Abstract
In vertebrates, Sonic hedgehog (Shh) and transforming growth factor-β (TGF-β) signaling pathways occur in an overlapping manner in many morphogenetic processes. In vitro data indicate that the two pathways may interact. Whether such interactions occur during embryonic development remains unknown. Using embryonic lung morphogenesis as a model, we generated transgenic mice in which exon 2 of the TβRII gene, which encodes the type II TGF-β receptor, was deleted via a mesodermal-specific Cre. Mesodermal-specific deletion of TβRII (TβRIIΔ/Δ) resulted in embryonic lethality. The lungs showed abnormalities in both number and shape of cartilage in trachea and bronchi. In the lung parenchyma, where epithelial-mesenchymal interactions are critical for normal development, deletion of mesenchymal TβRII caused abnormalities in epithelial morphogenesis. Failure in normal epithelial branching morphogenesis in the TβRIIΔ/Δ lungs caused cystic airway malformations. Interruption of the TβRII locus in the lung mesenchyme increased mRNA for Patched and Gli-1, two downstream targets of Shh signaling, without alterations in Shh ligand levels produced in the epithelium. Therefore, we conclude that TβRII-mediated signaling in the lung mesenchyme modulates transduction of Shh signaling that originates from the epithelium. To our knowledge, this is the first in vivo evidence for a reciprocal and novel mode of cross-communication between Shh and TGF-β pathways during embryonic development.
Transforming growth factor-β (TGF-β)2 ligands are multifunctional signaling proteins that exhibit a wide range of biological activities including regulation of cell proliferation and differentiation. TGF-β initiates its cellular actions by interacting with a heteromeric complex of transmembrane serine/threonine kinase receptors, the type I (TβRI) and type II (TβRII) receptors. The binding of TGF-β ligand to the receptor complex induces phosphorylation of type I by the type II receptors and activates Smads, a family of transcriptional factors that act as intracellular effectors of TGF-β signaling (1). Smad2 and/or Smad3 are phosphorylated in their C-terminal domain upon stimulation by either activin or TGF-β (2). Phosphorylation of Smad2 and Smad3 is accompanied by their association with Smad4 and translocation of the heteromeric complex to the nucleus where they affect transcription of target genes through interaction with promoter-specific transcriptional factors or by direct DNA binding (3).
Genetic manipulations of endogenous TGF-β signaling have revealed their important functions in vertebrate development. Targeted deletion of each of the three ligand isoforms causes severe abnormalities in morphogenesis of various organs including the lung. Tgf-β2-null mice exhibit perinatal mortality and a wide range of developmental abnormalities that include cardiac, lung, craniofacial, limb, spinal column, eye, inner ear, and urogenital defects (4). Tgf-β3 mutants die as neonates due to abnormal lung development and cleft palate (5). Targeted disruption of the Tgf-β1 gene results in abnormalities in the lung, manifested as dilation of the airways (6). Mice with targeted deletion of Smad3 are viable, but develop lung abnormalities akin to emphysema (7). Little is known about the role of other components of the TGF-β pathway and interactions with other signaling molecules in the lung.
Embryonic lung development represents a useful model in which to study complex tissue interactions in organ development. Lung morphogenesis is strictly dependent on cross-talk between two distinct tissues, the endodermal-derived epithelium and the mesodermal-derived lung mesenchyme (8). A major signaling pathway in this communication is Shh, the vertebrate homologue of Drosophila hh, which is highly expressed by embryonic lung epithelium. Patched (Ptc), the receptor for Shh is expressed by the lung mesenchyme, the site of highly focalized Fgf10 production. The role of Fgf10 in directing epithelial morphogenesis is central to lung development (9–12). In Fgf10(-/-) embryos, the lung tissue below the main stem bronchi is entirely absent (13, 14). Shh interacts with the Ptc/Smoothened (Smo) complex on the mesenchymal cell membrane and activates Gli-3, a 190-kDa transcription factor (15, 16). Activated Gli-3 binds directly to the Gli-1 promoter and induces its transcription in response to Shh (17). Gli-1 is a zinc transcription factor that activates the transcription of Ptc (18). Thus, increased transcription of Gli-1 & Ptc are reliable markers of Shh pathway activation. All three Gli family members are expressed in and are important for lung development (18, 19). The currently accepted model is that Shh both stimulates and restricts the level and spatial distribution of Fgf10 expression during lung morphogenesis. Consistent with this concept, deletion of Shh leads to diffused, but expanded Fgf10 mRNA throughout the mesenchyme as a consequence of which airways develop into large cystic structures (20). Precisely how Shh controls Fgf10 gene expression has hitherto remained unknown.
Because of its established role as a negative regulator of lung branching morphogenesis (21) TGF-β is a potential mediator in epithelial-mesenchymal cross-talk during lung development. Conventional deletion of TβRII lead to early embryonic lethality, and therefore was not informative for lung development (22). In the present study, we used a mesodermal-specific cre-loxP system to delete exon 2 in the TβRII locus in the lung mesenchyme. The lungs of TβRIIΔ/Δ mouse fetuses are abnormal with evidence of cystic airway malformations associated with alterations in Fgf10 gene expression, likely due to interruption of normal epithelial-mesenchymal cross-talk. Inactivation of TβRII and hence the specific TGF-β signaling pathway mediated through its normal activity in the lung mesenchyme results in alterations in Ptc and Gli mRNAs in the mutant lungs indicating interference with Shh signaling. Thus, TGF-β signaling, mediated via TβRII can modulate mesenchymal reception of Shh signaling, which originates from the epithelium, indicating cross-communication between the two signaling pathways during embryonic lung morphogenesis.
MATERIALS AND METHODS
Animals—Dermo1-cre, Rosa26-lacZ, and TβRIIfl/fl mice were generated and genotyped as previously described (23–25) and maintained on C57BL/6 genetic background. Dermo1-cre; Rosa26-lacZ mice were generated by crossing Dermo1- and Rosa26-lacZ mice. To generate TβRIIfl/fl; Dermo1-cre embryos (TβRIIΔ/Δ), TβRIIfl/+; Derom1-cre mice were crossed with TβRIIfl/fl mice.
Detection of β-Galactosidase (LacZ) Activity—LacZ activity was determined by X-gal staining as described (26). Whole mount staining was performed for embryonic lungs. Lungs of E15.5 and older were fixed and sectioned by cryostat, and the frozen sections were stained for LacZ activity.
Immunohistochemistry—For immunohistochemistry, samples were fixed in 4% paraformaldehyde and processed into serial paraffin sections using routine procedures. Immunostaining were performed as previously described (27). Primary antibodies that were used are: α-SMA (Sigma), PAI-1 (Abcam Cambridge, MA), PECAM (BD Pharmingen, San Diego, CA), Flk1 (Cell Signaling Technology, Beverly, MA), β-Tubulin (Biogenix, San Ramon, CA), Collagen1 (Abcam, Cambridge, MA), and TβRII (Abcam Cambridge, MA).
Western Blot Analysis—Protein extracts were prepared from E15.5 Dermo1-cre; TβRIIfl/fl and TβRIIΔ/Δ lungs in RIPA buffer (Sigma) from homogenizer, and then separated on 4–12% NuPAGE gels (Invitrogen). Proteins were then transferred onto Immobilon-P transfer membrane (Millipore Corp.). Membranes were probed with antibodies to TβRII (Abcam) and analyzed with the ECL Western blot analysis system as described by the manufacturer (GE Health, Memphis, TN).
In Situ Hybridization—Whole mount and section in situ hybridization were performed as previously described (27, 28). The digoxigenin-labeled RNA antisense and sense probes were prepared from following cDNA templates: a 0.4-kb fragment of SpC, a 1.4-kb fragment of Nkx2.1 (29), a 0.5-kb fragment of Foxj1, a 0.6-kb fragment of Shh (Dr. Andrew P. McMahon, Harvard University), a 0.7-kb fragment of Ptc (Dr. Matthew P. Scott, Stanford University), a 0.7-kb fragment of Gli-1, a 0.7-kb fragment of Foxf1 (Dr. Peter Carlsson, Göteborg University), a 0.4-kb fragment of Fgf10, a 1.3-kb fragment of Tbx4, and a 1-kb fragment of Tbx5(Dr. Virginia Papaioannou, Princeton University).
RNA Extraction, Polymerase Chain Reaction (PCR), and Northern Blotting—Total RNA was isolated from embryonic lungs and MRC5 cells (ATCC) by using TRIzol (Invitrogen). Superscript First-Strand Synthesis System kit (Invitrogen) was used to generate cDNA. Quantification of the selected genes by real-time PCR was performed using a LightCycler (Roche Applied Sciences) as previously described (30). Sequence of the primers were as follows: Mouse TβrII:5′-ATG CAT CCA TCC ACG TAA G-3′(forward), 5′-GAC ACG GTA GCA GTA GAA GA-3′ (reverse); human TβrII:5′-CAC GTT CAG AAG TCG GAT GT-3′(forward), 5′-CAT CAG AGC TAC AGG AAC AC-3′(reverse); Mouse TβrII(qPCR): 5′-CAT GAA AGA CAG TGT GCT GAG A-3′(forward), 5′-CTC ACA CAC GAT CTG GAT GC-3′(reverse); Mouse Foxf1:5′-AGC ATC TCC ACG CAC TCC-3′(forward), 5′-TGT GAG TGA TAC CGA GGG ATG-3′(reverse); Mouse Tbx4:5′-GCA TGA GAA GGA GCT GTG G-3′(forward), 5′-TTA CCT TGT AGC TGG GGA ACA-3′(reverse); Human PAI-1: 5′-AAC GGC CAG TGG AAG ACT C-3′(forward), 5′-GGG CGT GGT GAA CTC AGT AT-3′(reverse); Human Ptc:5′-AAC AAA AAT TCA ACC AAA CCT C-3′(forward), 5′-TGT CCT CGT TCC AGT TGA TGT G-3′(reverse); Human Gli-1:5′-CAG GGA GGA AAG CAG ACT GA-3′(forward), 5′-ACT GCT GCA GGA TGA CTG G-3′(reverse); Human Fgf10:5′-CGG GAC CAA GAA GGA GAA CT-3′(forward), 5′-ACG GCA ACA ACT CCG ATT-3′(reverse); Human GAPDH:5′-GAA GGT GAA GGT CGG AGT C-3′(forward), 5′-GAA GAT GGT GAT GGG ATT TC-3′(reverse). Ten micrograms of total RNA were electrophoresed in 1% RNA formaldehyde-agarose gel and blotted. Blots were hybridized with probes specific for TβRII, Shh, Ptc, Gli-1, Fgf10, Gapdh, and then autoradiographed or measured with Kodak Molecular Imaging Software Ver 4.0 to determine the quantity. 32P-labeled probes were synthesized from the following cDNA fragments: the TβRII probe was from a 0.4-kb PCR product of TβRII coding region. The Shh probe was from a 0.6-kb cDNA (Dr. Brigid L. M. Hogan, Vanderbilt University). The Ptc probe was from a 0.7-kb cDNA (Dr. Matthew P. Scott, Stanford University), The Gli-1 probe was from a 0.7-kb cDNA. The Fgf10 probe was from a 0.4-kb cDNA. The GAPDH probe was as reported before (31).
Cell Culture and TGF-β Treatment—Human pulmonary mesenchymal cell line MRC5 (ATCC, Manassas, VA) was maintained in EMEM medium (ATCC), containing 10% fetal bovine serum and 1% penicillin-streptomycin. MRC5 cells were treated with recombinant TGF-β (R&D systems, Minneapolis, MN) at 200 ng/ml for 2 h and then collected for RNA extraction.
RESULTS
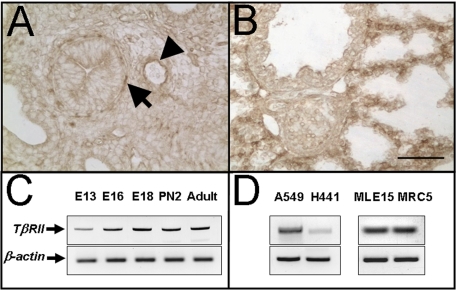
TβRII Expression during Lung Development—TβRII has been reported to be expressed in both the epithelium and the mesenchyme of the lung (32). To elucidate the expression pattern of TβRII in the murine lung, we used semi-quantitative RT-PCR and immunohistochemistry. Expression of TβRII mRNA can be detected by RT-PCR throughout lung development (Fig. 1 C). Commercially available antibody (Abcam) detects TβRII protein in mesenchymal cells localized around the airways (Fig. 1, arrows) and blood vessels (Fig. 1, arrowhead) in E15.5 lungs (Fig. 1A) and throughout the mesenchyme in E18 lungs (Fig. 1B). TβRII mRNA is clearly expressed in human lung epithelial carcinoma, H441 and A549 cell lines as well as mouse-immortalized epithelial MLE15 cells (Fig. 1D).
FIGURE 1.
TβRII expression in the murine lung. A and B show immunolocalization of TβRII protein in lungs from E15.5 and E18.5 embryos, respectively. The arrow shows TβRII protein localized in the subepithelial layer of proximal airways. The arrowhead points to endothelial localized TβRII around the blood vessel. B shows immunolocalized TβRII distributed throughout the parenchyma of E18.5 lungs, with highest expression in the interstitium, including cells of mesenchymal origin. C, semi-quantitative RT-PCR of TβRII mRNA during murine lung morphogenesis. E, embryonic day; PN, postnatal day. D, TβRII mRNA is also detectable in both human (A549 and H449) and murine (MLE15) transformed epithelial cell lines. MRC5 is a fetal human mesenchymal cell line. Scale bar, 100 μm.
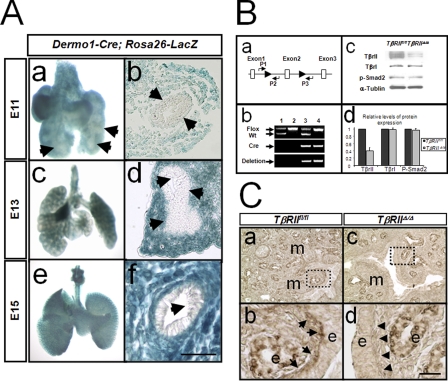
Recombination Mediated by Dermo1-cre in the Lung Mesenchyme—Recombination driven by Dermo1-cre in the lung was analyzed by crossing with the reporter mouse strain Rosa26-LacZ. Double transgenic, Rosa26-LacZ; Dermo1-cre fetuses were identified by PCR genotyping, and the lungs were excised and stained for LacZ. As shown in Fig. 2A, expression of Dermo1-cre resulted in nearly 100% recombination of the loxP sites in Rosa26-lacZ lung mesodermal cells (Fig. 2A, panels a–f). This recombination was entirely mesenchymal-specific in that no epithelial cells showed LacZ staining (Fig. 2A, panels a, b, d, and f, arrows).
FIGURE 2.
Generation and validation of TβRII conditional knock-out alleles. A, mesenchymal specific recombination induced by Dermo1-cre as determined by LacZ assay in murine lungs from various stages of embryonic development.Panelsa,c, and e are whole mount lung tissue. Panels b, d, and f are frozen saggital sections of the lungs in panels a, c, and e. Arrows in panels a, b, d, and f show the absence of LacZ in airway epithelial cells. Scale bar,50μm. B, evidence for deletion of TβRII gene by Dermo1-cre. Panel a, map of the mouse TβRII locus showing the relative position of the lox-P (flox) sequences and the primers used in identifying various genotypes. Primers for Cre were used as described by Yu et al. (23). Panel b, PCR analysis of TβRII gene using lung-extracted DNA and the primers P1, P2, and P3, as shown in panel a. Lane 1, TβRIIfl/+ lungs, showing two bands corresponding to the wild type (Wt) and the allele carrying the lox-P insertion. The absence of a PCR product using P1/P3 primers is due to the large distance between the two primers (absence of deletion). Lane2, TβRIIfl/fl lungs showing a single band corresponding to floxed allele. Lane 3, TβRIIΔ/+ lungs. Deletion of Exon 2 brings the sequences recognized by P1 and P3 primers sufficiently close to allow amplification of a PCR product (deletion). Lane 4, TβRIIΔ/Δ lungs. Panel c, Western blot analysis of TβRII, TβRI, and p-Smad2 in total lung protein from TβRIIfl/fl (lane 1) and TβRIIΔ/Δ (lane 2) mice. Panel d, densitometric quantification of the Western blot shown in panel c. C, immunohistochemical analysis for PAI-1 in TβRIIΔ/Δ and TβRIIfl/fl lungs. Arrowheads in panel d show the reduced expression of PAI-1 in the PBSMCs of mutant lungs compared with arrows in panel b (control). m, mesenchyme; e, epithelium. Scale bar, 100μm for panels a and c,23μm for panels b and d.
Inactivation of TβRII by Dermo1-cre-driven Recombination—Conventional deletion of TβRII led to early embryonic lethality (22). Thus, we used a conditional Cre-LoxP approach to specifically delete exon 2 in the TβRII locus in mesodermal lineages, including those of the lung. Accordingly, we crossed TβRIIfl/fl females with Dermo1-cre male mice and backcrossed the heterozygotes to generate TβRIIfl/fl; Dermo1-cre progeny. The latter genotype is referred to simply as TβRIIΔ/Δ. To validate Dermo1-cre-induced recombination and deletion of exon 2 within the TβRII gene, we used three approaches. First, we utilized lung DNA with PCR primers that could distinguish between deleted and intact TβRII alleles. The deletion in TβRII gene was verified by genomic PCR analysis shown in Fig. 2B, panel b. Second, we measured TβRII protein content by Western blot analysis in protein extracts of total lung tissue from control and TβRIIΔ/Δ embryos. These studies showed greater than 60% decrease in total lung TβRII protein, the remainder indicating non-mesenchymal (e.g. epithelial and endothelial) TβRII protein (Fig. 2B, panels c and d). In contrast, the protein level of TβRI and phosphorylated Smad2 (p-Smad2) remained the same. Therefore mesenchymal deletion of TβRII in the lung does not alter the level of phosphorylated Smad2 and TβRI. Finally, functional deletion of TβRII was verified by immunohistochemistry using antibodies to Plasminogen Activator Inhibitor (PAI-1), which is a downstream target of TGF-β signaling. In the control lungs, diffuse PAI-1 was detected throughout both the epithelium and the mesenchyme, but particularly in the progenitor of parabronchial smooth muscle cells (PBSMC) in the subepithelial mesoderm (Fig. 2C, panel b, arrows). In contrast, PAI-1 in the TβRIIΔ/Δ lungs was found only in the epithelium. As expected, there was virtually no PAI-1 staining in the mesoderm, nor in the PBSMC progenitors in TβRIIΔ/Δ lungs (Fig. 2C, panel d, arrowheads).
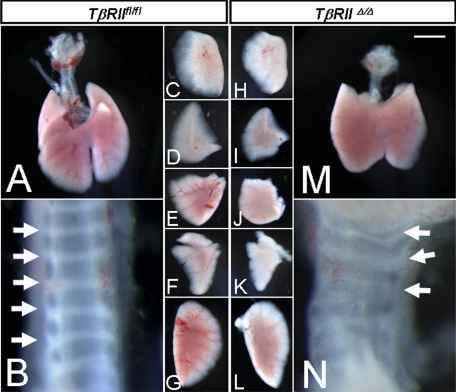
Ultrastructure and Cellular Differentiation in TβRIIΔ/Δ Lungs—Dermo1-cre-induced deletion of exon 2 within the TβRII gene was embryonically lethal by day 16–17 of gestation. E16–17 fetuses developed gross abnormalities in multiple organs that caused fetal demise. We therefore, collected and characterized lungs from TβRIIΔ/Δ E15.5 fetuses. Gross morphological assessment of the proximal lung structure showed a readily discernible phenotype manifested as disorganized formation of tracheal cartilage (Fig. 3N). Both the number as well as the shape of the tracheal cartilage was altered. The first and the second generation bronchi in the mutant lungs were devoid of cartilage altogether (not shown).
FIGURE 3.
Gross morphology of TβRIIΔ/Δ and TβRIIfl/fl trachea and lungs from E15.5 embryos. B and N, tracheas. Arrows show abnormal number and shape of tracheal cartilage. C and H, right apical lobes. D and I, right middle lobes. E and J, right caudal lobes. F and K, accessory lobes. G and L, left lobes. Scale bar, 1.0 mm for A and M; 0.2 mm for B and N; 0.8 mm for C–F, H–K, and 1.2 mm for G and L.
Mesodermal deletion of TβRII also impacted the shape and the size of the various lung lobes as shown in Fig. 3. However, the overall process of lobation and the number of lobes were normal (Fig. 3, H–L). Histological assessment showed a distinct phenotype in TβRIIΔ/Δ lungs, characterized by the presence of large, dilated airways, lined with columnar epithelial cells in the proximal lung (Fig. 4A).
FIGURE 4.
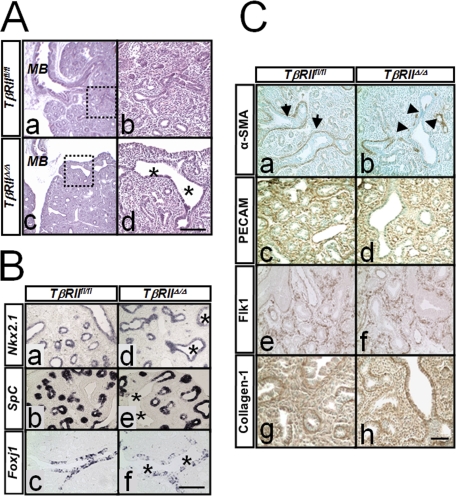
H & E staining and immunohistochemical analysis for lung developmental markers in TβRIIΔ/Δ and control lungs. A, gross histology of lungs from TβRIIfl/fl (panels a and b) and TβRIIΔ/Δ (panels c and d) E15.5 embryos. Saggital sections of lungs were analyzed by H & E staining. MB, mainstem bronchus. Asterisks show dilated proximal airways. Panels b and d are high magnification of areas within dotted squares in panels a and c, respectively. Scale bar, 330 μm for panels a and c; 100 μm for panels b and d. B, cell differentiation in TβRIIΔ/Δ embryonic lungs. In situ hybridization for Nkx2.1 (panels a and d), SpC (panels b and e), and Foxj1 (panels c and f). Asterisks, dilated airways. Scale bar, 100 μm. C, immunohistochemical analysis for α-SMA (panels a and b), PECAM (panels c and d), Flk1 (panels e and f), and Collagen1 (panels g and h) in E15.5 TβRIIfl/fl and TβRIIΔ/Δ lungs. Arrowheads in panel b show reduced expression of α-SMA surrounding the dilated airways of mutant lungs compared with arrows in panel a (control). Scale bar, 100 μm.
The epithelial cells throughout the TβRIIΔ/Δ lungs showed expression of NKX2.1, a transcription factor associated with onset of lung epithelial morphogenesis and normal lung structural development(Fig. 4B, panels a and d) (33). Transcripts for Surfactant Protein C, SPC, a marker of distal epithelial cells and a target of NKX2.1 was also expressed in the mutant lungs. Also, differentiation of ciliated cells as evidenced by expression of Foxj1 appeared to be normal in the absence of epithelial TβRII activity (Fig. 4B). The normal level and distribution of Surfactant Protein B, SPB, expressed in proximal and distal, differentiated epithelium, as well as β-tubulin, an established marker of airway ciliated cells were also observed by immunohistochemistry (data not show). Collectively, these data indicate that the absence of TβRII in the lung mesenchyme does not alter lung epithelial cell identity and differentiation.
Expression of TGF-β Targets in TβRIIΔ/Δ Lungs—In wild-type embryonic lungs, α-smooth muscle actin-positive cells are found as rings of smooth muscle progenitor cells surrounding the columnar epithelium of the proximal airways (Fig. 4C, panel a). In contrast, we found reduced α-SMA-positive cells surrounding the dilated airways in the mutant lungs (Fig. 4C, panel b). This is the same layer of smooth muscle cells in which PAI-1 was found to be abundantly expressed in the wild-type lung and drastically reduced in TβRIIΔ/Δ lungs (Fig. 2C). Immunohistochemistry was also performed to determine potential changes in the expression or spatial localization of other mesenchymal genes including collagen type I and platelet/endothelial cell adhesion molecule (PECAM) known to be modulated by TGF-β (6, 34). Reduced levels of PECAM were found in the mutant lungs (Fig. 4C, compare panels c and d). Another marker of endothelial cell differentiation, Flk1 appeared to have the same level and distribution in the mutant lungs as wild type (Fig. 4C, panels e and f). Collagen production is also under TGF-β control (6). Both in the wild-type control and the mutant lungs, collagen Type I was localized to the extracellular matrix surrounding both the distal and the proximal airways (Fig. 4C, panels g and h). No drastic alteration in collagen type I was observed in TβRIIΔ/Δ lungs suggesting that either TGF-β is signaling through utilization of type I receptor (homotetramer) or that collagen production and deposition may not be entirely TGF-β-dependent.
Expression of Fgf10 in TβRIIΔ/Δ Lungs—Whole mount and section in situ hybridization were performed for a number of genes with established roles during lung morphogenesis. Because the shape and size of the airways can be regulated by FGF10 activity and its expression domain, we investigated the expression pattern of Fgf10 in TβRIIΔ/Δ lungs. Whole mount in situ hybridization revealed increased expression, and expansion of the Fgf10 domain in the mutant embryonic lungs (Fig. 5A, panels e and f). A clearer demonstration of this alteration was observed by in situ hybridization experiments on tissue sections (Figure 5A, compare panels c and g). In the control lungs, Fgf10 mRNA was localized to mesenchymal cells adjacent to the growing tip of the peripheral airways (Fig. 5A, panels c and d) consistent with previously reported results (12). In the mutant lungs, however, we found an expanded Fgf10 expression domain and likely increased mRNA in the peripheral mesenchyme adjacent to the branching airways (Fig. 5A, panels e–h). This alteration in Fgf10 expression domain may explain the phenotype of the TβRIIΔ/Δ lungs in which airways are significantly dilated. Two transcription factors, Foxf1 and Tbx4 have been found to stimulate Fgf10 expression in the lung mesenchyme (35, 36). Consistent with this finding, in situ hybridization on tissue sections (Fig. 5B, panels a, b, d, and e) and real-time PCR (Fig. 5C) revealed increased transcripts for both in TβRIIΔ/Δ lungs compared with controls. Expression of another Tbx gene, Tbx5 remained unchanged (Fig. 5B, panels c and f). These data support the finding that Fgf10 is both increased and expanded in its expression domain in TβRIIΔ/Δ lungs.
FIGURE 5.
In situ hybridization analysis of Fgf10 and its upstream transcriptional regulators in TβRIIΔ/Δ and control lungs. A, whole mount (panels a, b, e, and f) and section (panels c, d, g, and h) in situ hybridization. Note expansion and increased Fgf10 expression in TβRIIΔ/Δ (panels e–h) compared with TβRIIfl/fl (panels a–d) lungs. Scale bar, 2.2 mm (panels a and c); 1 mm (panels b and d); 100 μm (panels e–h). B, section in situ hybridization for Foxf1 (panels a and d), Tbx5 (panels b and e), and Tbx4 (panels c and f) in TβRIIΔ/Δ and TβRIIfl/fl (control) E15.5 lungs. Scale bar, 100 μm. C, quantification of Foxf1 and Tbx4 mRNA by real-time PCR. Values are fold induction or repression, compared with TβRIIfl/fl controls (arbitrarily adjusted to 1). p value, 0.004.
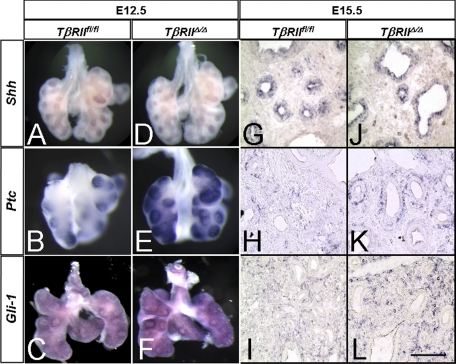
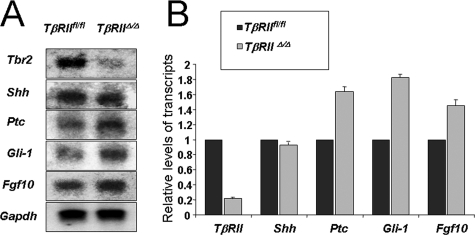
Cross-talk between TGF-β and Shh Pathways—Shh is thought to negatively regulate Fgf10 magnitude and distribution, thereby, controlling lung branching morphogenesis (20, 37). The mechanistic connection between Fgf10 and Shh remained unknown. We therefore examined Shh signaling by investigating the level and distribution of Shh and its downstream targets Ptc and Gli-1 in the mutant lungs compared with the TβRIIfl/fl controls. Whole mount and tissue section in situ hybridization showed similar levels and spatial localization of Shh mRNA in the control and mutant lungs (Fig. 6, A, D, G, and J). In contrast, there was a significant increase in the level of mRNA for both Ptc (Fig. 6, B, E, H, and K) and Gli-1 (C, F, I, and L) in the TβRIIΔ/Δ mutant lungs compared with the control. To validate the above in situ hybridization results, we used Northern blot analysis as shown in Fig. 7. Transcripts for TβRII were decreased by nearly 80% in TβRIIΔ/Δ lung tissue, compared with controls. Other changes included a ∼1.8-fold increase in Ptc and Gli-1 mRNA and a 1.5-fold increase in Fgf10. The magnitude of Shh remained nearly the same in the mutant and control lungs confirming the in situ hybridization findings.
FIGURE 6.
Whole mount and section in situ hybridization for components of the Shh pathway in TβRIIfl/fl and TβRIIΔ/Δ E12.5 (A–F) and E15.5 (G–L) lungs. Abundance of mRNA for both Ptc and Gli-1 is increased in the mutant lungs (E, F, K, and L) compared with the control lungs (B, C, H, and I). Little if any change is detectable in the epithelial-localized Shh (A, D, G, and J). Scale bar, 2.2 mm (A, B, E, F, I, J); 100 μm (C, D, G, H, K, L).
FIGURE 7.
Quantification of mRNA changes in TβRIIΔ/Δ lungs. Total RNA from E15.5 TβRIIΔ/Δ and TβRIIfl/fl (control) embryonic lungs was used for Northern blot analysis (A), and the results were quantified by densitometry and normalized by GAPDH (B). Values are fold induction or repression, compared with TβRIIfl/fl controls (arbitrarily adjusted to 1).
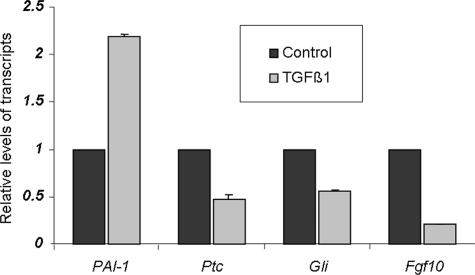
Because deletion of TβRII occurs specifically in the lung mesenchyme, one potential hypothesis is that TGF-β signaling through TβRII normally represses Gli-1 and Ptc mRNA levels in the lung mesenchymal cell layer. To determine the validity of this hypothesis, we used cultured MRC5 cells, which are derived from normal lung mesenchymal cells of a 14-week-old male fetus, and assessed the impact of exogenous TGF-β treatment on Gli-1 and Ptc mRNA levels by real-time PCR. In support of our in vivo observations, the results clearly showed that TGF-β represses steady state levels of both Gli-1 and Ptc mRNAs by nearly 50% (Fig. 8). Fgf10 has been proposed to be a target of Shh signaling in the lung (36) although this regulation has not been experimentally explored or demonstrated. In MRC5 cells, TGF-β treatment repressed Fgf10 mRNA consistent with our in vivo observations in Fig. 5A. Thus, these in vitro results validate our in vivo-based conclusions and support a model for interactions between TGF-β and Shh signaling pathways (Fig. 9).
FIGURE 8.
In vitro verification of the in vivo evidence that TGF-β is a negative regulator of Shh pathway. MRC5 cells were treated with either 200 ng/ml of TGF-β1 or equivalent bovine serum albumin (control). Real-time PCR-quantified mRNA for PAI-1 (positive control), Ptc and Gli-1. Values are fold induction or repression, compared with controls (adjusted to 1).
FIGURE 9.
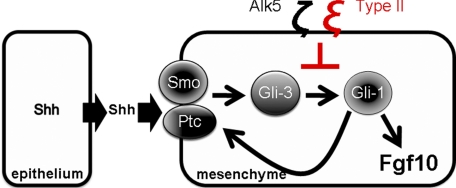
A hypothetical model for TGF-β∼Shh cross-talk in the developing lung. Shh is made in the lung epithelium. The Shh receptor complex, Ptc/smoothened is expressed on the cell surface of lung mesenchymal cells. Activation of Shh pathway leads to increased Gli-3 activity, which in turn stimulates Gli-1 and Ptc transcription. TGF-β ligand acts through TβRII and as yet unknown intracellular mediators (e.g. Smads) to interfere with Shh signal transduction in mesenchymal cells, evidenced by reductions in both Ptc and Gli-1 observed in this study.
DISCUSSION
The purpose of the current study was to examine the consequences of interruption in mesenchymal TβRII-mediated TGF-β signaling on lung morphogenesis. To this end, we generated mice carrying mesodermal-specific deletion of the TβRII exon 3 by Dermo1-cre-driven recombination. TβRIIΔ/Δ fetuses died in utero due to multi-organ abnormalities. Examination of embryonic lungs revealed that mesenchymal abrogation of endogenous TGF-β signaling caused abnormalities in epithelial morphogenesis, indicating disruption of normal epithelial-mesenchymal communication that is central to lung development. Analysis of key mediators of this cross-talk showed alterations in components of the Shh pathway. Both Ptc and Gli-1 as well as the transcription factor Foxf1 were increased in the mesenchyme of the mutant lungs. Shh mRNA in the lung epithelium was unchanged. Thus, abrogation of TβRII-mediated signaling in the lung mesenchyme results in increased expression of Shh downstream targets (i.e. Shh signaling). To our knowledge, this is the first demonstration of cross-communication between the TGF-β and the Shh pathways during vertebrate embryonic development.
At least two major phenotypic abnormalities were readily observable in TβRII mutant lungs. First, the trachea of the mutant lungs showed abnormalities in both structure and number of cartilage. Both TGF-β and Shh are known to be involved in the induction of early cartilaginous differentiation of mesenchymal cells in the limb and in the spine. In vitro, treatment of human bone marrow-derived mesenchymal stem cells (MSCs) with either TGF-β or recombinant Shh induced expression of cartilage markers aggrecan, Sox9, CEP-68, and collagen type II and X (38). Thus, the abnormalities observed in tracheal cartilage formation in TβRIIΔ/Δ lungs can be explained as either a direct result of mesenchymal TβRII deletion or indirect effect of interruption in Shh signaling.
Mesodermal inactivation of TβRII also caused abnormal epithelial morphogenesis manifested as cystic, dilated bronchi (Figure 4A). Lung branching morphogenesis is strictly dependent on epithelial-mesenchymal communication between the foregut endoderm and the mesodermal-derived splanchnic mesenchyme. The lung mesenchyme, via Fgf10 provides instructional signaling that directs epithelial morphogenesis. Targeted deletion of Fgf10 results in profoundly abnormal lungs that lack structures distal to the main stem bronchi (13, 14). Dynamic changes in the magnitude and spatial distribution of Fgf10 are critical to normal branching morphogenesis and are thought to be controlled by diffusible signals originating from the epithelial cells themselves. Shh is expressed in the distal epithelium, from where it activates signaling in the corresponding mesenchyme via its receptor, the patched (Ptch1)/smoothened (Smo) complex and their transcriptional effectors Gli-1, Gli-2, and Gli-3 (9, 11). Results from both in vitro and in vivo studies indicate that Shh negatively regulates Fgf10 production in the lung mesenchyme (20, 37). Thus, a current model of embryonic lung development is centered on epithelial Shh as playing a dual role in both activating and limiting mesenchymal signaling thereby “fine-tuning” the process of branching morphogenesis. In Shh(-/-) lungs, Fgf10 mRNA is found diffusely throughout the mesenchyme, as a consequence of which the airways develop into large cystic structures and branching morphogenesis is severely disrupted (20). Although not as severe, the phenotypically abnormal, cystic bronchi observed in TβRII mutant lungs are similar to those observed in Shh(-/-) lungs (Fig. 4A). In situ hybridization revealed a clear expansion of the Fgf10 domain in E15.5 TβRIIΔ/Δ lungs (Fig. 5A). Consistent with these results, Foxf1 and Tbx4, two transcription factors that stimulate Fgf10 production in the lung mesenchyme (35, 36), were also increased in the TβRIIΔ/Δ lungs (Fig. 5, B and C).
An abnormal characteristic of the cystic bronchial airways in TβRII lungs was the absence of PBSMCs (Fig. 4C). During development and in adult tissues, mesenchymal cells serve as precursors to diverse cell lineages, including PBSMCs. The function of TGF-β in promoting myofibroblast differentiation is well recognized (39, 40). Therefore, the absence of PBSMCs may be a direct impact of mesenchymal TβRII inactivation, suggesting that TGF-β signaling via the Type II receptor is required for PBSMC differentiation. The relatively high level of endogenous TβRII protein and the TGF-β target, PAI-1 in the PBSMCs (Figs. 1 and 2C) and drastic reduction of PAI-1 in response to mesodermal deletion of TβRII in these cells in the mutant lungs (Fig. 2C, panel d) supports the above hypothesis. In addition however, we showed previously that PBSMC progenitors express Fgf10, and the transcription factor Pitx2, both of which may be important in maintaining their undifferentiated status (41). These proliferating cells cease to express Fgf10 before onset of differentiation into PBSMCs (42). Thus, increased levels, or spatial expansion of Fgf10 distribution in TβRII mutants, may provide another, alternative mechanistic explanation for the absence of PBSMCs in the dilated bronchial airways; high levels of Fgf10 may inhibit PBSMC differentiation.
TGF-β signaling is inhibitory to branching morphogenesis (21). Activation of TGF-β in mesenchymal cells markedly inhibits Fgf10 expression (37, 43, 44). Positive transcriptional regulators of Fgf10, Tbx4, and Foxf1 are controlled by Shh signaling that emanates from the branching epithelium. Recently, potential interactions between Shh and TGF-β pathways have been examined in in vitro settings. Shh promotes motility and invasiveness of gastric cancer cells through TGF-β-mediated activation of the ALK5-Smad3 pathway (45). In contrast to our findings, TGF-β was shown to induce Gli-1 and Gli-2 expression in various human cell lines (46). Also, in transgenic mice overexpressing TGF-β1 in the skin, Gli-1 and Gli-2 were elevated in a Smad3-dependent manner (46). This apparent discrepancy may suggest both cell type (skin versus lung) and dose-dependent specificity of TGF-β effect on adult and embryonic tissue. The relationship between TGF-β and Shh during organogenesis, the focus of the present study had remained unknown. In particular whether the inhibition of Fgf10 in the lung mesenchyme in response to TGF-β involves the Shh pathway remained unknown. We found increased expression of Ptc and Gli-1, as well as the transcription factors Tbx4 and Foxf1 in the mesenchyme of TβRII mutant lungs, suggesting that endogenous TGF-β signaling via the type II receptor in the wild-type lung regulates Shh signal transduction in the mesenchyme. In previous studies, we found that Wnt5a alters Shh signaling by modulating Shh mRNA in the lung epithelium (30). In contrast, increased Shh signaling in the mesenchyme in response to TβRII inactivation occurrs in the absence of changes in epithelial Shh mRNA (Figs. 6 and 7). Therefore, TGF-β does not directly interfere with Shh originating from the lung epithelium, but dampens its transduction within the target tissue, the lung mesenchyme. This interference by TGF-β may provide a potential mechanism by which Shh limits Fgf10 expression domain in the lung mesenchyme during branching morphogenesis. In vitro studies using MRC5 cells, showed that treatment with TGF-β reduces the steady state level of Fgf10, concomitant with decreases in Ptc and Gli-1 mRNAs (Fig. 8) thereby validating the in vivo observations. Based on the collective in vivo and in vitro findings, a hypothetical model depicting the mechanisms of TGF-β∼Shh cross-talk is proposed in Fig. 9. In the wild-type lung, TGF-β fine-tunes Shh signaling through modulation of its downstream targets, Gli-1 and Ptc, which in turn may control the magnitude and spatial distribution Fgf10. In this manner, TGF-β signaling via TβRII participates in the response of mesenchymal cells to Shh signaling that originates from the lung epithelium.
Acknowledgments
We thank Dr. Yang Chai for providing the homozygous TβRII mutant mice, Dr. Andrew P. McMahon for Shh cDNA, Dr. Matthew P. Scott for Ptc cDNA, Dr. Peter Carlsson for Foxf1 cDNA, and Dr. Virginia Papaioannou for Tbx4 and Tbx5 cDNA.
This work was supported, in whole or in part, by NHLBI, National Institutes of Health. This work was also supported by the Hastings Foundation. The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The abbreviations used are: TGF, transforming growth factor; X-gal, 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside; Shh, Sonic hedgehog; PBSMC, parabronchial smooth muscle cells; GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
References
- 1.Massague, J. (1998) Annu. Rev. Biochem. 67 753-791 [DOI] [PubMed] [Google Scholar]
- 2.Souchelnytskyi, S., Tamaki, K., Engstrom, U., Wernstedt, C., ten Dijke, P., and Heldin, C. H. (1997) J. Biol. Chem. 272 28107-28115 [DOI] [PubMed] [Google Scholar]
- 3.Wrana, J. L. (2000) Sci STKE 2000, 2000 RE1. [DOI] [PubMed] [Google Scholar]
- 4.Sanford, L. P., Ormsby, I., Gittenberger-de Groot, A. C., Sariola, H., Friedman, R., Boivin, G. P., Cardell, E. L., and Doetschman, T. (1997) Development 124 2659-2670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kaartinen, V., Voncken, J. W., Shuler, C., Warburton, D., Bu, D., Heisterkamp, N., and Groffen, J. (1995) Nat. Genet. 11 415-421 [DOI] [PubMed] [Google Scholar]
- 6.Zhou, L., Dey, C. R., Wert, S. E., and Whitsett, J. A. (1996) Dev. Biol. 175 227-238 [DOI] [PubMed] [Google Scholar]
- 7.Chen, H., Sun, J., Buckley, S., Chen, C., Warburton, D., Wang, X. F., and Shi, W. (2005) Am. J. Physiol. Lung Cell Mol. Physiol. 288 L683-L691 [DOI] [PubMed] [Google Scholar]
- 8.Shannon, J. M., and Hyatt, B. A. (2004) Annu. Rev. Physiol. 66 625-645 [DOI] [PubMed] [Google Scholar]
- 9.Tabin, C. J., and McMahon, A. P. (1997) Trends Cell Biol. 7 442-446 [DOI] [PubMed] [Google Scholar]
- 10.Miller, L. A., Wert, S. E., and Whitsett, J. A. (2001) J. Histochem. Cytochem. 49 1593-1604 [DOI] [PubMed] [Google Scholar]
- 11.Bellusci, S., Furuta, Y., Rush, M. G., Henderson, R., Winnier, G., and Hogan, B. L. (1997) Development 124 53-63 [DOI] [PubMed] [Google Scholar]
- 12.Bellusci, S., Grindley, J., Emoto, H., Itoh, N., and Hogan, B. L. (1997) Development 124 4867-4878 [DOI] [PubMed] [Google Scholar]
- 13.Sekine, K., Ohuchi, H., Fujiwara, M., Yamasaki, M., Yoshizawa, T., Sato, T., Yagishita, N., Matsui, D., Koga, Y., Itoh, N., and Kato, S. (1999) Nat. Genet. 21 138-141 [DOI] [PubMed] [Google Scholar]
- 14.Min, H., Danilenko, D. M., Scully, S. A., Bolon, B., Ring, B. D., Tarpley, J. E., DeRose, M., and Simonet, W. S. (1998) Genes Dev. 12 3156-3161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ingham, P. W. (1995) Curr. Opin. Genet. Dev. 5 492-498 [DOI] [PubMed] [Google Scholar]
- 16.McMahon, A. P. (2000) Cell 100 185-188 [DOI] [PubMed] [Google Scholar]
- 17.Dai, P., Akimaru, H., Tanaka, Y., Maekawa, T., Nakafuku, M., and Ishii, S. (1999) J. Biol. Chem. 274 8143-8152 [DOI] [PubMed] [Google Scholar]
- 18.Grindley, J. C., Bellusci, S., Perkins, D., and Hogan, B. L. (1997) Dev. Biol. 188 337-348 [DOI] [PubMed] [Google Scholar]
- 19.Motoyama, J., Liu, J., Mo, R., Ding, Q., Post, M., and Hui, C. C. (1998) Nat. Genet. 20 54-57 [DOI] [PubMed] [Google Scholar]
- 20.Pepicelli, C. V., Lewis, P. M., and McMahon, A. P. (1998) Curr. Biol. 8 1083-1086 [DOI] [PubMed] [Google Scholar]
- 21.Xing, Y., Li, C., Hu, L., Tiozzo, C., Li, M., Chai, Y., Bellusci, S., Anderson, S., and Minoo, P. (2008) Dev. Biol. 320 340-350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oshima, M., Oshima, H., and Taketo, M. M. (1996) Dev. Biol. 179 297-302 [DOI] [PubMed] [Google Scholar]
- 23.Yu, K., Xu, J., Liu, Z., Sosic, D., Shao, J., Olson, E. N., Towler, D. A., and Ornitz, D. M. (2003) Development 130 3063-3074 [DOI] [PubMed] [Google Scholar]
- 24.Soriano, P. (1999) Nat. Genet. 21 70-71 [DOI] [PubMed] [Google Scholar]
- 25.Chytil, A., Magnuson, M. A., Wright, C. V., and Moses, H. L. (2002) Genesis 32 73-75 [DOI] [PubMed] [Google Scholar]
- 26.Pan, Q., Li, C., Xiao, J., Kimura, S., Rubenstein, J., Puelles, L., and Minoo, P. (2004) Gene 331 73-82 [DOI] [PubMed] [Google Scholar]
- 27.Li, C., Xiao, J., Hormi, K., Borok, Z., and Minoo, P. (2002) Dev. Biol. 248 68-81 [DOI] [PubMed] [Google Scholar]
- 28.Li, M., Wu, X., Zhuang, F., Jiang, S., Jiang, M., and Liu, Y. H. (2003) Dev. Dyn. 228 273-280 [DOI] [PubMed] [Google Scholar]
- 29.Minoo, P., Hu, L., Xing, Y., Zhu, N. L., Chen, H., Li, M., Borok, Z., and Li, C. (2007) Mol. Cell. Biol. 27 2155-2165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li, C., Hu, L., Xiao, J., Chen, H., Li, J. T., Bellusci, S., Delanghe, S., and Minoo, P. (2005) Dev. Biol. 287 86-97 [DOI] [PubMed] [Google Scholar]
- 31.Hamdan, H., Liu, H., Li, C., Jones, C., Lee, M., deLemos, R., and Minoo, P. (1998) Biochim. Biophys. Acta 1396 336-348 [DOI] [PubMed] [Google Scholar]
- 32.Zhao, Y., and Shah, D. U. (2000) Exp. Mol. Pathol. 69 67-78 [DOI] [PubMed] [Google Scholar]
- 33.Minoo, P., Su, G., Drum, H., Bringas, P., and Kimura, S. (1999) Dev. Biol. 209 60-71 [DOI] [PubMed] [Google Scholar]
- 34.Zeng, X., Gray, M., Stahlman, M. T., and Whitsett, J. A. (2001) Dev. Dyn. 221 289-301 [DOI] [PubMed] [Google Scholar]
- 35.del Moral, P. M., De Langhe, S. P., Sala, F. G., Veltmaat, J. M., Tefft, D., Wang, K., Warburton, D., and Bellusci, S. (2006) Dev. Biol. 293 77-89 [DOI] [PubMed] [Google Scholar]
- 36.Cardoso, W. V., and Lu, J. (2006) Development 133 1611-1624 [DOI] [PubMed] [Google Scholar]
- 37.Lebeche, D., Malpel, S., and Cardoso, W. V. (1999) Mech. Dev. 86 125-136 [DOI] [PubMed] [Google Scholar]
- 38.Warzecha, J., Gottig, S., Bruning, C., Lindhorst, E., Arabmothlagh, M., and Kurth, A. (2006) J. Orthop. Sci. 11 491-496 [DOI] [PubMed] [Google Scholar]
- 39.Cohen, P., Rajah, R., Rosenbloom, J., and Herrick, D. J. (2000) Am. J. Physiol. Lung Cell Mol. Physiol. 278 L545-L551 [DOI] [PubMed] [Google Scholar]
- 40.Black, P. N., Young, P. G., and Skinner, S. J. (1996) Am. J. Physiol. 271 L910-L917 [DOI] [PubMed] [Google Scholar]
- 41.De Langhe, S. P., Carraro, G., Tefft, D., Li, C., Xu, X., Chai, Y., Minoo, P., Hajihosseini, M. K., Drouin, J., Kaartinen, V., and Bellusci, S. (2008) PLoS ONE 3 e1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mailleux, A. A., Kelly, R., Veltmaat, J. M., De Langhe, S. P., Zaffran, S., Thiery, J. P., and Bellusci, S. (2005) Development 132 2157-2166 [DOI] [PubMed] [Google Scholar]
- 43.Beer, H. D., Florence, C., Dammeier, J., McGuire, L., Werner, S., and Duan, D. R. (1997) Oncogene 15 2211-2218 [DOI] [PubMed] [Google Scholar]
- 44.Tomlinson, D. C., Grindley, J. C., and Thomson, A. A. (2004) Endocrinology 145 1988-1995 [DOI] [PubMed] [Google Scholar]
- 45.Yoo, Y. A., Kang, M. H., Kim, J. S., and Oh, S. C. (2008) Carcinogenesis 29 480-490 [DOI] [PubMed] [Google Scholar]
- 46.Dennler, S., Andre, J., Alexaki, I., Li, A., Magnaldo, T., ten Dijke, P., Wang, X. J., Verrecchia, F., and Mauviel, A. (2007) Cancer Res. 67 6981-6986 [DOI] [PubMed] [Google Scholar]