Abstract
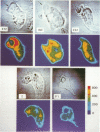
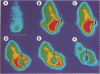
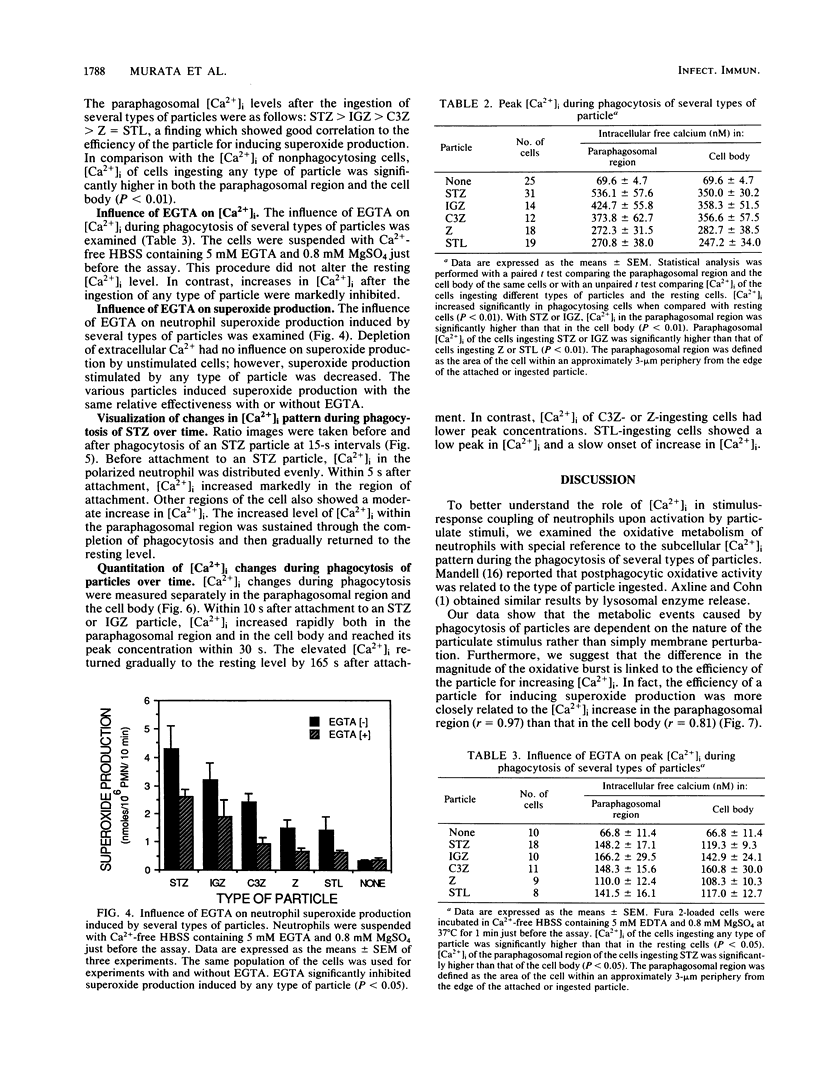
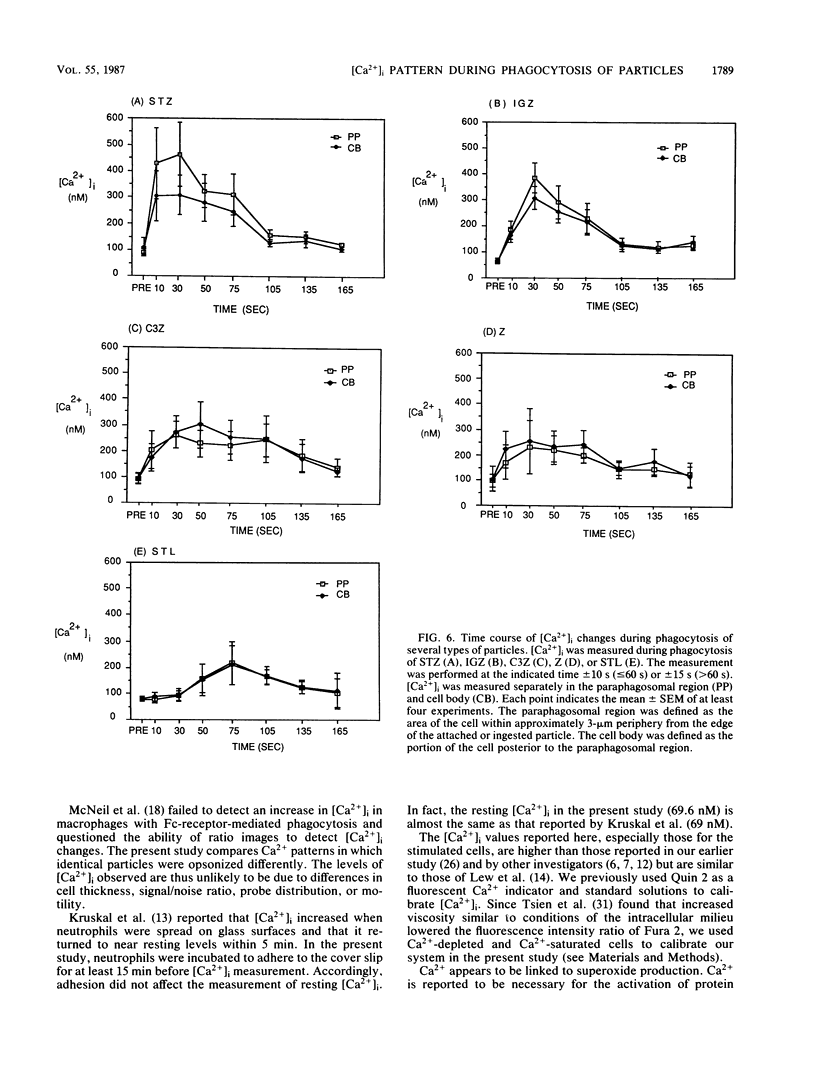
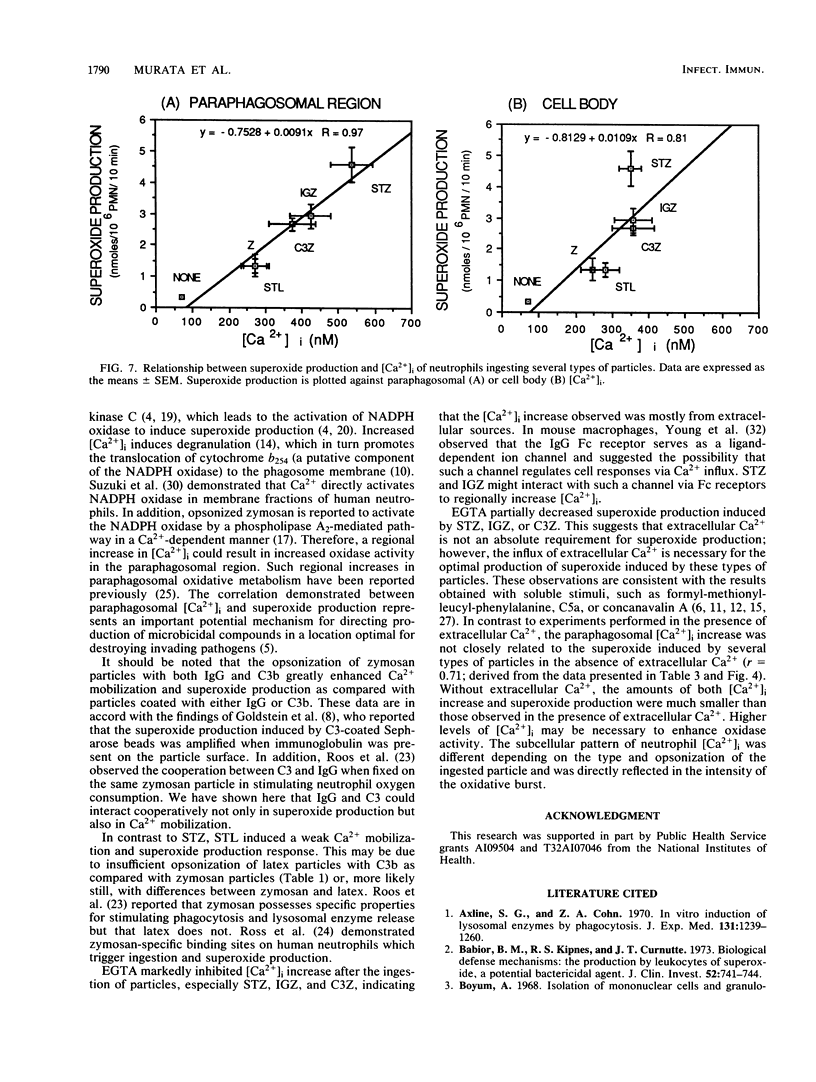
Intracellular free calcium concentration [( Ca2+]i) was measured with the fluorescent Ca2+ indicator Fura 2 within individual human neutrophils during the phagocytosis of several types of particles, including serum-treated zymosan (STZ), immunoglobulin G (IgG)-coated zymosan (IGZ), C3b-coated zymosan (C3Z), nontreated zymosan (Z), and serum-treated similarly sized latex particles (STL). STZ was coated with both IgG and C3b. IGZ was coated with only IgG, and C3Z was coated with only C3b. STL was coated with only C3b but to a lesser extent than C3Z. The ingestion of particles was greatest for STZ and somewhat lower for C3Z. Ingestion of IGZ and STL was much less than ingestion of C3Z. The relative efficiencies of the particles for inducing superoxide production were as follows: STZ greater than IGZ = C3Z greater than Z = STL. [Ca2+]i significantly increased from the resting level of approximately 70 to greater than 240 nM (P less than 0.01) during phagocytosis of the particles. The increment in [Ca2+]i was greater in the paraphagosomal region than in the cell body after the ingestion of STZ or IGZ. The mean peak [Ca2+]i values in the paraphagosomal cytoplasm of neutrophils ingesting one particle of STZ, IGZ, C3Z, Z and STL were 536.1 +/- 57.6, 424.7 +/- 55.8, 373.8 +/- 62.7, 272.3 +/- 31.5, and 270.8 +/- 38.0 nM, respectively, which showed good correlation (r = 0.97) with the efficiency of the particles for inducing superoxide production. Depletion of extracellular Ca2+ by EGTA [ethylene glycol-bis(beta-aminoethyl ether)-N,N,N'N'-tetraacetic acid] attenuated both [Ca2+]i increase and superoxide production induced by particles. Thus [Ca2+]i increased after ingestion of several types of particles, and the subcellular pattern of [Ca2+]i was different depending on the type of particle ingested. Greater increases in paraphagosomal [Ca2+]i were closely associated with greater increases in superoxide production by neutrophils.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Axline S. G., Cohn Z. A. In vitro induction of lysosomal enzymes by phagocytosis. J Exp Med. 1970 Jun 1;131(6):1239–1260. doi: 10.1084/jem.131.6.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babior B. M., Kipnes R. S., Curnutte J. T. Biological defense mechanisms. The production by leukocytes of superoxide, a potential bactericidal agent. J Clin Invest. 1973 Mar;52(3):741–744. doi: 10.1172/JCI107236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christiansen N. O., Larsen C. S., Juhl H. Ca2+ and phorbol ester activation of protein kinase C at intracellular Ca2+ concentrations and the effect of TMB-8. Biochim Biophys Acta. 1986 Jun 3;882(1):57–62. doi: 10.1016/0304-4165(86)90055-3. [DOI] [PubMed] [Google Scholar]
- Densen P., Mandell G. L. Gonococcal interactions with polymorphonuclear neutrophils: importance of the phagosome for bactericidal activity. J Clin Invest. 1978 Dec;62(6):1161–1171. doi: 10.1172/JCI109235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gennaro R., Pozzan T., Romeo D. Monitoring of cytosolic free Ca2+ in C5a-stimulated neutrophils: loss of receptor-modulated Ca2+ stores and Ca2+ uptake in granule-free cytoplasts. Proc Natl Acad Sci U S A. 1984 Mar;81(5):1416–1420. doi: 10.1073/pnas.81.5.1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman D. W., Gifford L. A., Olson D. M., Goetzl E. J. Transduction by leukotriene B4 receptors of increases in cytosolic calcium in human polymorphonuclear leukocytes. J Immunol. 1985 Jul;135(1):525–530. [PubMed] [Google Scholar]
- Goldstein I. M., Kaplan H. B., Radin A., Frosch M. Independent effects of IgG and complement upon human polymorphonuclear leukocyte function. J Immunol. 1976 Oct;117(4):1282–1287. [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Higson F. K., Durbin L., Pavlotsky N., Tauber A. I. Studies of cytochrome b-245 translocation in the PMA stimulation of the human neutrophil NADPH-oxidase. J Immunol. 1985 Jul;135(1):519–524. [PubMed] [Google Scholar]
- Korchak H. M., Rutherford L. E., Weissmann G. Stimulus response coupling in the human neutrophil. I. Kinetic analysis of changes in calcium permeability. J Biol Chem. 1984 Apr 10;259(7):4070–4075. [PubMed] [Google Scholar]
- Korchak H. M., Vienne K., Rutherford L. E., Wilkenfeld C., Finkelstein M. C., Weissmann G. Stimulus response coupling in the human neutrophil. II. Temporal analysis of changes in cytosolic calcium and calcium efflux. J Biol Chem. 1984 Apr 10;259(7):4076–4082. [PubMed] [Google Scholar]
- Kruskal B. A., Shak S., Maxfield F. R. Spreading of human neutrophils is immediately preceded by a large increase in cytoplasmic free calcium. Proc Natl Acad Sci U S A. 1986 May;83(9):2919–2923. doi: 10.1073/pnas.83.9.2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lew P. D., Monod A., Waldvogel F. A., Dewald B., Baggiolini M., Pozzan T. Quantitative analysis of the cytosolic free calcium dependency of exocytosis from three subcellular compartments in intact human neutrophils. J Cell Biol. 1986 Jun;102(6):2197–2204. doi: 10.1083/jcb.102.6.2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lew P. D., Wollheim C. B., Waldvogel F. A., Pozzan T. Modulation of cytosolic-free calcium transients by changes in intracellular calcium-buffering capacity: correlation with exocytosis and O2-production in human neutrophils. J Cell Biol. 1984 Oct;99(4 Pt 1):1212–1220. doi: 10.1083/jcb.99.4.1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandell G. L. Influence of type of ingested particle on human leukocyte metabolism. Proc Soc Exp Biol Med. 1971 Sep;137(4):1228–1230. doi: 10.3181/00379727-137-35761. [DOI] [PubMed] [Google Scholar]
- Maridonneau-Parini I., Tringale S. M., Tauber A. I. Identification of distinct activation pathways of the human neutrophil NADPH-oxidase. J Immunol. 1986 Nov 1;137(9):2925–2929. [PubMed] [Google Scholar]
- McNeil P. L., Swanson J. A., Wright S. D., Silverstein S. C., Taylor D. L. Fc-receptor-mediated phagocytosis occurs in macrophages without an increase in average [Ca++]i. J Cell Biol. 1986 May;102(5):1586–1592. doi: 10.1083/jcb.102.5.1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melloni E., Pontremoli S., Michetti M., Sacco O., Sparatore B., Salamino F., Horecker B. L. Binding of protein kinase C to neutrophil membranes in the presence of Ca2+ and its activation by a Ca2+-requiring proteinase. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6435–6439. doi: 10.1073/pnas.82.19.6435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers M. A., McPhail L. C., Snyderman R. Redistribution of protein kinase C activity in human monocytes: correlation with activation of the respiratory burst. J Immunol. 1985 Nov;135(5):3411–3416. [PubMed] [Google Scholar]
- Naccache P. H., Volpi M., Showell H. J., Becker E. L., Sha'afi R. I. Chemotactic factor-induced release of membrane calcium in rabbit neutrophils. Science. 1979 Feb 2;203(4379):461–463. doi: 10.1126/science.760200. [DOI] [PubMed] [Google Scholar]
- Romeo D., Zabucchi G., Miani N., Rossi F. Ion movement across leukocyte plasma membrane and excitation of their metabolism. Nature. 1975 Feb 13;253(5492):542–544. doi: 10.1038/253542a0. [DOI] [PubMed] [Google Scholar]
- Roos D., Bot A. A., van Schaik M. L., de Boer M., Daha M. R. Interaction between human neutrophils and zymosan particles: the role of opsonins and divalent cations. J Immunol. 1981 Feb;126(2):433–440. [PubMed] [Google Scholar]
- Ross G. D., Cain J. A., Lachmann P. J. Membrane complement receptor type three (CR3) has lectin-like properties analogous to bovine conglutinin as functions as a receptor for zymosan and rabbit erythrocytes as well as a receptor for iC3b. J Immunol. 1985 May;134(5):3307–3315. [PubMed] [Google Scholar]
- Salata R. A., Sullivan J. A., Mandell G. L. Visualization of hydrogen peroxide in living polymorphonuclear neutrophils utilizing leucodiacetyl 2',7'-dichlorofluorescin: photomicrographic and microphotometric studies. Trans Assoc Am Physicians. 1983;96:375–383. [PubMed] [Google Scholar]
- Sawyer D. W., Sullivan J. A., Mandell G. L. Intracellular free calcium localization in neutrophils during phagocytosis. Science. 1985 Nov 8;230(4726):663–666. doi: 10.1126/science.4048951. [DOI] [PubMed] [Google Scholar]
- Smolen J. E., Korchak H. M., Weissmann G. The roles of extracellular and intracellular calcium in lysosomal enzyme release and superoxide anion generation by human neutrophils. Biochim Biophys Acta. 1981 Nov 5;677(3-4):512–520. doi: 10.1016/0304-4165(81)90267-1. [DOI] [PubMed] [Google Scholar]
- Stossel T. P., Field R. J., Gitlin J. D., Alper C. A., Rosen F. S. The opsonic fragment of the third component of human complement (C3). J Exp Med. 1975 Jun 1;141(6):1329–1347. doi: 10.1084/jem.141.6.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stossel T. P. Quantitative studies of phagocytosis. Kinetic effects of cations and heat-labile opsonin. J Cell Biol. 1973 Aug;58(2):346–356. doi: 10.1083/jcb.58.2.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki H., Pabst M. J., Johnston R. B., Jr Enhancement by Ca2+ or Mg2+ of catalytic activity of the superoxide-producing NADPH oxidase in membrane fractions of human neutrophils and monocytes. J Biol Chem. 1985 Mar 25;260(6):3635–3639. [PubMed] [Google Scholar]
- Tsien R. Y., Rink T. J., Poenie M. Measurement of cytosolic free Ca2+ in individual small cells using fluorescence microscopy with dual excitation wavelengths. Cell Calcium. 1985 Apr;6(1-2):145–157. doi: 10.1016/0143-4160(85)90041-7. [DOI] [PubMed] [Google Scholar]
- Young J. D., Unkeless J. C., Young T. M., Mauro A., Cohn Z. A. Role for mouse macrophage IgG Fc receptor as ligand-dependent ion channel. Nature. 1983 Nov 10;306(5939):186–189. doi: 10.1038/306186a0. [DOI] [PubMed] [Google Scholar]