Abstract
The neurodegenerative disorder spinocerebellar ataxia 12 (SCA12) is caused by CAG repeat expansion in the non-coding region of the PPP2R2B gene. PPP2R2B encodes Bβ1 and Bβ2, alternatively spliced and neuron-specific regulatory subunits of the protein phosphatase 2A (PP2A) holoenzyme. We show here that in PC12 cells and hippocampal neurons, cell stressors induced a rapid translocation of PP2A/Bβ2 to mitochondria to promote apoptosis. Conversely, silencing of PP2A/Bβ2 protected hippocampal neurons against free radical-mediated, excitotoxic, and ischemic insults. Evidence is accumulating that the mitochondrial fission/fusion equilibrium is an important determinant of cell survival. Accordingly, we found that Bβ2 expression induces mitochondrial fragmentation, whereas Bβ2 silencing or inhibition resulted in mitochondrial elongation. Based on epistasis experiments involving Bcl2 and core components of the mitochondrial fission machinery (Fis1 and dynamin-related protein 1), mitochondrial fragmentation occurs upstream of apoptosis and is both necessary and sufficient for hippocampal neuron death. Our data provide the first example of a proapoptotic phosphatase that predisposes to neuronal death by promoting mitochondrial division and point to a possible imbalance of the mitochondrial morphogenetic equilibrium in the pathogenesis of SCA12.
Mitochondrial morphology and assembly of mitochondria into a contiguous network is a consequence of opposing fission and fusion events (1–3). In neurons, physiological levels of mitochondrial fission are necessary for axonal and dendritic transport of mitochondria and the proper development and function of synapses (4, 5). However, mitochondrial fragmentation is also an integral aspect of apoptosis because enhancing and inhibiting fission can promote and delay apoptosis, respectively (6, 7). Mitochondrial fission and fusion are separate processes catalyzed by large GTPases that were initially identified in yeast (8). The principal fission enzyme is dynamin-related protein 1 (Drp1),5 which, in analogy to the endocytosis motor dynamin, is thought to utilize GTP hydrolysis to mechanically constrict and sever mitochondria. Drp1 is recruited from the cytosol to the outer mitochondrial membrane (OMM) by a multiprotein complex that includes Fis1. In mammals, outer mitochondrial membrane fusion is carried out by two transmembrane GTPases, mitofusin 1 and 2 (Mfn1/2), which act in concert with the inner membrane fusion enzyme optic atrophy 1 (Opa1) (8).
The fundamental importance of a healthy mitochondrial fission/fusion balance in neurons is documented by the discovery that two common neurodegenerative disorders, Charcot-Marie-Tooth disease type 2A and dominant optic atrophy, are caused by mutations in mitochondrial fusion GTPases, Mfn2 and Opa1, respectively (9–11). Similarly, a dominant-inactivating mutation in Drp1 was recently linked to microcephaly and other neurological birth defects (12). Despite the clear relevance of mitochondrial morphogenesis to neuronal survival (6, 13–15), we know very little about the signal transduction pathways that impinge on mitochondrial remodeling processes and their physiological and pathophysiological sequelae.
Protein phosphatase 2A (PP2A) is a major, evolutionarily conserved family of Ser/Thr phosphatases with many essential functions in the cell (16). The predominant form of PP2A is a heterotrimer of a catalytic C and scaffolding A and a variable regulatory or B subunit. Regulatory subunits define substrate specificity and subcellular localization of the PP2A holoenzyme and are often expressed in a tissue- and cell type-specific pattern. As one of the 12 mammalian PP2A regulatory subunit genes, Bβ (PPP2R2B) gives rise to several neuron-specific splice variants (17, 18). Indicating a role for PP2A/Bβ in neuronal survival, a CAG repeat expansion in a non-coding region of PPP2R2B was shown to be responsible for spinocerebellar ataxia type 12, SCA12 (19). SCA12 is a relatively rare, late-onset neurodegenerative disorder characterized by diffuse cerebral and cerebellar atrophy (20). It is not known whether and how the CAG repeat expansion affects expression of the different Bβ splice variants in humans, and animal models of SCA12 have yet to be established.
We previously reported that the differentially spliced N terminus of Bβ2 recruits PP2A to the OMM by a transient and reversible interaction with receptor subunits of the translocase of the outer membrane (TOM) complex. Furthermore, overexpression of Bβ2 was shown to promote apoptosis in growth factor-deprived neuronal PC12 cells (18, 21). Here, we demonstrate that Bβ2 redistributes from the cytosol to mitochondria in dying PC12 cells and hippocampal neurons and that apoptosis induction by Bβ2 requires recruitment of the PP2A holoenzyme to the OMM. RNA interference-mediated knockdown of Bβ2 in hippocampal cultures protects neurons from excitotoxic, ischemic, and metabolic insults. Multiple lines of gain- and loss-of-function evidence indicate that PP2A/Bβ2 antagonizes survival by driving Drp1- and Fis1-dependent mitochondrial fragmentation. The survival and morphogenetic activities of PP2A/Bβ2 can be dissociated by Bcl2 overexpression, indicating that mitochondrial restructuring occurs upstream of apoptosis. Our results identify an outer mitochondrial PP2A holoenzyme as a critical regulator of the mitochondrial morphogenetic equilibrium, which determines the susceptibility of neurons to diverse injuries.
EXPERIMENTAL PROCEDURES
cDNA and shRNA Vectors—Wild-type and mutant Bβ1 and Bβ2-GFP expression vectors were described previously (18, 21). To target the protein to the OMM, N-terminally GFP-tagged PfARP32–239 (22) was modified by the addition of the first 29 residues of the mitochondrial import receptor MAS70p. Bβ2 and Fis1 were silenced by H1 promoter-driven expression of small hairpin RNAs (shRNAs) (pSUPER plasmid (23) or lentivirus (24)). 19-b target sites in the mRNAs were as follows (numbering relative to translation start site in rat mRNAs): Bβ2 (number 1), 7–25; Bβ2 (number 3), (–41)-(–23); Fis1 (number 3), 315–333; Fis1 (number 4), 421–439. The nonspecific control shRNA had a similar base composition but was randomized for no more than 14 consecutive matches to any mammalian mRNA.
Antibodies—A mouse monoclonal antibody directed against Bβ2 was generated at the University of Iowa Hybridoma Facility, using the N-terminal peptide KCFSRYLPYIFRPPNTILSS coupled to maleimide-activated keyhole limpet hemocyanin (Pierce) as an antigen. This antibody specifically recognized ectopically expressed Bβ2, but not Bβ1, by immunoblotting. As further confirmation of specificity, immunohistochemical labeling of brain sections with the Bβ2 antibody was nearly abolished in homozygous Bβ2 knock-out mice.6 Other antibodies were obtained commercially: sheep anti-cytochrome c (Sigma), rabbit anti-GFP (AbCam), mouse anti-neurofilament (2H3, Developmental Studies Hybridoma Bank, University of Iowa), and rabbit anti-β-galactosidase (Invitrogen).
Immunofluorescence Staining and Quantification—Hippocampal neurons from E18 rat embryos (25) were cultured in Neurobasal medium with B27 supplement (Invitrogen). At 18–21 days in vitro, cultures were challenged as indicated, fixed in 4% paraformaldehyde after 2 h, and processed for Bβ2 and cytochrome c immunofluorescence. The cytochrome c antibody was visualized by a Alexa Fluor 488-coupled secondary antibody (Invitrogen), whereas Bβ2 was detected with the Cy3 tyramide signal amplification kit (PerkinElmer Life Sciences) according to the manufacturer's instructions. Digital images of randomly selected neurons were captured at constant camera settings using the 40× objective of a Leica epifluorescence microscope. Clustering of Bβ2 in dendrites was quantified by image analysis using a macro custom-written for the ImageJ program (National Institutes of Health). To this end, images were background-subtracted using the rolling ball algorithm, and an autosegmentation mask was applied outlining regions of above threshold pixel intensity in the dendritic field. Average Bβ2 immunofluorescence (8-bit pixel intensity) in outlined dendritic clusters was compiled for 50–100 neurons per condition. For confocal microscopy of endogenous Bβ2 in the soma of hippocampal neurons, cultures were infected with lentivirus expressing mitochondria-targeted GFP (matrix import sequence from cytochrome oxidase subunit VIII) and challenged 5 days later with 2.5 μm rotenone or DMSO vehicle. After 2 h, cultures were paraformaldehyde-fixed, subjected to antigen retrieval (10 mm Tris, 1 mm EDTA, 0.05% Tween 20, pH 9.0, for 30 min at 80 °C), and then immunofluorescently labeled for Bβ2 and GFP as described above. Confocal image stacks (3-μm interval) were acquired with an Olympus FluoView 3000 microscope, and colocalization of Bβ2 with mitochondria was assessed by blinded comparison with a set of reference images.
Neuronal Survival Assays—Hippocampal cultures (25) were transduced with lentivirus or transfected using Lipofectamine 2000 (0.15%, 2 μg/ml DNA) at 10–21 days in vitro. For survival assays based on counting transfected neurons or apoptotic nuclei, GFP- or β-galactosidase-expressing plasmids were cotransfected. For oxygen-glucose deprivation assays, a plasmid expressing luciferase driven by the neuron-specific calcitonin gene-related peptide promoter was included (20% of total plasmid mass). After 3–5 days, cultures were challenged with glutamate (250 μm, 20 min), rotenone (400 nm continuous), or oxygen-glucose deprivation (25 min). Glutamate- and rotenone-treated cultures (and their vehicle controls) were fixed with 3.7% paraformaldehyde and processed for immunofluorescence staining for the transfection marker (GFP, β-galactosidase) and neurofilament protein as a neuronal marker. To score survival, transfection marker-positive neurons with intact processes were counted in quadruplicate wells of a 24-well plate. Apoptosis was quantified as the percentage of transfected neurons with condensed, irregular, or fragmented nuclei (labeled with 1 μg/ml Hoechst 33342). All survival assays based on counting neurons were performed blind to the experimental conditions.
For oxygen-glucose deprivation assays, medium was exchanged with custom Neurobasal medium without glucose (formula 05-0128DJ, Invitrogen), and cultures were placed for 25 min in a Billups-Rothenberg (Del Mar, CA) modular incubator chamber, which was flushed with 95% N2 and 5% CO2. After adding back glucose-containing Neurobasal medium, cultures were returned to the standard incubator. Control cultures were mock-treated under normoglycemic/normoxic conditions. 72 h later, neuronal survival was determined by luciferase assays.
Mitochondrial Morphology Assay—PC12 cells and hippocampal neurons cultured on, respectively, collagen- and poly-d-lysine-coated, chambered Number 1 cover glasses (20-mm2 chamber, Nalge Nunc), were infected with lentivirus or transfected using Lipofectamine 2000 as above. 24–96 h after transfection, cells were incubated with 100 nm tetramethylrhodamine methyl ester (TMRM, Invitrogen) for 30 min at 37 °C to visualize mitochondria, and live cell images through the midplane of the soma were captured using a Zeiss LSM 510 laser-scanning confocal microscope. Mitochondrial morphology was scored by a reference image-based method. Coded confocal images were assigned scores from 0 to 4 by comparison with a set of reference images with increasing degrees of mitochondrial elongation. Non-transfected or non-transduced cells were analyzed in some experiments, yielding mitochondrial morphology scores that were statistically indistinguishable from cells expressing control shRNA or control proteins (e.g. outer mitochondrial GFP).
Statistical Analysis—Data were analyzed by Student's t test (two-tailed) for single comparisons, and by one-way analysis of variance followed by pairwise Bonferroni post hoc tests for multiple comparisons.
RESULTS
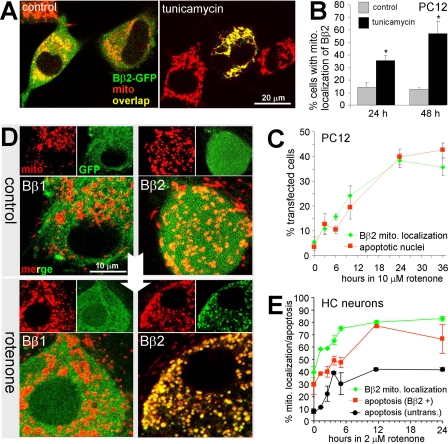
PP2A/Bβ2 Translocates to Mitochondria to Promote Neuronal Death—We investigated a possible redistribution of Bβ2-GFP in healthy versus stressed PC12 cells. Under basal conditions, Bβ2 localized to both cytosolic and mitochondrial compartments (the latter visualized by MitoTracker or TMRM) as reported previously (18). In contrast, Bβ1-GFP localization was exclusively cytosolic, excluding areas of high mitochondrial density (18). When cells were incubated with the glycosylation inhibitor tunicamycin (1.4μm), which induces apoptosis through the endoplasmic reticulum stress pathway, Bβ2 assumed an exclusively mitochondrial localization (Fig. 1A). Tunicamycin-dependent mitochondrial redistribution of Bβ2 was quantified at 24- and 48-h time points (Fig. 1B). Rotenone is a pesticide that blocks complex I of the electron transport chain and that has been used as a chemical model of Parkinson disease (26). Similar to tunicamycin, rotenone (10 μm) induced a quantitative translocation of Bβ2 from the cytosolic to the mitochondrial compartment, which correlated in time and on a cell-by-cell basis with nuclear condensation and fragmentation, hallmarks of apoptosis (Fig. 1C).
FIGURE 1.
The PP2A regulatory subunit Bβ2 translocates to mitochondria to promote neuronal death. A–C, PC12 cells transfected with Bβ2-GFP (∼50% efficiency) were treated for up to 48 h with vehicle (control), tunicamycin (1.4 μm), or rotenone (10 μm), stained with MitoTracker and the DNA dye DRAQ5, and analyzed live by confocal microscopy for colocalization of Bβ2(GFP) and mitochondria (mito) and for apoptotic nuclei (representative images shown in A, quantification shown in B and C) (means ± S.E. of 3 experiments with 40–60 transfected cells per condition, *, p < 0.01 by Student's t test). D and E, primary hippocampal neurons infected with lentivirus (feline immunodeficiency virus) expressing Bβ1-GFP or Bβ2-GFP (80–100% efficiency) were treated with vehicle or 2 μm rotenone followed by live confocal imaging of GFP and mitochondria (mito, stained with TMRM). Shown are representative images at 24 h ± rotenone (D) and a time course of rotenone-induced mitochondrial translocation of Bβ2-GFP and apoptosis in hippocampal neurons (E) (means ± S.E. of n = 3 independent experiments). Mitochondrial localization of Bβ2 was blindly scored on a reference image-based scale (0–4) and converted to a percent value to facilitate comparison with apoptosis (the percentage of infected neurons with DRAQ5-stained apoptotic nuclei).
We extended these results to primary hippocampal neurons transduced with lentivirus expressing Bβ1- or Bβ2-GFP. As in PC12 cells, Bβ1-GFP was excluded from mitochondria (labeled with the potential-sensitive dye TMRM). Confocal imaging of Bβ2-GFP-expressing neurons revealed a modest degree of colocalization with mitochondria (Fig. 1D). Treatment of hippocampal cultures with 2 μm rotenone resulted in a rapid relocalization of Bβ2, but not Bβ1, from the cytosolic to the mitochondrial compartment (Fig. 1D). As in PC12 cells, mitochondrial translocation of Bβ2 was tightly correlated with the appearance of apoptotic nuclei and occurred concomitantly (Fig. 1E). We also observed increased association of Bβ2-GFP with mitochondria of PC12 cells following growth factor deprivation (data not shown).
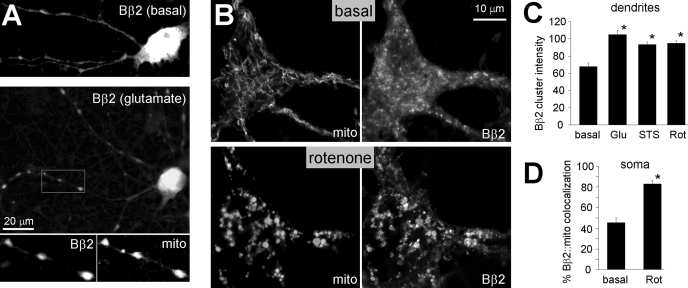
To investigate the localization of endogenous Bβ2, a monoclonal antibody was generated against a unique N-terminal sequence. This antibody is specific for Bβ2 as it labels various neuronal populations in brain sections of wild-type, but not homozygous Bβ2 null mice.6 Hippocampal cultures were challenged with glutamate (200 μm, 15 min), rotenone (2.5 μm, 2 h), or the classical apoptosis inducer staurosporine (0.5 μm, 2 h), fixed after 2 h, and immunofluorescently labeled for endogenous Bβ2 and mitochondrial markers. We used epifluorescence microcopy and computer-aided image analysis to quantify Bβ2 translocation to cytochrome c-positive areas in dendrites and detected significant increases with all insults (Fig. 2, A and C). To assess stress-induced association of Bβ2 with mitochondria in the soma of hippocampal neurons, cultures infected with lentivirus expressing mitochondrial matrix-targeted GFP were subjected to a 2-h DMSO or rotenone treatment, immunolabeled for Bβ2, and analyzed by confocal microscopy. Paralleling the behavior of GFP-tagged Bβ2 (Fig. 1, D and E), rotenone-induced mitochondrial swelling and fragmentation was accompanied by a transition of endogenous Bβ2 from a cytosolic to a largely mitochondrial staining pattern (Fig. 2, B and D). These data indicate that mitochondrial redistribution of this neuron-specific PP2A regulatory subunit may be a general consequence of neuronal injury.
FIGURE 2.
Endogenous PP2A/Bβ2 translocates to mitochondria in stressed hippocampal neurons. Primary hippocampal cultures were treated with glutamate (Glu, 200 μm for 15 min), rotenone (Rot, 2.5 μm for 2 h), staurosporine (STS, 0.5 μm for 2 h), or vehicle only (basal), fixed after 2 h, and processed for immunofluorescence with Bβ2 antibodies. For epifluorescence microscopy (A and C), mitochondria (mito) were visualized with antibodies against cytochrome c, whereas cultures used for confocal microscopy (B and D) were virally transduced with mitochondria-targeted GFP. A, representative epifluorescence images of control and glutamate-treated neurons, with the inset showing colocalization of Bβ2 and cytochrome c in dendritic varicosities. B, representative confocal sections through the soma of vehicle versus rotenone-treated hippocampal neurons labeled for Bβ2 and mitochondrial GFP. C, quantification of Bβ2 clustering in dendrites by image analysis using the ImageJ software. Dendritic clusters were outlined by automatic segmentation of images captured with constant camera settings, and their fluorescence intensities (8-bit pixel value) were averaged for each neuron. D, colocalization of somatic Bβ2 and mitochondria quantified by blinded comparison with a set of reference images. Bar graphs show means ± S.E. of 50–100 (C) or 19 and 23 neurons (D) from experiments representative of three, *, p < 10–5.
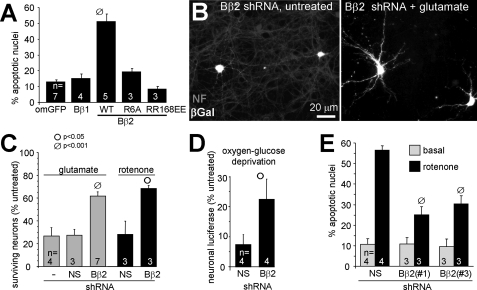
Mitochondrial Targeting of the PP2A Holoenzyme by Bβ2 Is Neurotoxic—When compared with non-transduced neurons in the same culture, Bβ2-GFP-overexpressing hippocampal neurons displayed increased vulnerability to rotenone (Fig. 1E). Therefore, translocation of Bβ2 to mitochondria appears to be a positive feedback mechanism that accelerates neuronal death. In support of this conclusion, long term (≥5 days) expression of Bβ2-, but not Bβ1-GFP, in hippocampal neurons resulted in massive apoptosis (Fig. 3A). We previously identified critical salt bridge interactions between Arg-168/Arg-169 of Bβ2 and Glu-100/Glu-101 of the scaffolding A subunit and characterized in detail the determinants for outer mitochondrial localization of Bβ2 (18, 21, 27). In further overexpression experiments, we found that the proapoptotic activity of Bβ2 requires recruitment of the PP2A holoenzyme to the mitochondrial surface, because mutations that disrupt association with mitochondria (R6A) or with the scaffolding and catalytic subunit (RR168EE) blocked the ability of Bβ2 to kill hippocampal neurons (Fig. 3A).
FIGURE 3.
Mitochondrial targeting of PP2A/Bβ2 is neurotoxic, whereas silencing is neuroprotective. A, hippocampal neurons were transfected with the indicated GFP fusion proteins (om, outer mitochondrial; WT, wild-type) and scored for Hoechst 33342-stained apoptotic nuclei 5–6 days later (means ± S.E. of n = 3–7 experiments with 85–90 neurons each). Bβ2 mutants that block mitochondrial localization (R6A) or PP2A dimer recruitment (RR168EE) also block apoptosis induction. Wild-type and mutant Bβ2 were expressed at similar levels as judged by GFP fluorescence and immunoblotting (data not shown). B–D, hippocampal cultures were cotransfected with Bβ2-directed (Bβ2(#1)) or nonsense (NS) shRNAs and expression marker plasmids, challenged after 3–5 days with glutamate (250 μm for 20 min), oxygen-glucose deprivation (25 min), or rotenone (400 nm continuously), and analyzed 2–3 days later for survival. Glutamate and rotenone-treated cultures were processed for β-galactosidase (βGal) or GFP expression marker and neurofilament (NF) protein immunofluorescence, and the number of transfected, surviving neurons was expressed relative to unchallenged cultures. Representative fields in B show glutamate-dependent degeneration (loss of neurofilament staining, gray) of untransfected, but not Bβ2 shRNA transfected (β-galactosidase-positive, white) neurons. Survival in oxygen-glucose-deprived cultures (D) was assessed by cotransfection with luciferase under control of the neuron-specific calcitonin gene-related peptide promoter and luciferase assays. E, hippocampal neurons transfected with vectors expressing the indicated shRNAs and GFP were treated ± 400 nm rotenone for 48 h, fixed, and stained to visualize apoptotic nuclei (means ± S.E. of 3–7 (C), 4 (D), and 3–4 (E) experiments; Student's t test comparisons between nonsense and Bβ2 shRNA transfections within each challenge group).
PP2A/Bβ2 Silencing Is Neuroprotective—We employed RNA interference to investigate whether endogenous Bβ2 is a negative regulator of neuronal survival. Two shRNAs targeting the splice-specific 5′-untranslated region and N-terminal coding sequence effectively silenced a Bβ2-luciferase fusion protein in hippocampal cultures and stably expressed Bβ2 in PC12 cells (supplemental Fig. 1). Bβ2 knockdown conferred robust neuroprotection in hippocampal cultures exposed to a brief excitotoxic glutamate treatment as determined by counting the number of transfected neurons with intact cell bodies and processes (Fig. 3, B and C). The representative images in Fig. 3B illustrate the enhanced glutamate resistance of Bβ2 shRNA-expressing neurons when compared with their untransfected neighbors. Secondly, in an in vitro model of ischemia (oxygen/glucose deprivation), Bβ2 knockdown attenuated hippocampal neuron loss, which in these experiments was measured by luciferase expression from a neuron-specific promoter (Fig. 3D). Lastly, Bβ2 silencing by two shRNAs increased the number of surviving neurons and decreased apoptosis following inhibition of mitochondrial electron transport with rotenone (Fig. 3, C and E). In aggregate, these results implicate the SCA12 disease-associated PP2A/Bβ2 regulatory subunit as a neuron-specific component of the cell death machinery.
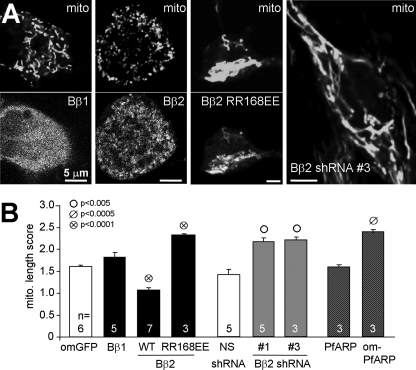
PP2A/Bβ2 Drives Mitochondrial Fission—Mitochondrial fragmentation is an early, obligatory step in the intrinsic, mitochondria-dependent apoptosis program (6, 7, 28). In hippocampal cultures stained with the mitochondrial voltage-sensitive dye TMRM, we observed that neurons expressing Bβ2-GFP showed more punctate mitochondria than Bβ1-expressing neurons (Fig. 4A). Similar results were obtained in dividing and differentiated PC12 cells, as well as in dissociated dorsal root ganglion neurons (supplemental Fig. Fig. 2, B–D). To quantify mitochondrial shape changes, coded confocal images through the midplane of the neuronal soma were compared with a set of reference images with increasing degrees of mitochondrial length and interconnectivity (supplemental Fig. 2A, score 0–4).
FIGURE 4.
PP2A/Bβ2 induces mitochondrial fragmentation. The indicated cDNAs (GFP fusion proteins) and shRNAs (with GFP expression marker) were either transduced via lentivirus or transfected into hippocampal neurons. After 2–3 days, morphology of TMRM-stained mitochondria (mito) was assessed by blinded comparison with a set of reference images (0–4). A, representative live confocal images. B, summary (means ± S.E. of n = 3–7 experiments with 25–30 neurons each; om, outer mitochondrial; WT, wild-type; Student's t test comparisons between GFP fusion proteins and omGFP and between nonsense (NS) and Bβ2 shRNAs).
Although expression of wild-type Bβ2 resulted in significant mitochondrial fragmentation, the monomeric Bβ2 mutant (RR168EE (18)) had an apparent dominant-negative effect, elongating and often clustering mitochondria near the nucleus (Fig. 4, A and B). RNA interference-mediated silencing of endogenous Bβ2 with two different shRNAs resulted in robust mitochondrial elongation to an extent similar to dominant-negative inhibition of the PP2A-targeting subunit (Fig. 4, A and B).
To support a role for PP2A activity in mitochondrial morphogenesis, we expressed Plasmodium falciparum aspartate-rich protein (PfARP) in hippocampal neurons. PfARP is a predominantly cytosolic member of the I2PP2A family of PP2A catalytic subunit inhibitors (22). Expression of an untargeted PfARP-GFP fusion protein had no effect on mitochondrial morphology. However, redirecting the PP2A inhibitor to mitochondria by fusing the OMM anchor sequence of the mitochondrial import receptor MAS70p to its N terminus generated longer mitochondria (Fig. 4B). These results suggest that OMM targeting of PP2A activity via the neuron-specific Bβ2 regulatory subunit promotes cell death by shifting the mitochondrial fission/fusion balance toward fission.
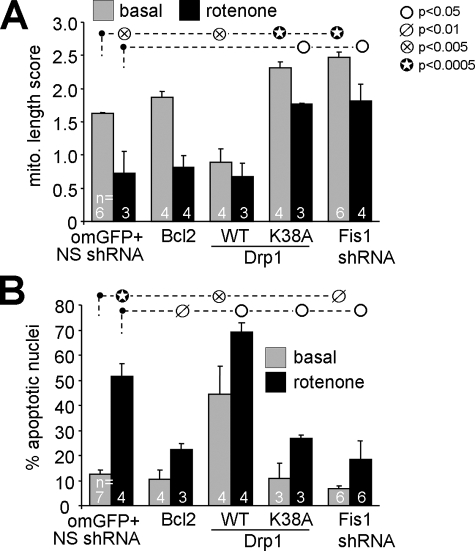
Mitochondrial Remodeling Is Critical for Neuronal Survival Regulation—Mitochondrial fragmentation induced by Bβ2 expression generally preceded loss of mitochondrial membrane potential (TMRM staining) and the appearance of apoptotic nuclei. Our results are therefore consistent with a model in which death is epistatic to fission. As a first test, we showed that rotenone-induced apoptosis, but not mitochondrial fragmentation, can be prevented by expressing the anti-apoptotic Bcl2 protein in hippocampal neurons (Fig. 5). Transfection of the fission GTPase Drp1 exacerbated both basal and rotenone-induced apoptosis. In agreement with a previous report (13), expression of a dominant-negative Drp1 mutant (K38A) or shRNA-mediated silencing of the adaptor protein Fis1 (supplemental Fig. 1A), which recruits Drp1 to the OMM, not only prevented mitochondrial fragmentation but also apoptosis induced by rotenone (Fig. 5). Thus, Fis1/Drp1-mediated mitochondrial fission is necessary and sufficient for at least some forms of neuronal cell death.
FIGURE 5.
Mitochondrial fission is necessary and sufficient for neuronal apoptosis. Hippocampal neurons were transfected with the indicated plasmids (and a GFP marker if applicable), treated after 3–5 days with vehicle (basal) or 400 nm rotenone, and analyzed after an additional 2 days. Mitochondrial morphology (A) was determined after live staining with TMRM, whereas apoptosis (B) was scored in fixed and Hoechst 33342-stained cultures as the percentage of transfected neurons with condensed/fragmented nuclei (means ± S.E. of 3–7 experiments). Driving mitochondrial fragmentation by wild-type Drp1 expression promotes, whereas inhibiting fragmentation (dominant-negative Drp1 K38A, Fis1 shRNA) stalls apoptosis. om, outer mitochondrial; WT, wild-type; NS, nonsense.
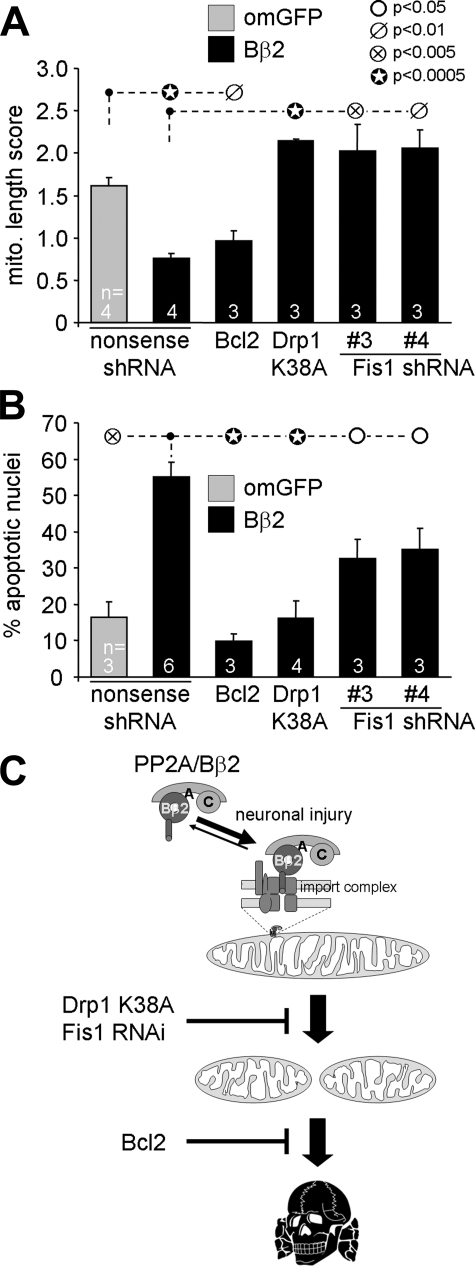
We next investigated the involvement of mitochondrial fission in neuronal apoptosis induced by expression of Bβ2. Bcl2 coexpression completely blocked cell death associated with recruiting PP2A to mitochondria but did not restore normal mitochondrial morphology (Fig. 6). Therefore, PP2A-mediated mitochondrial restructuring is upstream of and distinct from the disintegration of various organelles that accompanies cell death.
FIGURE 6.
Mitochondrial restructuring underlies survival regulation by outer mitochondrial PP2A/Bβ2. Hippocampal neurons were transfected with the indicated plasmid combinations and scored after 2–3 days for mitochondrial morphology (A, live TMRM stain) or after 5–6 days for apoptosis (B, the percentage of transfected neurons with condensed/fragmented nuclei). Means ± S.E. of n = 3–6 experiments are shown. om, outer mitochondrial. C, model illustrating the findings in this study (see “Discussion” for details).
To address whether mitochondrial morphogenetic events are required for the death-promoting activity PP2A/Bβ2, we expressed Bβ2 together with dominant-negative Drp1- or Fis1-directed shRNA. Interfering with Fis1 and Drp1 not only reinstated normal mitochondrial morphology (Fig. 6A) but also at least partially restored viability of hippocampal neurons challenged by Bβ2 overexpression (Fig. 6B).
DISCUSSION
We have identified the SCA12 gene product Bβ2 as a neuron-specific, OMM-localized PP2A regulatory subunit that regulates neuronal survival through the mitochondrial fission/fusion balance. RNA interference-mediated silencing of PP2A/Bβ2 was shown to confer neuroprotection against excitotoxic, ischemic, and free radical-mediated injury. Because OMM targeting is necessary for the proapoptotic activity of Bβ2, agents that interfere with the injury-induced mitochondrial translocation of this neuron-specific PP2A regulatory subunit should also be neuroprotective.
The CAG repeat expansion in SCA12 maps close to the transcript initiation site of the Bβ1 splice form (20), which lacks the mitochondrial localization and proapoptotic activity of Bβ2. Because Bβ2-specific exons are located upstream of the Bβ1 transcript start site, the Bβ2 pre-mRNA necessarily includes the CAG repeat. Although as yet untested, it is conceivable that the repeat expansion increases Bβ2 mRNA levels, resulting in late-onset neurodegeneration. Ultimately, however, animal models are required to address whether a disturbance of the mitochondrial fission/fusion equilibrium is the primary cause of neuron loss in SCA12.
Tilting the kinase/phosphatase balance toward dephosphorylation by recruiting PP2A to the OMM (via wild-type Bβ2 expression) resulted in smaller mitochondria and compromised neuronal viability, whereas silencing PP2A/Bβ2 had the opposite effect. Neuronal death induced by favoring outer mitochondrial dephosphorylations could be mitigated by expression of Bcl2, dominant-negative Drp1, and Fis1 knockdown. Importantly, Bcl2 did not prevent mitochondrial fragmentation, supporting a linear model in which PP2A-mediated mitochondrial remodeling is epistatic to apoptosis (Fig. 6C).
In agreement with similar results from other laboratories (13–15, 29), we found that inhibition of Fis1 and Drp1 also potently attenuates neurotoxicity of the electron transport inhibitor rotenone. Although the contribution of mitochondrial shape changes to apoptosis in immortalized cell lines is currently a matter of controversy (30, 31), Fis1/Drp1-dependent mitochondrial fragmentation appears to be a critical checkpoint in the demise of primary neurons induced by either proapoptotic proteins or environmental toxins.
The role of PP2A in programmed cell death is well established (3, 32, 33), and PP2A can dephosphorylate both proapoptotic and antiapoptotic Bcl2-family proteins (34–36). Here, we provide evidence for a novel mechanism of cell death regulation by PP2A (Fig. 6C). We hypothesize that in healthy neurons, as yet to be identified kinases drive mitochondrial fusion and/or inhibit fission by phosphorylation of the enzymes involved. Upon cell stress (e.g. glutamate, rotenone, staurosporine), the PP2A/Bβ2 holoenzyme translocates from the cytosol to the OMM via an interaction of a cryptic mitochondrial import sequence in the alternatively spliced N terminus of Bβ2 and receptor components of the TOM import complex (21). Outer mitochondrial PP2A, perhaps in conjunction with kinase inactivation, proceeds to dephosphorylate mitochondria-shaping proteins, thus inhibiting fusion and/or stimulating fission activities. Excessive mitochondrial fragmentation could lead to cell death either by fostering the release of apoptogenic proteins from the mitochondrial intermembrane space or by altering mitochondrial reactive oxygen species and Ca2+ homeostasis (1–3, 6, 28).
Drp1 was recently shown to be phosphorylated at two sites by cyclin-dependent kinase 1, protein kinase A, and calcium/calmodulin-dependent kinase I, with somewhat controversial effects on the GTPase and mitochondria-severing activities of the enzyme (37–41). Rather than PP2A, however, the calcium/calmodulin-dependent phosphatase PP2B/calcineurin has been implicated in the dephosphorylation and activation of Drp1 (39, 40). Thus, further studies are needed to identify the relevant PP2A substrates and dephosphorylation sites among mitochondria restructuring enzymes and their known and unknown modulators (8).
Although Bβ2 is a neuron-specific PP2A regulatory subunit, other mitochondrial PP2A holoenzymes have been reported in non-neuronal cells (34). It is therefore likely that similar survival signaling mechanisms operate outside of the nervous system. In summary, our study points to the mitochondrial fission/fusion equilibrium as a pivotal determinant for neuronal life and death decisions, identifies the phosphatase PP2A/Bβ2 as an important modulator, and suggests dysregulation of mitochondrial morphogenesis as a plausible pathogenic mechanism in SCA12.
Supplementary Material
Acknowledgments
We thank Yisang Yoon (University of Rochester) and Michael Knudson (University of Iowa) for Drp1 and Bcl2 expression plasmids, respectively, and Susan Holmes, Elizabeth O'Hearn, and Russell Margolis (The Johns Hopkins University) and members of the Strack laboratory for stimulating discussions.
This work was supported, in whole or in part, by National Institutes of Health Grant NS043254, NS056244 (to S. S.), NS054614 (to Y. M. U.), and NS046450 (to Y. W. H.). This work was also supported by American Heart Association Grant-in-Aid 0455653Z (to S. S.) and United Mitochondrial Disease Foundation Grant 04-65 (to S. S.). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
The on-line version of this article (available at http://www.jbc.org) contains two supplemental figures.
Footnotes
The abbreviations used are: Drp1, dynamin-related protein 1; PP2A, protein phosphatase 2A; SCA12, spinocerebellar ataxia 12; shRNA, short hairpin RNA; TMRM, tetramethylrhodamine methylester; DMSO, dimethyl sulfoxide; GFP, green fluorescent protein; OMM, outer mitochondrial membrane; PfARP, P. falciparum aspartate-rich protein.
R. A. Merrill, X. Zhou, and S. Strack, unpublished data.
References
- 1.Dimmer, K. S., and Scorrano, L. (2006) Physiol. (Bethesda) 21 233–241 [DOI] [PubMed] [Google Scholar]
- 2.Chan, D. C. (2006) Cell 125 1241–1252 [DOI] [PubMed] [Google Scholar]
- 3.McBride, H. M., Neuspiel, M., and Wasiak, S. (2006) Curr. Biol. 16 R551–560 [DOI] [PubMed] [Google Scholar]
- 4.Verstreken, P., Ly, C. V., Venken, K. J., Koh, T. W., Zhou, Y., and Bellen, H. J. (2005) Neuron 47 365–378 [DOI] [PubMed] [Google Scholar]
- 5.Li, Z., Okamoto, K., Hayashi, Y., and Sheng, M. (2004) Cell 119 873–887 [DOI] [PubMed] [Google Scholar]
- 6.Cheung, E. C., McBride, H. M., and Slack, R. S. (2007) Apoptosis 12 979–992 [DOI] [PubMed] [Google Scholar]
- 7.Suen, D. F., Norris, K. L., and Youle, R. J. (2008) Genes Dev. 22 1577–1590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoppins, S., Lackner, L., and Nunnari, J. (2007) Annu. Rev. Biochem. 76 751–780 [DOI] [PubMed] [Google Scholar]
- 9.Zuchner, S., Mersiyanova, I. V., Muglia, M., Bissar-Tadmouri, N., Rochelle, J., Dadali, E. L., Zappia, M., Nelis, E., Patitucci, A., Senderek, J., Parman, Y., Evgrafov, O., Jonghe, P. D., Takahashi, Y., Tsuji, S., Pericak-Vance, M. A., Quattrone, A., Battaloglu, E., Polyakov, A. V., Timmerman, V., Schroder, J. M., and Vance, J. M. (2004) Nat. Genet. 36 449–451 [DOI] [PubMed] [Google Scholar]
- 10.Alexander, C., Votruba, M., Pesch, U. E., Thiselton, D. L., Mayer, S., Moore, A., Rodriguez, M., Kellner, U., Leo-Kottler, B., Auburger, G., Bhattacharya, S. S., and Wissinger, B. (2000) Nat. Genet. 26 211–215 [DOI] [PubMed] [Google Scholar]
- 11.Delettre, C., Lenaers, G., Griffoin, J. M., Gigarel, N., Lorenzo, C., Belenguer, P., Pelloquin, L., Grosgeorge, J., Turc-Carel, C., Perret, E., Astarie-Dequeker, C., Lasquellec, L., Arnaud, B., Ducommun, B., Kaplan, J., and Hamel, C. P. (2000) Nat. Genet. 26 207–210 [DOI] [PubMed] [Google Scholar]
- 12.Waterham, H. R., Koster, J., van Roermund, C. W., Mooyer, P. A., Wanders, R. J., and Leonard, J. V. (2007) N. Engl. J. Med. 356 1736–1741 [DOI] [PubMed] [Google Scholar]
- 13.Barsoum, M. J., Yuan, H., Gerencser, A. A., Liot, G., Kushnareva, Y., Graber, S., Kovacs, I., Lee, W. D., Waggoner, J., Cui, J., White, A. D., Bossy, B., Martinou, J. C., Youle, R. J., Lipton, S. A., Ellisman, M. H., Perkins, G. A., and Bossy-Wetzel, E. (2006) EMBO J. 25 3900–3911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jahani-Asl, A., Cheung, E. C., Neuspiel, M., Maclaurin, J. G., Fortin, A., Park, D. S., McBride, H., and Slack, R. S. (2007) J. Biol. Chem. 282 23788–23798 [DOI] [PubMed] [Google Scholar]
- 15.Meuer, K., Suppanz, I. E., Lingor, P., Planchamp, V., Goricke, B., Fichtner, L., Braus, G. H., Dietz, G. P., Jakobs, S., Bahr, M., and Weishaupt, J. H. (2007) Cell Death Differ. 14 651–661 [DOI] [PubMed] [Google Scholar]
- 16.Janssens, V., and Goris, J. (2001) Biochem. J. 353 417–439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schmidt, K., Kins, S., Schild, A., Nitsch, R., Hemmings, B., and Götz, J. (2002) Eur. J. Neurosci. 16 2039–2048 [DOI] [PubMed] [Google Scholar]
- 18.Dagda, R. K., Zaucha, J. A., Wadzinski, B. E., and Strack, S. (2003) J. Biol. Chem. 278 24976–24985 [DOI] [PubMed] [Google Scholar]
- 19.Holmes, S. E., O'Hearn, E. E., McInnis, M. G., Gorelick-Feldman, D. A., Kleiderlein, J. J., Callahan, C., Kwak, N. G., Ingersoll-Ashworth, R. G., Sherr, M., Sumner, A. J., Sharp, A. H., Ananth, U., Seltzer, W. K., Boss, M. A., Vieria-Saecker, A. M., Epplen, J. T., Riess, O., Ross, C. A., and Margolis, R. L. (1999) Nat. Genet. 23 391–392 [DOI] [PubMed] [Google Scholar]
- 20.Holmes, S. E., O'Hearn, E., Cortez-Apreza, N., Hwang, H. S., Ross, C. A., Strack, S., and Margolis, R. L. (2006) in Genetic Instabilities and Neurological Diseases (Wells, R., and Ashizawa, T., eds) pp. 461–474, Academic Press, New York
- 21.Dagda, R. K., Barwacz, C. A., Cribbs, J. T., and Strack, S. (2005) J. Biol. Chem. 280 27375–27382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dobson, S., Kumar, R., Bracchi-Ricard, V., Freeman, S., Al-Murrani, S. W., Johnson, C., Damuni, Z., Chakrabarti, D., and Barik, S. (2003) Mol. Biochem. Parasitol. 126 239–250 [DOI] [PubMed] [Google Scholar]
- 23.Brummelkamp, T. R., Bernards, R., and Agami, R. (2002) Science 296 550–553 [DOI] [PubMed] [Google Scholar]
- 24.Gonzalez-Alegre, P., Bode, N., Davidson, B. L., and Paulson, H. L. (2005) J. Neurosci. 25 10502–10509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lim, I. A., Merrill, M. A., Chen, Y., and Hell, J. W. (2003) Neuropharmacology 45 738–754 [DOI] [PubMed] [Google Scholar]
- 26.Betarbet, R., Sherer, T. B., MacKenzie, G., Garcia-Osuna, M., Panov, A. V., and Greenamyre, J. T. (2000) Nat. Neurosci. 3 1301–1306 [DOI] [PubMed] [Google Scholar]
- 27.Strack, S., Ruediger, R., Walter, G., Dagda, R. K., Barwacz, C. A., and Cribbs, J. T. (2002) J. Biol. Chem. 277 20750–20755 [DOI] [PubMed] [Google Scholar]
- 28.Martinou, J. C., and Youle, R. J. (2006) Cell Death Differ. 13 1291–1295 [DOI] [PubMed] [Google Scholar]
- 29.Yuan, H., Gerencser, A. A., Liot, G., Lipton, S. A., Ellisman, M., Perkins, G. A., and Bossy-Wetzel, E. (2007) Cell Death Differ. 14 462–471 [DOI] [PubMed] [Google Scholar]
- 30.James, D. I., and Martinou, J. C. (2008) Dev. Cell 15 341–343 [DOI] [PubMed] [Google Scholar]
- 31.Sheridan, C., Delivani, P., Cullen, S. P., and Martin, S. J. (2008) Mol. Cell 31 570–585 [DOI] [PubMed] [Google Scholar]
- 32.Klumpp, S., and Krieglstein, J. (2002) Curr. Opin. Pharmacol. 2 458–462 [DOI] [PubMed] [Google Scholar]
- 33.Van Hoof, C., and Goris, J. (2003) Biochim. Biophys. Acta 1640 97–104 [DOI] [PubMed] [Google Scholar]
- 34.Ruvolo, P. P., Clark, W., Mumby, M., Gao, F., and May, W. S. (2002) J. Biol. Chem. 277 22847–22852 [DOI] [PubMed] [Google Scholar]
- 35.Chiang, C. W., Kanies, C., Kim, K. W., Fang, W. B., Parkhurst, C., Xie, M., Henry, T., and Yang, E. (2003) Mol. Cell. Biol. 23 6350–6362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ray, R. M., Bhattacharya, S., and Johnson, L. R. (2005) J. Biol. Chem. 280 31091–31100 [DOI] [PubMed] [Google Scholar]
- 37.Taguchi, N., Ishihara, N., Jofuku, A., Oka, T., and Mihara, K. (2007) J. Biol. Chem. 282 11521–11529 [DOI] [PubMed] [Google Scholar]
- 38.Chang, C. R., and Blackstone, C. (2007) J. Biol. Chem. 282 21583–21587 [DOI] [PubMed] [Google Scholar]
- 39.Cribbs, J. T., and Strack, S. (2007) EMBO Rep. 8 939–944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cereghetti, G. M., Stangherlin, A., Martins de Brito, O., Chang, C. R., Blackstone, C., Bernardi, P., and Scorrano, L. (2008) Proc. Natl. Acad. Sci. U. S. A. 105 15803–15808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Han, X. J., Lu, Y. F., Li, S. A., Kaitsuka, T., Sato, Y., Tomizawa, K., Nairn, A. C., Takei, K., Matsui, H., and Matsushita, M. (2008) J. Cell Biol. 182 573–585 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.