Abstract
Vav proteins participate in the assembly of a multibranched signal transduction pathway in lymphocytes, including the stimulation of the phosphatidylinositol 3-kinase/protein kinase B and the phospholipase C-γ/Ras GDP-releasing protein/Ras/Erk routes. In the present work, we used a genetic approach in chicken DT40 B-cell lines to investigate additional elements of the Vav route, the synergisms existing among the different Vav signaling branches, and the activities exerted by wild-type and oncogenic Vav proteins in B-lymphocytes. We show here that the Vav pathway is ramified in B-lymphocytes in additional diacylglycerol-dependent signaling branches such as those involving protein kinase C, protein kinase D, and phospholipase D. By using side-by-side comparisons of the activation levels of those signal transduction pathways in inhibitor-treated and knockout DT40 cells, we show that B-cells have different requirements regarding Vav proteins for the activation of antigen receptor downstream elements. Furthermore, we have detected interpathway cross-talk at the level of the most proximal elements but not among the most distal effector molecules of the Vav route. Finally, we show that the oncogenic versions of Vav1 and RhoA can activate alternative routes that could contribute to signal amplification and diversification events in transformed lymphocytes.
The Vav family is a group of signal transduction proteins involved in tyrosine kinase-regulated pathways that has single representatives in invertebrate species and usually three members (Vav1, Vav2, and Vav3) in vertebrates. The main known function of these proteins is to work as GDP/GTP exchange factors for Rho/Rac proteins, a family of Ras-related GTPases involved in a wide variety of intracellular functions such as cytoskeletal regulation, vesicle trafficking, and cell proliferation (1, 2). Under normal conditions, the enzyme activity of Vav proteins is stimulated by phosphorylation on tyrosine residues (3–5). However, this fine-tuned physiological regulation is lost when specific deletions or point mutations are created in the N termini of Vav proteins, leading to the generation of highly active proteins whose biochemical activities are independent of upstream signals (4, 5). Wild-type Vav proteins can also become spuriously activated by overexpression (6, 7), by tyrosine phosphorylation through autocrine loops (7, 8), or through binding to virally encoded molecules (9–11). The alteration of this regulatory cycle may be of interest for human pathologies, because it has been shown that constitutively active Vav proteins are oncogenic (4, 6, 12–14). Furthermore, Vav proteins are essential for the latency cycle of γ-herpesvirus (9, 10).
Whereas the biological activities associated to Vav proteins in non-hematopoietic cells can by ascribed to canonical Rho/Rac-dependent effects, the signaling role of Vav proteins in lymphocytes appears to be more complex. Thus, Vav proteins can promote the downstream activation of the Ras route via the PLC3-γ-dependent activation of RasGRP1, a DAG-regulated Ras protein activator (15–17). Likewise, Vav family proteins can trigger the activation of the Rap pathway via the F-actin-dependent translocation of the Rap exchange factor RasGRP2 (16). Vav proteins are also involved in the stimulation of PI3K and of NF-AT in lymphocytes (18–21). Finally, it has been shown that Vav proteins can bind to a number of signal transduction proteins via physical interactions (22, 23), suggesting that they can assemble catalytic-independent functions. In favor of this view, the activation of NF-AT by Vav proteins has been attributed to both Rac1-dependent and Rac1-independent pathways (24, 25).
The activation of enzymes involved in the generation of intracellular second messengers such as DAG, inositol triphosphate, Ca2+, and phosphatidylinositol 3,4,5-triphosphate by Vav proteins raises the possibility that these exchange factors may contribute to signal amplification and diversification events downstream of antigenic receptors. If so, it is also possible that the engagement of multiple signal transduction pathways could originate in turn synergistic and/or negative feedback mechanisms that would favor the modification of the signaling output of specific Vav downstream routes by other Vav-dependent pathways. Other related questions in this area include the relative dependence (total or partial) of B-cell signal transduction pathways on Vav function, the hierarchical position of Vav downstream elements in the assembly of this multibranched route, and the level of overlap between the downstream pathways activated in lymphocytes by wild-type and oncogenic forms of both Vav and Rho/Rac proteins.
To address these questions, we decided to conduct a multifaceted experimental approach based on the use of knockout cell lines, genetic rescue experiments, and the analysis of the effect of chemical inhibitors for enzymes located downstream of Vav proteins. Because mammalian B-cells contain the three Vav family members with overlapping functions that preclude an easy observation of signaling defects in single knockout animals (26, 27), these studies were conducted using DT40 B-cells. This chicken cell line expresses primarily Vav3 (15, 19), so Vav-dependent effects can be monitored in this system using DT40 cells lacking this Vav family member. Another advantage of using DT40 cells is that there is a wide collection of DT40 cell clones deficient for different signal transduction molecules, thus facilitating the genetic dissection of the signal transduction pathways under study. Previous results have shown that this is an optimal experimental system for the functional characterization of both B-cell signaling and Vav family-dependent responses (15, 19, 28, 29).
EXPERIMENTAL PROCEDURES
Antibodies and Reagents—Mouse monoclonal antibodies to GFP, HA, and AU5 were obtained from Covance. Antibodies to PKD, phospho-PKD, Erk, phospho-Erk, PKB, and phospho-PKB were purchased from Cell Signaling. Antibodies to PKC family members were from BD Transduction Laboratories. The rabbit polyclonal antiserum to the mouse Vav1 DH domain has been described previously (30). The mouse monoclonal antibody to chicken IgMs was from Southern Biotech. Horseradish peroxidase-conjugated secondary antibodies to rabbit and mouse IgGs were obtained from GE Healthcare. U73122, U73343, GF109203X, PD98059, Wortmannin, BAPTA, and Dantrolene were all purchased from Calbiochem. p110α inhibitor 2, TG-X221, and AS-252424 were from Cayman Chemical. Phorbol myristate acetate, t-butanol, and n-butanol were from Sigma.
Plasmids—The plasmid encoding human PKD1 tagged at its C terminus with EGFP (pEGFP-N1-PKD1) was obtained from Dr. A. Hausser (Institute for Cell Biology and Immunology, University of Stuttgart, Stuttgart, Germany). The expression vector encoding PKD1-HA (pcDNA3-HA-PKD) was provided by Dr. A. Toker (Harvard Medical School, Boston, MA). Plasmids encoding either wild-type (pCIneo-PLC-γ1) or palmitoylated (pCIneo-palm PLC-γ1) HA-tagged PLC-γ1 were obtained from Dr. E. Bonvini (MacroGenics, Rockville, MD). Plasmids encoding the active versions of PKCα (pEF-PKCαA/E) and PKCε (pcDNA3-HA-PKCεA/E) were provided by Dr. J. Moscat (Genome Research Center, Cincinnati, OH). Plasmids encoding the EGFP-tagged versions of wild-type (pEGFP-C1-hPLD1b and pEGFP-C1-mPLD2) and mutant PLDs (pEGFP-C1-hPLD1bK898R and pEGFP-C1-mPLD2K750R) were obtained from Dr. M. Frohman (Stony Brook University, Stony Brook, NY). Plasmids encoding mouse wild-type Vav1 (pJC11), mouse oncogenic Vav1 (Δ1–186 mutation, pKES12), HA-H-Ras, AU5-Rac1, AU5-RhoA, and AU5-Cdc42 proteins have been previously described (4, 6, 13, 15, 31).
Cell Culture, DNA Transfections, and Cell Stimulation—Wild-type and mutant DT40 cells were obtained from the Riken Bioresource Center (Ibaraki, Japan) and cultured and electroporated as described before (15). For cell stimulation, DT40 cells were resuspended in RPMI1640 media at a density of 5 × 106 cell/ml and then treated with either anti-IgM (5 μg/ml) or PMA (1 μg/ml) for the indicated periods of time. Inhibition of specific signaling pathways was achieved by incubation of DT40 cells with indicated inhibitors for 30 min at 37 °C prior to cell stimulation. To inhibit PKC activities, DT40 cells were incubated with GF109203X (5 μm). To inhibit PLC-γ2 activity, DT40 cells were incubated with U73122 (10 μm) and, as negative control, with the inactive U73343 compound (10 μm). For MEK inhibition, cells were treated with PD98059 (50 μm). For the inhibition of PI3K activities, cells were incubated with Wortmannin (100 nm), p110α inhibitor 2 (0.1 μm), TGX221 (0.5 μm), or AS252424 (0.5 μm). Inhibition of PLD activities was achieved by maintaining cells in RPMI media supplemented with 0.3% n-butanol. As negative control, we used parallel cultures preincubated with 0.3% t-butanol as above. To chelate intracellular Ca2+ and to block its release from intracellular stores, DT40 cells were incubated with BAPTA (50 μm) and Dantrolene (50 μm), respectively. After the indicated stimulation conditions, cells were disrupted by vortexing in a lysis buffer containing 20 mm Tris-HCl (pH 7.4), 150 mm NaCl, 1% Nonidet P-40, 1 mm NaF, 100 μm Na2VO4, and a mixture of protease inhibitors (Complete, Roche Applied Science). The resulting cell lysates were cleared by centrifugation, diluted 1:1 in SDS-PAGE sample buffer, and subjected to Western blot analysis with appropriate antibodies. Immunoreactive bands were visualized using a chemiluminescence detection system (Pierce). Activation of H-Ras was analyzed by pulldown assays using the H-Ras binding domain of c-Raf1 (15).
RESULTS
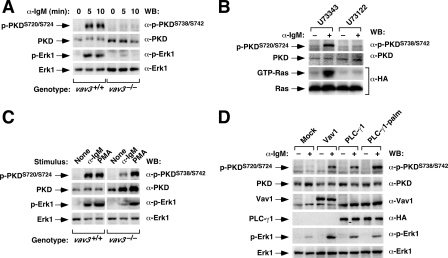
The Vav Pathway Is Essential for Proper Activation of the PKC/PKD Pathway in B-cells—The activation by Vav proteins of enzymes that generate intracellular second-messengers indicates that they may have a major role in signal amplification and diversification in lymphocytes. To verify this possibility, we decided to monitor the activation of PKD in B-cells in either the presence or absence of Vav3 protein. We selected this signaling target, because PKD is dependent on DAG for membrane translocation and, in addition, requires the activity of PKC family members to achieve catalytic activation and adequate subcellular localization kinetics (32). As previously described (33), the stimulation of wild-type DT40 cells by BCR cross-linking with anti-IgM antibodies resulted in a rapid and stable phosphorylation of PKD at the PKC-dependent (serine residues 720 and 724) and autophosphorylation sites (serine residue 898) (Figs. 1A and data not shown, respectively). By contrast, BCR-stimulated vav3-/- DT40 cells were inefficient at promoting PKD phosphorylation in any of those sites (Figs. 1A and data not shown). These defects were linked to improper activation of PLC-γ2, because the phenotype of vav3-/- DT40 cells could be mimicked in wild-type cells using treatments with U73122 (Fig. 1B), a general PLC-γ family inhibitor. Moreover, the defects found in vav3-/- DT40 cells could be rescued by stimulating them with DAG analogs (PMA) (Fig. 1C) or, alternatively, by overexpressing in them either wild-type or palmitoylated, membrane-anchored versions of PLC-γ1 (Fig. 1D). The rescue of PKD phosphorylation by overexpressed PLC-γ1 proteins still required the engagement of the BCR (Fig. 1D), indicating that the activation of PLC-γ1 in B-cells needs both membrane translocation and tyrosine phosphorylation. The defective activation of PKD in vav3-/- DT40 cells was also rescued by the overexpression of wild-type Vav1 (Fig. 1D), confirming that the defects observed in these cells are directly derived from the elimination of Vav function on those cells. The stimulation conditions of PKD were very similar to those required for the stimulation of RasGRP1 (15), Erk1 (Fig. 1, A, C, and D) (15), and H-Ras (Fig. 1B) (15) in the same set of experiments, further suggesting that these RasGRP/Ras and PKC/PKD cascades are under the regulation of the same Vav3/PLC-γ2 route during BCR signaling.
FIGURE 1.
Vav3 is required for PKD activation in chicken B-cells. A, defective activation of PKD in Vav3-deficient lymphocytes. DT40 cells of the indicated genotypes (bottom) were stimulated with anti-IgM antibodies (10 μg/ml). At the indicated times (top), cells were lysed and the protein extracts obtained were subjected to immunoblot analysis with the indicated antibodies (right). B, PLC-γ2-dependent activation of PKD. Wild-type DT-40 cells were preincubated with the indicated drugs (top, 10 μm each) for 30 min and then either left unstimulated (-) or stimulated (+) with anti-IgM antibodies for 5 min. Total cellular extracts were then obtained and subjected to immunoblot analysis (first, second, and fourth panels from top) with the indicated antibodies (right) or, alternatively, to pulldown experiments with a GST-c-Raf1 RBD fusion protein (see “Experimental Procedures”). After the pull down, bound proteins were released from beads by boiling, and Ras proteins were detected by anti-HA immunoblot (third panel from top). C, phorbol esters rescue the activation of PKD in Vav3-deficient cells. DT40 cells of the indicated genotypes (bottom) were stimulated with either anti-IgM or PMA (1 μm) as indicated (top). After 5 min, cells were lysed and the total cell extracts obtained were analyzed by immunoblot using the indicated antibodies (right). D, overexpression of PLC-γ2 promotes optimal activation of PKD in the absence of Vav3 upon BCR cross-linking. vav3-/- DT40 cells were transfected with expression vectors encoding the indicated proteins (top). 24 h after transfection, cells were either left unstimulated (-) or stimulated (+) with anti-IgM antibodies for 5 min. After cell lysis, total protein extracts were subjected to Western blot analysis with the indicated antibodies (right). The mobility of the indicated protein or phosphoprotein is indicated on the left of each panel with arrows. PLC-γ1 palm, palmitoylated version of PLC-γ1; WB, Western blot.
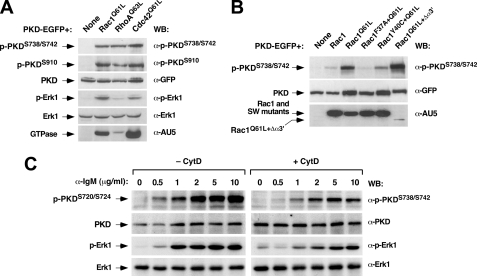
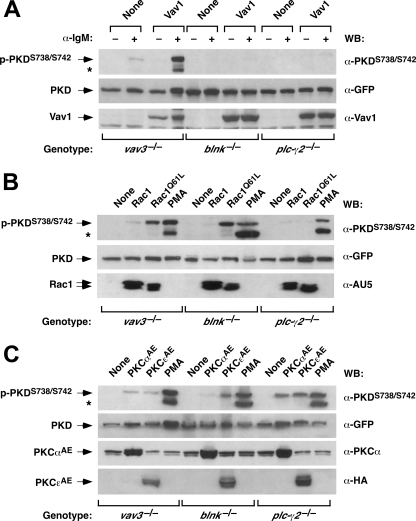
To verify this possibility, we next performed rescue experiments in vav3-/- DT40 cells with constitutively active versions of Rho/Rac family members, because, in the case of the Ras-GRP/Ras pathway, the engagement of this route has been shown to be dependent on Vav1 GDP/GTP exchange activity and Rac1 signals in T- and B-cells (15, 31). To this end, we expressed in vav3-/- DT40 cells human PKD-EGFP either alone or in combination with AU5-tagged versions of Rac1Q61L, RhoAQ63L, or Cdc42Q61L and then measured the phosphorylation levels of ectopically expressed PKD and endogenous Erk1 proteins by immunoblotting. These experiments indicated that Rac1Q61L, RhoAQ63L, and Cdc42Q61L could effectively rescue the phosphorylation of human PKD on residues 738, 742 (Fig. 2A, upper panel), and 910 (Fig. 2A, second panel from top). Those three sites are the counterparts of the phosphosites located at positions 720, 724, and 898 previously described in the chicken PKD ortholog. This pan-specific effect of Rho/Rac proteins on PKD phosphorylation was somewhat different in the case of the stimulation of endogenous Erk1, because, as previously described (15), RhoAQ63L cannot restore the activation of this Ras downstream element in vav3-/- DT40 cells (Fig. 2A, fourth panel from top).
FIGURE 2.
PKD activation is in a downstream position relative to Rho/Rac GTPases in the Vav pathway. A, constitutively active versions of Rac1, RhoA, and Cdc42 rescue the activation of PKD in Vav3-deficient cells. vav3-/- DT40 cells were transfected with a plasmid encoding PKD-EGFP either alone or in combination with vectors encoding AU5-tagged versions of Rho/Rac proteins (top). After transfection, the phosphorylation levels of PKD-EGFP protein were measured by Western blot using phosphospecific antibodies (right, two top panels). As a comparative control, we also determined the levels of phosphorylation of endogenous Erk1 protein under the same conditions using phosphospecific antibodies to Erk1 (fourth panel from top). The levels of expression of PKD-EGFP (third panel from top), endogenous Erk1 (fifth panel from top), and the AU5-tagged GTPases (bottom panel) were determined by immunoblot using the indicated antibodies (right). B, effect of Rac1 effector mutants on PKD activation in DT40 cells. vav3-/- DT40 cells were transfected with plasmids encoding PKD-EGFP either alone or in combination with vectors encoding the indicated Rac1 proteins (top). After transfection, the levels of phosphorylation of the ectopically expressed PKD protein were measured using a phosphospecific antibody (top panel). The levels of expression of PKD and Rac1 proteins were determined by immunoblot analysis with anti-GFP (middle panel) and anti-AU5 (bottom panel) antibodies, respectively. C, optimal PKD activation by the BCR requires the assembly of the F-actin cytoskeleton. Exponentially growing cells were cultured in the absence (- CytD) or presence (+ CytD) of cytochalasin D (10 μm) for 2 h and then stimulated for 10 min with the indicated amounts of anti-IgM antibodies (top). Cells were then lysed, and the resulting extracts were analyzed by immunoblot using either anti-phospho-PKD (upper panel) or anti-phospho-Erk1 (third panel from top) antibodies. Aliquots of the same lysates were subjected to Western blot with anti-PKD (second panel from top) and anti-Erk1 (bottom panel) antibodies to detect the total amount of these two proteins in those samples.
We next used Rac1Q61L effector mutants partially impaired in their signaling properties to identify the downstream routes implicated in PKD activation. These proteins included Rac1F37A+Q61L, a mutant that can activate p21-activated kinase and JNK without inducing detectable effects on the F-actin cytoskeleton or in cell transformation (34); Rac1Y40C+Q61L, a mutant that triggers cytoskeletal change and tumorigenesis without stimulating p21-activated kinase or JNK (34); and Rac1Q61L+Δα3′, a mutant that lacks the α3′ insert region that promotes p21-activated kinase, JNK, and cytoskeletal change but is incapable of inducing superoxide production or cell transformation (35). As shown in Fig. 2B (upper panel), the expression of Rac1Q61L+Δα3′ restored the phosphorylation of the ectopically expressed PKD fusion protein in vav3-/- DT40 cells to levels similar to those induced by Rac1Q61L. Instead, Rac1F37A+Q61L and Rac1Y40C+Q61L were totally or partially ineffective in restoring PKD phosphorylation, respectively. The inefficacy of these mutants in rescuing PKD phosphorylation is further highlighted by the observation that their expression levels are significantly higher than those of the Rac1Q61L+Δα3′ mutant protein (Fig. 2B, bottom panel). As a control, we observed that the co-transfection of wild-type Rac1 with the human PKD-EGFP protein was also ineffective in rescuing the phosphorylation of that kinase in vav3-/- DT40 cells (Fig. 2B, upper panel). To confirm the requirements of F-actin polymerization for the effective activation of PKD, we decided to study the effect of cytochalasin D, a known F-actin disrupting agent, on the BCR-dependent activation of PKD. As a control, we also monitored the phosphorylation of Erk1, because the stimulation of this kinase has been shown to be F-actin-dependent in DT40 cells (15). These experiments indicated that the pre-treatment of wild-type DT40 cells with cytochalasin D does influence negatively the phosphorylation of endogenous PKD and Erk1 proteins (Fig. 2C). Taken together, these results indicate that the activation of PKD in this system requires, like the RasGRP/Ras route (15, 31), the Rac1 and the F-actin cytoskeleton. However, unlike the case of that latter pathway, the activation of PKD appears to be also facilitated by additional Rho/Rac family members and Rac1 effectors.
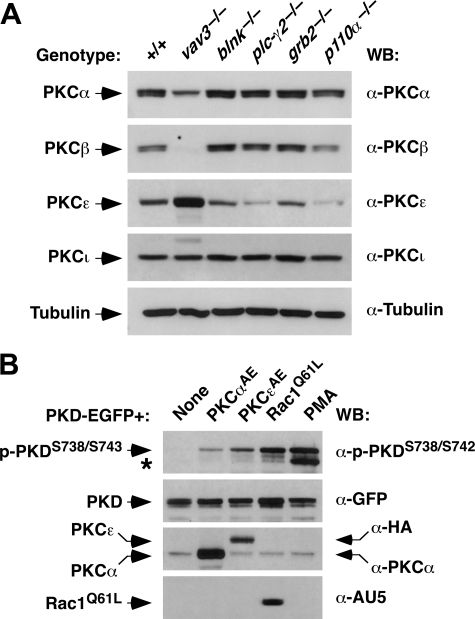
Vav3-mediated PKD Phosphorylation Is Mediated Preferentially by PKCε—To identify the kinase directly involved in PKD phosphorylation, we performed immunoblot analysis to characterize the expression of a number of PKC family members in DT40 cells. PKCδ was not detected in those cells (data not shown). DT40 cells expressed, however, a large number of other PKC family members, although the expression of some of them was genotype-dependent. For example, we found that vav3-/- cells expressed PKCα, PKCε, and PKCι but not PKCβ (Fig. 3A). This lack of expression seemed to be compensated by the overexpression of PKCε in vav3-/- DT40 cells (Fig. 3A, third panel from top). Unlike this cell line, the parental and other knockout DT40 cell lines showed expression of the other PKC isoforms tested (Fig. 3A). The absence of PKCβ was not the consequence of the lack of activation of PKD in vav3-/--deficient cells, because PKD phosphorylation can be rescued by upstream elements to PKCs such as Vav1, PLC-γ1, or phorbol esters (see above, Fig. 1B).
FIGURE 3.
PKCα and PKCε are involved in PKD activation in B-cells. A, expression of PKC isoforms. Total cellular extracts derived from DT40 cells of the indicated genotypes (top) were subjected to immunoblot analysis with antibodies to different PKCs (right). As control, aliquots of the same lysates were analyzed by anti-tubulin Western blot to demonstrate equal loading of all samples. B, constitutively active forms of PKCα and ε rescue PKD activation in Vav3-deficient cells. vav3-/- DT40 cells were transfected with an expression vector encoding PKD-EGFP alone or in combination with plasmids encoding the constitutively active versions of PKCα, PKCε, and Rac1 (top). 24 h post-transfection, cells were lysed, and the resulting extracts were analyzed by immunoblot with the indicated antibodies (right). As a control, an aliquot of lysates obtained from PMA-stimulated vav3-/- DT40 cells was analyzed in parallel in these studies. The asterisk shows the phosphorylated band of the endogenous PKD protein present in DT40 cells.
We then resorted to transient transfection experiments using constitutively active versions of PKCs to identify the PKC isoform(s) responsible for PKD phosphorylation in vav3-/- DT40 cells. To this end, the human PKD-EGFP fusion protein was expressed either alone or in combination with activated versions (A → E mutants) of PKCα and PKCε. We excluded PKCι in these analysis, because this protein, although expressed in DT40 cells (see above), is not regulated by either DAG or Ca2+ (36). As shown on Fig. 3B (upper panel), PKCεAE and, to a much marginal level PKCαAE promoted the phosphorylation of the ectopically expressed human PKD on Ser738 and Ser743. Hence, PKCε is probably the main kinase that mediates PKD phosphorylation in DT40 cells.
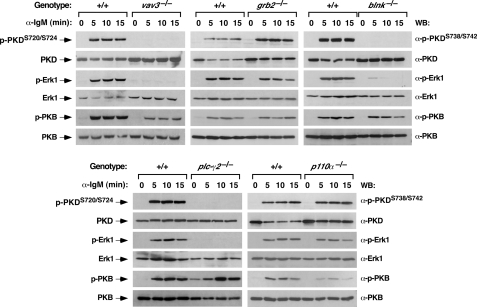
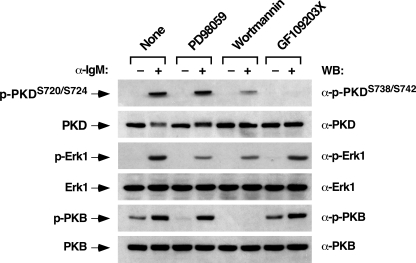
Influence of Proximal Vav Signaling Elements in the Activation of Distal Effectors of the Vav Pathway—It has been previously shown that Vav signaling in B-lymphocytes requires the participation of both adaptor molecules (Grb2 and Blnk) and downstream enzymes such as PLC-γ2 and PI3K (15, 19, 28). The availability of knockout cell clones deficient for all these signal transduction proteins allowed us to conduct comparative studies to revealing the signaling requirements for PKD phosphorylation by the BCR, the relative dependence of different BCR downstream pathways on Vav3 activity, and the possible cross-talk among the identified Vav3 downstream responses. To this end, we analyzed by immunoblot analysis the kinetics of phosphorylation of PKD, Erk1, and PKB (also known as Akt) in wild-type and Vav3-, Grb2-, Blnk-, PLC-γ2-, and p110α PI3K-deficient DT40 cells during BCR signal transduction. As previously shown (see above), the elimination of Vav3 expression led to the total elimination of the activation of PKD and Erk1 (Fig. 4, first block of panels). In contrast, these cells still displayed lower, but significant levels of PI3K activity, as assessed by the moderate levels of PKB phosphorylation observed upon BCR cross-linking (Fig. 4). This result suggests that while the PKD and Ras/Erk1 pathways are strictly dependent on Vav3, the activation of the PI3K/PKB route requires the engagement of both Vav3-dependent and independent routes by the BCR. The elimination of Grb2, a protein essential for the activation of Ras GDP/GTP exchange factors of the Sos1 family and with proposed roles in Vav3 translocation (28, 37), did not influence the activation of PKD or PKB (Fig. 4, second block of panels). In fact, the only defect observed in this cell line was slightly shorter kinetics of Erk1 phosphorylation (Fig. 4), a result consistent with the subsidiary role of the Sos GDP/GTP exchange factor family in the activation of Ras in lymphocytes (38). Thus, Grb2 seems to be totally dispensable for the stimulation of Vav3, PLC-γ2, or PI3K in this cell setting. The elimination of Blnk, an adaptor molecule implicated in the translocation of Vav3 to lipid rafts and in PLC-γ2 phosphorylation (39), resulted in the abrogation of PKD phosphorylation. In the case of Erk1, we could observe a consistent small and very transient induction Erk1 phosphorylation at the earliest stimulation time point (Fig. 4, third block of panels). Instead, the Blnk deficiency only affected marginally the phosphorylation kinetics of PKB (Fig. 4). Because Vav3 does affect PKB phosphorylation, these results indicate that the role of Blnk in this route is probably downstream of Vav3. The genetic deletion of the plc-γ2 gene resulted in the total elimination of the phosphorylation of PKD and Erk1 (Fig. 4, fourth block of panels), a result that confirms that PLC-γ2 is downstream of Vav3 and upstream of PKCs, RasGRP family members, and PKD. Instead, with the exception of a small delay in its kinetics, the phosphorylation of PKB was maintained (Fig. 4). Finally, we observed that the knockout of the p110α gene resulted in a decreased efficiency of PKB activation by the BCR (Fig. 4, fifth block of panels). This reduction was similar to that observed in the case of vav3-/- DT40 cells (Fig. 4, first block of panels). In contrast, the elimination of this PI3K isoform did not induce any significant defects in the phosphorylation of PKD and Erk1 (Fig. 4). Because PI3K activity is required for optimal PLC-γ2 activation by the BCR (29), these results indicate that PI3K family members different from p110α are involved in the Vav3-mediated activation of PLC-γ2. Likewise, the lower, but significant detection of PKB phosphorylation in p110α-/- DT40 cells also indicates that the stimulation of this route entails the utilization of other PI3K family members. To test this possibility, we measured the levels of phosphorylation of PKD, Erk1, and PKB in wild-type DT40 cells treated with Wortmannin, a PI3K inhibitor of wide specificity. We observed a total abrogation of the BCR-induced PKB phosphorylation (Fig. 5, fifth panel from top) and a significant reduction in the phosphorylation levels of both PKD and Erk1 in Wortmannin-treated ells (Fig. 5, first and third panels from top). The effect of this drug on PKD and Erk1, but not on PKB, was counteracted by the administration of PMA to DT40 cells, further confirming that the inhibitory effect of the PI3K inhibitor was probably due to inefficient PLC-γ2 activation by the BCR (data not shown).
FIGURE 4.
Role of Vav3, Grb2, Blnk, PLC-γ2, and p110α PI3K in the activation of PKD in chicken B-lymphocytes. DT40 cells of the indicated genotypes (top) were stimulated with anti-IgM antibodies during the indicated periods of time (top). After cell disruption, the activation and expression levels of PKD (top panels), Erk1 (third panels from top), and PKB (fifth panels from top) were determined by Western blot using appropriate antibodies (right). A similar analysis was performed to reveal the total amount of PKD (second panels from top), Erk1 (fourth panels from top), and PKB (bottom panels).
FIGURE 5.
Role of Erk1, PI3Ks, and PKCs in the activation of PKD in chicken B-cells. Wild-type DT40 cells were pre-treated for 30 min with the indicated inhibitors (top) and then either left unstimulated (-) or stimulated (+) with anti-IgM antibodies for 5 min. After cell lysis, the levels of activation and expression of PKD, Erk1, and PKB were determined by immunoblot analysis using appropriate antibodies (right).
To assess possible reciprocal dependences of Vav3 downstream elements, we also evaluated the effect of the chemical inhibition of the Erk1 and PKC routes on the phosphorylation of PKD, Erk1, and PKB. The treatment of DT40 cells with the MEK inhibitor PD98059 had no effect on PKD (Fig. 5, upper panel) or PKB phosphorylation (Fig. 5, fifth panel from top) while it induced a significant blockage of Erk1 phosphorylation (Fig. 5, third panel from top). Instead, the general PKC family inhibitor (GF109203X) blocked the phosphorylation of PKD but did not alter the phosphorylation levels of Erk and PKB (Fig. 5, first, third, and fifth panels, respectively). These results, together with those obtained with the knockout cell lines, indicate that Vav3 action contributes to the assembly of three coordinated, but mutually independent signal transduction cascades. Moreover, they indicate that Vav3 is required for the effective assembly of PLC-γ2-dependent pathways by the BCR but only partially needed for the stimulation routes lying downstream of PI3K.
Hierarchical Position of Vav Signaling Elements in the PKC/PKD Route—To dissect the signaling hierarchy between Vav, Rho/Rac proteins, Blnk, and PLC-γ2 during PKD activation, we resorted to reconstitution experiments in vav3-/-, blnk-/-, and plc-γ2-/- DT40 cells. As functional readout for these experiments, we used the trans-phosphorylation of PKD by PKCs. These experiments revealed that wild-type Vav1 rescued the Vav3 but not the Blnk or PLC-γ2 deficiencies (Fig. 6A, upper panel). Consistent with the phosphorylation-dependent exchange activity of Vav proteins, the rescue of wild-type Vav1 was only observed upon stimulation of DT40 cells by BCR cross-linking (Fig. 6A, upper panel). The overexpression of Rac1Q61L rescued, without the need of BCR stimulation, the lack of PKD phosphorylation in both vav3-/- and blnk-/- cells. This rescue was comparable to that observed after the stimulation of those cells with PMA (Fig. 6B, upper panel). However, Rac1Q61L could not rescue the PLC-γ2 deficiency (Fig. 6B, upper panel), further demonstrating that this GTPase is downstream of Vav3 and Blnk but upstream of that phospholipase. In agreement with our previous data (Fig. 2B), wild-type Rac1 did not rescue any of these deficiencies (Fig. 6B, upper panel). Finally, the constitutively active mutants of PKCα and PKCε restored the phosphorylation in the three cell lines, confirming the downstream position of these enzymes relative to Vav3, Blnk, and PLC-γ2.
FIGURE 6.
Vav1, Rac1, and PKCs show different signaling requirements for the activation of PKD during BCR signal transduction. A, Vav1 requires both Blnk and PLC-γ2 for PKD activation. DT40 cells of the indicated genotypes (bottom) were transfected with a vector encoding PKD-EGFP either alone or in combination with an expression plasmid encoding wild-type Vav1, as indicated (top). 24 h later, cells were either left unstimulated (-) or stimulated (+) with anti-IgM antibodies for 5 min. Cellular extracts were then obtained and analyzed by immunoblotting with the indicated antibodies (right) to detect the phosphorylation of PKD (top panel) and the expression levels of both PKD-EGFP (middle panel) and Vav1 (bottom panel). B and C, Rac1Q61L and PKCs activate PKD through a PLC-γ2-dependent and independent manner, respectively. DT40 cells of the indicated genotypes (B and C, bottom) were transfected with an expression vector encoding PKD-EGFP either alone or in combination with vectors expressing the indicated Rac1 (B) and PKC (C) proteins. 24 h post-transfection, cell extracts were obtained and analyzed by immunoblotting with the indicated antibodies (B and C, right) to detect the level of PKD-EGFP phosphorylation (B and C, upper panels) and the expression levels of PKD-EGFP (B and C, second panels from top), Rac1 GTPases (B, bottom panel), PKCα (C, third panel from top), and PKCε (C, bottom panel). In A–C, the asterisk shows the phosphorylated band of the endogenous PKD.
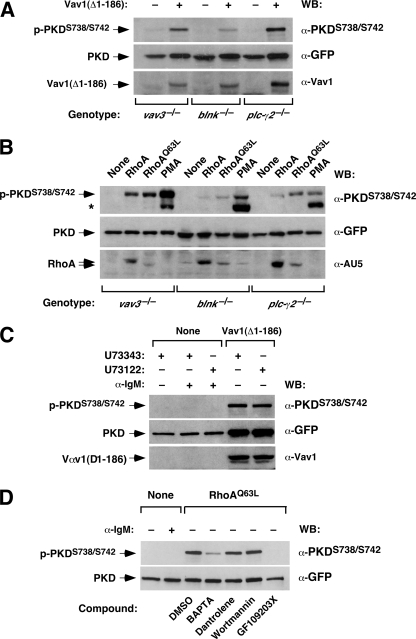
To further characterize the PKD signal transduction pathway in B-cells, we next performed rescuing transfection experiments in vav3-/-, blnk-/-, and plc-γ2-/- DT40 cells with oncogenic versions of Vav1 protein (Δ1–186 mutant) and RhoA. Vav1 (Δ1–186) is constitutively active independently of its phosphorylation status due to the removal of the inhibitory, N-terminal sequences present in the wild-type protein (4, 25, 40). Unexpectedly, we observed that this mutant, in addition to rescuing the Vav3 deficiency without the need of upstream BCR stimulation (Fig. 7A, upper panel), could restore PKD phosphorylation in PLC-γ2-deficient cells (Fig. 7A, upper panel). Likewise, the wild-type and constitutively active versions of RhoA restored PKD phosphorylation in both Vav3- and PLC-γ2-deficient cells (Fig. 7B, upper panel). High activity of wild-type RhoA under overexpression conditions is also observed in other experimental systems, such as the oncogenic transformation of rodent fibroblasts.4 Vav1 (Δ1–186) and RhoA proteins were less efficient in promoting PKD phosphorylation in blnk-/- cells (Fig. 7, A and B).
FIGURE 7.
RhoA and an oncogenic version of Vav1 can promote PKD activation in a PLC-γ2-independent manner. A and B, DT40 cells of the indicated genotypes (A and B, bottom) were transfected with an expression plasmid encoding PKD-EGFP either alone or in combination with vectors expressing the Vav1 (Δ1–186) oncoprotein (A), wild-type RhoA (B), and constitutively active RhoA (B) proteins. 24 h post-transfection, cell extracts were obtained and analyzed by immunoblotting with the indicated antibodies (A and B, right) to detect the level of PKD-EGFP phosphorylation (A and B, upper panels) and the expression levels of PKD-EGFP (A and B, middle panels), Vav1 (Δ1–186) (A, bottom panel), and RhoA proteins (B, bottom panel). In B, the asterisk shows the phosphorylated band of the endogenous PKD protein present in DT40 cells. C, the activation of PKD by Vav1 (Δ1–186) in plc-γ2-/- cells is not due to residual PLC-γ activity. PLC-γ2-deficient DT40 cells were transfected with a plasmid encoding PKD-EGFP either alone or in combination with a vector encoding the Vav1 (Δ1–186) oncoprotein (top). 24 h after transfection, cells were preincubated with the indicated drugs (top) for 30 min and then stimulated with anti-IgM antibodies for 5 min. The phosphorylation levels of PKD-EGFP protein were then measured by Western blot using phosphospecific antibodies (upper panel). The expression levels of PKD-EGFP and Vav1 (Δ1–186) were stimulated in parallel by immunoblotting with anti-GFP (middle panel) and anti-Vav1 (bottom panel) antibodies, respectively. The asterisk marks the position of the phosphorylated band of endogenous PKD. D, the activation of PKD by RhoAQ63L in plc-γ2-/- cells is PKC-dependent but Ca2+- and PI3K-independent. PLC-γ2-deficient DT40 cells were transfected with a plasmid encoding PKD-EGFP either alone or in combination with a vector encoding the RhoAQ63L (top). 24 h after transfection, cells were preincubated with the indicated drugs (bottom) for 30 min and left non-stimulated (-) or stimulated (+) with anti-IgM antibodies for 5 min. The phosphorylation levels of PKD-EGFP protein were then measured as above (upper panel). The expression levels of PKD-EGFP were determined in parallel by immunoblotting with anti-GFP antibodies (bottom panels).
The rescue of PKD phosphorylation in plc-γ2-/- DT40 cells by both Vav1 (Δ1–186) and RhoAQ63L was not due to residual levels of PLC-γ activities, because PKD phosphorylation was not abolished by the incubation of transfected cells with the U73122 inhibitor (Fig. 7C, upper panel, and data not shown). To characterize further this new pathway, we analyzed the effect of a number of inhibitors in this response. To this end, we determined the PKD phosphorylation levels in plc-γ2-/- cells co-expressing human PKD-EGFP and RhoAQ63L that had been treated with a general Ca2+chelator (BAPTA), a blocker of Ca2+ fluxing from the endoplasmic reticulum (Dantrolene) and with inhibitors for PI3K (Wortmannin) or PKCs (GF109203X). Under these conditions, the RhoAQ63L-mediated PKD phosphorylation could only be blocked by the PKC inhibitor (Fig. 7D). The treatment with BAPTA also induced some inhibition, but additional control experiments indicated that this was a non-specific event (data not shown). Similar results were obtained in plc-γ2-/- DT40 cells co-expressing PKD-EGFP and Vav1 (Δ1–186) (data not shown). These results suggest that there is a surrogate pathway involved in PKD phosphorylation in B-cells. This alternative pathway is PLC-γ2-independent but PKC-dependent.
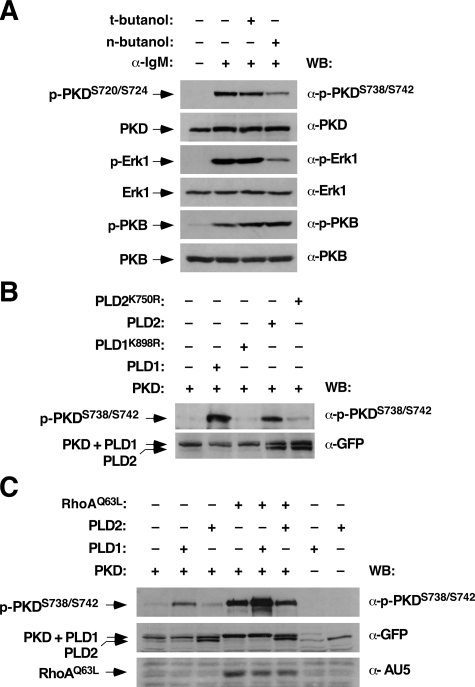
Because the RhoAQ63L- and Vav1 (Δ1–186)-mediated PKD phosphorylation was inhibited by PKC inhibitors, we surmised that this alternative route had to involve still the generation of DAG in plc-γ2-/- cells. An independent source of DAG is supplied by the PLD/phosphatidic acid phosphatase axis, a signaling route that mediates the stepwise conversion of phosphatidylcholine in phosphatidic acid and DAG (41). Indeed, it has been shown before that PLD1 is expressed and functional in BCR signaling events of DT40 cells (42, 43). To verify whether this route could be involved in the rescue of PKD phosphorylation by RhoAQ63L and Vav1 (Δ1–186), we first analyzed the effect of an inhibitor of PLD activity (n-butanol) in BCR-stimulated wild-type DT40 cells. As negative control, we used an analogous molecule to n-butanol (t-butanol) that does not inhibit PLD. The preincubation of DT40 cells with n-butanol decreased the phosphorylation levels induced by BCR cross-linking on PKD and Erk1 (Fig. 8A, first and third panels from top, respectively). In contrast, this inhibitor had no detectable effect on PKB phosphorylation (Fig. 8A, fifth panel from top). t-Butanol did not block the phosphorylation of any of those BCR downstream molecules (Fig. 8A). Given the above results, we decided to analyze the effect of overexpressing PLD family members in PLC-γ2-deficient DT40 cells on PKD phosphorylation. As shown in Fig. 8B (upper panel), the overexpression of EGFP-PLD1 and, to a lower extent, EGFP-PLD2 induced the phosphorylation of PKD on Ser738 and Ser742. The PLD-dependent phosphorylation of PKD was abolished when the wild-type PLD versions were replaced in the transfections with catalytically inactive phospholipase mutants (Fig. 8B, upper panel). PKD phosphorylation was enhanced when EGFP-PLD1 was co-expressed with AU5-RhoAQ63L (Fig. 8C, upper panel). This synergistic effect was limited to PLD1, because the co-expression of the GTPase with EGFP-PLD2 did not result in higher PKD phosphorylation levels (Fig. 8B, upper panel). Similar results were obtained using transfections in wild-type DT40 cells (data not shown).
FIGURE 8.
PLD1 triggers PKD phosphorylation in PLC-γ2-deficient DT40 cells. A, inhibition of PKD phosphorylation by the PLD inhibitor n-butanol during BCR stimulation. Wild-type DT40 cells were preincubated with the indicated molecules (top) and then either left unstimulated or stimulated with anti-IgM antibodies for 5 min. After stimulation, total cell extracts were obtained and analyzed by immunoblotting using the indicated antibodies (right). B, PLD family members induce phosphorylation on the PKD activation loop in a PLC-γ2-independent manner. plc-γ2-/- DT40 cells were transfected with PKD-EGFP plus either wild-type or mutant forms of EGFP-tagged PLD1 and PLD2 (top). After 24 h, cell extracts were obtained and analyzed by Western blot using the indicated antibodies (right). C, RhoAQ63L synergizes with PLD1 for PKD phosphorylation. Total cellular lysates from plc-γ2-/- DT40 cells expressing the indicated combinations of ectopically expressed proteins (top) were immunoblotted with the antibodies shown on the right.
DISCUSSION
In this work, we have analyzed the spectrum of downstream signals activated by Vav proteins in B-cells and, in addition, the relative contribution of them to the overall signaling program of these exchange factors. Our results indicate that, in addition to the expected activation of the PI3K/PKB and the PLC-γ2/RasGRP/Ras routes (15, 19), Vav proteins can induce the stimulation of additional signal transduction branches (Fig. 9). Thus, we have shown that Vav proteins are required for the activation of the PKC/PKD pathway and, possibly, for PLD proteins, a pathway that in turn can promote further amplification and diversification of BCR-dependent signals via the generation of second messengers such as DAG and phosphatidic acid, respectively (41, 44). All these downstream responses are the result of the GDP/GTP exchange activity of Vav proteins, because they can be mimicked and rescued by expressing in B-cells constitutively active versions of either Rac1 (in the case of PI3K- and PLC-γ2-dependent pathways) or RhoA (in the case of PLDs) GTPases. This is in contrast to other downstream routes elicited by Vav proteins that, as the case of the stimulation of NF-AT in T-lymphocytes (24, 25), seem to require the coalescence of both catalytic-dependent and independent Vav functions.
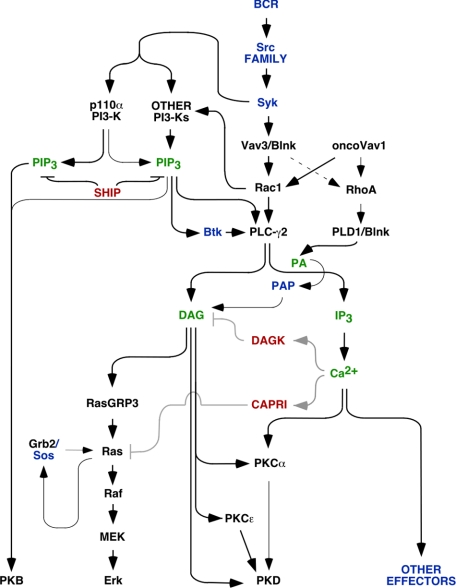
FIGURE 9.
A schematic view of the Vav family-regulated signaling pathways of DT40 B-cells. The main signal transduction molecules with positive roles in downstream signaling are indicated in black (the ones analyzed in this work) and blue (the ones not analyzed in our experiments). Putative negative regulators of B-cell signaling that could be operating in this pathway are shown in red. The main second messengers are indicated in green. Black thick arrows highlight the prevalence of a signaling pathway over the rest of the signaling connections presented in the figure. Gray arrows indicate pathways that have not been demonstrated yet in DT40 cells. See main text for further information.
By comparing the stimulation of effector pathways in wild-type and vav3-/- DT40 cell clones, we have observed that the need of Vav function for the stimulation of specific pathways by the BCR is signaling branch-dependent: DT40 cells are fully dependent on the function of these GDP/GTP exchange factors to trigger all DAG-mediated pathways, including the RasGRP/Ras/Erk cascade and the PKC/PKD route. Instead, these cells are only partially dependent on Vav function to stimulate the PI3K/PKB route upon BCR stimulation. These results indicate that additional signal transduction elements must exist to channel the activation of the latter pathway downstream of the BCR.
We have also used Grb2- and Blnk-deficient DT40 cells to address the role of these adaptor molecules in the engagement of Vav-dependent pathways. Previous results have shown that both Grb2 and Blnk associate physically with Vav3 in DT40 cells, a step required for the optimal localization of this exchange factor at the plasma membrane upon B-cell stimulation (28). Instead, Grb2 and Blnk appear to be dispensable for Vav3 tyrosine phosphorylation (28), a key post-translational modification that allows the activation of Vav catalytic activity (23, 26, 45, 46). Because Grb2 and Blnk have also roles in the activation of the Sos/Ras and PLC-γ2 pathways (37, 39), respectively, the use of these mutant cell lines also made it possible to compare the relative contribution of these routes and the Vav-dependent pathways to the stimulation of downstream BCR signaling targets. We observed that the lack of Grb2 had no major effect in any of the signal transduction pathways analyzed in this study with the exception of a very minor effect in the phosphorylation levels of Erk1 upon BCR stimulation. These results are consistent with the very limited role of the Grb2/Sos route in the activation of Ras in mature lymphocytes (15, 31, 47). Moreover, the lack of effect of the Grb2 deficiency on the activation of other Vav-dependent routes such as the PI3K/PKB and the PKC/PKD pathways rules out any direct upstream or downstream role for this adaptor molecule on the Vav pathway. Whether Grb2 plays roles in other Vav-dependent routes not analyzed here remains to be determined. In contrast to these results, we have observed that Blnk does play a major role in the Vav pathway, although its influence is limited to specific signaling branches rather than being of general importance to all Vav downstream effectors. Thus, we observed that the Blnk deficiency severely affects the PLC-γ2-dependent routes but had very subtle consequences at best in the case of the stimulation of the PI3K/PKB route downstream of the BCR. Our data are therefore more consistent with a downstream rather than an upstream role of Blnk in the Vav route (Fig. 9). Blnk also appears to contribute to the adequate activation of PLD by RhoAQ63L and Vav1 (Δ1–186) (Fig. 9).
The stimulation of different signaling branches by Vav proteins could induce synergistic or negative feedback interactions among them, leading to potential alterations in the relative signaling output of each of them depending on the relative activity of the other parallel pathways. Indeed, previous studies had revealed functional cross-talk among some of the pathways stimulated by Vav proteins (29, 42, 43, 48–53). Our present results indicate that synergistic interactions do exist, but that they are only operative at specific strata of the Vav signal transduction pathway. At the level of the most proximal elements, we have observed a unidirectional influence of PI3K on PLC-γ2 activity (Fig. 9). Accordingly, the blockage of PI3K activity in DT40 cells compromises the stimulation of the PLC-γ2- and DAG-dependent signaling branches. However, unlike the case of the activation of direct downstream targets of PI3Ks such as PKB, we observed that stimulated, Wortmannin-treated cells still keep a very significant activity of both PKD and Erk1. Moreover, the signaling outputs from the PKC/PKD and Ras/Erk routes were either not affected (in the case of PKD activation) or only mildly decreased (in the case of Erk1 phosphorylation) when these experiments were performed in p110α-/- DT40 cells instead of in Wortmannin-treated cells, indicating that other PI3K family members are involved in the cross-talk established between PI3K and PLC-γ2 (Fig. 9). The presence of additional PI3K family members downstream of the BCR receptor in DT40 cells is also supported by the observation that PKD phosphorylation is more affected by Wortmannin than by the vav3 and p110α gene deletion. Treatment of cells with inhibitors for either PI3Kβ (TGX-221) or PI3Kγ (AS-252424) do not affect PKD, Erk, or PKB phosphorylation (data not shown), indicating that these isoforms do not contribute to the stimulation of these pathways in DT40 cells. It will be interesting in the future to identify the specific family members that, in addition to the α isoform, contribute to the stimulation of PLC-γ2 and PKB pathways in this cell lineage.
The synergistic interaction between PI3K and PLC-γ2 is mostly unidirectional, because we observed that the PLC-γ2 deficiency does not trigger major defects in the overall levels of activation of the PI3K/PKB route in DT40 cells. Finally, we have observed that PLD activity is totally dispensable for PI3K activation, as assessed by the normal phosphorylation of PKB in stimulated, n-butanol-treated DT40 cells. However, this phospholipase is required for the optimal activation of both the PLC-γ2/RasGRP/Ras/Erk and the PKC/PKD routes by DT40 cells (Fig. 9). We believe that this effect is probably mediated by the generation of an additional surplus of DAG that amplifies the PLC-γ2-generated DAG pool rather than being due to a direct modulation of PLC-γ2 activity by PLDs. In favor of this view, we have observed that PLD proteins can activate DAG-dependent pathways in both wild-type and plc-γ2-/- DT40 cells. Furthermore, the activation of PLD induced by RhoA is totally PLC-γ2-independent.
In contrast to these interconnections at the level of the most proximal elements of the Vav route, we have unexpectedly seen that the blockage of more distal effector molecules does not have any major influence in the signal outputs of other Vav-dependent effectors. For example, the inhibition of the PKC/PKD route did not result in any detectable effect on Erk or PKB phosphorylation levels. Likewise, the use of MEK inhibitors did not have any impact on the overall phosphorylation levels of PKD or PKB. These results are somewhat surprising, because previous studies have indicated that PKC and PKD can favor the activation of the Ras/Erk route via phosphorylation of Ras-GRPs and Rin, respectively (51–53). Taken together, these results indicate a limited synergistic interpathway interaction at the level of proximal elements of the Vav route. It cannot be ruled out the presence of cross-talk further downstream in the Vav pathway, for example, at the level of transcriptomal dynamics. In this regard, it will be interesting to expand in the future the current studies to the analysis of Vav-dependent transcriptional factors to fully explore the physiological meaning of this multibranched signaling for the effector program of lymphocytes. We cannot exclude either the possibility that additional signaling proteins not contemplated in this work may be regulating the signaling output of the different branches of the Vav pathway. For example, it has been shown before that the knockout of ship, a gene encoding an inositol polyphosphate 5′-phosphatase, enhances Vav3-dependent responses in DT40 cells (19) (Fig. 9). Likewise, chimaerins, a group of DAG-dependent Rac1 GTPase-activating proteins, have been shown to inhibit Vav-mediated responses in T-lymphocytes (54).
Finally, the results presented here illustrate that the signaling routes induced by wild-type and oncogenic versions of signaling proteins do not have to be necessarily identical. By using genetic rescue experiments in DT40 cells, we have observed a clear signaling divergence between the wild-type and oncogenic forms of Vav1 for the activation of the PKC/PKD pathway. Whereas the former one could not promote PKD activation in the absence of Blnk or PLC-γ2, the latter version could elicit PKC/PKD activation even in the absence of Blnk and PLC-γ2. These results indicate that, at least in the case of this signaling response, oncogenic proteins will promote enhanced signals not only through the constitutive activation of effectors downstream of membrane receptors but, at the same time, by creating new signaling circuits that offer alternative ways for achieving the stimulation of those effectors.
Acknowledgments
We thank M. T. Blázquez for technical assistance.
This work was supported, in whole or in part, by National Institutes of Health Grant 5R01-CA73735-12 (to X. R. B.) from NCI. All Spanish funding is co-sponsored by Fondo de Desarrollo Regional of the European Union. The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The abbreviations used are: PLC, phospholipase C; PLD, phospholipase D; BAPTA, 1,2-bis(o-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid; BCR, B-cell receptor; DAG, diacylglycerol; EGFP, enhanced green fluorescent protein; Erk, extracellular regulated kinase; GFP, green fluorescent protein; MEK, MAPK/Erk kinase; NF-AT, nuclear factor of activated T-cells; PI3K, phosphatidylinositol-3 kinase; PKB, protein kinase B; PKC, protein kinase C; PKD, protein kinase D; PMA, phorbol myristate acetate; RasGRP1, Ras GDP releasing protein 1; HA, hemagglutinin.
M. J. Caloca, J. L. Zugaza, and X. R. Bustelo, unpublished observations.
References
- 1.Etienne-Manneville, S., and Hall, A. (2002) Nature 420 629-635 [DOI] [PubMed] [Google Scholar]
- 2.Bustelo, X. R., Sauzeau, V., and Berenjeno, I. M. (2007) BioEssays 29 356-370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Crespo, P., Schuebel, K. E., Ostrom, A. A., Gutkind, J. S., and Bustelo, X. R. (1997) Nature 385 169-172 [DOI] [PubMed] [Google Scholar]
- 4.Schuebel, K. E., Movilla, N., Rosa, J. L., and Bustelo, X. R. (1998) EMBO J. 17 6608-6621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Movilla, N., and Bustelo, X. R. (1999) Mol. Cell. Biol. 19 7870-7885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coppola, J., Bryant, S., Koda, T., Conway, D., and Barbacid, M. (1991) Cell Growth & Differ. 2 95-105 [PubMed] [Google Scholar]
- 7.Fernandez-Zapico, M. E., Gonzalez-Paz, N. C., Weiss, E., Savoy, D. N., Molina, J. R., Fonseca, R., Smyrk, T. C., Chari, S. T., Urrutia, R., and Billadeau, D. D. (2005) Cancer Cell 7 39-49 [DOI] [PubMed] [Google Scholar]
- 8.Patel, V., Rosenfeldt, H. M., Lyons, R., Servitja, J. M., Bustelo, X. R., Siroff, M., and Gutkind, J. S. (2007) Carcinogenesis 28 1145-1152 [DOI] [PubMed] [Google Scholar]
- 9.Rodrigues, L., Pires de Miranda, M., Caloca, M. J., Bustelo, X. R., and Simas, J. P. (2006) J. Virol. 80 6123-6135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pires de Miranda, M., Alenquer, M., Marques, S., Rodrigues, L., Lopes, F., Bustelo, X. R., and Simas, J. P. (2008) PLoS ONE 3 e1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fackler, O. T., Luo, W., Geyer, M., Alberts, A. S., and Peterlin, B. M. (1999) Mol. Cell 3 729-739 [DOI] [PubMed] [Google Scholar]
- 12.Katzav, S., Martin-Zanca, D., and Barbacid, M. (1989) EMBO J. 8 2283-2290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schuebel, K. E., Bustelo, X. R., Nielsen, D. A., Song, B. J., Barbacid, M., Goldman, D., and Lee, I. J. (1996) Oncogene 13 363-371 [PubMed] [Google Scholar]
- 14.Couceiro, J. R., Martin-Bermudo, M. D., and Bustelo, X. R. (2005) Exp. Cell Res. 308 364-380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Caloca, M. J., Zugaza, J. L., Matallanas, D., Crespo, P., and Bustelo, X. R. (2003) EMBO J. 22 3326-3336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Caloca, M. J., Zugaza, J. L., Vicente-Manzanares, M., Sanchez-Madrid, F., and Bustelo, X. R. (2004) J. Biol. Chem. 279 20435-20446 [DOI] [PubMed] [Google Scholar]
- 17.Reynolds, L. F., de Bettignies, C., Norton, T., Beeser, A., Chernoff, J., and Tybulewicz, V. L. (2004) J. Biol. Chem. 279 18239-18246 [DOI] [PubMed] [Google Scholar]
- 18.Vigorito, E., Bardi, G., Glassford, J., Lam, E. W., Clayton, E., and Turner, M. (2004) J. Immunol. 173 3209-3214 [DOI] [PubMed] [Google Scholar]
- 19.Inabe, K., Ishiai, M., Scharenberg, A. M., Freshney, N., Downward, J., and Kurosaki, T. (2002) J. Exp. Med. 195 189-200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reynolds, L. F., Smyth, L. A., Norton, T., Freshney, N., Downward, J., Kioussis, D., and Tybulewicz, V. L. (2002) J. Exp. Med. 195 1103-1114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu, J., Katzav, S., and Weiss, A. (1995) Mol. Cell. Biol. 15 4337-4346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bustelo, X. R. (2001) Oncogene 20 6372-6381 [DOI] [PubMed] [Google Scholar]
- 23.Bustelo, X. R., and Couceiro, J. R. (2008) UCSD Nat. Mol. Pages, DOI: 10.1038/mp.a002362.002301 [DOI]
- 24.Kuhne, M. R., Ku, G., and Weiss, A. (2000) J. Biol. Chem. 275 2185-2190 [DOI] [PubMed] [Google Scholar]
- 25.Zugaza, J. L., Lopez-Lago, M. A., Caloca, M. J., Dosil, M., Movilla, N., and Bustelo, X. R. (2002) J. Biol. Chem. 277 45377-45392 [DOI] [PubMed] [Google Scholar]
- 26.Bustelo, X. R. (2000) Mol. Cell. Biol. 20 1461-1477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fujikawa, K., Miletic, A. V., Alt, F. W., Faccio, R., Brown, T., Hoog, J., Fredericks, J., Nishi, S., Mildiner, S., Moores, S. L., Brugge, J., Rosen, F. S., and Swat, W. (2003) J. Exp. Med. 198 1595-1608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Johmura, S., Oh-hora, M., Inabe, K., Nishikawa, Y., Hayashi, K., Vigorito, E., Kitamura, D., Turner, M., Shingu, K., Hikida, M., and Kurosaki, T. (2003) Immunity 18 777-787 [DOI] [PubMed] [Google Scholar]
- 29.Shinohara, H., and Kurosaki, T. (2006) Subcell. Biochem. 40 145-187 [DOI] [PubMed] [Google Scholar]
- 30.Bustelo, X. R., Suen, K. L., Michael, W. M., Dreyfuss, G., and Barbacid, M. (1995) Mol. Cell. Biol. 15 1324-1332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zugaza, J. L., Caloca, M. J., and Bustelo, X. R. (2004) Oncogene 23 5823-5833 [DOI] [PubMed] [Google Scholar]
- 32.Zugaza, J. L., Sinnett-Smith, J., Van Lint, J., and Rozengurt, E. (1996) EMBO J. 15 6220-6230 [PMC free article] [PubMed] [Google Scholar]
- 33.Wood, C. D., Marklund, U., and Cantrell, D. A. (2005) J. Biol. Chem. 280 6245-6251 [DOI] [PubMed] [Google Scholar]
- 34.Joneson, T., McDonough, M., Bar-Sagi, D., and Van Aelst, L. (1996) Science 274 1374-1376 [DOI] [PubMed] [Google Scholar]
- 35.Joneson, T., and Bar-Sagi, D. (1998) J. Biol. Chem. 273 17991-17994 [DOI] [PubMed] [Google Scholar]
- 36.Suzuki, A., Akimoto, K., and Ohno, S. (2003) J. Biochem. 133 9-16 [DOI] [PubMed] [Google Scholar]
- 37.Bos, J. L., Rehmann, H., and Wittinghofer, A. (2007) Cell 129 865-877 [DOI] [PubMed] [Google Scholar]
- 38.Roose, J. P., Mollenauer, M., Ho, M., Kurosaki, T., and Weiss, A. (2007) Mol. Cell. Biol. 27 2732-2745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fu, C., Turck, C. W., Kurosaki, T., and Chan, A. C. (1998) Immunity 9 93-103 [DOI] [PubMed] [Google Scholar]
- 40.Llorca, O., Arias-Palomo, E., Zugaza, J. L., and Bustelo, X. R. (2005) EMBO J. 24 1330-1340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jenkins, G. M., and Frohman, M. A. (2005) Cell Mol. Life Sci. 62 2305-2316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hitomi, T., Yanagi, S., Inatome, R., Ding, J., Takano, T., and Yamamura, H. (2001) Genes Cells 6 475-485 [DOI] [PubMed] [Google Scholar]
- 43.Hitomi, T., Yanagi, S., Inatome, R., and Yamamura, H. (1999) FEBS Lett. 445 371-374 [DOI] [PubMed] [Google Scholar]
- 44.Exton, J. H. (2002) FEBS Lett. 531 58-61 [DOI] [PubMed] [Google Scholar]
- 45.Bustelo, X. R. (2002) Front. Biosci. 7 d24-d30 [DOI] [PubMed] [Google Scholar]
- 46.Bustelo, X. R. (2008) UCSD Nature Mol. Pages, DOI: 10.1038/mp.a002361.002301 [DOI]
- 47.Oh-hora, M., Johmura, S., Hashimoto, A., Hikida, M., and Kurosaki, T. (2003) J. Exp. Med. 198 1841-1851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yeo, E. J., Kazlauskas, A., and Exton, J. H. (1994) J. Biol. Chem. 269 27823-27826 [PubMed] [Google Scholar]
- 49.Hess, J. A., Ji, Q. S., Carpenter, G., and Exton, J. H. (1998) J. Biol. Chem. 273 20517-20524 [DOI] [PubMed] [Google Scholar]
- 50.Van Lint, J., Ni, Y., Valius, M., Merlevede, W., and Vandenheede, J. R. (1998) J. Biol. Chem. 273 7038-7043 [DOI] [PubMed] [Google Scholar]
- 51.Rykx, A., De Kimpe, L., Mikhalap, S., Vantus, T., Seufferlein, T., Vandenheede, J. R., and Van Lint, J. (2003) FEBS Lett. 546 81-86 [DOI] [PubMed] [Google Scholar]
- 52.Roose, J. P., Mollenauer, M., Gupta, V. A., Stone, J., and Weiss, A. (2005) Mol. Cell. Biol. 25 4426-4441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Teixeira, C., Stang, S. L., Zheng, Y., Beswick, N. S., and Stone, J. C. (2003) Blood 102 1414-1420 [DOI] [PubMed] [Google Scholar]
- 54.Caloca, M. J., Delgado, P., Alarcon, B., and Bustelo, X. R. (2008) Cell Signal. 20 758-770 [DOI] [PMC free article] [PubMed] [Google Scholar]