Abstract
The Sulfolobus solfataricus Y-family DNA polymerase Dpo4 is a model for translesion replication and has been used in the analysis of individual steps involved in catalysis. The role of conformational changes has not been clear. Introduction of Trp residues into the Trp-devoid wild-type protein provided fluorescence probes of these events, particularly in the case of mutants T239W and N188W. With both mutants, a rapid increase in Trp fluorescence was observed only in the case of normal base pairing (G:C), was saturable with respect to dCTP concentration, and occurred in the absence of phosphodiester bond formation. A subsequent decrease in the Trp fluorescence occurred when phosphodiester bond formation was permitted, and these rates were independent of the dCTP concentration. This step is relatively slow and is attributed to a conformational relaxation step occurring after pyrophosphate release, which was measured and shown to be fast in a separate experiment. The measured rate of release of DNA from Dpo4 was rapid and is not rate-limiting. Overall, the measurements provide a kinetic scheme for Dpo4 different than generally accepted for replicative polymerases or proposed for Dpo4 and other Y-family polymerases: the initial enzyme·DNA·dNTP complex undergoes a rapid (18 s-1), reversible (21 s-1) conformational change, followed by relatively rapid phosphodiester bond formation (11 s-1) and then fast release of pyrophosphate, followed by a rate-limiting relaxation of the active conformation (2 s-1) and then rapid DNA release, yielding an overall steady-state kcat of <1 s-1.
Replicative DNA polymerases insert dNTPs with high efficiency and fidelity but lose much of this capacity when they encounter DNA lesions that do not closely resemble the four canonical bases (A, T, C, or G), leading to deleterious miscoding and/or blocks to further polymerization (1). In the past decade the slower and less efficient Y-family and other translesion DNA polymerases have been characterized and found to often replace replicative polymerases at these blocked junctions (2). Based on biochemical and structural analysis, a popular consensus is that abnormal substrate geometry forms the basis for this “switch” to a translesion polymerase (3-5). Whereas replicative DNA polymerases use numerous residues to bind tightly to properly shaped substrates to bring about rapid and processive polymerization, Y-family polymerases make fewer protein-substrate contacts and allow aberrant shapes within their active sites (6).
Whether these two major enzyme classes catalyze polymerization similarly is not yet clear. Generally it is believed that replicative polymerases bind DNA tightly, followed by binding a correct dNTP (sorted from the mixture of all four that the DNA polymerase encounters) that instigates an “induced-fit” conformational change to form an active ternary complex leading to high efficiency and fidelity polymerization, e.g. (7). Subsequent to catalysis, a “relaxing” of the complex and the release of PP 4i both must occur, followed by either the dissociation of the product complex (i.e. in steady-state experiments) or translocation along the DNA to continue another round of catalysis. The induced-fit conformational change has been shown by crystallography to involve a large closing of the “O” helix of the fingers domain around the nascent base pair (8, 9), although recent studies indicate that the one or more conformational changes most responsible for high efficiency and fidelity polymerization must involve more subtle events that occur afterward and/or before this large change (10-12).
What remains unclear is whether or not there are similarly subtle events, unseen by crystallographic and other methods (e.g. atomic level dynamics simulations performed by Schlick and colleagues (13)), which comprise an induced-fit conformational change prior to selective dNTP polymerization by the Y-family DNA polymerases. Crystal structures of Sulfolobus solfataricus P2 DNA polymerase IV (Dpo4), a model Y-family DNA polymerase, have not shown obvious closing of residues around an incipient base pair; however, some discreet stepwise “shifts” of the enzyme are seen upon dNTP binding (forward movement of residues within the fingers, palm, and little finger domains, at the distance of a base pair) and following catalysis (the thumb follows suit (14)). Whether these changes are coupled to productive catalysis is not clear. Fiala and Suo (15) demonstrated a low α-phosphothioate elemental (“thio”) effect for correct dNTP phosphodiester bond formation by Dpo4, consistent with important conformational change(s) existing within the catalytic cycle, but the conclusiveness of the thio effects in elucidation of mechanisms has been questioned (16). Fiala and Suo (15) also compared product amplitudes in pulse-chase and pulse-quench experiments and concluded that Dpo4 is bound more tightly in a ternary complex, insinuating that there must be a conformational change to “lock” the complex, although the amplitudes did not appear to be statistically different. DeLucia et al. (17) used fluorescence measurements with 2-aminopurine in the substrate template to demonstrate that the Dpo4 homolog Dbh undergoes what the authors conclude to be multiple protein and DNA conformational states during the catalytic cycle, although no evidence was presented to show that it was the enzyme that was involved in the fluorescence changes. A similar study with the non-processive rat pol β and 2-aminopurine oligonucleotides was recently reported by Roettger et al. (18).
We sought evidence for a critical conformational change in Y-family DNA polymerases from the protein point of view, i.e. by fluorescent labeling of the enzyme rather than the DNA substrate to directly follow environmental changes of protein domains and avoid the complexity of base pairing, stacking, and other phenomena seen with fluorescent oligonucleotides. Others have used protein-based fluorescence to study replicative polymerases, e.g. Tsai and Johnson (19) observed fluorescence changes with a coumarin-labeled “Cys-light” bacteriophage T7 DNA polymerase and identified two conformational states prior to catalysis, one for correct dNTP positioning and another for incorrect dNTP positioning. The absence of Trp in wild-type Dpo4 provided an opportunity to prepare and utilize several single Trp mutants, based on available crystal structures. Two Dpo4 Trp mutants (Fig. 1), having full catalytic activity, revealed fluorescence changes that we interpret as conformational changes associated with (correct) dNTP positioning prior to catalysis and post-catalytic “relaxing” of the complex following rapid release of PPi, documented using independent kinetic experiments. We used the measured rates of fluorescence changes in developing minimal kinetic models and obtained satisfactory fitting of the catalytic data.
FIGURE 1.
Model of Trp residues mutated within a Dpo4 ternary structures. Adapted from PDB code 2ASD (14). A, Trp modeled into Dpo4 at residue 188; color scheme: Trp (green), Dpo4 (gray), DNA (yellow), and the incoming dCTP (blue). B, Trp modeled into Dpo4 at residue 239 (same color scheme as in A).
EXPERIMENTAL PROCEDURES
Materials—All reagents were of the highest quality commercially available. Unlabeled dNTPs were purchased from New England Biolabs (Beverly, MA). MDCC, PNPase, PPase, and 7-MeGuo were purchased from Sigma-Aldrich. High-performance liquid chromatography-purified synthetic oligonucleotides were purchased from Integrated DNA Technologies (Coralville, IA) or Midland Certified Reagant Co. (Midland, TX), and concentrations were determined spectrally using the nearest-neighbor method (20). [γ-32P]ATP was purchased from PerkinElmer Life Sciences.
DNA Sequences—Primer-template sequences used in this study
are as follows: the 13-mer primer strand 5′-GGGGGAAGGATTC-3′ and
18-mer template strand 5′-TCACXGAATCCTTCCCCC-3′, where X
= G or 8-oxoG, were annealed together and are designated in the text as either
DNAG or  ,
respectively. For analysis of fluorescence changes in the absence of
chemistry, the 3′-end of the primer strand was rendered incapable of
phosphoryl transfer by replacing the 3′-OH with a proton and is termed
,
respectively. For analysis of fluorescence changes in the absence of
chemistry, the 3′-end of the primer strand was rendered incapable of
phosphoryl transfer by replacing the 3′-OH with a proton and is termed
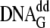 in the text. In
some experiments, the 14-mer primer sequence 5′-GGGGGAAGGATTCC-3′
was annealed to the 18-mer template 5′-TCACGGAATCCTTCCCCC-3′ to
investigate the fluorescence changes that occur, using a slightly different
sequence context.
in the text. In
some experiments, the 14-mer primer sequence 5′-GGGGGAAGGATTCC-3′
was annealed to the 18-mer template 5′-TCACGGAATCCTTCCCCC-3′ to
investigate the fluorescence changes that occur, using a slightly different
sequence context.
Dpo4 Trp Mutants—Wild-type Dpo4 contained a 6-histidine tag on the N terminus and was expressed in Escherichia coli and purified to electrophoretic homogeneity using nickel-nitrilotriacetic acid permeation and Mono-S chromatography as described previously (21). Purified Dpo4 was stored in small aliquots at -80 °C in 50 mm Tris-HCl buffer (pH 7.7 at 22 °C) containing 50 mm NaCl, 1 mm dithiothreitol, and 50% glycerol (v/v).
Mutagenesis was performed using a QuikChange site-directed mutagenesis kit (Stratagene, La Jolla, CA), and sequences were verified in the Vanderbilt DNA Sequencing Facility prior to bacterial expression. The Dpo4 T239W mutant was made using the following primers: sense, 5′-AGCTAGAGACGAGTATAACGAGCCTATAAGATGGAGAGTACGAAAGAGTA-3′; antisense, 5′-TACTCTTTCGTACTCTCCATCTTATAGGCTCGTTATACTCGTCTCTAGCT-3′. For Dpo4 N188W: sense, 5′-GCGGATGTACCCGGAATAGGTTGGATAACTGCGGAGAAACTAAAG-3′; antisense, 5′-CTTTAGTTTCTCCGCAGTTATCCAACCTATTCCGGGTACATCCGC-3′. For Dpo4 Y12W: sense, 5′-TGATTGTTCTTTTCGTTGATTTTGACTACTTTTGGGCTCAAGTTGAAGAAGT; antisense, 5′-ACTTCTTCAACTTGAGCCCAAAAGTAGTCAAAATCAACGAAAAGAACAATCA. Mutant proteins were expressed, purified, and stored using the same procedure as for wild-type enzyme (21), except that the 80 °C heat step was omitted because of the possibility of decreased mutant stability.
MDCC-PBP—The plasmid containing E. coli PBP with an A197C mutation, competent cells, and protocols for expression and labeling by MDCC were generously provided by C. E. Cameron (Pennsylvania State University, University Park, PA) and K. D. Raney (University of Arkansas Medical Center, Little Rock, AR). PBP was expressed from a pET-Ub-based plasmid in E. coli BL21(DE3)pCG1 competent cells in NZCYM media supplemented with 25 μg of kanamycin ml-1 and 20 μg of chloramphenicol ml-1. The pCG1 vector expresses a yeast ubiquitin protease that frees the fused protein from ubiquitin to produce mature protein (22). Overnight cultures (100 ml) were grown in NZCYM media supplemented with 25 μg of kanamycin ml-1, 20 μg of chloramphenicol ml-1, and 0.1% dextrose (w/v) at 30 °C (overnight) and used to inoculate 500 ml of NZCYM media containing 10 ml of a sugar solution (250 g of glycerol, 25 g of dextrose, and 100 g of lactose diluted to 1 liter), 25 ml of NPS solution (66 g of (NH4)2SO4, 136 g of monobasic potassium phosphate, and 142 g of dibasic sodium phosphate diluted to 1 liter), and 5 ml of a metal mixture (100 mm FeCl3, 1 m CaCl2, 1 m MnCl2, 1 m ZnSO4, 200 mm CoCl2, 100 mm CuCl2, 200 mm NiCl2, 100 mm Na2MoO4, 100 mm Na2SeO3, and 100 mm H3BO3). The cells were grown at 37 °C with aeration at 250 rpm to A600 = 1.0 and then cooled to 20 °C for overnight growth (12-16 h). Cells were harvested by pelleting at 6 × 103 × g in a Sorvall Model RC3B-Plus centrifuge (swinging bucket rotor) for 10 min (4 °C), washed once in 100 ml of 10 mm Tris-HCl buffer (pH 8.0, containing 1 mm EDTA), pelleted again, and stored at -80 °C. Each 500-ml culture yielded ∼10 g of wet cell weight.
Cells were suspended at a concentration of 4 mg of protein ml-1 in 50 mm sodium HEPES buffer (pH 7.5) containing 500 mm NaCl and 20% glycerol (v/v) and supplemented with protease-inhibitor mixture tablets (Roche Applied Biosciences, Indianapolis, IN) prior to lysis by sonication; the lysed cells were pelleted in a Beckman 45Ti rotor at 7 × 104 × g for 30 min at 4 °C. Polyethylene glycol 8000 was added to the supernatant to a final concentration of 6% (w/v) while stirring at 4 °C, followed by centrifugation at 104 × g for 10 min. Solid (NH4)2SO4 was added to the supernatant to 60% saturation, followed by centrifugation at 104 × g for 10 min; more solid (NH4)2SO4 was added to the supernatant to 80% saturation, followed by centrifugation at 7 × 104 × g for 20 min. The resulting pellet contained primarily the protein of interest, which was dissolved in and dialyzed against 10 mm Tris-HCl buffer (pH 8.0) containing 1.0 mm MgCl2. Further purification was by Q-Sepharose chromatography as described by Brune et al. (23).
Purified PBP was labeled with MDCC in the presence of a “phosphate mop,” as described by C. E. Cameron and K. D. Raney (personal communication). The phosphate mop removes trace Pi from solution by covalently coupling N7-MeGuo with Pi enzymatically using PNPase. To 1 ml of 100 μm Q-Sepharose-purified PBP was added N7-MeGuo to a final concentration of 2 mm, PNPase to 103 unit ml-1, and Tris-HCl buffer (pH 8.0) to 10 mm. After 30 min of incubation time (to allow for any free Pi to be eliminated via the phosphate mop), MDCC (25 mm stock, dissolved in dimethylformamide) was added to a final concentration of 2 mm and the sample was slowly rotated overnight at 22 °C. Subsequently, the mixture was centrifuged at 1.5 × 104 × g for 60 s to pellet undissolved MDCC, and the resulting supernatant was dialyzed against 10 mm Tris-HCl buffer (pH 8.0) containing 100 μm sodium phosphate in preparation for chromatography on a Mono-Q column equilibrated with the same buffer solution. As described by Brune et al. (23), the resulting preparation of PBP-MDCC contains a mixture of Pi-responsive and -unresponsive protein. Two major peaks were eluted from the Mono-Q column: the protein in the first peak was highly Pi-responsive, producing a 10-fold increase in fluorescence emission at 465 nm (excitation at 435 nm) at a rate of 450 s-1 at saturating Pi concentration, measured by stopped-flow fluorescence measurements at 10 °C (data not shown).
5′-End Labeling of Primers and Annealing to Primer-Template Complexes—The 13-mer DNA primer was 5′-labeled with [γ-32P]ATP using T4-polynucleotide kinase and annealed to the 18-mer templates as described previously (24, 25).
Reaction Buffer and Conditions—Unless stated otherwise, reactions were performed at 37 °C in 50 mm Tris-HCl buffer (pH 7.4 at 37 °C) containing 50 mm NaCl, 1 mm dithiothreitol, and 5% glycerol (v/v). Although bovine serum albumin is often used in DNA polymerase assays, it was not added here because it contains two tryptophan residues that could interfere with the fluorescence of the Trp-Dpo4 mutants. Deletion of albumin was found not have an effect on the rate of DNA polymerization.
Chemical Quench Analysis—In the determination of the steady-state parameters kcat and Km for single nucleotide incorporation, typical assays included (all concentrations are final) 1 μm 32P-primer-labeled DNAG, 5 mm MgCl2, and various concentrations of dNTP mixed together prior to adding 1-5 nm enzyme, in a final volume of 10 μl. Reactions were initiated by the addition of enzyme, incubated for 5 min at 37 °C, and then quenched by the addition of 10 μl of gel loading dye (90% formamide in 90 mm Tris-HCl buffer (pH 8.3) containing 90 mm boric acid, 2 mm EDTA, and 0.05% bromphenol blue, w/v). Products were separated by gel electrophoresis (20% polyacrylamide (w/v), 7 m urea) and analyzed by phosphorimaging using Quantity One software (Bio-Rad, Hercules, CA). Data were fit to the Michaelis-Menten equation to obtain the needed parameters using non-linear regression with the program Prism (GraphPad, San Diego, CA). In these steady-state determinations, the concentration of product DNA did not exceed 25% of the total concentration of substrate DNA.
A KinTek RQF-3 model chemical quench-flow apparatus (KinTek Corp., Austin, TX) was used to perform pre-steady-state experiments. Typically, excess wild-type or mutant Dpo4 (1-5 μm), incubated with 300 nm primer-labeled DNAG, was rapidly mixed with 5 mm MgCl2 and various concentrations of dNTP to obtain the nucleoside triphosphate-dependent rate of polymerization, kp. Incubations proceeded for a set amount of time (5 ms to 20 s) before samples were quenched with 0.37 m EDTA, and the substrate and product primers were separated by electrophoresis and analyzed by phosphorimaging.
Fluorescence Emission Spectra—An OLIS DM-45 spectrofluorometer (On-Line Instrument Systems, Bogart, GA) was used to record emission spectra of Dpo4 Trp mutants and PBP-MDCC (at 22 °C). For Dpo4 Trp mutants (excitation at 290 nm), the emission spectra of various complexes of Dpo4 Trp mutants were recorded before and after addition of substrates (e.g. DNA or dNTP to form binary or ternary complexes, respectively), scanning from 320 to 420 nm. For PBP-MDCC, detection of fluorescence intensities with and without Pi was achieved by exciting the chromophore at 435 nm and detecting emission over a wavelength range of 435-590 nm. Reaction buffer alone was used as a reference standard for subtraction.
Stopped-flow Fluorescence Measurements—An OLIS RSM-1000
spectrofluorometer was used in measurements involving transient fluorescent
assays. For optimal signal/noise ratios for observing changes in Trp
fluorescence, 3.16-mm slits (20 nm bandwidth) were employed along with both
335 nm long-pass (CVI Laser Corp., Albuquerque, NM) and 355 nm bandpass
filters (Newport, Irvine, CA) attached to the sample photomultiplier tube.
MgCl2 (5 mm) was included in all syringes. In typical
experiments for measuring the effect of dNTP binding on fluorescence of the
Dpo4 Trp mutants, one syringe contained mutant Dpo4 plus
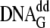 , and the second
syringe contained various concentrations (up to 900 μm) of the
correct dNTP. After rapid mixing, the final concentration of Dpo4:DNA was 1
μm. For determination of DNA off-rates, Dpo4 T239W (in one
syringe, 1 μm) was present in an equimolar complex with DNA and
rapidly mixed with a 5-fold excess of wild-type protein from the second
syringe. Alternatively, the DNA was added to wild-type Dpo4 and was rapidly
mixed with a 5-fold excess of mutant enzyme. For analysis of ternary complex
, and the second
syringe contained various concentrations (up to 900 μm) of the
correct dNTP. After rapid mixing, the final concentration of Dpo4:DNA was 1
μm. For determination of DNA off-rates, Dpo4 T239W (in one
syringe, 1 μm) was present in an equimolar complex with DNA and
rapidly mixed with a 5-fold excess of wild-type protein from the second
syringe. Alternatively, the DNA was added to wild-type Dpo4 and was rapidly
mixed with a 5-fold excess of mutant enzyme. For analysis of ternary complex
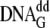 off-rates, Dpo4
mutant Y12W was added in 5-fold excess to a 1 μm wild-type
Dpo4·
off-rates, Dpo4
mutant Y12W was added in 5-fold excess to a 1 μm wild-type
Dpo4·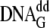 ·dCTP
complex. Both syringes contained 500 μm dCTP. In all cases,
standard assays were performed, including all components except the reagent
producing a change (dNTP in the case of nucleotide dependence on fluorescence
changes, or DNA in the case of DNA off-rate determinations).
·dCTP
complex. Both syringes contained 500 μm dCTP. In all cases,
standard assays were performed, including all components except the reagent
producing a change (dNTP in the case of nucleotide dependence on fluorescence
changes, or DNA in the case of DNA off-rate determinations).
Observation of PPi release during DNA polymerization was measured as described by Hanes and Johnson (26, 27). Briefly, MDCC-PBP fluorescence was observed (OLIS RSM-1000) using 1.24-mm slits (8 nm bandwidth) along with an Oriel 455 nm long-pass filter (Oriel, Stratford, CT) coupled to the photomultiplier tube. To ensure that free Pi was minimal prior to mixing of syringe contents, a mixture of 200 μm N7-MeGuo, 0.2 unit of PNPase ml-1, and 0.005 unit of yeast PPase ml-1 was present in both syringes, along with reaction buffer. A mixture of 500 μm dCTP, 5 mm MgCl2, and 1.1 μm PBP-MDCC from one syringe was rapidly mixed with a mixture of 1.2 μm Dpo4 T239W and 200 nm DNAG from the other, and the resulting Pi binding to PBP-MDCC (following release of PPi due to DNA polymerization and rapid hydrolysis of PPi to Pi by PPase) was observed by monitoring the increase in fluorescence emission at 465 nm (455 nm long-pass Oriel filter, excitation at 435 nm).
Data Analysis—The data obtained from gel electrophoresis and fluorescence experiments was analyzed by non-linear regression using Equation 1,
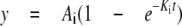 |
(Eq.1) |
where y is the concentration or signal produced over time, Ai is the amplitude of the signal, ki is the observed rate constant, and c is the starting point. Kinetic parameters obtained using Equation 1 were fit to the following hyperbolic function to describe the concentration dependence,
 |
(Eq.2) |
where S is the substrate concentration, ki is the maximum rate constant, Kd is the apparent dissociation constant with S, and c is the starting point.
To obtain fits to fluorescence decays, Equation 3 was used,
 |
(Eq.3) |
where kr is the rate of decay.
Global Data Fitting—Pre-steady-state and steady-state data were fitted using the program DynaFit (28). Best fits were obtained to plots of time courses or rates versus substrate concentrations using least-squares curve fitting by Levenberg-Marquardt methods, based upon integration of ordinary differential equations.
RESULTS
Construction of Dpo4 Trp Mutants—Wild-type Dpo4 is devoid of Trp. On the basis of available crystal structures of Dpo4 (6, 14, 21, 29-35), we selected seven potential sites that might report different conformational changes: Tyr-12, Phe-37, Tyr-48, Asn-188, Thr-239, Arg-332, and Tyr-274. Constructs with Trp substitutions at each of these sites were prepared, and the proteins were expressed in E. coli and purified. Five of the seven mutants had only low catalytic activity in preliminary assays and were not used for catalytic experiments (Y12W, F37W, Y48W, R332W, and Y274W). Two mutants, T239W and N188W (Fig. 1), were highly active (Table 1) and were utilized. Dpo4 T239W has the Trp residue in a region near the “little finger” domain, which has roles in fidelity and lesion bypass (36). Dpo4 N188W reported similar fluorescence changes and was used to validate and supplement results obtained with Dpo4 T239W.
TABLE 1.
Kinetic parameters describing polymerization of dCTP opposite template G by wild-type and Dpo4-Trp mutants
Steady-state and transient analysis of product formation were done using
radiolabeled DNAG to compare mutant and wild-type Dpo4
polymerization rates and dissociation constants
(Table 1 and supplemental Fig.
S1). The specificity constants kcat/Km
and kpol/Kd were both similar (within
a factor of four) to those routinely found for wild-type Dpo4 protein in this
laboratory (31,
34,
35,
37). The reported
DNAG Kd for wild-type Dpo4 is 50 nm
(37) and was found to be
similar for these mutants. A third Dpo4 mutant, Y12W, was found to be
relatively inactive (see above) and was used only to report conformational
changes associated with DNA release from the
Dpo4· ·dCTP
complex.
·dCTP
complex.
Fluorescence Properties of Dpo4 Mutants and Complexes—Fluorescence emission spectra were recorded for these mutants under multiple states and in complex with DNAG (Fig. 2, A-C). A red shift (∼10 nm) of the fluorescence maximum and a large overall quenching of fluorescence were observed (the fluorescence was somewhat intensified at higher wavelengths), upon adding DNAG to T239W. A large quenching of the fluorescence was also observed upon the addition of DNAG to Dpo4 N188W or Y12W. The addition of saturating concentrations of dNTP (500 μm) produced a strong inner filter effect (i.e. quenching of fluorescence observed after the addition of 500 μm dNTPs to the free amino acid Trp, Fig. 2D). However, upon closer observation, a significant increase in fluorescence of the Dpo4 T239W ternary complex was observed upon addition of the correct dNTP (dCTP) as compared with the free amino acid Trp control, e.g. note the switch in intensity maxima between dCTP (blue trace) and dTTP (green trace) comparing control and Dpo4 T239W scans (Fig. 2, D and E). Additionally, a red shift of ∼6 nm occurred upon the addition of dCTP to Dpo4 T239W-DNAG as well (Fig. 2D). A red shift of ∼6 nm was also seen with the addition of dGTP, but this is attributed to a C base immediately upstream of the templating G (see below).
FIGURE 2.
Steady-state fluorescence spectra of substrate binding to Dpo4-Trp mutants. A, spectra of 1 μm Dpo4 T239W before (black trace) and after (blue trace) addition of 1 μm DNAG was added, respectively. B and C, same as in A but with Dpo4 N188W (B) and Dpo4 Y12W (C), respectively. D, free Trp control. Spectra were obtained after adding 500 μm of each dNTP separately to 1 μm free Trp: dATP (red), dTTP (green), dCTP (blue), dGTP (brown), and no dNTP (black). E, same as in part D, except 1 μm free Trp was replaced by a 1 μm Dpo4 T239W·DNAG complex.
Despite the inner filter effects, stopped-flow fluorescence kinetic measurements showed increases in fluorescence over time that were dependent upon the identities of both the incoming and template bases. Depending on the sequence context, only the addition of the correct dNTP (500 μm) to 1 μm Dpo4 T239W-DNAG produced a rapid increase in fluorescence (Fig. 3, A-C). When phosphodiester bond formation was possible (3′-OH-terminated primer DNA was added instead of dideoxy-terminated primer), this increase was followed by a slower “decay” of fluorescence that ended at varying intensities (Fig. 3B). Using DNAG in complex with Dpo4 T239W, increased fluorescence was also observed upon addition of an incorrect dNTP, in this case dGTP opposite G. However, because the “+1” template base in this case was a C, this phenomenon is likely due to “slippage” of the template followed by insertion of a dNTP opposite the +1 template base, corresponding to single base deletions, a frequent occurrence during polymerization with Y-family DNA polymerases (38, 39).
FIGURE 3.
Fluorescence changes of Dpo4 mutants T239W and N188W observed upon binding of dNTPs and catalysis. The relevant portion of the DNA sequence within the region of dNTP binding is shown above the graphs. All dNTPs were added to a final concentration of 500 μm: dATP (red), dTTP (black), dCTP (blue), and dGTP (green). A, G as the incipient template base and C the next, +1 base. The addition of dCTP or dGTP increased the fluorescence of Dpo4 T239W in the absence of phosphodiester bond formation (3′-deoxy primer terminus). B, when phosphodiester bond formation occurred (3′-OH primer terminus), the fluorescence increase seen with Dpo4 T239W was followed by a decrease (adding dCTP opposite G). C, an experiment similar to that of C was done, but the sequence context was changed to insert dNTPs opposite C (instead of G). D, with Dpo4 N188W as the enzyme (using the same sequence context as in part A), fluorescence changes were only seen when the correct dNTP was added.
Similar fluorescence changes were observed with Dpo4 N188W, i.e. a fast increase in fluorescence intensity upon binding of the correct dNTP followed by a slower fluorescence decay as phosphodiester bond formation proceeded (Fig. 3D). Addition of the +1 dNTP (dGTP) to the Dpo4 N188W-DNAG complex did not yield a change in fluorescence as in the case of Dpo4 T239W-DNAG (Fig. 3B), implying that position (Trp) 239 is in a more ideal position to directly observe the cause(s) of single base deletions.
DNA Dissociation Rates from Binary and Ternary Dpo4 Complexes—Fiala and Suo (15) used the results of (Sp)-dNTP α-thiophosphate product burst/DNA trapping experiments to determine that the rate of release of DNA from a (wild-type) Dpo4·DNA·dNTP complex (0.41 s-1) is rate-limiting in steady-state catalysis, although this conclusion is based on a small difference in burst amplitudes. In contrast, other groups have used fluorescence measurements with the closely related Dbh polymerase of Sulfolobus acidocaldarius (both Dbh and Dpo4 are members of the DinB polymerase family) and reported that Dbh releases DNA from a binary complex at a rate of 100 s-1, 103-fold faster than suggested by Fiala and Suo for Dpo4 (15, 17, 40).
DNA off-rates were measured using several Trp fluorescence approaches (Table 2). A complex of equimolar amounts of Dpo4 T239W and DNAG was mixed with a 5-fold excess of unlabeled wild-type Dpo4 (trap), and the fluorescence change was monitored, i.e. the DNA was released from the Trp-labeled enzyme and bound to the excess trap enzyme (Fig. 4A). Dpo4 T239W fluorescence decreased when DNA was released (as shown with the longer range of wavelengths relevant to this stopped-flow analysis, Fig. 2A), and the decay rate was fit (single-exponential) to obtain a DNAG koff = 73 ± 3 s-1. A reciprocal analysis was done to determine the off-rate of the DNAG duplex from wild-type Dpo4 by mixing a wild-type Dpo4·DNAG complex with a 5-fold molar excess of Dpo4 T239W (trap); the rate of fluorescence increase (i.e. released DNA rapidly bound to Dpo4 T239W) was koff = 70 ± 4 s-1 (Fig. 4B). In control experiments, mixing of DNAG with Dpo4 T239W was done to exclude the possibility that the fluorescence change is not simply reporting the DNA on-rate; the on-rate was too fast to be observed under these conditions at the enzyme concentration used (black trace, Fig. 4B).
TABLE 2.
DNA off-rates from various Dpo4 complexes as determined by Trp fluorescence
| Enzyme complex | koff |
|---|---|
| s−1 | |
| Dpo4·DNAG | ~70 |
| Dpo4·DNAG·dCTP | 16 ± 3 |
| Dpo4·DNAG | 17 ± 3 |
FIGURE 4.
DNA off-rates estimated by changes in Trp fluorescence of Dpo4
mutants. A, a Dpo4·DNAG complex was rapidly
mixed with a 5-fold molar excess of wild-type Dpo4 as a trap and the change in
fluorescence upon release of DNAG from T239W was observed,
koff = 73 ± 3 s-1. B, a
wild-type Dpo4·DNAG complex (blue trace) or
DNAG alone (black trace) was rapidly mixed with a 5-fold
molar excess of Dpo4 T239W as the trap, and the fluorescence increase observed
upon Dpo4 T239W binding of the released DNA from wild-type Dpo4 was recorded,
koff = 70 ± 4 s-1. C, as in
B, DNA off-rates from wild-type Dpo4 binary
(Dpo4·DNAG, blue trace) and ternary
(Dpo4· ·dCTP,
red trace) complexes upon
·dCTP,
red trace) complexes upon
 binding to Dpo4
Y12W were recorded, koff(binary) = 99 ± 6
s-1 and koff(ternary) = 16 ± 2
s-1.
binding to Dpo4
Y12W were recorded, koff(binary) = 99 ± 6
s-1 and koff(ternary) = 16 ± 2
s-1.
With a DNA off-rate of ∼70 s-1, Dpo4 can only exhibit a burst during the pre-steady state if the rate of DNAG release is considerably reduced after the phosphodiester bond formation event. A ternary complex might lower substrate off-rates. To determine the off-rate of DNA (3′-dd terminated primer) from ternary complexes by fluorescence, a different approach was needed. Dpo4 T239W and N188W report fluorescence changes upon binding of both the DNAdd and dNTP substrates, and this approach is problematic. A third Dpo4 mutant, Y12W, was used, which records a fluorescence decrease upon binding of DNA but not dNTPs (data not shown). Using Dpo4 Y12W as the trap enzyme, we determined the off-rate of DNAG from a ternary·wild-type Dpo4 complex by observing DNA binding to the labeled trap, with comparison to a binary control, koff(binary) = 99 ± 6 s-1 and koff(ternary) = 16 ± 2 s-1 (Fig. 4C). The fluorescence-derived DNA off-rates are summarized in Table 2.
Kinetics of Nucleotide Binding—In the absence of
phosphodiester bond formation, apparent ground-state dNTP binding constants
and rates of dNTP-induced conformational changes can be estimated according to
the following model (Reaction
1).
To define the kinetics of enzyme conformational change, data (Fig. 5A) were fit to single exponentials to obtain rates, plotted as a function of dCTP concentration opposite an unmodified G (Fig. 5B). The data were fit to Equation 2, with kobs = k3[dNTP]/(Kd(G) + [dNTP]) + k-3, with a ground-state dissociation constant (Kd(G) = 210 ± 46 μm), a forward conformational change rate (k3 = 18 ± 1 s-1), and a reverse conformational change rate (k-3 = 21 ± 1 s-1) (41) (see supplemental Fig. S3).5 Kd(G) was within error of the value obtained by analyzing product formation, Kd (dCTP opposite G) = 190 ± 40 μm (Table 1). The net equilibrium dissociation constant, defined as the product of all equilibrium constants prior to the catalytic step, was obtained by plotting the maximal fluorescence intensity as a function of dCTP concentration and fit to a hyperbola to obtain Keq,G = 41 ± 7 μm (Fig. 5C). Because Keq,G is a function of both the dNTP ground-state dissociation constant and the isomerization constant (defined as K3 = k3/k-3), Keq,G can also be obtained using the equation Keq,G = Kd(G)/(1 + K3) = 110 μm (similar to the independently obtained value, 41 ± 7 μm). The kinetic constants obtained by this analysis are summarized in Fig. 6A.
FIGURE 5.
Fluorescence changes observed upon dCTP binding to a Dpo4
T239W· complex. A, stopped-flow fluorescence changes induced by addition
of dCTP opposite template G: 15 μm (red); 30
μm (orange); 50 μm (green); 120
μm (blue); 240 μm (violet); and
500 μm (brown). B, rates of dCTP-induced
fluorescence changes and the ground state Kd,dCTP opposite
template G were estimated by fitting the observed rates as a function of dCTP
concentration to a hyperbola (Equation 2, see “Experimental
Procedures”), k3 = 18 ± 1 s-1,
k-3 = 21 ± 1 s-1, and
Kd,dCTP = 210 ± 46 μm. C,
the maximal amplitude reached at each concentration of dCTP in A was
plotted and fit to a hyperbola to obtain an equilibrium dissociation constant,
Keq(G) = 41 ± 7 μm.
complex. A, stopped-flow fluorescence changes induced by addition
of dCTP opposite template G: 15 μm (red); 30
μm (orange); 50 μm (green); 120
μm (blue); 240 μm (violet); and
500 μm (brown). B, rates of dCTP-induced
fluorescence changes and the ground state Kd,dCTP opposite
template G were estimated by fitting the observed rates as a function of dCTP
concentration to a hyperbola (Equation 2, see “Experimental
Procedures”), k3 = 18 ± 1 s-1,
k-3 = 21 ± 1 s-1, and
Kd,dCTP = 210 ± 46 μm. C,
the maximal amplitude reached at each concentration of dCTP in A was
plotted and fit to a hyperbola to obtain an equilibrium dissociation constant,
Keq(G) = 41 ± 7 μm.
FIGURE 6.
Kinetic scheme of steps prior to phosphodiester bond formation. dNTP dissociation constants and conformational change rates were based on Dpo4 T239W fluorescence measurements. E, enzyme (Dpo4); E*, enzyme conformation posed for catalysis. A,dCTP opposite a template G; B, opposite 8-oxoG (vide infra). See Figs. 5 and 11.
Slow Fluorescence Decay Step—When phosphodiester bond formation was possible, the increase in fluorescence intensity of the Trp was followed by a slower rate of decay (e.g. Fig. 3C). The rate of product formation was dependent upon a ground-state dissociation constant of ∼200 μm for dCTP incorporation opposite G (Table 1); thus if the decay is a measure of the actual rate of phosphodiester bond formation, one would expect the rate to be sub-maximal at concentrations <200 μm. However, varying the dCTP concentrations from 15 to 500 μm did not significantly alter the decay rate, ∼1.5 s-1, opposite G (Fig. 7).
FIGURE 7.
Kinetics of fluorescence decay following phosphodiester bond formation. Initial time points (i.e. Fig. 3) were excluded to show the fit of the data to a single decay exponential (Equation 3, see “Experimental Procedures”). A, Dpo4 T239W·DNAG (1 μm) was mixed with dCTP: 15 μm (red), 30 μm (orange), 50 μm (green), 120 μm (blue), 240 μm (purple), and 500 μm (brown), and the resulting fluorescence changes were recorded. Inset: decay rates plotted as a function of dCTP concentration.
PPi Release Kinetics—The fluorescence decay rates (Figs. 3 and 7) report on a specific step within the latter part of the catalytic cycle, i.e. following phosphodiester bond formation. The phosphodiester bond formation step was ruled out as a possible assignment, because the decay rate did not depend on the dNTP concentration. PPi release was hypothesized as likely to be too fast to be rate-limiting, on the order of >104 s-1 assuming a ground-state dissociation constant of >100 μm and PPi binding close to a diffusion limit (108 m-1 s-1). An attempt to observe the reverse reaction, PPi binding, did not result in any observable fluorescence change even at high mm PPi concentrations. Also, DNA release from a post-phosphodiester bond formation binary complex was >10-fold too fast, based on the fluorescence work (Fig. 4). The fluorescence decay rate was attributed to relaxation of the ternary complex, which must occur after phosphodiester bond formation.
A coupled system was utilized that indirectly reports the rate of PPi release by directly monitoring the fluorescence change resulting from hydrolyzed PPi (Pi) binding to PBP-MDCC (26). In this system, the PPi released during Dpo4 catalysis is rapidly hydrolyzed by PPase, and the resulting Pi binds to PBP-MDCC, causing a rapid conformational change that can be monitored fluorometrically. The fastest rate of PPi release that could be monitored (without observing a lag in the kinetics) was ∼25 s-1, measured after mixing the Dpo4·oligonucleotide substrate complex with dCTP (27). The rate for the fluorescence change indicative of Pi binding to PBP-MDCC (following Dpo4 T239W polymerization of dCTP opposite a template G (Fig. 8)) was 2.1 s-1. The rate of product formation for dCTP opposite G was performed under identical conditions; both data sets were compared directly and found to be nearly identical (Fig. 8). Because these rates match, the actual release of PPi must be much faster, at least an order of magnitude, in that all of the prior steps involved in the initial fluorescence changes and phosphodiester bond formation contribute to the observed rate. Therefore, the release of PPi must occur before the fluorescence decay.
FIGURE 8.
Comparison of rate of PPi release with incorporation of C opposite G. Dpo4 T239W (1.2 μm) was preincubated with 200 μm DNAG prior to rapid mixing in the stopped-flow apparatus with 500 μm dCTP and 1.1 μm PBP-MDCC with 0.005 unit of PPase ml-1, 5 mm MgCl2, and a phosphate mop (200 μm N7-MeGuo and 0.2 unit of PNPase ml-1) present in both syringes. PPi dissociation was monitored by stopped-flow fluorescence measurements upon binding of the released Pi to PBP-MDCC, k = 2.1 s-1 (the residuals trace is shown in the inset). Superimposed are data from rapid quench experiments (○) monitoring product formation using a 32P-labeled primer under identical conditions.
Simulation of Polymerization Kinetics—All modeling was done using the program DynaFit (28). For polymerization of unmodified DNA by Dpo4 (dCTP inserted opposite G), rate constants were added to a classic minimal model (42), with an adjustment to have a conformational change occur after the release of PPi (Fig. 9A). This latter modification of the order of the catalytic cycle was made to accommodate the observed fast release of PPi relative to the slow fluorescence decay rate (Fig. 8). Typical DNA polymerase initial values were given to steps presently uncharacterized for Y-family polymerases (Fig. 9A), primarily based on work performed previously using exonuclease-deficient replicative polymerases (43, 44). Similar substrate and product DNA off-rates were used (43, 44), and substrate binding was assumed to occur at a near diffusion-limited rate constant of ∼108 m-1 s-1. The PPi dissociation constant was set at ∼500 μm and k-4, the rate constant for pyrophosphorolysis, at 1 s-1 (26, 42, 45). The rate constant for the reverse of the conformational change following PPi release (k-6) was initially assumed to be unfavorable, e.g. ∼0.01 s-1, but after performing iterations to best fit the data sets, was adjusted to a larger value (see below).
FIGURE 9.
Fitting of pre-steady-state and steady-state rates for dCTP polymerization opposite a template G by Dpo4 T239W to a minimal kinetic model. See supplemental Figs. S4-S6 for data, script, and result files. A, minimal model, with rate constants. E, Dpo4 T239W; Dn, substrate DNA; Dn + 1, product DNA; and E*, Dpo4 T239W in active conformation. B, plot of the rate of phosphodiester bond formation by Dpo4 T239W kobs values (•) versus dCTP concentration (Fig. S1B) by fitting to the minimal model (solid line). C, model fit to plots of product formation (•) versus time dependence on dCTP concentration (Fig. S1A). D, model fit to steady-state plot of v (kobs) (•) versus dCTP concentration plot (Fig. S1C). The lines (C and D) were fit to the rate constants in the model (A) using the program DynaFit. See supplemental Figs. S4-S6 for DynaFit scripts and results.
Initial modeling was done with the data set in which kp was measured as a function of dCTP concentration (supplemental Fig. S1B) to obtain a reasonable value for the rate constant for phosphodiester bond formation, k4 (Fig. 9B). Previously, hyperbolic fitting of the data had yielded an estimate, kpol = 5.7 s-1 (Table 1), but fitting to this scheme gave a higher value of k4 = 11 s-1, which is attenuated to produce the observed rates. k4 must be higher than the observed rate, because k4 is a function of both kpol and the forward conformational change rate k3, i.e. k4 = k3kpol/(k3 - kpol) (19). Using the adjusted value of k4 as the rate constant for phosphodiester bond formation, data sets of product formation versus time at varying dCTP concentrations (supplemental Fig. S1A) and a data set of steady-state v versus dCTP concentration (supplemental Fig. S1C) were fit to the model and adjusted using reiterative processes with the software (Fig. 9, C and D, respectively). Multiple combinations of rate adjustments were attempted by “floating” the rate constants, but the only value that needed a large adjustment was the rate constant for the reverse of the conformational change following PPi release (k-6), to a final value of 300 s-1, to obtain optimal fits for both the pre-steady-state and steady-state data plots.
Although the fits are reasonable, the overall amount of product formed was somewhat overestimated by the model in the pre-steady-state data plots (Fig. 9C). The model predicts a complete conversion of substrate DNAG to product at each dCTP concentration, but the possibility exists that an additional, unproductive step within the catalytic cycle negatively impacts product formation in the pre-steady-state. There is no evidence to suggest that there is an inactive complex formed during correct dNTP polymerization by DNA polymerases (43, 44), but crystallography has revealed that Dpo4 makes minimal contacts with a correct dNTP within a ternary complex (compared with replicative polymerases, e.g. (6)). A step in which an active ternary complex loses substrate, i.e. E*·DNAG·dCTP↔E*·DNAG + dCTP, was added to the minimal mechanism without changing the rate constants in Fig. 9A. The forward rate was optimized to 11 s-1, with a reverse rate of 0.005 s-1 (Fig. 10A), and the modified model better fit the pre-steady-state data. The adjusted model (Fig. 10A) was applied to the data sets of Fig. 10, B and D, and provided better fits (see supplemental Figs. S4-S6). Alternative models were also considered, e.g. adding an inactive complex (E·DNAG·dCTP↔E′·DNAG·dCTP (ternary) or E·DNAG↔E′·DNAG (binary)) but found to fit the pre-steady-state data or the steady-state data poorly (supplemental Figs. S5 and S6).
FIGURE 10.
Kinetic model with an additional step representing a dissociated active complex. A, adjusted minimal model (Fig. 9A) with the added step E*·DNAG·dCTP Δ E*·DNAG + dCTP. B, model fit (solid line) to plots of product formation (•) versus time at various dCTP concentrations (Fig. 9C), with the added step. The model was also fit to a steady-state plot of v versus dCTP concentration with a result virtually identical to that in Fig. 9D (results not shown).
Kinetics of DNA Release and Nucleotide Binding Opposite a Modified Template—We also analyzed dCTP incorporation opposite template 8-oxoG, an oxidative lesion that causes some Dpo4 miscoding during replicative DNA polymerization, in possible contrast to normal base pair formation (35). Binding of dCTP opposite 8-oxoG yielded a large fluorescence increase similar to G. Dpo4 primarily treats 8-oxoG as a G mimic, i.e. by incorporating dCTP opposite the lesion ∼95% of the time (steady-state analysis) (31, 35), but wild-type Dpo4 incorporates dCTP more rapidly opposite 8-oxoG than opposite G; kpol/Kd ∼ 20-fold higher (35).
With Dpo4 T239W, rates were measured as before with G as the template base.
Dpo4 T239W fluorescence decreased upon
 release
(Fig. 11A), and the
decay rate was fit (single exponential) to obtain a
release
(Fig. 11A), and the
decay rate was fit (single exponential) to obtain a
 koff = 17 ± 3 s-1
(Table 2). Data
(Fig. 11B) were fit
(single exponentials), and the rates were plotted as a function of dCTP
concentration to obtain a ground-state dissociation constant,
Kd(G*) = 170 ± 42 μm, and
conformational change rates, k3 = 34 ± 3
s-1 and k-3 = 4.5 ± 0.9 s-1
(Fig. 11C). Fitting
the fluorescence amplitudes (Fig.
11D) as a function of dCTP concentration to a hyperbola
yielded a net equilibrium dissociation constant,
Keq(G*) = 11 ± 1 μm.
Keq(G*) was also calculated using the equation
Keq(G*) =
Kd(G*)/(1 + K3) = 20
μm. The kinetic constants obtained in these analyses are
summarized in Fig.
6B.
koff = 17 ± 3 s-1
(Table 2). Data
(Fig. 11B) were fit
(single exponentials), and the rates were plotted as a function of dCTP
concentration to obtain a ground-state dissociation constant,
Kd(G*) = 170 ± 42 μm, and
conformational change rates, k3 = 34 ± 3
s-1 and k-3 = 4.5 ± 0.9 s-1
(Fig. 11C). Fitting
the fluorescence amplitudes (Fig.
11D) as a function of dCTP concentration to a hyperbola
yielded a net equilibrium dissociation constant,
Keq(G*) = 11 ± 1 μm.
Keq(G*) was also calculated using the equation
Keq(G*) =
Kd(G*)/(1 + K3) = 20
μm. The kinetic constants obtained in these analyses are
summarized in Fig.
6B.
FIGURE 11.
Fluorescence changes observed upon DNA release from Dpo4 T239W and
nucleotide binding to a Dpo4 T239W·DNAdd complex with
an 8-oxoG template base. A, the experiment of
Fig. 4A was repeated,
and fluorescence changes were recorded upon release of
 from Dpo4 T239W,
koff = 17 ± 3 s-1. B,
fluorescence changes induced by addition of dCTP opposite the template
from Dpo4 T239W,
koff = 17 ± 3 s-1. B,
fluorescence changes induced by addition of dCTP opposite the template
 containing 8-oxoG:
5 μm (black), 15 μm (red), 30
μm (orange), 50 μm (green), 120
μm (blue), 240 μm (violet), and
500 μm (brown). C, rates of dCTP-induced
fluorescence changes and ground state Kd,dCTP opposite
template 8-oxoG were estimated, k3 = 34 ± 2.5
s-1, k-3 = 4.5 ± 0.9 s-1, and
Kd,dCTP = 170 ± 42 μm. D,
the maximal amplitudes measured in C were plotted and fit to a
hyperbola to obtain an estimate of the equilibrium dissociation constant of
dCTP bound opposite 8-oxoG, Keq(G*) = 11
± 1 μm.
containing 8-oxoG:
5 μm (black), 15 μm (red), 30
μm (orange), 50 μm (green), 120
μm (blue), 240 μm (violet), and
500 μm (brown). C, rates of dCTP-induced
fluorescence changes and ground state Kd,dCTP opposite
template 8-oxoG were estimated, k3 = 34 ± 2.5
s-1, k-3 = 4.5 ± 0.9 s-1, and
Kd,dCTP = 170 ± 42 μm. D,
the maximal amplitudes measured in C were plotted and fit to a
hyperbola to obtain an estimate of the equilibrium dissociation constant of
dCTP bound opposite 8-oxoG, Keq(G*) = 11
± 1 μm.
DISCUSSION
The absence of Trp residues in the Y-family DNA polymerase Dpo4 (wild-type) provided an opportunity to place individual fluorophores in selected parts of the protein and use these to detect conformational changes. Two of the mutants, T239W and N188W, had somewhat higher catalytic activities than wild-type Dpo4. Strong fluorescence changes were observed upon DNA binding, and further fluorescence changes were associated with the binding of a correctly paired dNTP. The rates of this latter change were measured as a function of the dCTP concentration and were saturable in a hyperbolic fashion, and we conclude that this binding step involves a conformational change. Coupling the fluorescence rate data with rates of product formation allowed estimation of the rate constants for the conformational change (k3), the reverse reaction (k-3), and phosphodiester bond formation (k4) (Fig. 9A). When phosphodiester bond formation was allowed to proceed, the fluorescence decreased (opposite direction of the change prior to bond formation), and this step is considered to involve relaxation of the initial conformational change (observed prior to phosphodiester bond formation). The rate of this step was relatively invariant with dCTP concentration, as would be expected (Fig. 7). Separate experiments (monitoring PPi detection) showed that PPi release is fast and must necessarily precede the slower conformational relaxation step (Fig. 8). Release of the DNA product from Dpo4 is fast. Collectively these results yield a scheme in which the conformational change steps before and after phosphodiester bond formation can be observed, and the latter of these appears to be the most “rate-limiting” step in the overall cycle. This model differs from the common paradigm that has the DNA “off-rate” being rate-limiting in the steady state. Although this model was developed with Trp-containing mutants of Dpo4, we believe that the conclusions also apply to wild-type Dpo4, which has similar overall kinetic parameters (Table 1), although differences in rate-limiting steps cannot be unambiguously ruled out.
The selection of placement of the Trp residues was based upon inspection of crystal structures that we and others have collected (6, 14, 21, 29-35). Several of the original Trp mutants (Fig. 1) did not show fluorescence changes upon dNTP binding (Y12W, F37W, Y48W, Y274W, and R332W), and all of these showed severely attenuated polymerization efficiency relative to wild-type Dpo4. Exactly why these mutants were inactive is not clear. Regarding the active mutants, the position Trp-188 is within the thumb domain and Trp-239 is in the region linking the little finger and thumb domains (Fig. 1). The red shift in the emission wavelength was observed for the Dpo4 T239W mutant but not Dpo4 N188W. Quenching was observed in both cases. In the crystallographic comparison of the apo-enzyme and the “binary” DNA-bound form (46) the only large domain shift (upon binding DNA) is in the “little finger” domain, and Trp-239 (being nearby in the linker region between the little finger and “thumb” domains) may be responding by undergoing a rotamer shift into the solvent (47). The quenching of Trp-188 may imply a change of the environment (upon DNA binding) but not increased polarity (near Trp-188 or, for that matter, Trp-12 (Fig. 2C)). In a recent publication, Wong et al. (46) reported the Dpo4 mutant Y274W. The (Trp) fluorescence was decreased upon binding to DNA, but no other properties were reported.
The details of functional conformational changes in DNA polymerases are not understood well. All DNA polymerases must be able to bind all four canonical dNTPs but need to use other forces to position the appropriate one for each replication event, and the free energy change involved in correct Watson-Crick pairing is insufficient to explain the fidelity results (48, 49). A large conformational change from an open to a closed structure is associated with correct dNTP binding in many replicative polymerases, i.e. the change is seen only after the binding of the correct dNTP, but the measured rates are too fast to justify a role as the critical conformational change leading directly to catalysis (12, 50). This conclusion implies that less obvious structural changes are more critical. The available structures of Y-family DNA polymerases have not shown major structural changes upon dNTP binding, and the question about whether any changes exist that could be associated with catalysis has remained open. Some relatively small changes in the crystal structures of Dpo4 have been detected (14, 46), but these have not been directly associated with productive activity. The lack of a thio effect on kpol may be consistent with the existence of a non-covalent rate-limiting step prior to bond formation (15) but cannot be considered proof (16). Fiala and Suo (15) concluded that a difference in product yield amplitude obtained in pulse-chase and pulse-quench experiments constituted evidence for a step before bond formation, but the difference does not appear to be statistically different. The fluorescence changes associated with Dpo4 T239W and N188W are concluded to be associated with conformational steps prior to and following bond formation (Fig. 3), based largely on the fact that the two observed fluorescence changes, the large, fast increase followed by a slower decrease, fit within this catalytic model and behave appropriately (i.e. only the fluorescence increase posited to occur prior to phosphodiester bond formation is dNTP concentration-dependent (Fig. 7)). Monitoring a fluorophore in the enzyme has advantages in that the phenomena is more likely associated with the protein changes; many fluorescence kinetic studies have been done with fluorophores in the DNA only (e.g. 2-aminopurine) (17, 18, 40, 51), but the spectral changes are often complicated by the effects of base pairing and stacking.
The order of the steps in the catalytic cycle can be defined (Figs. 9A and 10A). The rate of increase in Trp fluorescence was dNTP concentration-dependent but is too slow to be the direct binding of a dNTP. This step must precede phosphodiester bond formation, in that this fluorescence change was seen with dideoxy-terminated primers. The decrease in fluorescence, the opposite of the earlier step, was only seen when phosphodiester bond formation could occur, and the rate was invariant with dNTP concentration. Therefore this change must follow phosphodiester bond formation. We measured PPi release using a new coupled method developed by Hanes and Johnson (27). The rate of PPi formation was virtually identical to that of formation of DNA product under single turnover conditions (Fig. 8). This experiment does not directly measure PPi, but the fact that PPi appears to be immediately available argues that the PPi release step must be very fast and therefore must necessarily precede the much slower conformational relaxation step 6 in the catalytic cycle (Figs. 9A and 10A). This sequence of post-bond formation steps is opposite that we (and many others) have previously presented for other DNA polymerases (15, 42-45, 52). However, the kinetic models and simulations will generally yield mathematically similar results for both cases because the step is fast. If PPi release necessarily followed the slow conformational change associated with the fluorescence decrease, then the PPi release rate would have been much slower (Fig. 8). Hanes and Johnson (26) also found what appears to be very rapid PPi release in the mechanism of bacteriophage pol T7.
The issue of which of the one or more steps limits rates of catalysis in DNA polymerases has been discussed extensively in the literature (11, 52, 53). Most DNA polymerases exhibit burst kinetics, at least in incorporations involving the four normal bases, necessarily implicating a rate-limiting step after product formation. Historically this step has generally been presumed to be the release of the DNA product. In some cases (replicative DNA polymerases) the rates have been measured and are consistent with the steady-state rates (44, 45). With Dpo4, we found fast rates of DNA product release (Table 2) and a slow conformational relaxation (Fig. 3), which appears to be the rate-limiting step in steady-state catalysis. Recent DNA fluorescence studies have shown that Dbh, a close homolog of Dpo4, also releases DNA rapidly and therefore may follow a similar mechanism (17, 40).
Although much of the general enzymology literature is replete with discussion of a “rate-limiting step,” the point has been raised by Johnson that what limits observed rates (and particularly the specificity) is not the speed of a given step but the extent to which particular steps are reversible (19, 54). Much of this analysis was based on work with a fluorescent conjugate of bacteriophage pol T7, which showed different fluorescent responses depending upon whether base pairing was correct or incorrect. In particular, the reversibility of conformational steps during pol T7 binding of dNTPs could be probed using fluorescence kinetics (19). On the basis of rates of conformational steps measured by fluorescence kinetics, the rate of phosphodiester bond formation by pol T7 was concluded not to contribute to the overall rate of correct dNTP incorporation, because the isomerization leading to substrate release is relatively slow, essentially committing all bound substrate to product formation. Incorrect dNTP binding was found to have the opposite effect, with the phosphodiester bond formation step being much slower than substrate release. Dpo4 appears to be a somewhat different case, in that correct dNTP binding yields a conformational change that resembles that of incorrect dNTP binding by pol T7, i.e. release of substrate is faster than the rate of phosphodiester bond formation (Fig. 9A), although the ratio of substrate release (k8) versus phosphodiester bond formation (k4) is much higher in the former case.
The concept of rates of conformational changes leading to specificity has been expanded from the pol T7 conjugate to ribosome and dihydrofolate reductase systems (54). In a sense, these concepts are not totally novel in that the kinetic isotope effect literature contains discussions of the same concepts in terms of “commitment to forward (or reverse) catalysis” etc. (55), although this is not always in the vein of conformational changes.
The ability to directly probe events involved in conformational changes linked with pairing of DNA bases raises the prospect of extension to abnormal systems, such as the incorporation of dNTPs opposite carcinogen-modified bases (49). We have initiated some studies with the template 8-oxoG (Fig. 11), a lesion that shows some misincorporation with Dpo4 (31, 35). The conformational changes that form an active complex with dCTP opposite 8-oxoG are actually more favorable, with k3/k-3 ∼ 10-fold higher than opposite G (Fig. 10C). The specificity constant, kpol/Kd, for dCTP incorporation opposite 8-oxoG was previously found to be over 20-fold higher than with G for Dpo4 (31, 35), so the results are not surprising. The reason for the increased efficiency and highly favored conformational change may due to an increased number of contacts Dpo4 makes with the 8-oxoG template compared with G, specifically one involving a hydrogen bond between the 8-oxoG carbonyl oxygen (C-8) and Arg-332 (14, 31, 35).
Given that single base deletions are relatively uncommon, it is interesting that Dpo4 T239W yields strong fluorescence changes upon dNTP binding that leads to these events (e.g. dGTP opposite the +1 template base C (Fig. 3, C and D)). The results are consistent with a model where Dpo4 responds to a potential to form a correct base pair by instigating a conformational change, whether or not this leads to rapid catalysis. Studies using the fluorescent base analog 2-aminopurine (17) showed that Dbh likely utilizes the classic Streisinger model of base slippage when instigating a deletion, i.e. that the template base “slips” to pair with the 3′ base of the primer strand upon binding of a dNTP that correctly pairs with the next template base. The sequence DNAG fits the model but the sequence used in Fig. 3C does not, and Dpo4 T239W reported fluorescence changes that would be predicted by the model. Other DNA sequences, where the +1 template base within DNAG has been replaced with other bases, also gave increases in fluorescence upon binding of their respective dNTP pairing partners (data not shown). A decrease in fluorescence following phosphodiester bond formation was not detected (e.g. Fig. 3B), reflecting the relatively slow rate of bond formation. Dpo4 crystal structures have shown some support for a classic Streisinger model for base slippage, i.e. with a templating abasic site (30), but other crystal structures have not, i.e. with a templating benzo[a]pyrene diol epoxide-G or 1,N2-ethenoguanine adduct (21, 29). However, in the latter cases, the active sites were largely distorted due to the extra bulk of the base lesions, and therefore models describing base deletion mutations may vary depending on the lesion.
The fact that Trp-239 fluorescence changes were not observed upon binding of an incorrect dNTP supports the conclusion that a conformational change only occurs during correct base pair alignment, including base pairs formed after slip-page. However, because slightly different sequence contexts lead to varying fluorescence intensities upon correct dNTP binding (e.g. compare Fig. 3, C and D), Trp-239 is probably uniquely sensitive to each base pair. Thus, the Trp might have to be positioned elsewhere to detect other putative changes related to misincorporation.
The results presented here raise a number of questions that cannot be fully addressed yet. One is how subtle the change in the environments of Trp-188 and Trp-239 (of the mutants) is in these conformational events. In some cases (with Dpo4) we have observed substantial kinetic thio effects, but it is not clear as to whether these can be attributed to a rate-limiting phosphodiester bond formation step or not (37). Another open question is how many of these findings are applicable to other Y-family DNA polymerases. Future studies will attempt to answer the question of what happens with other DNA adducts (other than 8-oxoG), in which phosphodiester bond formation is much slower.
In conclusion, we have used site-directed Trp mutants of the Y-family DNA polymerase Dpo4 that report what we believe is a protein conformational change associated with correct base pairing, preceding the phosphodiester bond formation step. The step involving relaxation of the conformation (after phosphodiester bond formation occurs) can also be observed and measured. This step is separated from phosphodiester bond formation by a fast PPi release step. The second conformational change appears to be the most rate-limiting step in the cycle and is followed by a rapid release of the DNA product. The order and relative rates of these reactions with Dpo4 differ from other proposals with Dpo4 and with several well established examples of replicative polymerases.
Supplementary Material
Acknowledgments
We thank R. L. Eoff for assistance with initial selection of site of Trp substitution and for helpful discussions, C. E. Cameron for the reagents and procedures for preparing PBP, K. A. Johnson for the derivation in supplemental Fig. S3, and K. Trisler for assistance in preparation of the manuscript.
This work was supported, in whole or in part, by National Institutes of Health Grants R01 ES010375 (to F. P. G.) and P30 ES000267 (to F. P. G.) from the United States Public Health Service. The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1-S6.
Footnotes
The abbreviations used are: PPi, pyrophosphate; Dpo4, S. solfataricus P2 DNA polymerase IV; Dbh, DinB homolog of S. acidocaldarius (Dpo4 ortholog); MDCC, N-[2-(1-maleimidyl)ethyl]-7-(diethylamino)coumarin-3-carboxamide; 8-oxoG, 7,8-dihydro-8-oxo-2′-deoxyguanosine; PBP, phosphate-binding protein; 7-MeGuo, 7-methylguanosine; Pi, inorganic phosphate; pol, (DNA) polymerase; PNPase, purine nucleoside phosphorylase; PPase, yeast pyrophosphatase; HEPES, N-(2-hydroxyethyl)-1-piperazine-N'(ethanesulfonate).
We set kobs = (k3[dNTP]/(Kd + [dNTP])) + k-3, relating this directly to Equation 2, with k3 dependent on [dNTP] (41). The equation for Keq,G was used by Tsai and Johnson (19) for a coumarin-labeled pol T7. The derivation for that equation was provided by K. A. Johnson, University of Texas, Austin (now included as supplemental Fig. S3). The equation is from “Case 2,” where it is assumed that the F signal (from the increase in fluorescence) is only due to the active complex (isomerized to “XDN”). The signal is dependent upon both Kd(dNTP) (1/K1 in the derived equation) and the isomerization constant K3 (K2 in the derived equation). The equation describing the dNTP concentration on the overall signal amplitude is a hyperbola: signal = [SK2/(1 + K2)]/[1/(K1(1 + K2)) + S]; Kd (i.e., Keq,G) = 1/(K1(1 + K2)) = Kd(dNTP)/(1 + K3).
References
- 1.Kunkel, T. A. (2004) J. Biol. Chem. 279 16895-16898 [DOI] [PubMed] [Google Scholar]
- 2.Prakash, S., Johnson, R. E., and Prakash, L. (2004) Annu. Rev. Biochem. 74 317-353 [DOI] [PubMed] [Google Scholar]
- 3.Kool, E. T. (2002) Annu. Rev. Biochem. 71 191-219 [DOI] [PubMed] [Google Scholar]
- 4.Lehmann, A. R., Niimi, A., Ogi, T., Brown, S., Sabbioneda, S., Wing, J. F., Kannouche, P. L., and Green, C. M. (2007) DNA Repair (Amst.) 6 891-899 [DOI] [PubMed] [Google Scholar]
- 5.McCulloch, S. D., Kokoska, R. J., Chilkova, O., Welch, C. M., Johansson, E., Burgers, P. M., and Kunkel, T. A. (2004) Nucleic Acids Res. 32 4665-4675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ling, H., Boudsocq, F., Woodgate, R., and Yang, W. (2001) Cell 107 91-102 [DOI] [PubMed] [Google Scholar]
- 7.Sawaya, M. R., Prasad, R., Wilson, S. H., Kraut, J., and Pelletier, H. (1997) Biochemistry 36 11205-11215 [DOI] [PubMed] [Google Scholar]
- 8.Li, Y., Korolev, S., and Waksman, G. (1998) EMBO J. 17 7514-7525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Doublié, S., Tabor, S., Long, A. M., Richardson, C. C., and Ellenberger, T. (1998) Nature 391 251-257 [DOI] [PubMed] [Google Scholar]
- 10.Purohit, V., Grindley, N. D., and Joyce, C. M. (2003) Biochemistry 42 10200-10211 [DOI] [PubMed] [Google Scholar]
- 11.Showalter, A. K., and Tsai, M. D. (2002) Biochemistry 41 10571-10576 [DOI] [PubMed] [Google Scholar]
- 12.Rothwell, P. J., Mitaksov, V., and Waksman, G. (2005) Mol. Cell 19 345-355 [DOI] [PubMed] [Google Scholar]
- 13.Wang, Y., Arora, K., and Schlick, T. (2006) Protein Sci. 15 135-151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rechkoblit, O., Malinina, L., Cheng, Y., Kuryavyi, V., Broyde, S., Geacintov, N. E., and Patel, D. J. (2006) PLoS Biol. 4 e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fiala, K. A., and Suo, Z. (2004) Biochemistry 43 2116-2125 [DOI] [PubMed] [Google Scholar]
- 16.Herschlag, D., Piccirilli, J. A., and Cech, T. R. (1991) Biochemistry 30 4844-4854 [DOI] [PubMed] [Google Scholar]
- 17.DeLucia, A. M., Grindley, N. D., and Joyce, C. M. (2007) Biochemistry 46 10790-10803 [DOI] [PubMed] [Google Scholar]
- 18.Roettger, M. P., Bakhtina, M., and Tsai, M. D. (2008) Biochemistry 47 9718-9727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tsai, Y. C., and Johnson, K. A. (2006) Biochemistry 45 9675-9687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Borer, P. N. (1975), in Handbook of Biochemistry and Molecular Biology, 3rd. Ed. (Fasman, G. D., ed) pp 589-590, CRC Press, Cleveland, OH
- 21.Zang, H., Goodenough, A. K., Choi, J.-Y., Irminia, A., Loukachevitch, L. V., Kozekov, I. D., Angel, K. C., Rizzo, C. J., Egli, M., and Guengerich, F. P. (2005) J. Biol. Chem. 280 29750-29764 [DOI] [PubMed] [Google Scholar]
- 22.Gohara, D. W., Ha, C. S., Kumar, S., Ghosh, B., Arnold, J. J., Wisniewski, T. J., and Cameron, C. E. (1999) Protein Expr. Purif. 17 128-138 [DOI] [PubMed] [Google Scholar]
- 23.Brune, M., Hunter, J. L., Howell, S. A., Martin, S. R., Hazlett, T. L., Corrie, J. E., and Webb, M. R. (1998) Biochemistry 37 10370-10380 [DOI] [PubMed] [Google Scholar]
- 24.Maniatis, T., Fritsch, E. F., and Sambrook, J. (1982) Molecular Cloning: A Laboratory Manual, Cold Spring Harbor Laboratories, Cold Spring Harbor, NY
- 25.Kuchta, R. D., Mizrahi, V., Benkovic, P. A., Johnson, K. A., and Benkovic, S. J. (1987) Biochemistry 26 8410-8417 [DOI] [PubMed] [Google Scholar]
- 26.Hanes, J. W., and Johnson, K. A. (2007) Nucleic Acids Res. 35 6973-6983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hanes, J. W., and Johnson, K. A. (2008) Anal. Biochem. 372 125-127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kuzmic, P. (1996) Anal. Biochem. 237 260-273 [DOI] [PubMed] [Google Scholar]
- 29.Ling, H., Sayer, J. M., Plosky, B. S., Yagi, H., Boudsocq, F., Woodgate, R., Jerina, D. M., and Yang, W. (2004) Proc. Natl. Acad. Sci. U. S. A. 101 2265-2269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ling, H., Boudsocq, F., Woodgate, R., and Yang, W. (2004) Mol. Cell 13 751-762 [DOI] [PubMed] [Google Scholar]
- 31.Eoff, R. L., Irimia, A., Angel, K., Egli, M., and Guengerich, F. P. (2007) J. Biol. Chem. 282 19831-19843 [DOI] [PubMed] [Google Scholar]
- 32.Irimia, A., Zang, H., Loukachevitch, L. V., Eoff, R. L., Guengerich, F. P., and Egli, M. (2006) Biochemistry 45 5949-5956 [DOI] [PubMed] [Google Scholar]
- 33.Irimia, A., Eoff, R. L., Pollon, P. S., Guengerich, F. P., and Egli, M. (2007) J. Biol. Chem. 282 36421-36433 [DOI] [PubMed] [Google Scholar]
- 34.Eoff, R. L., Angel, K. C., Egli, M., and Guengerich, F. P. (2007) J. Biol. Chem. 282 13573-13584 [DOI] [PubMed] [Google Scholar]
- 35.Zang, H., Irminia, A., Choi, J.-Y., Angel, K. C., Loukachevitch, L. V., Egli, M., and Guengerich, F. P. (2006) J. Biol. Chem. 281 2358-2372 [DOI] [PubMed] [Google Scholar]
- 36.Boudsocq, F., Kokoska, R. J., Plosky, B. S., Vaisman, A., Ling, H., Kunkel, T. A., Yang, W., and Woodgate, R. (2004) J. Biol. Chem. 279 32932-32940 [DOI] [PubMed] [Google Scholar]
- 37.Eoff, R. L., Irimia, A., Zang, H. K. C. A., Egli, M., and Guengerich, F. P. (2006) J. Biol. Chem. 282 1456-1467 [DOI] [PubMed] [Google Scholar]
- 38.Kokoska, R. J., Bebenek, K., Boudsocq, F., Woodgate, R., and Kunkel, T. A. (2002) J. Biol. Chem. 277 19633-19638 [DOI] [PubMed] [Google Scholar]
- 39.Kobayashi, S., Valentine, M. R., Pham, P., O'Donnell, M., and Goodman, M. F. (2002) J. Biol. Chem. 277 34198-34207 [DOI] [PubMed] [Google Scholar]
- 40.Cramer, J., and Restle, T. (2005) J. Biol. Chem. 280 40552-40558 [DOI] [PubMed] [Google Scholar]
- 41.Johnson, K. A. (2003), in Kinetic Analysis of Macromolecules. A Practical Approach (Johnson, K. A., ed) pp 1-18, Oxford University Press, Oxford, UK
- 42.Dahlberg, M. E., and Benkovic, S. J. (1991) Biochemistry 30 4835-4843 [DOI] [PubMed] [Google Scholar]
- 43.Furge, L. L., and Guengerich, F. P. (1999) Biochemistry 38 4818-4825 [DOI] [PubMed] [Google Scholar]
- 44.Woodside, A. M., and Guengerich, F. P. (2002) Biochemistry 41 1039-1050 [DOI] [PubMed] [Google Scholar]
- 45.Patel, S. S., Wong, I., and Johnson, K. A. (1991) Biochemistry 30 511-525 [DOI] [PubMed] [Google Scholar]
- 46.Wong, J. H., Fiala, K. A., Suo, Z., and Ling, H. (2008) J. Mol. Biol. 379 317-330 [DOI] [PubMed] [Google Scholar]
- 47.Royer, C. A. (2006) Chem. Rev. 106 1769-1784 [DOI] [PubMed] [Google Scholar]
- 48.Echols, H., and Goodman, M. F. (1991) Annu. Rev. Biochem. 60 477-511 [DOI] [PubMed] [Google Scholar]
- 49.Guengerich, F. P. (2006) Chem. Rev. 106 420-452 [DOI] [PubMed] [Google Scholar]
- 50.Joyce, C. M., Potapova, O., Delucia, A. M., Huang, X., Basu, V. P., and Grindley, N. D. (2008) Biochemistry 47 6103-6116 [DOI] [PubMed] [Google Scholar]
- 51.Luo, G., Wang, M., Konigsberg, W. H., and Xie, X. S. (2007) Proc. Natl. Acad. Sci. U. S. A. 104 12610-12615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wong, I., Patel, S. S., and Johnson, K. A. (1991) Biochemistry 30 526-537 [DOI] [PubMed] [Google Scholar]
- 53.Joyce, C. M., and Benkovic, S. J. (2004) Biochemistry 43 14317-14324 [DOI] [PubMed] [Google Scholar]
- 54.Johnson, K. A. (2008) J. Biol. Chem. 283 26297-26301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Northrop, D. B. (1975) Biochemistry 14 2644-2651 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.













