to allow expression of the PI(4,5)P2 reporter. Bombardment had little disruptive effect on cellular and monolayer integrity, as determined by maintenance of Na+ transport and a high trans-epithelial resistance.
Fluorescence emissions from the PI(4,5)P2 reporter at the apical membrane of mpkCCDc14 cells within a confluent monolayer were collected using TIRF (also called evanescent-field) microscopy (36-38). Upon expression of the PI(4,5)P2 reporter, 5 × 5-mm sections of the support were excised, inverted, and placed onto cover glass coated with poly-l-lysine. All TIRF experiments were performed in the TIRF microscopy core facility housed within the Department of Physiology at the University of Texas Health Science Center, San Antonio. We have previously described imaging the GFP-PLC-δ-PH reporter and other fluorophore-tagged proteins using this core facility (29, 39, 40). In brief, fluorescence emissions were collected using an inverted TE2000 microscope with through-the-lens (prismless) TIRF imaging (Nikon). Samples were viewed through a plain Apo TIRF ×60 oil-immersion, high-resolution (1.45 NA) objective. Fluorescence emissions were collected through a 535 ± 25 nm bandpass filter (Chroma Technology Corp.) by exciting GFP with an argon-ion laser with an acoustic optic tunable filter (Prairie Technology Inc.) used to restrict excitation wavelength to 488 nm. Fluorescence images were collected and processed with a 16-bit, cooled charge-coupled device camera (Cascade 512F, Roper Scientific Inc.) interfaced to a PC running Metamorph software. This camera uses a front-illuminated EMCCD with on-chip multiplication gain. Images were collected once a minute with a 100-ms exposure time. Images were not binned or filtered with pixel size corresponding to a square of 122 × 122 nm.
Statistics and Data Treatment—All summarized data are reported as mean ± S.E. and compared with the Student's (two-tailed) t test. p ≤ 0.05 was considered significant. For presentation, current data from some cell-attached patches were subsequently software filtered at 50 Hz and slow baseline drifts were corrected.
RESULTS
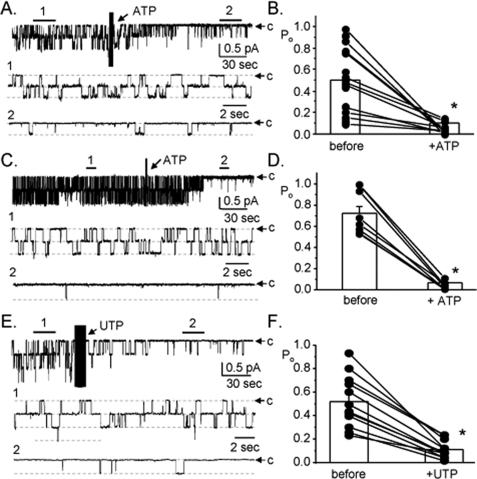
ATP Rapidly Decreases ENaC Open Probability—The first aim of this study was to assess the physiological regulation of ENaC by ATP, and other purinergic stimuli, in the mammalian collecting duct. Fig. 1, A and B, document the actions of 100 μm ATP on ENaC in native mouse collecting duct principal cells. As shown in the representative current trace in Fig. 1A, ATP rapidly and markedly decreases ENaC activity. As summarized in Fig. 1B, ATP significantly decreases ENaC open probability within 2 min from 0.50 ± 0.07 to 0.10 ± 0.05 (n = 12; n = 7 mice). Similar to the findings in murine collecting duct, we found that ATP rapidly and significantly decreases ENaC open probability in isolated, split-open rat collecting ducts (Figs. 1, C and D)(n = 9, n = 6 rats).
FIGURE 1.
Stimulation of purinergic receptors rapidly decreases ENaC open probability in the mouse collecting duct. Representative continuous current traces from cell-attached patches containing at least two ENaC before and after addition of 100 μm ATP (A and C) and 100 μm UTP (E) to the bath solution. Patches were formed on the apical membrane of principal cells within freshly isolated, split-open murine (A and E) and rat (C) collecting ducts. Collecting ducts were harvested from animals fed a nominally Na+-free diet. Patches were voltage clamped to -Vp = -60 mV. At this holding potential and with our recording solutions, inward Li+ current is downward. Areas under the bars over the continuous traces are shown below at expanded time scales. Dashed lines indicate the respective current levels with c denoting the closed state. Summary graphs of ENaC open probability changes in response to ATP (B and D) and UTP (F) from paired patch clamp experiments performed on isolated, split-open collecting ducts. Circles represent data from individual experiments with means shown as bars. *, significant decrease compared with before addition of ATP or UTP.
Other nucleotides also decrease ENaC activity in collecting duct principal cells. Fig. 1, E and F, shows a representative current trace and summary graph (n = 12, n = 6 mice), respectively, documenting the inhibitory effect of 100 μm UTP on ENaC in the split-open mouse collecting duct. As shown by these paired experiments, UTP, similar to ATP, rapidly and significantly decreases ENaC open probability (from 0.52 ± 0.06 to 0.11 ± 0.02). These results are consistent with metabotropic (P2Y) purinergic receptors mediating inhibition of ENaC.
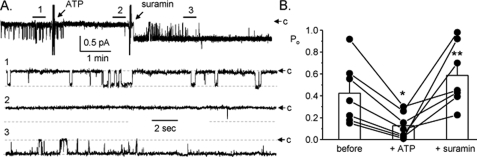
To further test this idea, we next assayed the effects of ATP on ENaC in the presence of the P2 receptor antagonist suramin. Fig. 2A shows a representative current trace for a patch made on the apical membrane of a principal cell in an isolated, split-open collecting duct from a mouse. This patch contained a single ENaC. As is clear in this trace and in the summary graph in Fig. 2B of a series of paired experiments (n = 7, n = 5 mice), addition of 100 μm suramin in the presence of 100 μm ATP reversed decreases in ENaC open probability. Inhibition of purinergic receptors often increased ENaC activity above starting levels even in the continued presence of inhibitory concentrations of ATP.
FIGURE 2.
Inhibition of P2 receptors reverses decreases in ENaC Po in response to ATP. A, continuous current trace from a cell-attached patch containing one ENaC before and after addition of ATP in the absence and presence of suramin. All other conditions are identical to those described in the legend to Fig. 1A. B, summary graph of ENaC open probability changes in response to ATP in the absence and presence of suramin from paired patch clamp experiments performed on isolated, split-open murine collecting ducts. Collecting ducts were harvested from animals fed a nominally Na+-free diet. *, significant decrease compared with before addition of ATP. **, significant increase compared with addition of ATP alone.
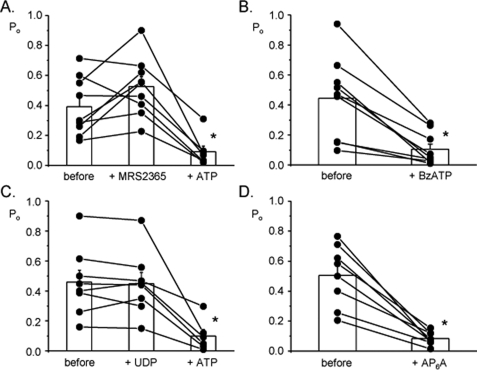
ATP Decreases ENaC Po via P2Y2 Receptors—We next probed which purinergic receptors transduce the inhibitory actions of ATP and UTP to ENaC. Because there is little evidence for P2Y12-14 receptors being in the collecting duct and these receptors do not respond to ATP, and because UTP, which does not stimulate P2X receptors, mimicked ATP actions, we focused our efforts on P2Y1,2,4,6&11.3 Summarized in Fig. 3 are results from paired patch clamp experiments on principal cells in isolated, split-open murine collecting ducts in which we tested the role of these receptors in regulation of ENaC. (Representative current traces are shown in supplemental Fig. S1.) The summary graph in Fig. 3A shows that 100 nm of the specific P2Y1 activator, MRS2365 (17, 18, 20), has little effect on ENaC open probability, although, subsequent addition of ATP decreases channel activity as expected (n = 9, n = 5 mice). Fig. 3B shows that similar to ATP, 100 μm BzATP rapidly and significantly decreases ENaC open probability (n = 11, n = 6 mice). BzATP activates both P2Y2 and P2Y11 receptors and is a partial inhibitor of P2Y4 receptors (17, 18, 20). UDP was used to probe the role of P2Y6 receptors; this receptor is preferentially activated by UDP (17, 18, 20). Fig. 3C demonstrates that 100 μm UDP has little effect on ENaC, although subsequent addition of ATP decreases channel activity (n = 8, n = 5 mice). Fig. 3D shows the effects of AP6Aon ENaC (n = 8, n = 4 mice). This dia-denosine polyphosphate, similar to ATP, rapidly and significantly decreases ENaC open probability. AP6A has been reported to be a specific agonist for P2Y2 receptors (11, 41). This pharmacological profile and the fact that both ATP and UTP, but not UDP, decrease ENaC activity excludes P2Y1,6,&12-14 and P2X receptors from playing a role in the observed decrease in ENaC activity, thereby implicating P2Y2 and possibly P2Y4&11 as the relevant receptors. Although the responses to BzATP and AP6A are most supportive of P2Y2 being the primary mediator of ATP and UTP action on ENaC in the mammalian collecting duct, it can be challenging to definitively isolate the actions of this receptor from those of P2Y4&11 using only pharmacologic approaches. Thus, we performed additional experiments using P2Y2 -/- mice.
FIGURE 3.
ATP decreases ENaC Po via P2Y2 receptors. Summary graphs of ENaC open probability changes in response to MRS2365 (A), BzATP (B), UDP (C), and AP6A(D) from paired patch clamp experiments performed on isolated, split-open murine collecting ducts. In some instances, ATP was subsequently added. Collecting ducts were harvested from animals fed a nominally Na+-free diet. All other conditions are identical to those described in the legend to Fig. 1. *, significant decrease compared with control period.
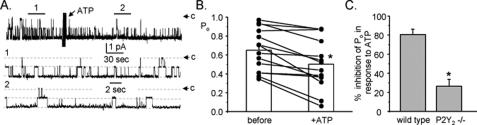
Fig. 4 reports the actions of 100 μm ATP on ENaC in principal cells in collecting ducts isolated from P2Y2 -/- mice. As shown in the representative current trace in Fig. 4A, ATP had a blunted effect on ENaC in this preparation. In contrast to the actions of ATP on ENaC in wild-type mice, where ATP decreased activity by ∼80% (see Fig. 1), in the paired experiments on P2Y2-/- mice, as summarized in Fig. 4B, ATP had only a modest effect with Po being 0.65 ± 0.06 and 0.50 ± 0.07 before and after addition of ATP, respectively (n = 14, n = 10 mice). These results agree with the pharmacological results above and are consistent with P2Y2 being the primary mediator of negative regulation of ENaC by ATP in the mammalian collecting duct. Although substantially muted, the residual action of ATP remaining in collecting ducts from P2Y2-/- mice suggest that other (likely P2) receptors may have some redundancy with P2Y2 receptors with respect to negative regulation of ENaC. This redundancy, although, must be weak and incomplete because the loss of P2Y2 receptors abolishes the bulk of regulation by ATP, as is apparent when comparing percent inhibition in response to ATP in the paired experiments for wild-type versus P2Y2-/- mice (Fig. 4C).
FIGURE 4.
ATP has a blunted effect on ENaC Po in collecting ducts from P2Y -/-2 mice. A, representative continuous current trace before and after addition of ATP from a cell-attached patch formed on a principal cell within a collecting duct isolated from a P2Y2-/- mouse. All other conditions are identical to those as described in the legend to Fig. 1. B, summary graph of ENaC open probability changes in response to ATP from paired patch clamp experiments performed on split-open collecting ducts isolated from P2Y2-/- mice. Collecting ducts were harvested from animals fed a nominally Na+-free diet. *, significantly decreased compared with before addition of ATP. C, summary graph comparing the percent inhibition of ENaC Po in response to ATP in collecting ducts from wild-type (n = 12) and P2Y2-/- (n = 14) mice. Collecting ducts harvested from animals fed a nominally Na+-free diet. *, significantly less compared with wild-type mice.
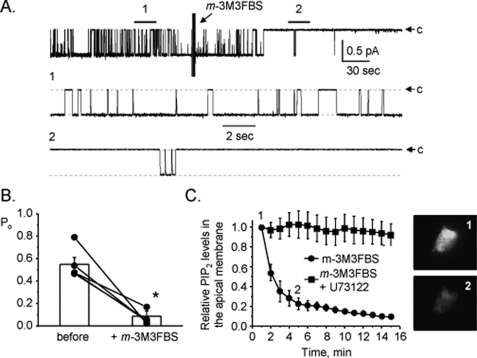
ATP Decreases ENaC Po via PLC Signaling—The P2Y2 receptor is a G protein-coupled receptor that increases PLC activity via Gq/11 (6, 17-20). We and others demonstrated in previous studies that ATP applied to the apical membrane of cultured, immortalized mouse principal cells rapidly decreases ENaC activity via activating PLC and promoting PI(4,5)P2 metabolism (28, 42, 43). Thus, we hypothesized that this signaling pathway couples P2Y2 receptors to ENaC in native principal cells. To test this, we asked whether direct stimulation of PLC mimics actions of ATP on ENaC in the mouse collecting duct. Shown in Fig. 5A is a representative current trace from a cell-attached patch made on the apical membrane of a principal cell in a split-open murine collecting duct before and after addition of 25 μm of the PLC activator m-3M3FBS. As is clear in this representative trace direct activation of PLC, akin to ATP, rapidly and markedly decreases ENaC open probability. Moreover, as summarized in Fig. 5B (n = 5, n = 4 mice), the robustness of the ENaC response to direct PLC stimulation is similar to its response to ATP with ENaC having significantly decreased open probability. Fig. 5C shows results from a positive control monitoring the time course of PLC activation in principal cells in response to m-3M3FBS alone and in the presence of the PLC inhibitor U73122. For these experiments, PLC activity was followed by monitoring the metabolism of apical membrane PI(4,5)P2 in polarized mpkCCDc14 principal cells. Changes in PI(4,5)P2 metabolism were measured by quantifying fluorescence emissions from a PI(4,5)P2 reporter with TIRF microscopy used to isolate emissions from the apical membrane. As is clear in this summary plot and in the representative micrographs shown to the right for the 0- and 5-min time points, direct activation of PLC quickly promotes apical membrane PI(4,5)P2 metabolism. These results are important because they confirm that m-3M3FBS activates PLC in principal cells. (We made a similar observation in studies with CHO cells; see supplemental Fig. S2.) Moreover, the findings demonstrate a close temporal coupling between changes in PLC activity and ENaC activity. They also are consistent with tight spatial coupling between apical membrane metabotropic P2Y2 receptors and ENaC with changes in apical membrane PI(4,5)P2 conveying information from the receptor to the channel.
FIGURE 5.
Direct activation of PLC decreases ENaC Po. A, continuous current trace from a cell-attached patch containing one ENaC before and after addition of m-3M3FBS. All other conditions are identical to those described in the legend to Fig. 1. B, summary graph of ENaC open probability changes in response to m-3M3FBS from paired patch clamp experiments performed on isolated, split-open murine collecting ducts. Collecting ducts were harvested from animals fed a nominally Na+-free diet. *, significant decrease compared with before addition of m-3M3FBS. C, summary graph showing apical membrane PI(4,5)P2 metabolism in mpkCCDc14 cells in response to m-3M3FBS alone (circles) and in the presence of U73122 (squares). Metabolism was quantified using a fluorescent PI(4,5)P2 reporter and TIRF microscopy. To the right are fluorescence micrographs showing emissions from the apical membrane PI(4,5)P2 reporter before (1) and 5 min after (2) treatment with m-3M3FBS.
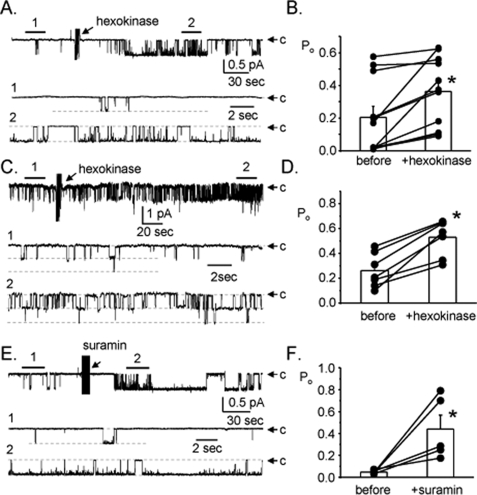
Tonic Release of ATP from Collecting Duct Cells Modulates ENaC—Because of the emerging idea that ATP functions as a paracrine signal in the distal nephron and our initial findings with suramin suggesting tonic regulation of ENaC by endogenous ATP (see Fig. 2), we decided to pursue this issue further. To test for tonic regulation of ENaC by endogenous ATP released from unstimulated collecting duct cells, we quantified changes in ENaC activity in cell-attached patches formed on principal cells in split-open murine collecting ducts before and after adding 10 units/ml of hexokinase to metabolize endogenous ATP. Fig. 6A shows a representative current trace for ENaC before and after addition of hexokinase. The results of this representative experiment and the summary graph of similar paired experiments (Fig. 6B; n = 11, n = 9 mice) show that addition of hexokinase rapidly increases ENaC open probability. We repeated these experiments in polarized mpkCCDc14 cells. As shown by the representative current trace for ENaC in the apical membrane of an mpkCCDc14 cell in Fig. 6C, and summarized in the graph in Fig. 6D, in these cells addition of hexokinase significantly increased ENaC open probability in a rapid manner. Direct inhibition of P2 receptors with suramin in isolated, split-open collecting ducts yielded a similar response. Fig. 6E shows a typical current trace for ENaC in a cell-attached patch formed on the apical membrane of a principal cell in a split-open murine collecting duct before and after addition of suramin in the absence of any other stimulus. As shown in Fig. 6F (n = 11, n = 9 mice), inhibition of P2 receptors alone with suramin significantly increased ENaC activity in this native preparation.
FIGURE 6.
Endogenous ATP released from collecting duct principal cells tonically decreases ENaC activity. A, representative continuous current trace for ENaC before and after addition of hexokinase. For these experiments, collecting ducts were harvested from mice fed a normal [Na+] diet to set initial ENaC activity low to maximize any observable increase in channel activity. All other conditions are identical to those described in the legend to Fig. 1A. B, summary graph of ENaC open probability changes in response to hexokinase from paired patch clamp experiments performed on isolated, split-open murine collecting ducts. *, significant increase compared with control period before addition. C, representative continuous current trace for ENaC in a cell-attached patch formed on the apical membrane of an mpkCCDc14 cell within a polar monolayer before and after addition of hexokinase. All other conditions are the same as described in the legend to Fig. 1A. D, summary graph of ENaC open probability changes in response to hexokinase from paired patch clamp experiments performed on mpkCCDc14 cells. *, significant increase compared with control period before addition. E, representative continuous current trace for ENaC before and after addition of suramin in the absence of any other stimulus. For these experiments, collecting ducts were harvested from mice fed a normal [Na+] diet. All other conditions are identical to those described in the legend to Fig. 1A. F, summary graph of ENaC open probability changes in response to suramin from paired patch clamp experiments performed on isolated, split-open murine collecting ducts. *, significant increase compared with before addition.
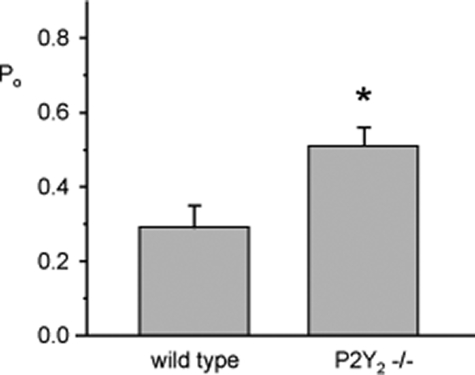
Loss of Paracrine Regulation by ATP in the Collecting Duct Increases ENaC Activity—If endogenous ATP acting in a paracrine manner exerts tonic inhibition on ENaC in the mammalian collecting duct via P2Y2 receptors, then ENaC should be more active in mice lacking this receptor compared with control mice that have this receptor. Indeed, this is what we observe, as shown in Fig. 7. When comparing resting ENaC activity in collecting ducts isolated from wild-type (n = 12, n = 6 mice) and P2Y2-/- (n = 18, n = 8 mice) mice fed a normal salt diet with all other conditions equal, we found that ENaC has significantly greater basal open probability in P2Y2-/- mice. This finding is in agreement with the data shown in Fig. 4, which indicates that exogenous ATP has little effect on ENaC in collecting ducts from P2Y2-/- mice. It is also consistent with tonic down-regulation of ENaC activity in response to endogenous ATP acting in an autocrine/paracrine manner.
FIGURE 7.
ENaC activity is greater in collecting ducts from P2Y -/-2 mice compared with wild-type mice. Summary graph comparing resting ENaC open probability measured by patch clamping in split-open mouse collecting ducts isolated from wild-type and P2Y2-/- mice maintained on a normal [Na+] diet. *, significantly greater compared with wild type.
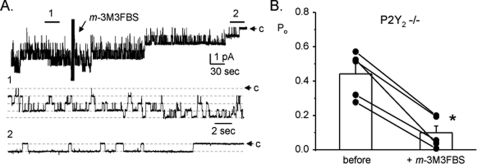
If loss of P2Y2 receptors is the primary defect leading to ENaC hyperactivity in knock-out mice, they ought to retain the ability to have inhibition by post-receptor mechanisms in the signal transduction cascade. Thus, we tested the effect of directly stimulating PLC on ENaC in P2Y2-/- mice. As shown by the representative current trace and summary graph in Fig. 8, A and B (n = 5, n = 4 mice), direct stimulation of PLC in P2Y2-/- mice by m-3M3FBS rapidly and significantly decreases ENaC open probability. This finding is similar to that in wild-type in response to m-3M3FBS (see Fig. 5), and to the effects of ATP and UTP on ENaC in collecting ducts harvested from wild-type mice (see Fig. 1).
FIGURE 8.
Stimulating PLC in P2Y -/-2 mice decreases ENaC Po. A, representative continuous current trace for ENaC in a cell-attached patch formed on the apical membrane of a principal cell in an isolated, split-open collecting duct from a P2Y2-/- mouse before and after addition of m-3M3FBS. All other conditions are identical to those described in the legend to Fig. 1. B, summary graph of ENaC open probability changes in response to m-3M3FBS from paired patch clamp experiments performed on isolated, split-open collecting ducts from P2Y2-/- mice maintained on a regular [Na+] diet. *, significant decrease compared with before addition of m-3M3FBS.
DISCUSSION
The current study of purinergic regulation of ENaC in the mammalian collecting duct provides several new insights regarding regulation and “basal” activity of ENaC in the collecting duct. Our data document dynamic decreases in ENaC open probability in response to ATP, showing directly with single channel patch clamp analysis that ENaC within the native collecting duct is a target of purinergic signaling. Moreover, by using a pharmacological approach in combination with knock-out mice, we obtained unequivocal evidence that P2Y2 receptors are the principal means by which purinergic signaling decreases ENaC activity. Additional findings place PLC as a key signaling event distal to P2Y2 receptors in the transduction cascade to ENaC. Our most important results are those implicating tonic regulation of ENaC by local ATP, which decreases channel activity through autocrine/paracrine signaling mediated by P2Y2 receptors. One ramification of losing this intrinsic regulation is inappropriately hyperactive ENaC, which likely contributes to the hypertension documented in mice lacking this receptor (30). The current description of tonic regulation of ENaC by local ATP signaling in the mammalian distal nephron leads us to propose that similar to renal filtration, renal Na+ handling, particularly that in the collecting duct, is under dynamic control by mechanisms both extrinsic and intrinsic to the kidney. This idea is akin to that of others who previously inferred such regulation primarily on the strength of data from cultured epithelial cells (7, 8, 13, 21, 44); however, our results provide compelling evidence that such intrinsic regulation exists in the native mammalian collecting duct. Moreover, the current results in combination with our earlier studies on P2Y2-/- mice (30) demonstrate that loss of this intrinsic control can, in some instances, lead to disease, suggesting that similar to extrinsic regulation of Na+ transport by the renin-angiotensin-aldosterone system, local control by ATP is physiologically important.
The current finding that ATP rapidly and dynamically decreases ENaC open probability in native collecting duct principal cells is consistent with similar findings, made previously by us and others, for ENaC activity in cell line models of collecting duct epithelium (28, 43, 44). Moreover, the results are also consistent with related findings regarding ATP-dependent decreases in the amiloride-sensitive Na+ current in native collecting ducts (11, 14, 24), and M-1 (42, 45, 46) and Madin-Darby canine kidney (7) cells, as well as in mouse and rabbit airway epithelium (42, 47) and mouse distal colon (48).
Our conclusion that the P2 receptor mediating regulation of ENaC resides on the apical plasma membrane is also consistent with the prevailing thinking about purinergic regulation of Na+ transport and ATP release in collecting duct cells (7, 10, 14, 24, 45-47, 49). Several studies using pharmacological approaches point to the P2Y2 receptor as possibly being the primary receptor involved in ATP regulation of Na+ transport (11, 14, 24, 45, 46, 48, 49). The pharmacology of purinergic regulation of ENaC in collecting ducts from the wild-type mice presented here, and our experiments on collecting ducts from P2Y2-/- mice adds unequivocal support for this view. An interesting addendum is that P2Y2 receptors, whereas carrying the bulk of this regulation, may have some overlap in function with other collecting duct receptors, possibly other P2 receptors because we found that a modest ATP response remained in collecting ducts from P2Y2-/- mice. Others have suggested that certain P2Y and possibly P2X receptors also play a role in modulating transport in epithelia and ENaC activity (11, 50, 51). Thus, P2Y4 and perhaps P2Y11 may have limited, redundant function with P2Y2 receptors in the mammalian collecting duct.
Investigation of tonic and stimulus-dependent ATP release from renal epithelial cells is an active area of study (7, 12, 16, 49). Of particular importance to the current investigation are recent findings documenting substantial ATP release from resting Madin-Darby canine kidney cells (7, 13). Madin-Darby canine kidney cells are a model for distal nephron epithelia. This released ATP was functionally available and capable of activating P2 receptors and decreasing amiloride-sensitive Na+ transport presumably mediated by ENaC. Native mouse medullary thick ascending limb cells also have been shown to release ATP (13). This released ATP is able to cause oscillations in intracellular Ca2+ in wild-type but not in P2Y2 knock-out mice. A different study recently documented flow-induced nucleotide release in the intact nephron that was able to mobilize intracellular Ca2+ in tubules from wild-type mice but less so in P2Y2-/- mice (12). This flow response was greatly reduced by scavenging endogenously released ATP with apyrase or block-ade of P2 receptors with suramin, maneuvers similar to those used in the current study to assess tonic regulation of ENaC by endogenously released ATP from unstimulated collecting ducts. Data from these previous studies, which demonstrated that ATP is tonically released in a functionally significant manner by resting and stimulated renal epithelial cells, fit nicely with the current findings, which show that endogenously released ATP tonically regulates ENaC activity in the collecting duct and that P2Y2 receptors play a critical role in this action of ATP.
Supplementary Material
This work was supported, in whole or in part, by National Institutes of Health Grants R01DK59594 and R01DK70571 (to J. D. S.), R01DK56248 and R01DK28602 (to V. V.), and R01GM66232 (to P. A. I.). This work was also supported by American Heart Association (AHA) Established Investigator Award 0640054N (to J. D. S.), AHA Grant-in-Aid 0655232Y (to V. V.), AHA Fellowship 085062F (to O. P.), German Research Foundation Grants RI1535/3-1 and 3-2 (to T. R.), a National Kidney Foundation Fellowship (to T. R.), and the Research Service of the Department of Veterans Affairs (to V. V.). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1 and S2.
Footnotes
The abbreviations used are: EnaC, epithelial Na+ channel; PLC, phospholipase C; PI(4,5)P2, phosphatidylinositol 4,5-bisphosphate; GFP, green fluorescent protein; PH, plekstrin homology; mpkCCDc14, immortalized mouse collecting duct principal cell line; -Vp, negative pipette potential; i, unitary current; N, number of active channels within a patch; Po, open probability; NPo, channel activity; TIRF, total internal reflection fluorescence; MRS2365, (N)-methanocarba-2MeSADP; AP6A, diadensoine hexaphosphate; BzATP, adenosine 5′-triphosphate; m-3M3FBS, 2,4,6-trimethyl-N-(meta-3-trifluoromethyl-phenyl)-benzenesulfonamide; U73122, [1-(6-[17β-3-methoxyestra-1,3,5-(10)triene-17-yl] amino/hexyl)1H-pyrroledione.
References
- 1.Garty, H., and Palmer, L. G. (1997) Physiol. Rev. 77 359-396 [DOI] [PubMed] [Google Scholar]
- 2.Hummler, E., and Horisberger, J. D. (1999) Am. J. Physiol. 276 G567-G571 [DOI] [PubMed] [Google Scholar]
- 3.Bonny, O., and Hummler, E. (2000) Kidney Int. 57 1313-1318 [DOI] [PubMed] [Google Scholar]
- 4.Lifton, R. P., Gharavi, A. G., and Geller, D. S. (2001) Cell 104 545-556 [DOI] [PubMed] [Google Scholar]
- 5.Hovater, M. B., Olteanu, D., Welty, E. A., and Schwiebert, E. M. (2008) Purinergic. Signal. 4 109-124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vallon, V. (2008) Am. J. Physiol. 294 F10-F27 [DOI] [PubMed] [Google Scholar]
- 7.Xie, Y., and Schafer, J. A. (2008) Purinergic. Signal. 4 125-137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schwiebert, E. M., and Kishore, B. K. (2001) Am. J. Physiol. 280 F945-F963 [DOI] [PubMed] [Google Scholar]
- 9.Hovater, M. B., Olteanu, D., Hanson, E. L., Cheng, N. L., Siroky, B., Fintha, A., Komlosi, P., Liu, W., Satlin, L. M., Bell, P. D., Yoder, B. K., and Schwiebert, E. M. (2008) Purinergic. Signal. 4 155-170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shirley, D. G., Bailey, M. A., and Unwin, R. J. (2005) Am. J. Physiol. 288 F1243-F1248 [DOI] [PubMed] [Google Scholar]
- 11.Wildman, S. S., Marks, J., Turner, C. M., Yew-Booth, L., Peppiatt-Wildman, C. M., King, B. F., Shirley, D. G., Wang, W., and Unwin, R. J. (2008) J. Am. Soc. Nephrol. 19 731-742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jensen, M. E., Odgaard, E., Christensen, M. H., Praetorius, H. A., and Leipziger, J. (2007) J. Am. Soc. Nephrol. 18 2062-2070 [DOI] [PubMed] [Google Scholar]
- 13.Geyti, C. S., Odgaard, E., Overgaard, M. T., Jensen, M. E., Leipziger, J., and Praetorius, H. A. (2008) Pflugers Arch. 455 1105-1117 [DOI] [PubMed] [Google Scholar]
- 14.Lehrmann, H., Thomas, J., Kim, S. J., Jacobi, C., and Leipziger, J. (2002) J. Am. Soc. Nephrol. 13 10-18 [DOI] [PubMed] [Google Scholar]
- 15.Woo, K., Dutta, A. K., Patel, V., Kresge, C., and Feranchak, A. P. (2008) J. Physiol. 586 2779-2798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vekaria, R. M., Unwin, R. J., and Shirley, D. G. (2006) J. Am. Soc. Nephrol. 17 1841-1847 [DOI] [PubMed] [Google Scholar]
- 17.Fields, R. D., and Burnstock, G. (2006) Nat. Rev. Neurosci. 7 423-436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burnstock, G. (2006) Pharmacol. Rev. 58 58-86 [DOI] [PubMed] [Google Scholar]
- 19.Burnstock, G. (2006) Novartis Found. Symp. 276 26-48 [PubMed] [Google Scholar]
- 20.Burnstock, G. (2007) Cell Mol. Life Sci. 64 1471-1483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Unwin, R. J., Bailey, M. A., and Burnstock, G. (2003) News Physiol. Sci. 18 237-241 [DOI] [PubMed] [Google Scholar]
- 22.Li, L., Jeanette, L. I., Zheng, W., Cash, M. N., Teng, X., Wingo, C. S., Verlander, J. W., and Xia, S. L. (2007) Biochem. Biophys. Res. Commun. 359 438-444 [DOI] [PubMed] [Google Scholar]
- 23.Ecelbarger, C. A., Maeda, Y., Gibson, C. C., and Knepper, M. A. (1994) Am. J. Physiol. 267 F998-F1006 [DOI] [PubMed] [Google Scholar]
- 24.Deetjen, P., Thomas, J., Lehrmann, H., Kim, S. J., and Leipziger, J. (2000) J. Am. Soc. Nephrol. 11 1798-1806 [DOI] [PubMed] [Google Scholar]
- 25.Lu, M., Macgregor, G. G., Wang, W., and Giebisch, G. (2000) J. Gen. Physiol. 116 299-310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haugh, J., Codazzi, F., Teruel, M., and Meyer, T. (2000) J. Cell Biol. 151 1269-1280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bens, M., Vallet, V., Cluzeaud, F., Pascual-Letallec, L., Kahn, A., Rafestin-Oblin, M. E., Rossier, B. C., and Vandewalle, A. (1999) J. Am. Soc. Nephrol. 10 923-934 [DOI] [PubMed] [Google Scholar]
- 28.Pochynyuk, O., Bugaj, V., Vandewalle, A., and Stockand, J. D. (2008) Am. J. Physiol. 294 F38-F46 [DOI] [PubMed] [Google Scholar]
- 29.Staruschenko, A., Pochynyuk, O., Vandewalle, A., Bugaj, V., and Stockand, J. D. (2007) J. Am. Soc. Nephrol. 18 1652-1661 [DOI] [PubMed] [Google Scholar]
- 30.Rieg, T., Bundey, R. A., Chen, Y., Deschenes, G., Junger, W., Insel, P. A., and Vallon, V. (2007) FASEB J. 21 3717-3726 [DOI] [PubMed] [Google Scholar]
- 31.Li, D., Wei, Y., Babilonia, E., Wang, Z., and Wang, W. H. (2006) Am. J. Physiol. 290 F806-F812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sun, P., Lin, D. H., Wang, T., Babilonia, E., Wang, Z., Jin, Y., Kemp, R., Nasjletti, A., and Wang, W. H. (2006) Am. J. Physiol. 291 F1192-F1200 [DOI] [PubMed] [Google Scholar]
- 33.Pochynyuk, O., Tong, Q., Medina, J., Vandewalle, A., Staruschenko, A., Bugaj, V., and Stockand, J. D. (2007) J. Gen. Physiol. 130 399-413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gamper, N., and Shapiro, M. S. (2006) Methods Mol. Biol. 337 27-38 [DOI] [PubMed] [Google Scholar]
- 35.Yuan, W., Burkhalter, A., and Nerbonne, J. M. (2005) J. Neurosci. 25 9185-9194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Taraska, J. W., Perrais, D., Ohara-Imaizumi, M., Nagamatsu, S., and Almers, W. (2003) Proc. Natl. Acad. Sci. U. S. A. 100 2070-2075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Steyer, J. A., and Almers, W. (2001) Nat. Rev. Mol. Cell. Biol. 2 268-275 [DOI] [PubMed] [Google Scholar]
- 38.Axelrod, D. (2001) Traffic 2 764-774 [DOI] [PubMed] [Google Scholar]
- 39.Tong, Q., Gamper, N., Medina, J. L., Shapiro, M. S., and Stockand, J. D. (2004) J. Biol. Chem. 279 22654-22663 [DOI] [PubMed] [Google Scholar]
- 40.Pochynyuk, O., Medina, J., Gamper, N., Genth, H., Stockand, J. D., and Staruschenko, A. (2006) J. Biol. Chem. 281 26520-26527 [DOI] [PubMed] [Google Scholar]
- 41.Wildman, S. S., Unwin, R. J., and King, B. F. (2003) Br. J. Pharmacol. 140 1177-1186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kunzelmann, K., Bachhuber, T., Regeer, R., Markovich, D., Sun, J., and Schreiber, R. (2005) FASEB J. 19 142-143 [DOI] [PubMed] [Google Scholar]
- 43.Ma, H. P., Saxena, S., and Warnock, D. G. (2002) J. Biol. Chem. 277 7641-7644 [DOI] [PubMed] [Google Scholar]
- 44.Ma, H. P., Li, L., Zhou, Z. H., Eaton, D. C., and Warnock, D. G. (2002) Am. J. Physiol. 282 F501-F505 [DOI] [PubMed] [Google Scholar]
- 45.Cuffe, J. E., Bielfeld-Ackermann, A., Thomas, J., Leipziger, J., and Korbmacher, C. (2000) J. Physiol. 524 77-90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thomas, J., Deetjen, P., Ko, W. H., Jacobi, C., and Leipziger, J. (2001) J. Membr. Biol. 183 115-124 [DOI] [PubMed] [Google Scholar]
- 47.Poulsen, A. N., Klausen, T. L., Pedersen, P. S., Willumsen, N. J., and Frederiksen, O. (2005) Pflugers Arch. 450 227-235 [DOI] [PubMed] [Google Scholar]
- 48.Matos, J. E., Sorensen, M. V., Geyti, C. S., Robaye, B., Boeynaems, J. M., and Leipziger, J. (2007) Pflugers Arch. 454 977-987 [DOI] [PubMed] [Google Scholar]
- 49.Woda, C. B., Leite, M., Jr., Rohatgi, R., and Satlin, L. M. (2002) Am. J. Physiol. 283 F437-F446 [DOI] [PubMed] [Google Scholar]
- 50.Wildman, S. S., Marks, J., Churchill, L. J., Peppiatt, C. M., Chraibi, A., Shirley, D. G., Horisberger, J. D., King, B. F., and Unwin, R. J. (2005) J. Am. Soc. Nephrol. 16 2586-2597 [DOI] [PubMed] [Google Scholar]
- 51.Matos, J. E., Robaye, B., Boeynaems, J. M., Beauwens, R., and Leipziger, J. (2005) J. Physiol. 564 269-279 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.