Abstract
Latent membrane protein 1 (LMP1) of Epstein-Barr virus (EBV) is a proven oncogene that is essential for transformation of human B cells by the virus. LMP1 induces constitutive activation of several signal transduction pathways involving nuclear factor κB, phosphatidylinositol 3-kinase/Akt, and the mitogen-activated protein kinases (MAPK) p38, c-Jun N-terminal kinase (JNK), and extracellular signal-regulated kinase (Erk). Sequencing of LMP1 isolated from a panel of EBV+ B cell lymphomas identified three different variants of LMP1, each distinct from the B95.8 prototype isoform. All tumor variants of LMP1 as well as the B95.8 LMP1 isoform were able to induce rapid p38 phosphorylation as well as Akt and JNK activation. Additionally all variants showed similar ability to activate nuclear factor κB. In contrast, only tumor-derived LMP1 variants induced prolonged Erk activation and c-Fos expression. Sequence analysis revealed only two amino acids, 212 and 366, shared by the tumor variants but distinct from B95.8. Point mutation of either amino acids 212 (glycine to serine) or 366 (serine to threonine) from the B95.8 isoform to the tumor variant version of LMP1 was sufficient for gain of function characterized by sustained activation of Erk and subsequent c-Fos induction and binding to the AP1 site. Our results indicate that the enhanced ability of tumor-derived LMP1 to induce and stabilize the c-Fos oncogene can be localized to two amino acids in the C terminus of LMP1.
LMP14 is an EBV-encoded oncoprotein that is essential for transformation of human B lymphocytes (1-3). In B cells LMP1 mimics the CD40 receptor, although unlike CD40, LMP1 functions in a ligand-independent manner and is constitutively active (4). LMP1 activates several cellular signaling pathways culminating in expression of downstream genes involved in cell transformation, survival, and proliferation. Thus, LMP1 plays a central role in EBV-associated tumorigenesis.
LMP1 is composed of a short cytoplasmic N-terminal tail required for insertion into the membrane, six membrane-spanning domains that facilitate oligomerization of the protein, and a C-terminal cytoplasmic tail. Within the C terminus of LMP1 are two major signaling domains, C-terminal activation region 1 (CTAR1) and CTAR2. The CTAR interact with tumor necrosis factor receptor-associated factors (TRAFs) and TRAF-associated death domain protein (TRADD) molecules and potentially other adapter molecules to activate NF-κB, Jun N-terminal kinase (JNK), p38 mitogen-activated protein (MAP) kinase, extracellular signal-regulated kinase (Erk), and phosphoinositide 3-kinase (PI3K). Several key sites within the C terminus of LMP1 are necessary for proper signaling including the PXQXT motif in the CTAR1 region which mediates binding of TRAFs 1, 2, 3, and 5, and tyrosines 384 and 385 of CTAR2 that are essential for TRADD interaction, the recruitment of TRAF2, and association of receptor-interacting protein (5-11).
Activation of NF-κB by LMP1 is critical to the survival of EBV-infected B cells. NF-κB activation is highly regulated through association with the IκB complex that prevents nuclear localization of NF-κB. In EBV-infected B cells the activation of NF-κB is initiated through interaction of LMP1 with TRAF2 at CTAR1 and TRAF6 at CTAR2, allowing for activation of the IκB kinase (12, 13). IκB kinase phosphorylates IκBα, thereby targeting it for proteasomal degradation. NF-κB is then free to translocate to the nucleus and to activate transcription of downstream genes that promotes cell survival (14, 15). Similar to the NF-κB pathway, interaction of the adapter protein TRAF2 with both CTAR1 and CTAR2 (indirectly through TRADD) is required for p38 MAPK activation by LMP1 (16). We (17) and others (18) have shown that activation of p38 by LMP1 contributes to the induction of IL-10, an important autocrine growth factor for EBV-infected B cells.
Whereas CTAR1 and CTAR2 both contribute to signaling of NF-κB and p38, the activation of JNK, PI3K/Akt, and Erk rely on a single CTAR. Specifically, the CTAR2 domain of LMP1 participates in activation of the JNK pathway through interaction with TRAF6, TRAF2, and TRADD (19-21). JNK kinase (JNKK/SEK) phosphorylates JNK, which in turn leads to phosphorylation of c-Jun and activation of the AP-1 transcription factor (20). Recent studies implicate LMP-mediated activation of JNK in the efficient progression of B cells through the G2/M phase of the cell cycle (22). The PI3K/Akt pathway, in contrast, is activated by LMP1 through its CTAR1 domain. The p85 regulatory subunit of PI3K associates with CTAR1 leading to Akt phosphorylation; however, it is not yet known whether TRAFs or other adapter molecules are involved in this interaction (23). The PI3K/Akt pathway is required for maximal production of IL-10 by LMP1-expressing B cells through inactivation of the inhibitory kinase glycogen synthase kinase β (17). Finally, Erk1/2 is activated by CTAR1 of LMP1 in a Raf/MEK1/2-dependent manner with the possible involvement of TRAF2 and TRAF5 (24, 25). The Raf/MEK/Erk pathway is important in relaying signals from extracellular ligands, including growth factors, from the membrane to the cytoplasm. Activation of Erk may contribute to transcriptional activity of AP-1 through induction and phosphorylation of c-Fos (26, 27). Erk activation can affect diverse cellular functions including, survival, proliferation, differentiation, and migration. How Erk signaling mediates these varied functions remains unclear, but the strength, duration, and location of Erk activation as well as the cellular context are likely to play a role. Erk activation by LMP1 is required for malignant transformation of Rat-1 fibroblasts, demonstrating the importance of this pathway in LMP1-driven pathogenesis (24). However, the role of LMP1-mediated Erk activation in B cells is not known.
The majority of studies characterizing LMP1 signaling have used LMP1 derived from the B95.8 strain of EBV, originally isolated from a patient with infectious mononucleosis (28). However, LMP1 is also expressed in association with other latent cycle EBV genes in several EBV-associated malignancies including Hodgkin disease, nasopharyngeal carcinoma (NPC), and post-transplant lym-phoproliferative disease (PTLD). The characteristic combination of the latent cycle genes expressed and the distinct cellular hosts associated with each of these diseases likely contribute to their unique pathogenesis. However, an additional layer of complexity must be considered as LMP1 molecules that have been isolated and sequenced from a variety of tumor specimens show significant diversity in their predicted amino acid sequence. Variations in LMP1 sequences may have evolved with the virus to evade the immune system and could contribute to changes in the function of this protein. Variants of LMP1 include an isoform derived from Chinese NPC patients that contains a 10 aa deletion in the CTAR2 region of LMP1 (29). This isoform of LMP1, termed CAO, has been shown to be more oncogenic than the B95.8 prototype of LMP1 in human epithelial cell lines (30). The same deletion was also observed in samples from South American patients with Hodgkin disease (31). Comparisons in LMP1 sequences have led to at least two classification schemes. Sandvej et al. (32) isolated and sequenced LMP1 from healthy European EBV carriers and patients with EBV disease and described a nomenclature for the grouping of these variants, termed A, B, C, and D, on the basis of common point mutations and deletions. Analysis of signaling pathways by these variants revealed differences in NF-κB and AP-1 activation, although these differences could not be attributed to specific mutation/deletions within the LMP1 gene (33). Mainou and Raab-Traub (34) proposed another classification scheme on the basis of six LMP1 variants isolated from clinical specimens that differed in their sequence from Groups A-D. All six LMP1 sequence variants could induce transformation of Rat-1 fibroblasts, and no major differences in PI3K and NF-κB signaling were identified (34). Neither of these studies analyzed the impact of LMP1 sequence variation on signaling in B cells nor has the effect upon the induction of the MAPK, p38, and Erk, been examined.
In this study we characterized LMP1 sequence variants in EBV+ B lymphoma cell lines derived from patients with PTLD (35, 36). Inducible, chimeric LMP1 molecules were created and expressed to directly compare the signaling properties of the tumor-derived variants of LMP1 with LMP1 derived from the B95.8 strain of EBV. Our results indicate that the PTLD tumor-derived variants of LMP1 and the B95.8 version of LMP1 induce comparable activation of NF-κB, PI3K, JNK, and p38. However, whereas Erk activation by B95.8-derived LMP1 was transient, tumor-derived LMP1 signaling led to sustained Erk activation, the induction of functional c-Fos, and AP-1 activation. Moreover, the gain of function by tumor-derived LMP1 was mapped to one amino acid within CTAR1 and a second amino acid within CTAR2. These results provide the first evidence that specific sites within CTAR1 and CTAR2 determine the nature of Erk signaling by LMP1 and suggest that tumor-derived LMP1 possesses unique properties that generate qualitatively different Erk signals to affect cell function.
EXPERIMENTAL PROCEDURES
Reagents—Anti-Erk, anti-phospho-Erk (Tyr204), anti-p38, and anti-c-Fos antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz, CA), whereas anti-phospho-p38 (Thr180/Tyr182), anti-phospho-JNK (Thr183/Tyr185), anti-JNK, anti-phospho Akt (Ser473), and anti-Akt antibodies were purchased from Cell Signaling Technologies (Boston, MA). Anti-IκB and anti-ICAM-PE antibodies, IgG1-PE isotype control, and streptavidin-PE were obtained from BD Pharmingen. Secondary antibodies including unconjugated goat anti-mouse IgG, horseradish peroxidase (HRP)-conjugated donkey anti-rabbit, and HRP-conjugated goat anti-mouse were purchased from Jackson ImmunoResearch Laboratories (West Grove, PA). Anti-nerve growth factor receptor (NGFR) (clone 20.4) and anti-β-actin antibodies were obtained from Sigma-Aldrich, and biotinylated anti-NGFR was obtained from Chromaprobe (Maryland Heights, MO).
Cell Lines—The BL41 Burkitt's lymphoma cell line, the B lymphoma cell line BJAB, and an EBV-infected version, BJAB_B95.8 were kindly provided by E. Kieff and E. Cahir-McFarland (Harvard Medical School, Boston, MA). The EBV+ spontaneous B lymphoblastoid cell lines (SLCL) MF4, JC62, VB5, and JB7 were derived from peripheral blood of four liver transplant recipients with PTLD, whereas the AB5 line was similarly generated from a lymph node biopsy of a kidney transplant recipient with PTLD as previously described (36). All cell lines were grown in RPMI 1640 supplemented with 10% fetal calf serum and 50 units/ml penicillin-streptomycin (Invitrogen).
LMP1 Sequencing—Genomic DNA was isolated from 5 × 106 cells of SLCL using DNAzol reagent according to the manufacturer's instructions. DNA was used as a template for sequencing of the C-terminal region of LMP1 representing aa 190-386. The following primers were used for C terminus sequencing: forward primer, 5′-TTCCTAGACCTCATCCTGCTCAT-3′, and reverse primer, 5′-CAAGCCTATGACATGGTAATG-3′.
Generation of BL41 Clones Expressing NGFR-LMP1—RNA was isolated from EBV+ B lymphoma cell lines using TRIzol reagent (Invitrogen). Three μg of RNA was used to generate first-strand cDNA with an oligo-dT primer (Invitrogen). Full-length LMP1 was cloned from cDNA and introduced into the pSG5 vector (Stratagene, La Jolla, CA). The C-terminal region of each LMP1 variant, starting from the HGQRH portion of the LMP1 C terminus and corresponding to aa 185-189 of B95.8 LMP1, was subcloned from the relevant pSG5.LMP1 vector.
The NGFR-LMP1 vector was generously provided by W. Hammerschmidt (GSF-National Research Center for Environment and Health, Munich, Germany). NGFR was cloned from the vector and modified to delete aa 272-276 of NGFR (FKRWN) and the 3-aa linker region (RGI). The C terminus portion of each LMP1 variant and NGFR were joined by splicing by overlapping extension PCR with introduction of Xba-1 and HindIII restriction sites. These sites were used to insert the chimera into the pCDNA3.1 expression vector. BL41 cells were electroporated at 210 V, 960 microfarads with 15 μg of NGFR-LMP1.pCDNA3 constructs. The cells were plated (10,000 cells/ml) in RPMI with 10% fetal calf serum, 50 units/ml penicillin-streptomycin, and 0.7 mg/ml of G418 (Invitrogen), and selected clones were expanded and screened for NGFR-LMP1 expression by fluorescence-activated cell sorter.
Generation and Expression of Point Mutants of NGFR-LMP1—NGFR-LMP1.pCDNA3 (B95.8 version) was used as a template for site-directed mutagenesis using PCR Quick site mutagenesis kit (Stratagene) according to the manufacturer's instructions. The following primers were used to introduce a Gly to Ser mutation at aa 212 (sense, 5′-CTCAACAAGCTACCGATGATTCTAGCCATGAATCTG-3′, and antisense, 5′-CAGATTCATGGCTAGAATCATCGGTAGCTTGTTGAG) and a Ser to Thr mutation at aa 366 (sense 5′-ACGCTGCTTTTGGGTACTTCTGGTTCCGGTG-3′, antisense 5′-CACCGGAACCAGAAGTACCCAAAAGCAGCGT-3′). PCR products were sequenced to check for proper substitutions. The new point-mutated NGFR-LMP1.pCDNA3 constructs were transfected into BL41 cells, and stable clones were selected as described above for generation of BL41 clones expressing wild type NGFR-LMP1.
Activation of LMP1 by Cross-linking of NGFR-LMP1 in BL41 Clones—0.5-5 × 106 cells were incubated with anti-NGFR antibodies (0.5 μg/1 × 106 cells) for 30 min on ice. Goat anti-mouse IgG antibodies were added (1.7 μg/1 × 106 cells) for various times ranging from 0 min to 24 h at 37 °C to activate LMP1 signaling. When necessary, excess cross-linking antibodies were blocked by the addition of mouse IgG.
Flow Cytometry—One million cells of the BL41 clones were washed with phosphate-buffered saline containing 0.1% sodium azide and 1% fetal calf serum and stained with biotinylated anti-NGFR mAb. The cells were washed again and incubated with streptavidin-PE. After two more washes the cells were analyzed for NGFR expression on a FACScan flow cytometer using CellQuest software (BD Biosciences). To analyze ICAM expression, NGFR-LMP1-expressing cells were cross-linked for 24 h as described above, and excess cross-linking antibodies were blocked with mouse IgG. The cells were then labeled with anti-CD54 (ICAM)-PE or isotype control and analyzed by flow cytometry.
Western Blotting—BL41 clones expressing NGFR-LMP1 were stimulated for 0-24 h by cross-linking with anti-NGFR mAb and goat anti-mouse IgG. The cells were harvested, washed with phosphate-buffered saline, 1 mm orthovanadate. and lysed in 1% Nonidet P-40, 0.5% deoxycholic acid phospholysis buffer containing 150 mm NaCl, 0.5 mm EDTA, 10 mm sodium fluoride, 2 mm phenylmethylsulfonyl fluoride, 50 μg/ml aprotinin, 50 μg/ml leupeptin, and 5 μg/ml pepstatin. Lysates were cleared by centrifugation. 40-60 μg of lysate protein was resolved on SDS-PAGE and transferred to nitrocellulose, and Western blotting with appropriate antibodies was performed. The membranes were developed using ECL (Amersham Biosciences). Densitometry was performed using an Alpha Imager 2000 (Alpha Innotech Corp., San Leandro, CA).
AP-1 Luciferase Assay—293T cells were transiently co-transfected with 1.6 μg of the firefly luciferase reporter plasmid (pAP1-luc), 0.16 μg of the control Renilla luciferase reporter plasmid (ppR6-Renilla), and 0.8 μg of an NGFR-LMP1 expression plasmid using FuGENE 6 (Roche Applied Science). After transfection, cultures were maintained in normal growth medium (Dulbecco's modified Eagle's medium with 10% fetal calf serum and 1% penicillin/streptomycin) for 48 h and then transferred (without trypsinizing) to 2 wells of a 24-well plate. To induce LMP1 signaling, cells were incubated with anti-NGFR antibodies (1 μg/ml) for 30 min at 37 °C, then incubated with goat anti-mouse antibodies (5 μg/ml) for 6 h at 37 °C. The Dual-Luciferase Reporter Assay system (Promega) was used as described to generate cell lysates and determine firefly and Renilla luciferase activities. Relative luciferase activity was calculated as the ratio of firefly luciferase activity to Renilla luciferase activity.
Measurement of Active c-Fos—BL41.NGFR-LMP1 cell clones were activated with anti-NGFR and goat anti-mouse antibodies for 0, 30 min, or 2 or 3 h. Cells were harvested, and nuclear extracts were isolated using the nuclear extract kit (Active Motif., Carlsbad, CA). Five μl of nuclear lysates were used in TransAM AP-1 c-Fos assay (Active Motif) according to the manufacturer's protocol. Briefly, nuclear lysates were added to 96-well plates that were precoated with wild type consensus AP-1 oligonucleotide. After four washes the plate was incubated with anti-c-Fos antibody followed by horseradish peroxidase-conjugated secondary antibody. Developing solution was then added, and the plate was analyzed at 450 nm. Nuclear lysates from 12-O-tetradecanoylphorbol-13-acetate-stimulated K-562 cells served as a positive control. Additionally, excess consensus AP-1 oligonucleotide was added to the 3-h time point lysates to demonstrate specificity.
RESULTS
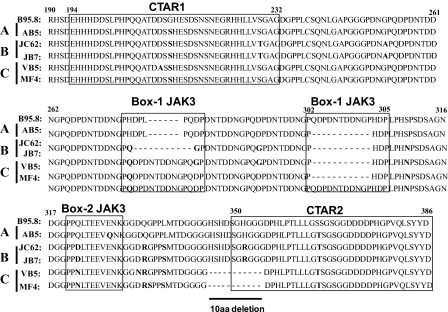
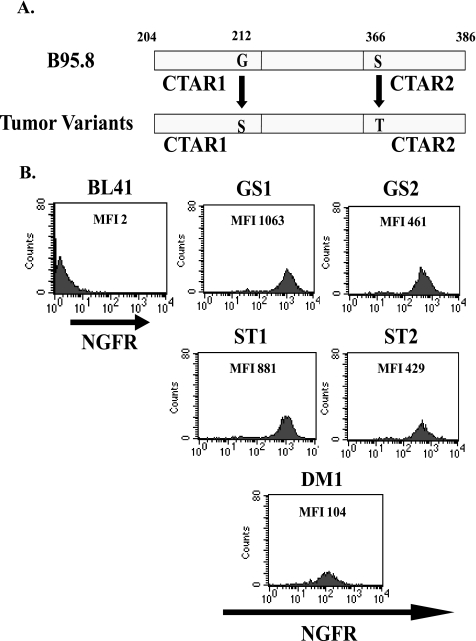
Identification of LMP1 Sequence Variants in PTLD-derived B Cell Lymphomas—The extent of sequence variation in LMP1 isolated from a panel of EBV+ B cell lines from patients with PTLD was determined. Genomic DNA from EBV+ B lymphoma cell lines from PTLD patients and the BJAB_B95.8 cell line were isolated, and the C terminus of LMP1 variants (aa 190-386) were sequenced and aligned. Using the nomenclature described by Sandvej et al. (32), variants obtained from the tumor cell lines were identified that represent Groups A (AB5), B (JC62, JB7), and C (MF4, VB5) (Fig. 1). Group A variants share the most sequence homology with B95.8 and are characterized by three mutations at aa 212 (Gly to Ser), aa 328 (Glu to Gln), and aa 366 (Ser to Thr). Group B variants are defined by nine amino acid substitutions that include aa 212 (Gly to Ser), aa 229 (Ser to Thr), aa 252 (Gly to Ala), aa 309 (Ser to Asn), aa 322 (Gln to Asp), aa 334 (Gln to Arg), aa 338 (Leu to Ser), aa 352 (His to Arg), and aa 366 (Ser to Thr). Group C clones contain mutations at aa 212 (Gly to Ser), aa 309 (Ser to Asn), aa 322 (Gln to Asn), aa 334 (Gln to Arg), and aa 338 (Leu to Ser) and share the 10-aa deletion overlapping the start of the CTAR2 region (aa 346-355) with the CAO variant of LMP1.
FIGURE 1.
LMP1 C terminus sequence alignment of B95.8 and tumor variants. Genomic DNA was isolated from BJAB_B95.8 and EBV+ B cell lymphoma lines from PTLD patients and used as a template for sequencing of both strands. Amino acid sequences of LMP1 were aligned and compared with the B95.8 variant using the DNAStar program. Amino acid numbers are indicated; note that B95.8 contains an extra repeat (QDPDNTDDNGP) beginning at position 303. Bold residues indicate point mutations relative to B95.8, and dashed lines represent deletions. CTAR1 and CTAR2 domains and putative JAK3 binding motifs are indicated with boxes. Groups of LMP1 variants were determined based on nomenclature described by Sandvej et al. (32) and are indicated on the left-hand side of the diagram.
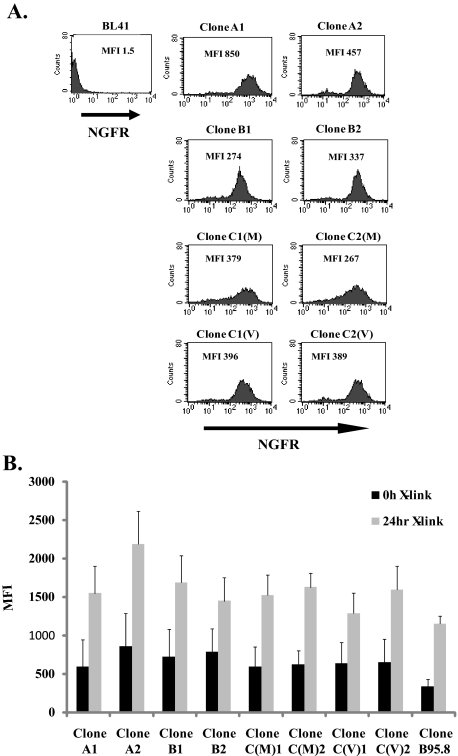
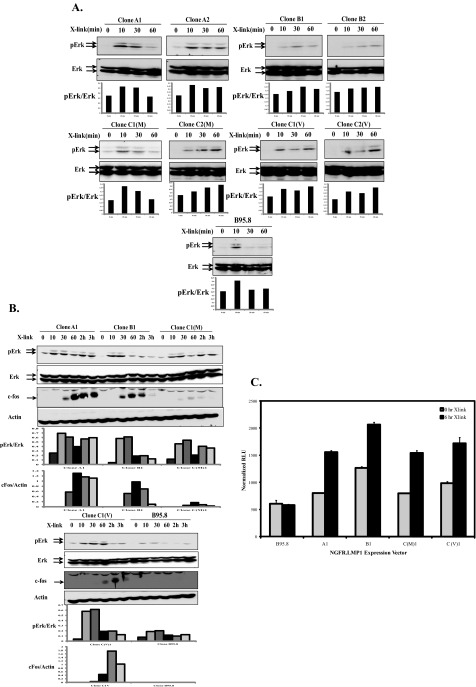
Chimeric NGFR-LMP1 Proteins Derived from PTLD B Cell Lymphomas Are Functional—To determine whether differences in LMP1 amino acid sequences correspond to differences in the ability of LMP1 to activate cellular signaling pathways, NGFR-LMP1 chimeric molecules were created so that the NGFR transmembrane domain was fused to the C terminus of each LMP1 variant. To create NGFR-LMP1 chimeric molecules representing Groups A-C, LMP1 derived from the AB5 cell line (Group A), the JB7 cell line (Group B) and MF4 and VB5 cell lines (Group C) were utilized. The NGFR-LMP1 chimeric proteins were expressed in EBV- BL41 cells. In cells expressing the chimeric proteins, LMP1 does not signal unless NGFR-LMP1 is cross-linked with anti-NGFR mAb and goat anti-mouse antibodies. This system permits controlled and inducible LMP1 signaling. Expression of NGFR-LMP1 in BL41 clones was confirmed by immunofluorescent staining and flow cytometry using anti-NGFR mAb. Two clones from each group were used in subsequent assays (Fig. 2A). The clones were named according to their sequence group and, for group C clones, the cell line from which they were derived, either VB5 (Clone C1(V) and C2 (V)) or MF4 (Clone C1(M) and C2(M)). All clones were tested for LMP1 function by analyzing surface expression of CD54 (ICAM) 24 h after NGFR-LMP1 cross-linking as ICAM expression is known to be induced by full-length LMP1 (37). Indeed all tumor-derived clones as well as the B95.8 derived clone were able to markedly up-regulate ICAM expression after NGFR-LMP1 cross-linking independent of the LMP1 variant group from which they were derived (Fig. 2B).
FIGURE 2.
BL41 clones express functional NGFR-LMP1 containing tumor-derived LMP1 variants. Stable BL41.NGFR-LMP1 clones expressing the C terminus of tumor LMP1 variants were generated by transfection of NGFR-LMP1.pCDNA3 constructs followed by G418 selection and limiting dilution cloning as described under “Experimental Procedures.” A, NGFR-LMP1 expression on the surface of BL41 clones was confirmed by flow cytometry after staining with anti-NGFR-biotin followed by streptavidin-R-phycoerythrin. Median fluorescent intensity (MFI) for each clone is indicated. The parental BL41 cell line (upper left) was used as a negative control. Clones were named according to the LMP1 Group nomenclature A-C. Clones C1(M) and C2(M) express the LMP1 variant from MF4 SLCL, and C1(V) and C2(V) express the LMP1 variant from VB5 SLCL. B, function of NGFR-LMP1 in each of the tumor-derived LMP1 clones as well as the B95.8 clone was tested by cross-linking with anti-NGFR followed by goat anti-mouse IgG for 24 h. ICAM (CD54) up-regulation was then tested by staining with anti-ICAM-PE or isotype control and analyzed by flow cytometry. Data are shown as mean fluorescent intensity of ICAM ± S.E. and are representative of four independent experiments.
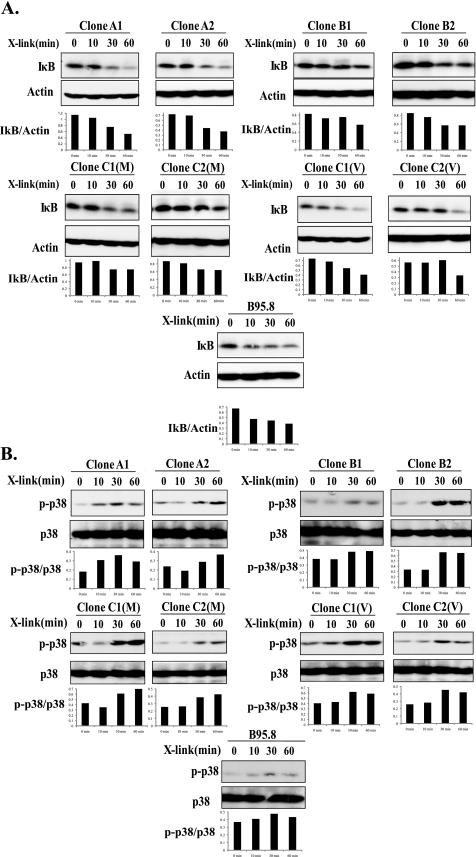
Analysis of NF-κB, p38, JNK, and Akt Activation by LMP1 Variants—LMP1 is known to activate multiple signaling pathways through interaction with TRAFs, TRADD, and receptor-interacting protein (9-11). As mentioned previously, both CTAR1 and CTAR2 regions of LMP1 can activate the NF-κB pathway; however, CTAR2 is responsible for 80% of NF-κB activation by LMP1 (10). Group C variants have a deletion within the CTAR2 region of LMP1, raising the possibility that this group may have altered activation of the NF-κB pathway. To assess activation of NF-κB by LMP1 variants, NGFR-LMP1 was cross-linked for 0, 10, 30, or 60 min to initiate LMP1 signaling in BL41.NGFR-LMP1 clones, and IκB degradation was analyzed as a readout for NF-κB activation. The BL41.NGFR-LMP1 clone expressing the B95.8-derived LMP1 C terminus was used as a control (17). All clones tested, including clones from group C, showed marked degradation of IκB by 30-60 min after LMP1 activation (Fig. 3A). Likewise, all tumor-derived LMP1 and the B95.8-derived LMP1 could activate NF-κB to similar extents in NF-κB luciferase reporter assays performed in 293 cells (data not shown). Therefore, the 10-aa region deleted in Group C is likely dispensable for NF-κB activation by LMP1.
FIGURE 3.
Tumor-derived LMP1 and B95.8-derived LMP1 variants activate the NF-κB and p38 pathways. Signaling was induced in 5 × 106 BL41 cells expressing NGFR-LMP1 chimeric proteins by cross-linking NGFR-LMP1 for 0, 10, 30, or 60 min using anti-NGFR antibody followed by goat anti-mouse IgG. Two clones for each LMP1 variant were utilized. The cells were harvested at the indicated time points and lysed. 60 μg of each lysate was resolved on 12% SDS-PAGE and transferred to nitrocellulose membranes. A, Western blotting was performed with anti-IκB and anti-β-actin antibodies. The corresponding densitometry graphs are shown below each blot. Data are representative of four independent experiments. B, p38 activation was analyzed by Western blotting with anti-phospho-p38 (p-p38) and total p38 antibodies. The corresponding densitometry graphs are shown below each blot. The blots are representative of four independent experiments.
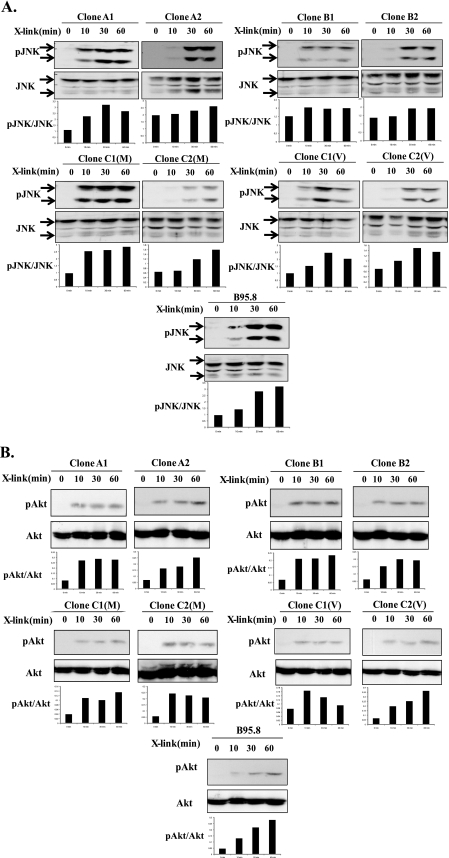
p38 activation by LMP1 is also attributed to both CTAR1 and CTAR2 regions through interaction with the TRAF2 adapter (16). Phosphorylation of p38 was observed in all BL41.NGFR-LMP1 clones within 10-30 min after cross-linking of NGFR-LMP1, indicating that neither the 10-aa deletion nor point mutations at amino acids 210, 212, or 229 within CTAR1 affect p38 activation (Fig. 3B). Another MAP kinase, JNK, is activated by LMP1 solely through CTAR2 and involves TRADD interaction (20). Therefore, we considered the possibility that deletions and point mutations in CTAR2 among the LMP1 variants could impact upon JNK phosphorylation after NGFR-LMP1 cross-linking (Fig. 1). However, all BL41.NGFR-LMP1 clones showed comparable activation of the JNK pathway as JNK (JNK1 and JNK2) becomes phosphorylated within 10-30 min after LMP1 activation in all clones tested (Fig. 4A). The delay in maximal activation of JNK observed in clones A2, B2, C2(M), and C2(V) was most likely due to lower expression of NGFR-LMP1 in these clones and not differences in LMP1 sequence.
FIGURE 4.
All LMP1 variants activate JNK and Akt. BL41 cells (5 × 106) expressing NGFR-LMP1 proteins were treated with anti-NGFR antibodies and goat anti-mouse IgG for the indicated times, and lysates were prepared. Proteins were resolved on 10% SDS-PAGE and transferred to nitrocellulose membranes. A, Western blotting with anti-phospho-JNK (pJNK) and total JNK antibodies was performed. Arrows indicate JNK1(bottom band) and JNK2 (top band). The corresponding densitometry graphs are shown below each blot. Data are representative of four independent experiments. B, lysates were evaluated for Akt activation by Western blotting with anti-phospho Akt and anti-Akt antibodies. The corresponding densitometry graphs are shown below each blot. The data are representative of four independent experiments.
PI3K/Akt activation by LMP1 is primarily initiated at the CTAR1 region (23). As the CTAR1 region of LMP1 from groups A, B, and C contains several point mutations near the TRAF interacting site PXQXT (aa 204-208) which include mutations at aa 210 (VB5), 212 (all SLCL), and 229 (Group B), it was considered possible that NGFR-LMP1 chimeric proteins containing these mutations would be less efficient in inducing Akt phosphorylation. However, Fig. 4B shows that all BL41.NGFR-LMP1 clones tested are equally able to induce activation of PI3K. Moreover, the kinetics of Akt activation are similar irrespective of LMP1 sequence, as Akt is phosphorylated within 10 min of NGFR-LMP1 cross-linking by all variants. Thus, LMP1 tumor variants as well as the B95.8 variant similarly activate NF-κB, p38, JNK, and Akt despite multiple point mutations in CTAR1 and CTAR2 and deletions within CTAR2 in a number of the variants. This implies that only TRAF/TRADD-interacting sites that are intact in all variants are necessary for the signaling of these pathways to occur properly.
Tumor Variants of LMP1 Induce Sustained Erk Activation and c-Fos Expression—LMP1 is sufficient to transform Rat-1 fibroblasts, and the activation of Erk is required for this process (24). Similarly, Erk activation by LMP1 increases the invasive properties of transformed cells as seen in NPC (38). We reasoned that tumor variants of LMP1 may have evolved to possess enhanced transforming and growth abilities in EBV-infected cells through increased Erk activation. Thus, Erk activation was analyzed by phospho-Erk Western blotting after NGFR-LMP1 cross-linking in BL41 cells expressing either tumor-derived variants or the B95.8 variant of LMP1. In Fig. 5A, both Erk1 (top band) and Erk2 (bottom band) were analyzed. All BL41.NGFR-LMP1 clones were able to induce Erk phosphorylation within 10 min of NGFR cross-linking. However, Erk activation persisted for up to 60 min in BL41 cells expressing the tumor variants of LMP1, whereas BL41 cells expressing the B95.8 version of LMP1 displayed more transient Erk activation (Fig. 5A). Moreover, constitutively active full-length tumor-derived LMP1 from groups A, B, and C each showed marked Erk activation in B lymphoma cells, whereas full-length B95.8 LMP1 showed only modest Erk activation (data not shown).
FIGURE 5.
LMP1 tumor-derived variants induce more sustained Erk activation, c-Fos, and AP-1 activity than B95.8 isoform. A, NGFR-LMP1 signaling was induced in BL41clones expressing tumor-derived LMP1 or B95.8-derived LMP1 by cross-linking with anti-NGFR and goat anti-mouse IgG for 0, 10, 30, or 60 min. Cells were lysed in phospholysis buffer, and lysates were analyzed for Erk activation by Western blot with anti-phospho-Erk (pErk) and total Erk antibodies. Arrows indicate Erk1 (top band) and Erk2 (bottom band). The corresponding densitometry graphs are shown below each blot. The blots are representative of four independent experiments. B, BL41 clones expressing NGFR-LMP1 proteins were activated by anti-NGFR and goat anti-mouse IgG cross-linking for 0, 10, 30, and 60 min and 2 and 3 h. The cells were harvested and lysed at the time point required and analyzed for Erk activation and c-Fos induction by Western blot with anti-pErk, anti-Erk, anti-c-Fos, and anti-β-actin antibodies. The corresponding densitometry graphs are shown below each blot. The blots are representative of three independent experiments. C, 293T cells were transiently transfected with firefly luciferase reporter plasmid, Renilla luciferase reporter plasmid, and NGFR-LMP1 expression plasmids. NGFR-LMP1 signaling was induced in 293T cells expressing tumor-derived LMP1 or B95.8 LMP1 by cross-linking with anti-NGFR and goat anti-mouse IgG for 0 or 6 h. Relative luciferase activity (RLU) was calculated as the ratio of firefly luciferase activity to Renilla luciferase activity as expressed as normalized RLU. Data represent the mean of triplicate samples and are representative of three independent experiments.
The duration of Erk activation may lead to differences in downstream signaling pathways regulated by immediate early genes (27). Along these lines, c-Fos is stabilized by sustained Erk signaling, whereas it is rapidly degraded during transient Erk activation (39). To determine whether Erk activation induced by the tumor-derived variants of LMP1 is more sustained than Erk signaling induced by B95.8-derived LMP1, Erk phosphorylation and c-Fos induction was monitored by Western blotting over a 3-h time course after NGFR-LMP1 cross-linking in BL41 clones. Overall, the tumor-derived variants of LMP1 were much more efficient in induction of Erk phosphorylation and sustained Erk activation than the B95.8-derived LMP1. Interestingly, c-Fos was induced by all tumor LMP1 variants within 30 min of signaling initiation, whereas there was no evidence of c-Fos induction by the B95.8 LMP1 variant at any time point tested (Fig. 5B). Because c-Fos is a component of the transcription factor, AP-1, we utilized a luciferase reporter assay in 293T cells to determine the ability of LMP1 variants to activate AP-1. As shown in Fig. 5C, cross-linking of tumor-derived NGFR-LMP1 molecules resulted in AP-1 activation. In contrast, cross-linking of B95.8-derived NGFR-LMP1 did not lead to AP-1 activation.
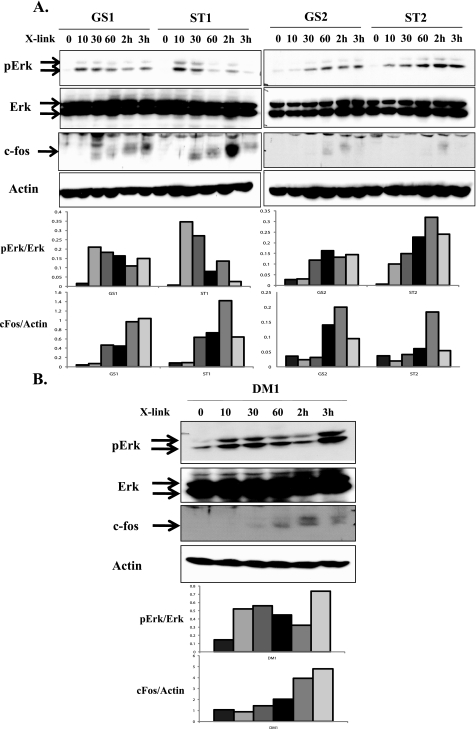
Two Amino Acids in the C Terminus of LMP1 Are Critical for c-Fos Induction and AP-1 Binding—To determine the specific residues of LMP1 that are important for sustained Erk activation and c-Fos induction, we compared the C terminus alignment of tumor-derived LMP1 variants and the B95.8-derived LMP1 (Fig. 1). Two amino acid residues were common to all the tumor variants but were not shared by the B95.8 variant. These differences consisted of a Gly to Ser mutation at 212 aa and a Ser to Thr mutation at 366 aa (Fig. 1). To elucidate a role for these residues in c-Fos induction, the B95.8 NGFR-LMP1.pCDNA3 construct was subjected to site-directed mutagenesis to create point mutations at either aa 212 (Gly to Ser, referred to as GS), aa 366 (Ser to Thr, referred to as ST), or both sites (referred to as DM for double mutant) (Fig. 6A). BL41 clones expressing the point-mutated NGFR-LMP1 constructs GS, ST, and DM were created and analyzed for NGFR-LMP1 expression by immunofluorescent staining and flow cytometry as previously described (Fig. 6B). BL41 clones expressing the GS, ST, and DM forms of NGFR-LMP1 were tested for Erk activation and c-Fos induction after cross-linking of NGFR-LMP1. Either the GS or the ST mutation alone was sufficient to confer gain of function through sustained (3 h) Erk activation and c-Fos induction (Fig. 7A); the DM mutation also initiated sustained Erk activation and c-Fos induction (Fig. 7B). The GS, ST, and DM mutations in B95.8-derived NGFR-LMP1 specifically affected Erk activation, as patterns of phosphorylation of p38 and Akt were not affected (data not shown).
FIGURE 6.
Generation of BL41 clones expressing NGFR-LMP1 containing point mutations. A, schematic representations of the C terminus of B95.8 LMP1 and tumor-derived LMP1 variants are shown. Point mutations at residues 212 and 366 in the tumor-derived LMP1 variants are indicated and compared with residues 212 and 366 of B95.8 LMP1. Gly-212 and Ser-366 within NGFR-LMP1 of the B95.8 variant were point-mutated to Ser and Thr, respectively, using a QuikChange site-directed mutagenesis kit. BL41 cells were then transfected with point mutant constructs of NGFR-LMP1.pCDNA3, and stable clones were selected. B, fluorescence-activated cell sorter analysis for NGFR-LMP1 expression in stable BL41 clones. The BL41 parental cell line is a negative control. Median fluorescent intensities (MFI) are shown. GS indicates a Gly to Ser point mutant at aa 212. ST indicates a Ser to Thr point mutant at aa 366, and DM indicates the presence of both mutations.
FIGURE 7.
Two point mutations in the C terminus of tumor-derived LMP1 variants account for c-Fos induction by LMP1. BL41 clones expressing NGFR-LMP1 molecules containing single GS and ST (A) and double point mutant (DM)(B) were incubated with anti-NGFR mAb followed by goat anti-mouse IgG for 0, 10, 30, or 60 min or 2 or 3 h. Cells were lysed, and 60 μg of lysates were resolved on SDS-PAGE followed by Western blotting with anti-phospho-Erk (pErk), anti-Erk, anti-c-Fos, and anti-β-actin antibodies. The corresponding densitometry graphs are shown below the blots. The blots are representative of three independent experiments.
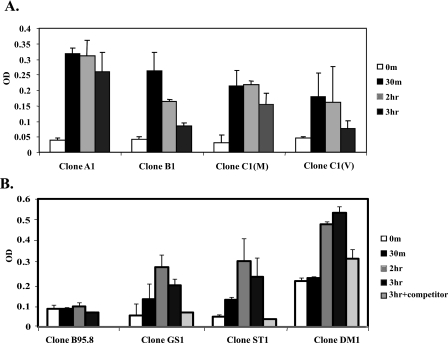
To further establish that c-Fos induced by LMP1 signaling is functional, c-Fos binding to the AP-1 site was analyzed. LMP1 signaling was induced in BL41.NGFR-LMP1 clones expressing either tumor-derived variant LMP1, B95.8 LMP1, or B95.8 single point-mutated LMP1, and nuclear lysates harvested over a time course up to 3 h were analyzed for binding to the AP-1 consensus site (Fig. 8). All nuclear lysates from BL41.NGFR-LMP1 clones expressing the tumor-derived variants of LMP1 showed c-Fos/AP-1 binding within 30 min (Fig. 8A), whereas nuclear lysates from BL41.NGFR-LMP1 clones expressing the B95.8-derived LMP1 did not exhibit c-Fos binding after prolonged cross-linking of NGFR-LMP1 (Fig. 8B). These data are consistent with the AP-1 luciferase reporter assay (Fig. 5C). Furthermore, both single mutants tested, GS and ST, as well as the double mutant DM, were able to induce rapid c-Fos/AP-1 binding after LMP1 activation by NGFR-LMP1 cross-linking, with the highest levels of binding observed at 2-3 h (Fig. 8B). These data demonstrate a role for aa 212 and 366 of LMP1 in activation of Erk and c-Fos and may account for functional differences in tumor-derived LMP1 compared with LMP1 derived from the B95.8 laboratory strain of EBV.
FIGURE 8.
c-Fos induced by tumor-derived LMP1 variants binds to the AP-1 site. A, BL41 clones expressing tumor-derived NGFR-LMP1 variants were incubated with anti-NGFR and goat anti-mouse IgG for 0 or 30 min or 2 or 3 h. The cells were harvested, and nuclear lysates were obtained using the Active Motif nuclear extract kit. 5 μl of nuclear lysates were used in the TransAM AP-1 c-Fos binding assay with the AP-1 target site. c-Fos binding to AP-1 was determined by anti-c-Fos antibody followed by horseradish peroxidase-goat anti-mouse IgG. The data are shown as the mean ± S.D. absorbance measurement of two independent experiments. B, BL41 clones expressing B95.8, point mutant (GS and ST), or the double mutant (Clone DM1), NGFR-LMP1 were cross-linked for 0 or 30 min or 2 or 3 h without or with excess consensus AP-1 oligonucleotide (3 h with competitor). Nuclear lysates were obtained and were analyzed in the TransAM AP-1 c-Fos binding assay as described in A.
DISCUSSION
LMP1 is a critical latent cycle protein of EBV that can promote activation, survival, growth, and transformation in infected cells. Characterization of the signaling pathways induced by LMP1 that lead to these outcomes have primarily relied upon the B95.8 prototype variant of LMP1 originally isolated from a patient with infectious mononucleosis. Other studies have utilized the CAO version of LMP1 that was isolated from a Chinese patient with NPC and contains a characteristic 10-aa deletion. The role that this deletion plays in tumorigenicity is still being questioned as some groups found the CAO variant of LMP1 to be more oncogenic than the B95.8 prototype (40), whereas other groups report no apparent differences between the CAO variant and the B95.8 variant or AG876, an EBV type II variant of LMP1 that is very similar to B95.8 but has a 10-aa deletion in CTAR2 identical to CAO (41, 42). In this study we present a systematic analysis of signal transduction by LMP1 variants expressed by a panel of PTLD-derived B cell lymphomas and compare their function to the B95.8 LMP1. By using NGFR-LMP1-inducible chimeras expressing the C terminus of LMP1, we were able to dissect the signaling pathways induced by LMP1 in more detail and to determine qualitative and quantitative differences in signaling associated with specific LMP1 variants.
The PTLD-derived LMP1 variants we identified could be assigned to Groups A, B, and C according to nomenclature previously applied to LMP1 variants isolated from Dutch, Swedish, and German patients (32). No group D variants were observed in our panel of PTLD tumors. Two of the PTLD tumor cell lines were assigned to Group C, as their LMP1 variants contained the characteristic 10-aa deletion overlapping CTAR2 that is also present in the CAO variant, but these two Group C variants contained additional distinct mutations. Thus, both Group C clones were included in the study. Our findings indicate that all tumor-derived LMP1 variants as well as the B95.8 variant can effectively activate NF-κB, PI3K, p38, and JNK pathways to the same extent, indicating that these pathways are not affected by the point mutations and deletions present in tumor-derived LMP1. However, we provide the first evidence that regulation of the Erk pathway and the downstream induction and stabilization of c-Fos in B cell lymphomas relies upon two specific amino acid residues in the LMP1 C terminus, Ser at position 212 and/or Thr at position 366. Interestingly, 60 of 64 LMP1 sequences in GenBank™obtained from NPC specimens also contain Ser at aa 212 and Thr at aa 366.
All PTLD-derived tumor variants of LMP1 were able to activate the NF-κB pathway to a similar extent and with similar kinetics as the B95.8-derived variant of LMP1. Notably, the 10-aa deletion that overlaps CTAR2 did not affect activation of NF-κB in Group C variants. These findings agree with the results of Fielding et al. (33) who reported that NF-κB activation was increased only in LMP1 variants representing Group D and CAO but not in LMP1 variants from Groups A-C. The observation that both the CAO variant and Group C variants share the 10-aa deletion but exhibit differences in NF-κB activation suggests that other regions of LMP1, such as the TRADD interacting site, may be more important for NF-κB activation. Interestingly, the B95.8 variant of LMP1 can interact with an E3 ubiquitin ligase, HOS, which has been shown to contribute to decreased NF-κB activation, whereas the CAO variant of LMP1 did not interact with HOS and had increased NF-κB activation. Though these differences were minor, three amino acids, Gly-212, Ser-350, and Ser-366, were described as important in the LMP1/HOS interaction observed in the study (43). In our hands, two of the four tumor-derived variants of LMP1 interacted with HOS in immunoprecipitation experiments (data not shown). However, differences in the ability to interact with HOS did not correspond to differences in NF-κB activation by these variants, as analyzed by IκB degradation. The disparity between our findings and those of Mainou and Raab-Traub (34) may lie in the assay used to analyze NF-κB activation or the cellular context. Their observed correlations between HOS binding and NF-κB activation were based on luciferase assays conducted in 293 epithelial cells, whereas our studies examined endogenous IκB levels in Burkitt's lymphoma B cell lines. Therefore, we conclude that point mutations and deletions present in tumor-derived variants of LMP1 do not influence the ability of LMP1 to activate the NF-κB pathway in B lymphocytes.
The p38 MAP kinase pathway is regulated by LMP1 through TRAF interactions with CTAR1 and CTAR2. Our study represents the first analysis of p38 activation by variants of LMP1 in B cells. Sequence analysis of the panel of LMP1 variants we utilized indicates that the TRAF/TRADD interaction sites are well conserved between variants of LMP1 isolated from PTLD tumors and the B95.8 variant of LMP1. Therefore, it is not surprising that p38 activation was indistinguishable among all the LMP1 variants we analyzed.
Activation of the PI3K/Akt pathway by LMP1 has been shown to be important for transformation of Rat-1 fibroblasts and to involve the CTAR1 domain of LMP1 (23, 44). Induction of this pathway by LMP1 has mainly been studied in epithelial cells or fibroblasts. We recently reported that the PI3K/Akt pathway is critically involved in LMP1-mediated induction of cellular IL-10 in B cells (17). The CTAR1 domain of the PTLD-derived LMP1 variants contains several point mutations relative to B95.8-derived LMP1; however, the TRAF interacting domain is conserved. In this study we conclude that the region containing these mutations must be dispensable for PI3K activation in the BL41 B cell line, as the PI3K pathway was activated by all tumor-derived variants of LMP1 at the same rate and intensity as by B95.8 LMP1. These findings are consistent with a report that all LMP1 variants tested were equally capable of causing transformation of Rat-1 fibroblasts, a PI3K-dependent process (44). The mutations within the CTAR1 domain tested in the Mainou et al. study (44) were similar to those we observed in PTLD-derived LMP1 variants. Our findings are consistent with the idea that PI3K activation by LMP1 depends largely on the integrity of the TRAF-interacting domain that has been preserved in the tumor-derived variants. Furthermore, these data suggest that the interaction of the p85 subunit of PI3K with LMP1 occurs through TRAF adapters or, alternately, directly at the TRAF interacting site of CTAR1, which is conserved among the group of LMP1 variants analyzed.
CTAR2 is the only region of LMP1 required for JNK activation and subsequent c-Jun phosphorylation in cells lacking TRAF1 expression. Other studies that have analyzed the ability of LMP1 variants to activate JNK examined AP-1 activity as an indirect measure of JNK activation (33, 34). In contrast, we directly analyzed JNK1/2 activation by the panel of LMP1 variants we isolated from PTLD-associated B lymphoma cell lines. We show that the 10-aa deletion overlapping the start of CTAR2 is dispensable for phosphorylation of JNK as LMP1 variants from all groups, including Group C, rapidly activated JNK after LMP1 activation. This pattern of JNK activation differs from the results of Fielding et al. (33), who found that Group A and C clones were less efficient than Group B clones in the activation of AP-1 transcription in a Jurkat T leukemic cell line. This disparity may be due to the expression of LMP1 variants in different cellular environments (T cells versus B cells) that may activate AP-1 through different protein complexes. Along these lines, LMP1 induces formation of c-jun/junB AP-1 complexes in NPC cells, whereas the constituents of the LMP1-induced AP-1 complex in B cell lymphomas have not yet been identified (45). The AP-1 transcription factor can consist of heterodimers formed from members of the c-Jun (c-jun, junB, junD) and the c-Fos (c-Fos, fosB, fra-1, fra-2) families. JNK is not the only kinase that contributes to AP-1 transcriptional activity, however. c-jun is regulated by JNK through phosphorylation, whereas both the Erk and p38 pathways have been shown to affect stability and induction of c-Fos family members. Erk activation by LMP1 variants has not been addressed previously, and considering that Erk has a role in AP-1 activation through c-Fos induction and stabilization, we suggest that differences in Erk activation by distinct variants can account for differences in AP-1 activation.
Indeed, we observed sustained Erk activation (up to 3 h) after NGFR-LMP1 cross-linking in all BL41.NGFR-LMP1 clones expressing tumor-derived LMP1 variants, whereas the B95.8 variant of LMP1 induced more transient phosphorylation of Erk. The c-Fos component of AP-1 is transcriptionally induced immediately upon mitogen stimulation; however, it is degraded within 45 min unless phosphorylated by Erk to extend its half-life to at least 2 h (46-48). Upon analysis of c-Fos induction by LMP1 variants, we found that tumor-derived variants of LMP1 were able to induce and stabilize c-Fos protein such that it was detectable for up to 2-3 h after LMP1 activation, whereas no significant induction of c-Fos by B95.8 LMP1 was observed. We show for the first time that PTLD-derived tumor variants of LMP1 corresponding to Groups A, B, and C have gain of function mutations that result in sustained Erk activation and stabilization of the c-Fos component of AP-1.
Our analysis of the C-terminal sequences of LMP1 revealed two amino acids (Ser-212 and Thr-366) that were common to all the tumor variants but differed from the B95.8 isoform (Gly-212 and Ser-366). These sites have been previously suggested as potential HOS interacting sites (43). By point mutating these two residues in the B95.8 LMP1 variant, either individually or together to match the residues in the tumor-derived LMP1 variants, we determined that these amino acids are important in c-Fos protein induction and stabilization likely through prolongation of Erk signaling. Interestingly, only one of these sites (Gly-212 to Ser) is located within the CTAR1 domain of LMP1 that has been linked to Erk activation. Mutation of the second site (Ser-366 to Thr), located in the CTAR2 region of LMP1, also allowed for prolonged Erk activation and c-Fos induction and stabilization. This implies that Erk activation by LMP1 in B cells may involve contributions from both CTAR1 and CTAR2. Also of interest is that neither of these amino acids is located within known TRAF or TRADD interacting regions of LMP1. Therefore, in B cell lymphomas the Erk pathway may be activated by LMP1 by additional, non-TRAF-dependent means. Studies performed with human epithelial cell lines SCC12F and HeLa suggest that the Raf/Mek1/2 pathway but not Ras is necessary for Erk activation by LMP1 (49). However, a separate study of LMP1-induced Erk activation in Rat-1 epithelial cell lines indicates Ras involvement (24). One possibility is that Raf or Mek1/2 interacts with LMP1 directly through regions containing G212S and/or S366T to activate downstream Erk activation. Point mutations in tumor-derived variants of LMP1 at these residues may allow for stronger, more efficient interaction with the members of Erk-MAPK pathway allowing for sustained signaling. Future studies examining interactions between LMP1 and members of this pathway will need to be performed to address this possibility.
In conclusion, we have analyzed LMP1 variants isolated from EBV+ B cell lymphomas and from the B95.8 lab strain of EBV for their ability to activate signal transduction pathways in B cells. Our data demonstrate that all tumor-derived LMP1 variants, even those containing a 10-aa deletion overlapping the CTAR2 domain, can activate the p38, JNK, and NF-κB pathways similarly to the B95.8 LMP1 isoform. Furthermore, all LMP1 variants analyzed can activate the CTAR1-dependent PI3K pathway and induce Akt phosphorylation possibly through TRAF interactions with CTAR1. Importantly, our results provide the first evidence that tumor-derived LMP1 variants differ from the B95.8 LMP1 isoform in their ability to activate the Erk pathway and promote downstream c-Fos stabilization that may contribute to the tumorigenicity of LMP1. Finally, we identified specific amino acid residues, corresponding to aa 212 and 366 of B95.8 LMP1, that are mutated in all tumor-derived LMP1 variants analyzed and account for the difference in Erk pathway activation as well as c-Fos induction and stabilization. The identification and characterization of unique functional properties of LMP1 variants expressed in EBV+ tumors may provide new insight into the pathogenesis of EBV-associated malignancies.
This work was supported, in whole or in part, by National Institutes of Health Grants RO1AI41789 (to O. M. M.) and RO1 CA105157 (to O. M. M.). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The abbreviations used are: LMP1, latent membrane protein 1; EBV, Epstein-Barr virus; CTAR, C-terminal activation region 1; TRAF, tumor necrosis factor receptor-associated factor; TRADD, TRAF-associated death domain protein; JNK, Jun N-terminal kinase; MAP, p38 mitogen-activated protein; Erk, extracellular signal-regulated kinase, PI3K, phosphoinositide 3-kinase; NPC, nasopharyngeal carcinoma; PTLD, post-transplant lymphoproliferative disease; PE, phosphatidylethanolamine; NGFR, nerve growth factor receptor; SLCL, spontaneous B lymphoblastoid cell lines; aa, amino acid(s); mAb, monoclonal antibody; DM, double mutant; AP-1, activator protein 1; ICAM, intercellular adhesion molecule; HOS, homologue of Slimb.
References
- 1.Kaye, K. M., Izumi, K. M., and Kieff, E. (1993) Proc. Natl. Acad. Sci. U.S.A. 90 9150-9154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang, D., Liebowitz, D., and Kieff, E. (1985) Cell 43 831-840 [DOI] [PubMed] [Google Scholar]
- 3.Moorthy, R. K., and Thorley-Lawson, D. A. (1993) J. Virol. 67 1638-1646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gires, O., Kohlhuber, F., Kilger, E., Baumann, M., Kieser, A., Kaiser, C., Zeidler, R., Scheffer, B., Ueffing, M., and Hammerschmidt, W. (1997) EMBO J. 16 6131-6140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brodeur, S. R., Cheng, G., Baltimore, D., and Thorley-Lawson, D. A. (1997) J. Biol. Chem. 272, 19777-19784 [DOI] [PubMed] [Google Scholar]
- 6.Devergne, O., Cahir McFarland, E. D., Mosialos, G., Izumi, K. M., Ware, C. F., and Kieff, E. (1998) J. Virol. 72 7900-7908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Devergne, O., Hatzivassiliou, E., Izumi, K. M., Kaye, K. M., Kleijnen, M. F., Kieff, E., and Mosialos, G. (1996) Mol. Cell. Biol. 16 7098-7108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mosialos, G., Birkenbach, M., Yalamanchili, R., VanArsdale, T., Ware, C., and Kieff, E. (1995) Cell 80 389-399 [DOI] [PubMed] [Google Scholar]
- 9.Sandberg, M., Hammerschmidt, W., and Sugden, B. (1997) J. Virol. 71 4649-4656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Izumi, K. M. and Kieff, E. D. (1997) Proc. Natl. Acad. Sci. U S A. 94 12592-12597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Izumi, K. M., Cahir McFarland, E. D., Ting, A. T., Riley, E. A., Seed, B., and Kieff, E. D. (1999) Mol. Cell. Biol. 19 5759-5767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huen, D. S., Henderson, S. A., Croom-Carter, D., and Rowe, M. (1995) Oncogene 10 549-560 [PubMed] [Google Scholar]
- 13.Mitchell, T., and Sugden, B. (1995) J. Virol. 69 2968-2976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haskill, S., Beg, A. A., Tompkins, S. M., Yurochko, A. D., Sampson-Johannes, A., Mondal, K., Ralph, P., and Baldwin, A. S., Jr. (1991) Cell 65 1281-1289 [DOI] [PubMed] [Google Scholar]
- 15.Karin, M., Cao, Y., Greten, F. R., and Li, Z. W. (2002) Nat. Rev. Canc. 2 301-310 [DOI] [PubMed] [Google Scholar]
- 16.Eliopoulos, A. G., Gallagher, N. J., Blake, S. M., Dawson, C. W., and Young, L. S. (1999) J. Biol. Chem. 274 16085-16096 [DOI] [PubMed] [Google Scholar]
- 17.Lambert, S. L., and Martinez, O. M. (2007) J. Immunol. 179 8225-8234 [DOI] [PubMed] [Google Scholar]
- 18.Vockerodt, M., Tesch, H., and Kube, D. (2001) Genes Immun. 2 433-441 [DOI] [PubMed] [Google Scholar]
- 19.Wan, J., Sun, L., Mendoza, J. W., Chui, Y. L., Huang, D. P., Chen, Z. J., Suzuki, N., Suzuki, S., Yeh, W. C., Akira, S., Matsumoto, K., Liu, Z. G., and Wu, Z. (2004) Mol. Cell. Biol. 24 192-199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eliopoulos, A. G., and Young, L. S. (1998) Oncogene 16 1731-1742 [DOI] [PubMed] [Google Scholar]
- 21.Kieser, A., Kilger, E., Gires, O., Yeffing, M., Kolch, W., and Hammerschmidt, W. (1997) EMBO J. 16 6478-6485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kutz, H., Reisbach, G., Schultheiss, U., and Kieser, A. (2008) Virology 371 246-256 [DOI] [PubMed] [Google Scholar]
- 23.Dawson, C. W., Tramoutanis, G., Eliopoulos, A. G., and Young, L. S. (2003) J. Biol. Chem. 278 3694-3704 [DOI] [PubMed] [Google Scholar]
- 24.Roberts, M. L., and Cooper, N. R. (1998) Virology 240 93-99 [DOI] [PubMed] [Google Scholar]
- 25.Chuang, H. C., Lay, J. D., Hsieh, W. C., Wang, H. C., Chang, Y., Chuang, S. E., and Su, I. J. (2005) Blood 106 3090-3096 [DOI] [PubMed] [Google Scholar]
- 26.Murphy, L. O., MacKeigan, J. P., and Blenis, J. (2004) Mol. Cell. Biol. 24 144-153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Murphy, L. O., Smith, S., Chen, R. H., Fingar, D. C., and Blenis, J. (2002) Nat. Cell. Biol. 4 556-564 [DOI] [PubMed] [Google Scholar]
- 28.Skare, J., Edson, C., Farley, J., and Strominger, J. L. (1982) J. Virol. 44 1088-1091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hu, L. F., Zabarovsky, E. R., Chen, F., Cao, S. L., Ernberg, I., Klein, G., and Winberg, G. (1991) J. Gen. Virol. 72 2399-2409 [DOI] [PubMed] [Google Scholar]
- 30.Dawson, C. W., Eliopoulos, A. G., Blake, S. M., Barker, R., and Young, L. S. (2000) Virology 272 204-217 [DOI] [PubMed] [Google Scholar]
- 31.Guiretti, D. M., Chabay, P. A., Valva, P., Stefanoff, C. G., Barros, M. H., De Matteo, E., Renault, I. Z., Preciado, M. V., and Hassan, R. (2007) J. Med. Virol. 79 1722-1730 [DOI] [PubMed] [Google Scholar]
- 32.Sandvej, K., Gratama, J. W., Munch, M., Zhou, X. G., Bolhuis, R. L., Andresen, B. S., Gregersen, N., and Hamilton-Dutoit, S. (1997) Blood 90 323-330 [PubMed] [Google Scholar]
- 33.Fielding, C. A., Sandvej, K., Mehl, A., Brennan, P., Jones, M., and Rowe, M. J. (2001) Virology 75 9129-9141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mainou, B. A., and Raab-Traub, N. (2006) J. Virol. 80 6458-6468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Beatty, P. R., Krams, S. M., and Martinez, O. M. (1997) J. Immunol. 158 4045-4051 [PubMed] [Google Scholar]
- 36.Nepomuceno, R. R., Snow, A. L., Beatty, P. R., Krams, S. M., and Martinez, O. M. (2002) Transplantation 74 396-402 [DOI] [PubMed] [Google Scholar]
- 37.Mehl, A. M., Floettmann, J. E., Jones, M., Brennan, P., and Rowe, M. (2001) J. Biol. Chem. 276 984-992 [DOI] [PubMed] [Google Scholar]
- 38.Liu, L. T., Peng, J. P., Chang, H. C., and Hung, W. C. (2003) Oncogene 22 8263-8270 [DOI] [PubMed] [Google Scholar]
- 39.Murphy, L. O., and Blenis, J. (2006) Trends. Biochem. Sci. 31 268-275 [DOI] [PubMed] [Google Scholar]
- 40.Hu, L. F., Chen, F., Zheng, X., Ernberg, I., Cao, S. L., Christensson, B., Klein, G., and Winberg, G. (1993) Oncogene 8 1575-1583 [PubMed] [Google Scholar]
- 41.Nicholson, L. J., Hopwood, P., Johannessen, I., Salisbury, J. R., Codd, J., Thorley-Lawson, D., and Crawford, D. H. (1997) Oncogene 15 275-283 [DOI] [PubMed] [Google Scholar]
- 42.Johnson, R. J., Stack, M., Hazlewood, S. A., Jones, M., Blackmore, C. G., Hu, L. F., and Rowe, M. (1998) J. Virol. 72 4038-4048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tang, W., Pavlish, O. A., Spiegelman, V. S., Parkhitko, A. A., and Fuchs, S. Y. (2003) J. Biol. Chem. 278 48942-48949 [DOI] [PubMed] [Google Scholar]
- 44.Mainou, B. A., Everly, D. N., Jr., and Raab-Traub, N. (2005) Oncogene 24 6917-6924 [DOI] [PubMed] [Google Scholar]
- 45.Song, X., Tao, Y. G., Deng, X. Y., Jin, X., Tan, Y. N., Tang, M., Wu, Q., Lee, L. M., and Cao, Y. (2004) Cell. Signal. 16 1153-1162 [DOI] [PubMed] [Google Scholar]
- 46.Curran, T., Miller, A. D., Zokas, L., and Verma, I. M. (1984) Cell 36 259-268 [DOI] [PubMed] [Google Scholar]
- 47.Okazaki, K., and Sagata, N. (1995) EMBO. J. 14 5048-5059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen, R. H., Juo, P. C., Curran, T., and Blenis, J. (1996) Oncogene 12 1493-1502 [PubMed] [Google Scholar]
- 49.Dawson, C. W., Laverick, L., Morris, M. A., Tramoutanis, G., and Young, L. S. (2008) J. Virol. 82 3654-3664 [DOI] [PMC free article] [PubMed] [Google Scholar]