Abstract
Expression of the BRCA2 tumor suppressor gene is tightly linked to its roles in DNA damage repair and maintenance of chromosomal stability and genomic integrity. Three transcription factors that activate (USF, NF-κB, and Elf1) and a single factor that represses (SLUG) BRCA2 promoter activity have been reported. In addition, a 67-bp region (–582 to–516) associated with inhibition of promoter activity has been identified. However, it remains unclear how the 67-bp region contributes to regulation of BRCA2 expression. Here, we describe the affinity purification of a 120-kDa protein that binds to a silencer-binding region within the 67-bp repression region of the BRCA2 promoter. Mass spectrometry revealed the identity of the protein as poly-(ADP-ribose) polymerase-1 (Parp-1). Gel shift, antibody super-shift, and chromatin immunoprecipitation (ChIP) assays demonstrated that Parp-1 is associated with the BRCA2 promoter both in vitro and in vivo. Furthermore, Parp-1 inhibitors (either 3-AB or NU1025) and Parp-1 gene specific siRNA resulted in increased levels of endogenous BRCA2 expression. Inhibition of Parp-1 activity (by 3-AB) reduced histone 3 lysine 9 acetylation and blocked Parp-1 binding to the BRCA2 promoter. These results indicate that Parp-1 down-regulates BRCA2 expression through an interaction with a repression region of the BRCA2 promoter.
Breast cancer is the second leading cause of cancer-related deaths and represents the leading cause of cancer in women. BRCA2 is a tumor suppressor gene associated with familial predisposition to breast and other cancers (1, 2). Germline mutations in BRCA2 account for about 25% of autosomal dominant familial breast cancers (3, 4). While the role of BRCA2 in sporadic breast cancer remains unclear, loss of heterozygosity of the BRCA2 locus has been detected in over 50% of sporadic breast tumors. This suggests a role for BRCA2 in sporadic breast tumor development. However, somatic mutations in BRCA2 (5, 6) and methylation of the BRCA2 promoter have not been detected in breast cancers (7). One possible mechanism of BRCA2 involvement in breast cancer progression is through deregulation of the BRCA2 gene.
The BRCA2 gene encodes a 3418-amino acid nuclear protein that has been implicated in maintenance of genomic integrity and in the cellular response to DNA damage (8). Absence of BRCA2 function is associated with centrosome amplification, chromosomal rearrangement, aneuploidy, and reduced efficiency of homologous recombination-mediated double-strand break repair. BRCA2 binds directly to proteins (such as RAD51, BCCIP, PALB2, and BRAF35) that are critical for meiotic and mitotic recombination, DNA double-strand break (DSB) repair, and chromosome segregation.
The expression of the BRCA2 gene is stringently regulated during the cell cycle. In proliferating cells, BRCA2 expression is increased relative to the rate of cell proliferation (8, 9). While BRCA2 expression is closely linked to its involvement in cell cycle checkpoints and DNA repair, the mechanisms that regulate BRCA2 expression are not well understood. Examination of the minimal promoter sequence of BRCA2 has revealed several canonical elements for the binding of transcription factors including E-box, E2F, and Ets recognition motifs (10). USF binds the E-box (10, 11), and Elf1, an Ets family protein binds the Ets recognition motif (10) and activates the expression of BRCA2 (10, 11). NF-κB has also been shown to bind the –144 to –59-bp region of the promoter and induce BRCA2 expression (11). SLUG negatively regulates BRCA2 expression by binding an E2-box flanked by two Alu sequences in the –701 to –921-bp region (12), while p53 represses the BRCA2 promoter by blocking the binding of USF (33).
We previously reported a potential silencer-binding region located –582 to –516 bp upstream of the BRCA2 transcription start site (11). Deletion of the sequence resulted in a 2.5-fold activation of the BRCA2 promoter. In this study we show that poly-(ADP-ribose) polymerase-1 (Parp-1)4 binds to the silencer-binding region and negatively regulates the BRCA2 promoter. We also demonstrate that Parp-1 specific inhibitors and Parp-1 siRNA induce BRCA2 transcription. Thus, Parp-1 appears to play a critical role in the regulation of BRCA2 transcription.
EXPERIMENTAL PROCEDURES
Cell Culture—Human breast tumor MCF-7 cells were obtained from the American Type Culture Collection and cultured in the Minimum Essential Medium (MEM, Invitrogen) supplemented with 10% bovine calf serum (HyClone) and maintained at 37 °C with 5% CO2. Cell culture reagents were obtained from Invitrogen.
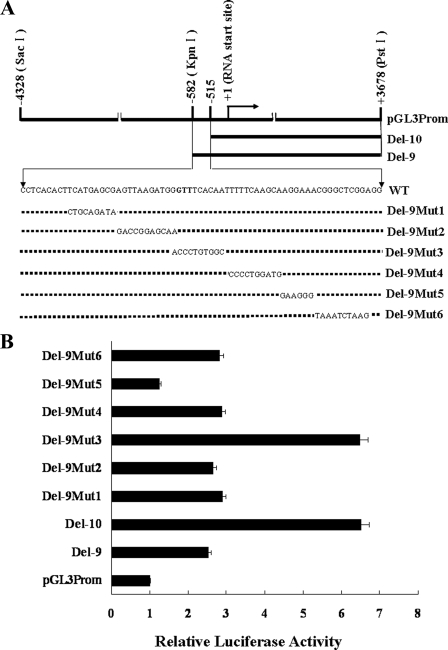
BRCA2 Reporter Constructs—Three BRCA2 promoter constructs, Del-9, Del-10, and pGL3Prom shown in Fig. 1A, were cloned into the pGL3 reporter vector as described previously (11). Six mutations were systematically introduced into the 67-bp region (–582 ∼–516) in Del-9 using the QuikChange site-directed mutagenesis kit (Stratagene). The six mutated constructs were confirmed by DNA sequencing and identified as Del-9mut1, Del-9mut2, Del-9mut3, Del-9mut4, Del-9mut5, and Del-9mut6, respectively.
FIGURE 1.
Activity profiles of human BRCA2 promoter luciferase reporter constructs in MCF-7 cells. A, schematic diagram of pGL3Prom, Del-9, Del-10, and a series of Del-9 mutants. B, luciferase activity profiles of the BRCA2 promoter reporter constructs in MCF-7 cells. To control for transfection efficiency, cells were co-transfected with pRL-TK, and the activity associated with each construct was normalized relative to Renilla luciferase activity. The luciferase activity for each construct is shown relative to the wild-type pGL3Prom construct.
Luciferase Reporter Assay—Plasmid DNAs for transient transfection were isolated using a plasmid maxi kit (Promega). MCF-7 cells were plated at a density of 2 × 105 cells/well in 6-well plates. All transfections were carried out using Lipofectamine™ 2000 (Invitrogen) according to the manufacturer's instructions. A total of 2 μg of the BRCA2 promoter construct and 0.1 μg of the pRL-TK Renilla luciferase vector were used for each transfection. The pRL-TK Renilla luciferase vector was used to control for transfection efficiency. Each transfection experiment was performed in duplicate and repeated a minimum of three times. Firefly luciferase and Renilla luciferase assays were performed using the Dual-Luciferase Reporter Assay System (Promega). Approximately 48-h post transfection, cells were washed twice with 1× phosphate-buffered saline and harvested with 600 μl of passive lysis buffer (Promega). Cell lysates were cleared by centrifugation, and 5 μl was added to 100 μl of firefly luciferase substrate. Light units were measured in a luminometer. Renilla luciferase activity was measured in the same tube after addition of 100 μl of Stop and Glo reagent. For Parp-1 inhibitor treatment experiments, transfected cells were first incubated in regular media for 12 h and then switched to the media containing 3-aminobenzamide (3-AB) (10 mm) or NU1025 (100 μm) for another 12 h before the cells were harvested.
Electrophoretic Mobility Shift Assays—Double-strand oligonucleotides were generated from single-strand oligonucleotides (Table 1) with biotinylated 5′-ends. The biotin-labeled double-strand oligonucleotides were then used as probes for electrophoretic mobility shift assays. Nuclear extracts were isolated from cultured MCF-7 cells using NE-PER® Nuclear and Cytoplasmic Extraction Reagents (Pierce). DNA-protein binding reactions were performed in a binding buffer (Pierce) according to the manufacturer's instructions. Binding reactions were initiated by addition of 0.05 pmol of DNA probe to 20 μg of the nuclear extracts. Electrophoresis was performed in a 5% native polyacrylamide gel in 0.5× TBE buffer at 100 V for 120 min. The gels were then incubated in 0.5× TBE buffer for 15 min at room temperature, and finally transferred to a Nylon membrane. Membranes were cross-linked at 120 mJ/cm2. The signals were visualized using the Light Chemiluminescent EMSA kit (Pierce). Competition experiments and antibody supershift experiments were carried out similarly except that the unlabeled competitor cold probes (5 pmol or 100×) and anti-Parp-1 antibody (5 μg) (Cell Signaling Technology) were added in the binding reaction, respectively. Electrophoretic gel mobility shift assays were also performed by using end-blocked probes. The biotinylated double-strand probes were modified by binding horseradish peroxidase-conjugated streptavidin (Pierce) to the DNA ends according to the manufacturer's instructions. 20 μg of MCF-7 nuclear extracts were incubated with the streptavidin-labeled probes in the binding buffer with a final volume of 20 μl at room temperature for 15 min. The DNA-protein complexes were separated by electrophoresis and transferred to a Nylon membrane as described above. Signals were developed by using an ECL detection system (Millipore).
TABLE 1.
List of oligonucleotides used in the construction of probes for EMSA and DNA affinity pull-down assays
| Name | Sequence of oligonucleotides |
|---|---|
| WT | 5-gatgggtttcacaatttttcaagca-3 (Sense) |
| 5-tgcttgaaaaattgtgaaacccatc-3 (Antisense) | |
| M | 5-gatgaccctgtggctttttcaagca-3 (Sense) |
| 5-tgcttgaaaaagccacagggtcatc-3 (Antisense) |
DNA Affinity Pull-down of a DNA-binding Protein—Biotin-labeled oligonucleotides were coated to Dynabeads M280 according to the manufacturer's instructions (Dynal Biotech), and then incubated with the MCF-7 nuclear extracts in a binding buffer (80 mm NaCl, 50 mm Tris-HCL, pH 7.5, 4% (v/v) glycerol, 5 mm MgCl2, 0.25 mg/ml poly(dI-dC), 1 mm phenylmethylsulfonyl fluoride (PMSF), 1 mm dithiothreitol (DTT), 2.5 mm EDTA) at 25 °C for 1 h. These beads were followed by washing three times with the binding buffer. Protein fractions were eluted by adding elution buffer (0.5 m NaCl, 50 mm Tris-HCL, pH 7.5, 4% (v/v) glycerol, 5 mm MgCl2, 0.25 mg/ml poly(dI-dC), 2.5 mm EDTA, 1 mm PMSF, 1 mm DTT) and stored at –80 °C. The protein fractions were separated on 10% Tris-glycine SDS-polyacrylamide gels and visualized by the SilverQuest Silver Staining Kit (Invitrogen). Discrete protein bands identified in the eluted fractions were characterized by MS/MS mass spectrometry (Bruker MALDI-TOF-TOF). Mass spectrometry data were analyzed by using MASCOT search engine software.
Western Blotting—MCF-7 cells were washed twice with 1× PBS, and cell lysates were prepared with RIPA buffer containing Complete Protease Inhibitor Mixture Tablets (Roche Applied Science). Approximately 100 μg of total protein from whole cell lysates were separated by SDS-PAGE and immunoblotted with antibodies against Parp-1 and BRCA2 (Cell Signaling Technology). Signals were developed by an ECL detection system.
Real-time RT-PCR—Real-time RT-PCR was carried out using an ABI PRISM 7500 Sequence Detector (Applied Biosystems). A total reaction volume of 25 μl was prepared containing 0.5 μl of template cDNA, 12.5 μl of 2× SYBR Green Master Mix (Takara), and 200 nm each of the forward and reverse PCR primers. The PCR conditions included an initial denaturation step at 95 °C for 10 s followed by 40 cycles of 95 °C for 5 s and 60 °C for 40 s. A non-template control was included in each experiment. Melting curve analysis and agarose electrophoresis were carried out to validate the specificity of the amplification products. Relative expression was determined using the Ct method (13): fold value = 2–[ΔΔCt], where ΔΔCt =ΔCt sample –ΔCt calibrator; ΔCt = (Ct gene of interest)–(Ct β-actin). All PCR reactions were performed in triplicate for each sample and were repeated three times. All experimental data were expressed as mean ± S.D.
siRNA against Parp-1—A pair of siRNA oligonucleotides (catalogue 111005) directed against human Parp-1 were purchased from Ambion Inc. (Austin, TX). A nonspecific negative control (NC) siRNA (sense: 5-uucuccgaacgugucacgutt, anti-sense: 5-acgugacacguucggagaatt) was obtained from Shanghai GenePharma Co., Ltd. siRNA oligonucleotides (200 nm) were transiently transfected into MCF-7 cells using Lipofectamine™ 2000. Forty-eight hours post-transfection, cell lysates were prepared and used for Western blotting, and RNA was prepared and used for RT-PCR and real time RT-PCR analyses. The PCR primers for BRCA2 and Parp-1 are listed in Table 2.
TABLE 2.
Nucleotide sequences of the primers used in RT-PCR and real-time RT-PCR
| Gene | Sense primer | Antisense primer | PCR size |
|---|---|---|---|
| bp | |||
| Parp-1 | 5-ctactcggtccaagatcgcc-3′ | 5-ttgaaaaagccctaaaggctca-3′ | 240 |
| BRCA2 | 5-caagcagatgatgtttcctgtcc-3′ | 5-agaactaagggtgggtggtgtagc-3′ | 243 |
Chromatin Immunoprecipitation (ChIP)—A total of 1 × 107 MCF-7 cells were fixed and lysed in a volume of 500 μl with ChIP lysis buffer (Upstate Biotechnology). DNA was sheered by sonication 10 × 20 s (amplitude 4 u), precleared with protein G-agarose, and immunoprecipitated with 5 μl of rabbit anti-Parp-1, histone H3 (acetyl K9) (Abcam Inc.), Histone H3 (Abcam Inc.) or normal rabbit serum according to the manufacturer's instructions. A target 119-bp fragment of the BRCA2 promoter was PCR-amplified using a pair of primers: 5′-agcgaaactccgtctcaaaa-3′ (F) and 5′-ccgtttccttgcttgaaaaa-3′ (R). A 166-bp GAPDH PCR fragment was also amplified with the following primers: 5′-tactagcggttttacgggcg-3′ (F) and 5′-tcgaacaggaggagcagagagcga (R) as a control. PCR conditions were the same as described above. A standard curve was constructed from known dilutions of input chromatin. For the quantitative ChIP studies, MCF-7 cells were pretreated with or without 10 mm 3-AB for 4 h, fixed, lysed, immunoprecipitated, and PCR-amplified as described above.
RESULTS
Identification of cis-Acting Regulatory Elements in a 67-bp Inhibitory Region of the BRCA2 Promoter—Our previous study showed that deletion of the –582 to –516-bp region resulted in 2.5-fold activation of the human BRCA2 promoter (11), suggesting that the 67-bp region contains cis-acting regulatory element(s) that are critical for negative regulation of basal transcription activity of the human BRCA2 promoter. To more accurately map the potential cis-acting regulatory element(s) within the 67-bp inhibitory region, a series of mutation constructs were generated as shown in Fig. 1A. Firefly luciferase activity was assayed following transient transfection of MCF-7 cells with these BRCA2 promoter constructs. Normalized luciferase activities for each mutant construct relative to 1) the construct containing the 67-bp region with no mutations (Del-9) and 2) the construct with the 8-kb BRCA2 promoter (pGL3Prom) (11) are shown in Fig. 1B. The results indicate that the BRCA2 promoter is regulated not only by a putative silencer but also by a potential enhancer within the 67-bp region. Mutation of the GTTTCACAAT element (Del-9mut3) caused a 2.5-fold activation of the promoter, whereas a 1.5-fold reduction in the activity was detected following mutation of the AGGAAA motif (Del-9mut5). Interestingly, the combination of this AGGAAA motif (Del-9mut5, 1.5-fold activation) and the previously reported Elf1 (Ets family member) binding site (–61 to –53, 2-fold activation) was associated with a 10-fold activation of the BRCA2 promoter.5
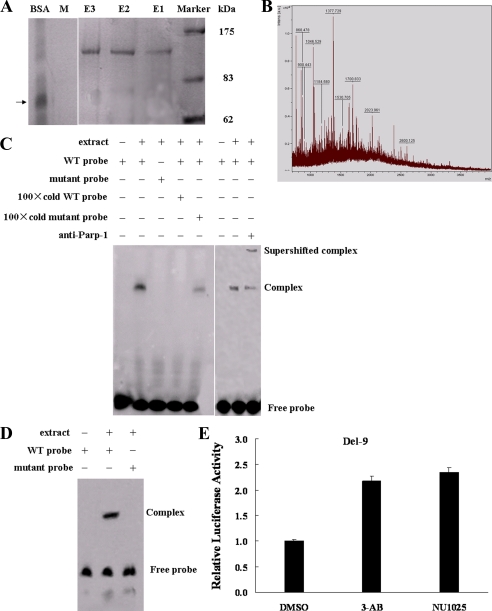
PARP-1 Specifically Binds and Negatively Regulates the BRCA2 Promoter—To identify the specific DNA-binding protein for the GTTTCACAAT motif, nuclear extracts from MCF-7 cells were incubated with a biotinylated wild-type (WT) probe. The biotinylated DNA-protein complex was bound to streptavidin-coated magnetic beads, eluted, and separated by sulfate-polyacrylamide gel electrophoresis. As shown in Fig. 2A, a ∼120-kDa protein band was detected. To control for nonspecific protein binding to DNA, we repeated the experiment utilizing a biotinylated mutant probe. As expected, the ∼120-kDa protein was not eluted. To identify the potential DNA-binding protein, the ∼120-kDa protein band was excised from the gel and subjected to mass spectrometry. Parp-1 was finally identified with a significant probability score of 174 (scores greater than 79 are significant (p < 0.05) (Fig. 2B).
FIGURE 2.
Parp-1 binds to the BRCA2 promoter. A, MCF-7 cell nuclear extracts were mixed with a biotinylated WT probe and a mutant probe (M). The DNA-protein complex was isolated with streptavidin-labeled magnetic beads. The magnetic bead column was washed and eluates were collected. Eluted fractions were separated on 10% acrylamide gels and visualized by silver staining. The 120-kDa protein band indicates a prominent DNA-protein complex. BSA was used as a control, and its position is indicated by a black arrow. The experiment was carried out three times. E1, E2, E3 (WT probe eluates); M, mutant probe eluate. B, mass spectrometry of the ∼120-kDa protein. C, EMSAs and antibody super-shifts were performed in MCF-7 nuclear extracts using WT, mutant probes, and Parp-1 antibody. D, EMSAs were carried out in MCF-7 nuclear extracts using the end-streptavidin blocked-biotin-labeled WT and mutant probes.E, luciferase activity of Del-9 in MCF-7 cells treated with 3-AB/NU1025, two Parp-1 inhibitors, or DMSO for 12 h.
To confirm Parp-1 binding to the BRCA2 promoter, we analyzed nuclear extracts from MCF-7 cells by EMSA using biotinylated WT and mutant probes (Table 1). As shown in Fig. 2C, incubation of the nuclear extracts with the WT probe gave a specific DNA-protein complex. The 100× non-biotinylated cold WT probe completely eliminated this complex whereas the non-biotinylated cold mutant probe had little influence. No DNA-protein complex was detected when the nuclear extracts were incubated with the biotinylated mutant probe (Fig. 2C). Antibody super-shift results further confirmed that the observed DNA-protein complex contained Parp-1. In the presence of the Parp-1 antibody, the complex was super-shifted and the intensity of the original complex was reduced (Fig. 2C). To exclude the possibility that Parp-1 binds the ends of the probe nonspecifically, we carried out gel shift assays using MCF-7 nuclear extracts and the labeled probes that had been end-blocked with streptavidin. Binding of nuclear extracts to an end-blocked WT probe produced a shifted complex that was not observed with an end-blocked mutant probe (Fig. 2D). In addition, BRCA2 promoter activity was induced in the cells treated with Parp-1 specific inhibitor, 3-AB at 10 mm or NU1025 at 100 μm concentrations (Fig. 2E). Taken together, the data indicate that Parp-1 associates with and negatively regulates the BRCA2 promoter.
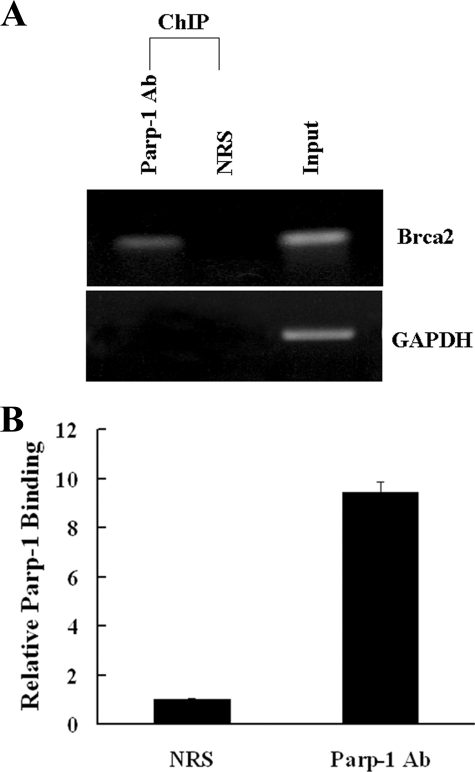
Parp-1 Binds to the BRCA2 Promoter in Vivo—To show that Parp-1 binds to the BRCA2 promoter in vivo, we carried out ChIP analysis. Fixed and sheared chromatin was immunoprecipitated with anti-Parp-1 antibody and PCR-amplified for BRCA2 promoter target sequences. The amplification of glyceradehyde-3-phosphate dehydrogenase (GAPDH) was used as a negative control. As shown in Fig. 3A, BRCA2 promoter sequences were only amplified in the anti-Parp-1-immunoprecipitated chromatin. Real-time PCR demonstrated that the BRCA2 promoter sequences were enriched ∼10-fold in anti-Parp-1 immunoprecipitated chromatin over chromatin immunoprecipitated with normal rabbit serum (NRS) (Fig. 3B), indicating that Parp-1 occupies the BRCA2 promoter in vivo.
FIGURE 3.
Parp-1 binds to the BRCA2 promoter in vivo. A, ChIP was carried out in MCF-7 cells. Parp-1 binds to the target region in the BRCA2 promoter. Anti-Parp-1 antibody and normal rabbit serum (NRS) were used as indicated. Parp-1 was not associated with the human GAPDH promoter. Five percent of input DNA was used as a template in the PCR amplifications as a positive control. PCR fragments were resolved on a 1.0% agarose gel. B, quantitative ChIP, shown as mean ± S.D.
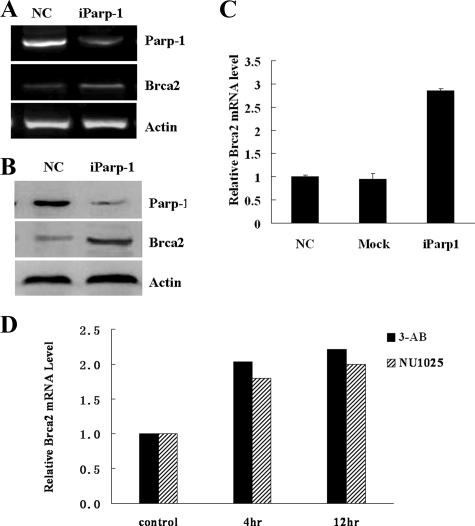
Depletion of Parp-1 Induces BRCA2 Expression—To establish whether the physical presence of Parp-1 was important for the regulation of BRCA2 expression, Parp-1 expression was knocked down by a Parp-1 specific siRNA in MCF-7 cells. To control for the effects of transfection and nonspecific siRNA, MCF-7 cells were also transfected with a nonspecific negative control siRNA. The BRCA2 mRNA and protein expression levels were analyzed in MCF7 cells 48-h post transfection. The BRCA2 mRNA levels were increased substantially following depletion of Parp-1 (Fig. 4A). Real-time RT-PCR analysis showed that depletion of Parp-1 resulted in a 2.5-fold induction of BRCA2 mRNA expression (Fig. 4B). However, no change in BRCA2 mRNA levels was detected in cells transfected with nonspecific negative control siRNA. In keeping with these observations, the BRCA2 protein expression levels were also increased in the cells transfected with the Parp-1 siRNA (Fig. 4C).
FIGURE 4.
Knockdown and inhibition of Parp-1 induces BRCA2 expression. A, siRNA knockdown of Parp-1 in MCF-7 cells. Transfections with negative control siRNA (NC) and with Parp-1 siRNA (iParp-1) are shown. Parp-1 and BRCA2 mRNA was measured by semi-quantitative RT-PCR 48-h post-transfection. B, BRCA2 mRNA expression was analyzed by real-time RT-PCR. C, Parp-1 and BRCA2 protein expression was measured by Western blotting. D, BRCA2 mRNA expression was induced in MCF-7 cells treated with 3-AB and NU1025 Parp-1 inhibitors. The real-time RT-PCR BRCA2 mRNA results were normalized to the amount of GAPDH mRNA.
Inhibition of Parp-1 Activity Also Induces BRCA2 mRNA Expression—To determine the effects of Parp-1 inhibitors on the production of BRCA2 in MCF-7 cells, the cells were treated with two different Parp-1 inhibitors, 3-AB (10 mm), and NU1025 (100 μm). The BRCA2 mRNA expression levels were examined after 4, 12, 24, and 48 h of treatment. As shown in Fig. 4D, a 2-fold induction of BRCA2 mRNA expression was detected at the 4-h time point, and this was maintained up to 12 h. However, the level of induction slowly declined after 24 and 48 h of treatment (data not shown). Thus, inhibition of Parp-1 enzymatic activity resulted in a significant increase in the level of BRCA2 mRNA that was consistent with the level of BRCA2 promoter activation induced by the same inhibitors (Fig. 2E). This suggests that Parp-1 negatively regulates BRCA2 expression predominantly at the level of transcription initiation.
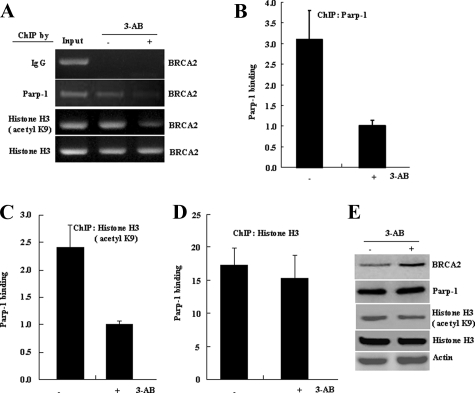
Inhibition of Parp-1 Activity Reduces Histone H3(K9) Acetylation and Blocks Parp-1 Binding to the BRCA2 Promoter—To further elucidate the role of Parp-1 in the regulation of the BRCA2 promoter, a correlation between the lysine 9 (K9) acetylation on histone H3 and DNA binding ability of Parp-1 at the BRCA2 promoter was assessed. In this study, Parp-1 enzymatic activity was inhibited by 10 mm 3-AB. The BRCA2 promoter target sequences were PCR-amplified from chromatin that was immunoprecipitated with the indicated antibodies. As shown in Fig. 5, amplification of the BRCA2 promoter target sequences was decreased 2.5–3.0-fold in both anti-Parp-1 (Fig. 5, A and B) and anti-histone H3 (acetyl K9) (Fig. 5, A and C) immunoprecipitated chromatin when the MCF-7 cells were treated with 3-AB for 4 h. However, a minimal change was observed in PCR-amplified BRCA2 promoter target sequences from anti-histone H3-immunoprecipitated chromatin (Fig. 5, A and D). 3-AB treatment also caused a mild reduction in the levels of acetylated lysine 9 on histone H3 (Fig. 5E). These data indicate that Parp-1 enzymatic activity may be required for the acetylation of the core histone H3 and relaxation of the chromatin in the promoter region of BRCA2. This would allow Parp-1 access to its specific binding sites and cause a down-regulation of BRCA2 expression.
FIGURE 5.
Inhibition of Parp-1 activity reduces histoneH3 (K9) acetylation and blocks Parp-1 binding to the BRCA2 promoter. A, ChIP was carried out in MCF-7 cells treated without (–) or with (+) 10 mm 3-AB for 4 h, by using anti-Parp-1, anti-histone H3 (acetyl K9), anti-histone H3 antibodies or normal rabbit serum (IgG) as indicated. A 119-bp BRCA2 promoter target fragment was PCR-amplified and resolved on a 1.0% agarose gel. Quantitative ChIPs from anti-Parp-1 (B), anti-Histone H3 (acetyl K9) (C), or anti-Histone H3 (D) immunoprecipitated chromatin (mean ± S.D.) are shown. E, Western blotting of Parp-1, Histone H3 (acetyl K9), Histone H3, and β-actin in MCF-7 cells treated without (–) or with (+) 10 mm 3-AB for 4 h.
DISCUSSION
Studies on BRCA2 indicate a pivotal role for this protein in the biology of most eukaryotic cells. In human mammary epithelial cells, the expression of BRCA2 protein appears to be regulated by a series of genetic and epigenetic mechanisms. The present study provides evidence of another mechanism for the regulation of BRCA2 gene expression. Our data demonstrate sequence-specific binding of Parp-1 to the BRCA2 promoter. In particular, we showed that transcription of BRCA2 is enhanced in the presence of Parp-1 inhibitors and Parp-1 siRNA. Overexpression of Parp-1 in MCF-7 cells did not demonstrate a significant change in BRCA2 expression. This is possibly due to the fact that Parp-1 is already highly expressed in these cells (data not shown). Collectively, our results demonstrate that Parp-1 activation and its physical presence play a critical role in BRCA2 transcription regulation.
Parp-1 is the founding member of a 17 protein family of structurally related molecules (14, 15). The Parp-1 gene is highly conserved, especially at amino acids comprising structural motifs and functional domains. There is an N-terminal DNA binding domain and a C-terminal β-nicotinamide adenine dinucleotide (NAD+) binding domain. The central section of Parp-1 contains an auto-modification domain, which also associates with other proteins (16). Parp-1 has important roles in DNA damage repair (17, 18), but more recent evidence has demonstrated a role for Parp-1 in transcriptional regulation (19). Parp-1 has been reported to regulate target genes via at least two non-exclusive mechanisms. First, Parp-1 is a constituent of chromatin and Parp-1 activity mediates chromatin relaxation thereby altering accessibility to transcription factors. In Drosophila melanogaster, Parp-1 is present at transcriptionally repressed chromatin regions. Parp-1 activation and its subsequent removal is a prerequisite for transcriptional activity to occur (20). This PAR-mediated chromatin loosening is observed at larval salivary gland polytene-chromosome puffs (21). At other promoters, Parp-1 is recruited to nucleosomes with topoisomerase IIb. Activation of Parp-1 by topoisomerase-induced DNA strand breaks induces gene transcription (22). The second mechanism by which Parp-1 or PAR can modulate transcription is by acting as part of gene-specific enhancer/promoter binding complexes. It can regulate transcription by modifying the activity of enhancer and promoter elements. Parp-1 acts to stimulate transcription with some activators, but can inhibit transcription with other factors, depending on the cell type and promoter context (23, 24). Parp-1 can bind DNA in a sequence-specific manner. Various binding sequences have been described, and although there is a shared tri-nucleotide sequence between two of these sites (“tgttg” (25) and “ttgannacaa” (26)), there is no significant match between the other known Parp-1 binding sequences. The probe we used contains the tri-nucleotide sequence in the BRCA2 promoter. Parp-1 can also contact DNA by binding to secondary hairpin structures (27).
Parp-1 also has a stimulatory effect on some transcription factors and co-factors, which does not necessarily require Parp-1 polymerase activity (28, 29, 30). Our results suggest that the catalytic activity of Parp-1 is important at the BRCA2 locus. Therefore, the transcriptional role of Parp-1 that is not required its polymerase activity likely does not account for the observed effects on BRCA2. Others have demonstrated a dual effect of Parp-1 at some promoters whereby Parp-1 inhibition represses expression and Parp-1 knockdown has the opposite effect (31). The proposed mechanism is that Parp-1 binding represses transcription but activation of Parp-1 followed by auto poly-(ADP-ribosy)lation and disassociation from DNA leads to induction of transcription. Our findings show that both Parp-1 inhibition and depletion induce BRCA2 expression. This argues that this dual mechanism does not occur at the BRCA2 locus. Ambrose et al. (32) have shown that Parp-1 inhibition and Parp-1 depletion by siRNA induces Bcl-6 mRNA expression in Bcl-6 expressing cell lines. Here, the Parp-1-binding site reported was flanked by upstream inhibitory Bcl-6 and STAT-5-binding sites, suggesting a model in which local Parp-1 activation and consequent chromatin relaxation causes increased accessibility to Bcl-6 and STAT-5 and reduced Bcl-6 transcription. Our data show that inhibition of Parp-1 activity is associated with induction of BRCA2 transcription and conversely that increased Parp-1 activation at the BRCA2 locus causes repression of BRCA2 transcription. We speculate that a substantial reduction in the amount of Parp-1 would have the same effect as inhibition of enzyme activity. According to the model that Ambrose et al. have proposed, locally activated Parp-1 at the BRCA2 locus and consequent chromatin relaxation causes accessibility to the putative repressor, causing a repression of BRCA2. Importantly, there is a “ggaa” (–532 ∼ –529) sequence that is an Ets recognition motif located downstream of the Parp-1 binding site in the BRCA2 promoter. Elf1, an Ets family member, has been found to bind the BRCA2 promoter (–61 ∼–53) near the USF binding site and activate BRCA2 transcription when the site is not occupied by USF (10, 11). Based on these observations, we predict that there may be an Ets family member binding to the “ggaa” (–532 ∼–529) motif, acting as an enhancer of Parp-1 activation and causing chromatin relaxation. Further studies are needed to evaluate this possibility.
Repression of the BRCA2 gene is important because accumulation of BRCA2 in the cell may potentially lead to cell death. Manish et al. have shown that SLUG represses the transcription of BRCA2 in cells expressing SLUG protein. However, since SLUG protein is not expressed in MCF-7 cells, it appears that there are other proteins that regulate the transcription of BRCA2 in MCF-7 cells. Our data suggest that Parp-1 accounts for this activity. In summary, we have found that Parp-1 binds to the BRCA2 promoter in a sequence-specific manner and negatively regulates BRCA2 expression. Further evaluation of the role of Parp-1 may reveal a very unique mechanism of BRCA2 gene expression regulation and help us to understand the possible causes of some sporadic breast cancer cases.
This work was supported, in whole or in part, by National Institutes of Health R01 Grant CA116167 and NCI Sponsored Program in Research Excellence (SPORE) in Breast Cancer to the Mayo Clinic (to F. J. C.) and a Ruth L. Kirschstein National Research Training Award (to K. W.). This work was also supported from the “863 Projects” of Ministry of Science and Technology of PR China Grant No. 2006AA02A109 (to R. C. Z.). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The abbreviations used are: Parp, poly-(ADP-ribose) polymerase; siRNA, short interfering RNA; CHiP, chromatin immunoprecipitation assay; EMSA, electrophoretic mobility shift assay; WT, wild type; 3-AB, 3-aminobenzamide; BSA, bovine serum albumin; PBS, phosphate-buffered saline.
R. C. Zhao, unpublished data.
References
- 1.Wooster, R., Bignell, G., Lancaster, J., Swift, S., Seal, S., Mangion, J., Collins, N., Gregory, S., Gumbs, C., and Micklem, G. (1995) Nature 378 789–792 [DOI] [PubMed] [Google Scholar]
- 2.Tavtigian, S. V., Simard, J., Rommens, J., Couch, F., Shattuck-Eidens, D., Neuhausen, S., Merajver, S., Thorlacius, S., Offit, K., Stoppa-Lyonnet, D., Belanger, C., Bell, R., Berry, S., Janecki, T., Jiang, P., Kehrer, R., Leblanc, J. F., Mitchell, J. T., McArthur-Morrison, J., Nguyen, K., Peng, Y., Samson, C., Schroeder, M., Snyder, S. C., Steel, L., Stringfellow, M., Stroup, C., Swedlund, B., Swensen, J., Teng, D., Thomas, A., Tran, T., Tran, T., Trnachant, M., Weaver-Feldhaus, J., Wong, A. K. C., Shixuya, H., Eyfjord, J. E., Cannon-Albright, L., Labrie, F., Skolnick, M. H., Weber, B., Kamb, A., and Goldgar, D. E. (1996) Nat. Genet. 12 333–337 [DOI] [PubMed] [Google Scholar]
- 3.Thorlacius, S., Struewing, J. P., Hartge, P., Olafsdottir, G. H., Sigvaldason, H., Tryggvadottir, L., Wacholder, S., Tulinius, H., and Eyfjörd, J. E. (1998) Lancet 352 1337–1339 [DOI] [PubMed] [Google Scholar]
- 4.Easton, D. (1997) Nat. Genet. 16 210–211 [DOI] [PubMed] [Google Scholar]
- 5.Teng, D. H., Bogden, R., Mitchell, J., Baumgard, M., Bell, R., Berry, S., Davis, T., Ha, P. C., Kehrer, R., Jammulapati, S., Chen, Q., Offit, K., Skolnick, M. H., Tavtigian, S. V., Jhanwar, S., Swedlund, B., Wong, A. K., and Kamb, A. (1996) Nat. Genet. 13 241–244 [DOI] [PubMed] [Google Scholar]
- 6.Lancaster, J. M. (1996) Nat. Genet. 13 238–240 [DOI] [PubMed] [Google Scholar]
- 7.Collins, N., Wooster, R., and Stratton, M. R. (1997) Br. J. Cancer. 76 1150–1156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bertwistle, D., Swift, S., Marston, N. J., Jackson, L. E., Crossland, S., Crompton, M. R., Marshall, C. J., and Ashworth, A. (1997) Cancer Res. 57 5485–5488 [PubMed] [Google Scholar]
- 9.Boulton, S. J. (2006) Biochem. Soc. Trans. 34 633–645 [DOI] [PubMed] [Google Scholar]
- 10.Davis, P. L., Miron, A., Andersen, L. M., Iglehart, J. D., and Marks, J. R. (1999) Oncogene. 18 6000–6012 [DOI] [PubMed] [Google Scholar]
- 11.Wu, K. J., Jiang, S. W., Thangaraju, M., Wu, G. J., and Couch, F. J. (2000) J. Biol. Chem. 275 35548–35556 [DOI] [PubMed] [Google Scholar]
- 12.Tripathi, K. M., Misra, S., Khedkar, S. V., Hamilton, N., Irvin-Wilson, C., Sharan, C., Sealy, L., and Chaudhuri, G. (2005) J. Biol. Chem. 280 17163–17171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gibson, U. E., Heid, C. A., and Williams, P. M. (1996) Genome Res. 6 995–1001 [DOI] [PubMed] [Google Scholar]
- 14.Ame, J. C., Spenlehauer, C., and de Murcia, G. (2004) Bioessays. 26 882–893 [DOI] [PubMed] [Google Scholar]
- 15.D'Amours, D., Desnoyers, S., D'Silva, I., and Poirier, G. G. (1999) Biochem. J. 342 249–268 [PMC free article] [PubMed] [Google Scholar]
- 16.Masson, M., Niedergang, C., Schreiber, V., Muller, S., Menissier-de Murcia, J., and de Murcia, G. (1998) Mol. Cell. Biol. 18 3563–3571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Satoh, M. S., and Lindahl, T. (1992) Nature 356 356–358 [DOI] [PubMed] [Google Scholar]
- 18.Durkacz, B. W., Omidiji, O., Gray, D. A., and Shall, S. (1980) Nature 283 593–596 [DOI] [PubMed] [Google Scholar]
- 19.Ju, B. G., Solum, D., Song, E. J., Lee, K. J., Rose, D. W., Glass, C. K., and Rosenfeld, M. G. (2004) Cell 119 815–829 [DOI] [PubMed] [Google Scholar]
- 20.Kim, M. Y., Mauro, S., Gevry, N., Lis, J. T., and Kraus, W. L. (2004) Cell 119 803–814 [DOI] [PubMed] [Google Scholar]
- 21.Tulin, A., and Spradling, A. (2003) Science 299 560–562 [DOI] [PubMed] [Google Scholar]
- 22.Bong-Gun, J. U., Lunyak, V. V., Perissi, V., Garcia-Bassets, I., Rose, D. W., Glass, C. K., and Rosenfeld, M. G. (2006) Science 312 1798–1802 [DOI] [PubMed] [Google Scholar]
- 23.Miyamoto, T., Kakizawa, T., and Hashizume, K. (1999) Mol. Cell. Biol. 19 2644–2649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Soldatenkov, V. A., Chasovskikh, S., Potaman, V. N., Trofimova, I., Smulson, M. E., and Dritschilo, A. (2002) J. Biol. Chem. 277 665–670 [DOI] [PubMed] [Google Scholar]
- 25.Huang, K., Tidyman, W. E., Le, K. U., Kirsten, E., Kun, E., and Ordahl, C. P. (2004) Biochemistry 43 217–228 [DOI] [PubMed] [Google Scholar]
- 26.Zhang, Z., Hildebrandt, E. F., Simbulan-Rosenthal, C. M., and Anderson, M. G. (2002) Virology 295 107–116 [DOI] [PubMed] [Google Scholar]
- 27.Lonskaya, I., Potaman, V. N., Shlyakhtenko, L. S., Oussatcheva, E. A., Lyubchenko, Y. L., and Soldatenkov, V. A. (2005) J. Biol. Chem. 280 17076–17083 [DOI] [PubMed] [Google Scholar]
- 28.Anderson, M. G., Scoggin, K. E., Simbulan-Rosenthal, C. M., and Steadman, J. A. (2000) J. Virol. 74 2169–2177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hassa, P. O., and Hottiger, M. O. (2002) Cell Mol. Life Sci. 59 1534–1553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cervellera, M. N., and Sala, A. (2000) J. Biol. Chem. 275 10692–10696 [DOI] [PubMed] [Google Scholar]
- 31.Amiri, K. I., Ha, H. C., Smulson, M. E., and Richmond, A. (2006) Oncogene 25 7714–7722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ambrose, H. E., Papadopoulou, V., Beswick, R. W., and Wagner, S. D. (2007) Oncogene 26 6244–6252 [DOI] [PubMed] [Google Scholar]
- 33.Wu, K., Jiang, S. W., and Couch, F. J. (2003) J. Biol. Chem. 278 15652–15660 [DOI] [PubMed] [Google Scholar]