Abstract
Nuclear matrix proteins (NMPs) are important diagnostic and prognostic markers in various human cancers. The hHpr1/p84/Thoc1 protein, a key NMP, resides in the nuclear matrix and is involved in the human TREX complex, which is required for regulation of transcription elongation, pre-RNA splicing, and mRNA export of a subset of human genes. Depletion of hHpr1/p84/Thoc1 decreases growth rates in multiple cancer cell lines, and the expression levels of hHpr1/p84/Thoc1 are strongly associated with tumor size and aggressiveness of several human cancers. Little is known about the expression of this protein in human non-small cell lung cancer (NSCLC) and its association with patients’ clinicopathologic characteristics and prognosis. We evaluated hHpr1/p84/Thoc1 expression in 133 NSCLC patients by immunohistochemistry of tissue microarrays using paraffin-embedded tumor tissue and we confirmed the tissue staining by Western blot analysis. The prognostic significance of hHpr1/p84/Thoc1 expression in tumor tissue was assessed by the Cox proportional hazards regression model. Expression of hHpr1/p84/Thoc1 was found in 51% of patients, and was more prevalent in males than females (59% vs 43%, p = 0.07) and in blacks than whites (91% vs 48%, p = 0.009). In survival analysis, hHpr1/p84/Thoc1 expression appeared to be weakly associated with elevated risk of death among patients with stage I tumors (RR = 1.53, 95% CI = 0.85-2.77, p = 0.16), squamous cell carcinomas (RR = 1.75, 95% CI = 0.73-4.21, p = 0.21), and family histories of lung cancer (RR = 1.55, 95% CI = 0.81-2.97, p=0.18), although none of these associations was statistically significant. Thus elevated expression of hHpr1/p84/Thoc1 is common in NSCLC and may have prognostic significance in subgroups of patients. Further studies with larger sample size are needed to elucidate the role of this critical nuclear matrix protein in NSCLC prognosis.
Keywords: nuclear matrix proteins, hHpr1/p84/Thoc1, non-small cell lung cancer, tissue microarray
Introduction
Changes in nuclear structure, which are largely determined by the nuclear matrix, are characteristics of human cancers. During carcinogenesis, alterations of nuclear structure usually manifest as deformation of the nuclear matrix architecture [1-3]. The nuclear matrix consists of the nuclear lamins, RNA, and a fibrogranular network of proteins that include more than 200 nuclear matrix proteins (NMPs) [3,4]. Many NMPs are involved in important cellular functions, including gene transcription, steroid hormone binding, and RNA processing. Functional changes in these proteins have been implicated in carcinogenesis [3,5-7], and many NMPs have been identified as unique “fingerprint” markers for cancers of the colon [1], bladder [8], kidney [9], prostate [7,10], and breast [11]. For example, Getzenberg et al [8] found 6 NMPs (BLCA1-6) that were unique to patients with transitional cell bladder carcinoma, and BLCA-4 expression was found before tumors were detectable, indicating the diagnostic significance of this NMP in transitional cell carcinoma [8]. Partin and colleagues [12] found that NMP PC-1 was specifically elevated in prostatic cancer tissue compared to benign prostatic hyperplasia and normal prostatic tissue.
hHpr1/p84/Thoc1 is an NMP that was originally isolated by a yeast two-hybrid screen using the retinoblastoma tumor suppressor protein amino terminal domain as “bait” [13]. It is expressed in most tissues throughout the cell cycle [13], except in the G0 phase [14]. Overexpression of hHpr1/p84/Thoc1 can trigger p53-independent apoptosis that is inhibited by binding of hHpr1/p84/Thoc1 to the retinoblastoma tumor suppressor protein [15,16]. We previously reported that depletion of hHpr1/p84/Thoc1 sensitized cancer cells to the cytotoxic effects of DNA damage [17]. Thus it seems likely that high levels of hHpr1/p84/Thoc1 expression may increase the resistance of cancer cells to anticancer treatment and affect prognosis.
Consistent with its localization in the nuclear matrix and RNA-processing center, hHpr1/p84/Thoc1 regulates the transcription elongation of a subset of genes by participating in the TREX protein complex [17,18], which is conserved from yeast to man and that couples transcription elongation, pre-RNA splicing, and mRNA export. Depletion of hHpr1/p84/Thoc1 decreases growth rates in multiple cancer cell lines, such as Hela, 293T, HCT116, U2OS, and MDA-MB-231 [17,18]. In humans, high levels of hHpr1/p84/Thoc1 have been observed in breast cancer cells and are strongly associated with tumor size and aggressiveness, implying potential significance of this protein in tumor transformation, progression, and metastasis (18). Recently, we compared the requirements for Thoc1 in the proliferation and survival of isogenic normal and oncogene-transformed cells [19]. We found that neoplastic cells rapidly lose viability via apoptotic cell death following depletion of pThoc1. In contrast, viability of normal cells is largely unaffected by pThoc1 loss, suggesting that THoc1 may provide a novel molecular target for cancer therapy [19].
The expression of hHpr1/p84/Thoc1 in non-small cell lung cancer (NSCLC), the number one cancer killer, and its relationships with patients’ clinicopathologic characteristics and prognosis have been unknown. We conducted this study in an attempt to characterize hHpr1/p84/Thoc1 expression in NSCLC and to assess its prognostic value.
Materials and Methods
Patient selection
We retrospectively selected patients diagnosed with NSCLC at Roswell Park Cancer Institute (RPCI) between January 1995 and December 1999. The selection criteria included pathological confirmation of primary NSCLC, no concurrent cancer diagnosed within 1 mo after the NSCLC diagnosis, complete surgical removal of the tumor (macroscopically and microscopically) as the initial treatment modality, adequate archival tissue available for immunohistochemical evaluation, and survival for at least 1 mo after surgery (to avoid the possible bias from perioperative mortality). Initially, 221 patients were identified. After examining tumor immunostaining and patient follow-up data, 88 patients were excluded for inadequate tumor specimen or poor staining (n = 48), or causes of death not related to lung cancer (n = 40), leaving a total of 133 patients in the analysis. This cohort of patients was followed through October 2003 (median follow-up = 59.2 mo). This study was approved under an institutional review board protocol at RPCI for the investigation of molecular markers relevant to lung cancer pathogenesis and prognosis.
Tumor and patient data
Patient demographic and clinicopathologic data were obtained from medical records (by J.Y.), including age, sex, ethnicity, Eastern Cooperative Oncology Group (ECOG) performance status [20], weight loss status (presence or absence, defined as loss of more than 5% of body weight within 3 mo prior to the current diagnosis), smoking history (current, former, or never, and pack-years of smoking, calculated as the multiplicative product of cigarettes smoked per day and years of smoking), family history of lung cancer (≥1 first-degree relative diagnosed with lung cancer), tumor pathologic stage (I, II, or IIIA), tumor histologic type (adenocarcinoma, squamous cell carcinoma, large cell carcinoma, or others), tumor grade (well, moderately, or poorly differentiated), vital status (dead or alive), and date of death or last date of follow-up. Because most patients were fully active at the time of diagnosis (ECOG status = 0), we combined ECOG scores of ≥1 for data analysis. Lung cancer-specific death was used as the outcome of interest in the survival analysis.
Histologic examination and tissue microarray construction
H&E-stained slides were reviewed to confirm the histopathologic diagnosis and select adequate specimens for analysis. All tumors were classified according to the most recent World Health Organization classification guidelines for lung cancer [21]. Pathologic stage was based on the revised international classification system [22]. Neutral-buffered, formalin-fixed (10% vol/formalin in water; pH 7.4), paraffin-embedded tissue blocks were retrieved from the paraffin archive of the Department of Pathology at RPCI.
Areas of viable tumor and non-neoplastic tissue were identified and marked by one of the investigators (D.T.) for the construction of microarrays. High-density tissue microarrays (TMAs) were assembled using a Beecher tissue puncher/array system (Beecher Instruments, Silver Spring, MD), as previously described [23]. This system consists of thin-walled stainless-steel needles with an inner diameter of approximately 1 mm and a stylet for transferring and emptying the needle contents. The assembly was held in an X-Y position guide that was manually adjusted by digital micrometers. Specimens were retrieved from selected regions of the donor paraffin block and precisely arrayed in a new recipient block. Tissue core samples were 1.0 mm in diameter and 1.0 to 3.0 mm long, depending on the depth of tissue available in the donor block. In each case, 2 or 3 core samples of normal and tumor tissue were acquired from 2 donor blocks, resulting in 336 samples. The samples were assembled in 3 high-density TMA blocks. Each sample contained up to 3200 neoplastic cells.
Immunohistochemical staining
Sections 5 μm thick were cut from the TMA blocks for immunohistochemical staining and processed within 1 wk of cutting to avoid oxidation of the antigens. Initial sections were stained with H&E to verify the histologic type. The avidin-biotin staining method was used as previously described [24]. In brief, antigen retrieval was carried out using Dako target retrieval solution (H1, pH 8.9, 10 min; Dako Corp., Carpinteria, CA). An antibody against hHpr1/p84/Thoc1 was used at a dilution of 1:100. The antibody was incubated with the TMA sections for 30 min at ambient temperature in an automatic immunostainer (Dako), and then with a detection kit in accordance with the manufacturer’s instructions. The immunoreactivity of normal lung tissue was used as a positive control. Negative control tissue was stained with monoclonal-negative antibody (Dako) instead of hHpr1/p84/Thoc1. Two investigators (D.T. and T.K.) independently reviewed the slides without knowledge of the patients’ identities or outcomes. The percentages of stained neoplastic cells in the TMA sections were assessed. Unequivocal nuclear staining of 10% or more tumor cells was counted as positive expression.
Western blot analysis
A subset of patients (n = 10) in the cohort had adequate frozen tissue for Western blot analysis. We cut 30-μm slices from frozen tissue samples and extracted them in 400 μl of lysis 250 solution (50 mM Tris, pH 7.4, 250 mM NaCl, 5 mM EDTA, and 0.1% NP-40) supplemented with a cocktail of protease inhibitors (Sigma, St. Louis, MO). Samples were sonicated using an ultrasonic dismembrator (Fisher Scientific, Pittsburgh, PA) at setting 7 for 7-8 strokes, followed by centrifugation at 4°C for 20 min. The protein concentration was measured by Bradford protein assay (Bio-Rad, Hercules, CA). Twenty μg of total protein for each sample was resolved by 7.5% SDS-PAGE. The proteins were transferred to a polyvinylidine fluoride membrane, and binding was blocked with 5% non-fat dry milk in 20 mM Tris (pH 7.5), 150 mM NaCl, and 0.1% Tween 20 (TBST) for 1 hr at ambient temperature. The blot was incubated with mouse monoclonal anti-p84N5 antibody (GeneTex, San Antonio, TX) in a 1:5000 dilution in fresh TBST and 5% milk, and the bound antibody was detected using horseradish peroxidase-conjugated secondary antibody and chemiluminescence (Amersham, Piscataway, NJ). The other primary antibody used was mouse monoclonal anti-β-actin (Calbiochem, San Diego, CA) at a dilution of 1:10,000.
Statistical analysis
The t-test and chi-square test were used to evaluate associations between hHpr1/p84/Thoc1 expression and patients’ demographic and clinicopathologic characteristics. The Cox proportional hazards regression model was used to estimate the relative risk (RR) of death and 95% confidence intervals (CIs) associated with hHpr1/p84/Thoc1 expression. Survival curves for hHpr1/p84/Thoc1 were generated by the Kaplan-Meier survival analysis. The log-rank test was used to determine the significant differences between the survival curves. Age and variables that were significantly associated with survival were included in a multivariate Cox regression model for adjusted analysis. Stratified analyses were performed for important clinicopathologic variables including tumor stage, tumor histologic characteristics, tumor grade, ECOG performance status, and weight loss. Values of p <0.05 were considered statistically significant. All analyses were performed using SPSS statistical software version 13.0 (SPSS, Inc., Chicago, IL).
Results
Patient data and hHpr1/p84/Thoc1 expression
As shown in Table 1, 53% of patients were men and 47% were women. The mean age was 65.1 yr. Ninety-three percent of patients were previous smokers (58%) or current smokers (35%); 7% were never-smokers. The histological classification included 81 patients (61%) with adenocarcinomas, 40 patients (30%) with squamous cell carcinomas, 3 patients (2%) with large cell carcinomas, and 9 patients (7%) with adenosquamous cell carcinomas. Forty-three percent of patients reported at least one first-degree relative diagnosed with lung cancer. Twelve percent of patients experienced weight loss (≥5%) within 3 mo prior to diagnosis. Sixty-two percent of patients were diagnosed with stage I tumors, 22% with stage II, and 16% with stage IIIA. Sixty-eight percent of patients died before the completion of the study. The mean and median survival durations among these patients were 35 and 32 mo, respectively, with a range of 2-89 mo. The mean and median follow-up times for the remaining patients were 76 and 75 mo, respectively, with a range of 50-102 mo.
Table 1.
Clinicopathologic characteristics of 133 NSCLC patients
| Characteristic | Number (%) |
|---|---|
| Sex | |
| Male | 70(53) |
| Female | 63 (47) |
| Ethnicity | |
| White | 122 (92) |
| Black | 11 (8) |
| Mean age, yr (SD) | 65.1 (10.9) |
| Smoking status | |
| Current | 46 (35) |
| Former | 77 (58) |
| Never | 10 (8) |
| Mean pack-yr of smoking (SD) | 41.9 (29.2) |
| Family history of lung cancer | |
| Yes | 57 (43) |
| No | 41 (31) |
| Unknown | 35 (26) |
| ECOG performance status | |
| 0 | 91 (68) |
| ≥1 | 42 (32) |
| Weight loss | |
| No | 117 (88) |
| Yes | 16 (12) |
| Vital status | |
| Alive | 43 (32) |
| Dead | 90 (68) |
| Tumor pathologic stage | |
| I | 83 (62) |
| II | 29 (22) |
| IIIA | 21 (16) |
| Tumor histologic type | |
| Adenocarcinoma | 81 (61) |
| Squamous cell carcinoma | 40 (30) |
| Large cell carcinoma | 3 (2) |
| Adenosquamous cell carcinoma | 9 (7) |
| Tumor grade | |
| Well differentiated | 5 (4) |
| Moderately differentiated | 48 (36) |
| Poorly differentiated | 80 (60) |
| hHpr1/p84/Thoc1 expression | |
| Negative | 65 (49) |
| Positive | 68 (51) |
Fig. 1 shows samples of expression of hHpr1/p84/Thoc1 in NSCLC tumor tissue from immunohistochemistry microarray. A Western blot analysis using matched frozen tissue samples confirmed the expression of hHpr1/p84/Thoc1 detected by immunohistochemical staining (Fig. 2). Relationships between hHpr1/p84/Thoc1 expression and patient epidemiologic and clinicopathologic characteristics are shown in Table 2. Men and blacks appeared more likely than women and whites to have positive hHpr1/p84/Thoc1 expression, respectively. No obvious associations of hHpr1/p84/Thoc1 expression were noted for the other epidemiologic and clinicopathologic factors.
Fig. 1.
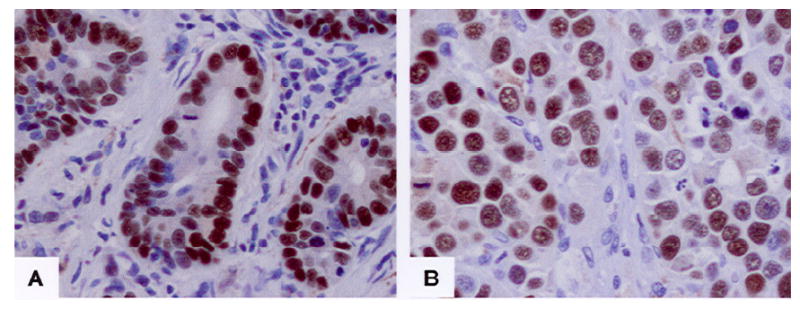
Samples of p84N5 in the NSCLC tumor tissue immunohistochemistry microarray. A: a moderately differentiated adenocarcinoma. B: a poorly differentiated squamous cell carcinoma. Notice sharp nuclear staining of tumor cells vs negative staining of intervening stromal cells.
Fig. 2.
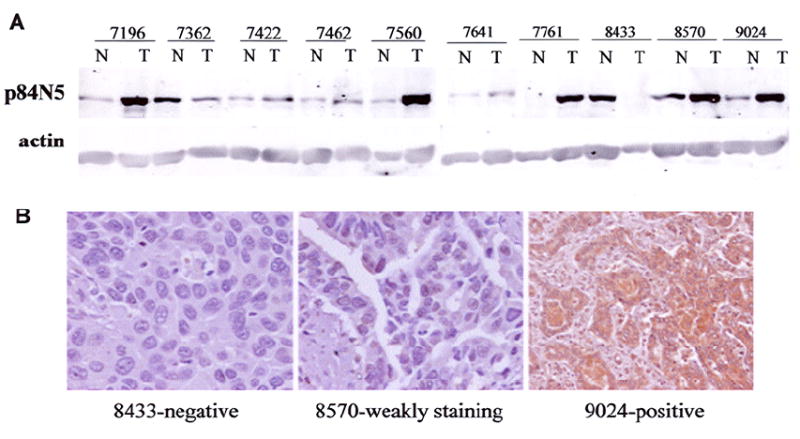
Expression of hHpr1/p84/Thoc1 in NSCLC. A: Western blot analysis of p84 levels from tumor (T) or adjacent normal (N) tissues in selected tumor samples. B: Corresponding immunohistochemical staining and scoring of 3 samples shown in (A).
Table 2.
Association between hHpr1/p84/Thoc1 expression and demographic and clinicopathologic characteristics in 133 patients with NSCLC
| Characteristic | HHpr1/p84/Thoc1 | ||
|---|---|---|---|
| Positive (n=68) | Negative (n=65) | p value | |
| Sex, n (%) | 0.07 | ||
| Male | 41/70 (59) | 29/70 (41) | |
| Female | 27/63 (43) | 36/63 (57) | |
| Ethnicity, n (%) | 0.009 | ||
| White | 58/122 (48) | 64/122 (53) | |
| Black | 10/11 (91) | 1/11 (9) | |
| Mean age, yr (SD) | 65.9 (10.3) | 64.3 (11.5) | 0.38 |
| Smoking status, n (%) | 0.86 | ||
| Current | 25/46 (54) | 21/46 (46) | |
| Former | 38/77 (49) | 39/77 (51) | |
| Never | 5/10 (50) | 5/10 (50) | |
| Mean pack-years (SD) | 41.3 (26.7) | 42.5 (31.8) | 0.81 |
| Family history, n (%) | 0.54 | ||
| No | 31/57 (54) | 26/57 (46) | |
| Yes | 18/41 (43) | 23/41 (56) | |
| Unknown | 19/35 (54) | 16/35 (46) | |
| Tumor pathologic stage, n (%) | 0.94 | ||
| I | 43/83 (52) | 40/83 (48) | |
| II | 15/29 (52) | 14/29 (48) | |
| IIIA | 10/21 (48) | 11/21 (52) | |
| Tumor histologic type, n (%) | 0.25 | ||
| Adenocarcinoma | 38/81 (47) | 43/81 (53) | |
| Squamous cell carcinoma | 25/40 (63) | 15/40 (38) | |
| Large cell carcinoma | 2/3 (67) | 1/3 (33) | |
| Adenosquamous cell carcinoma | 3/9 (33) | 6/9 (67) | |
| Tumor grade, n (%) | 0.87 | ||
| Well differentiated | 2/5 (40) | 3/5 (60) | |
| Moderately differentiated | 26/48 (52) | 22/48 (48) | |
| Poorly differentiated | 40/80 (50) | 40/80 (50) | |
| Performance status, n (%) | 0.58 | ||
| 0 | 48/91 (53) | 43/91 (47) | |
| ≥1 | 20/42 (48) | 22/42 (52) | |
| Weight loss, n (%) | 0.53 | ||
| No | 61/117 (52) | 56/117 (48) | |
| Yes | 7/16 (44) | 9/16 (56) | |
hHpr1/p84/Thoc1 expression and survival
Table 3 shows the relationship between hHpr1/p84/Thoc1 expression and patient survival. Among the 133 patients, no significant association was found between Hpr1/p84N5/Thoc1 expression and patient survival (RR = 1.16, 95% CI = 0.77-1.75, p = 0.49). In subgroup analyses stratified by stage, histology, and family history of lung cancer, positive hHpr1/p84/Thoc1 expression was appeared to be weakly associated with elevated risk of death among patients with stage I tumors (RR = 1.53, 95% CI = 0.85-2.77, p = 0.16), with squamous carcinomas (RR = 1.75, 95% CI = 0.73-4.21, p = 0.21), or with family history of lung cancer (RR = 1.55, 95% CI = 0.81-2.97, p = 0.18) (Table 3). Survival curves for positive hHpr1/p84/Thoc1 vs negative hHpr1/p84/Thoc1 among these subgroups of patients are shown in Fig. 3. No significant association was found between hHpr1/p84/Thoc1 expression and survival when stratified by gender, race, smoking status, grade, performance status, and weight loss status (data not shown).
Table 3.
Risk of death in hHpr1/p84/Thoc1-positive patients compared with negative patients.
| Characteristic | RR (95% CI) | p value |
|---|---|---|
| Overall (n = 133) | 1.16 (0.77-1.85) | 0.49 |
| Tumor stage | ||
| I (n = 85) | 1.53 (0.85-2.77) | 0.16 |
| II (n = 29) | 0.77 (0.36-1.67) | 0.51 |
| IIIA (n = 21) | 0.95 (0.38-2.35) | 0.91 |
| Tumor histologic type | ||
| Adenocarcinoma (n = 81) | 0.90 (0.54-1.52) | 0.70 |
| Squamous cell carcinoma (n = 40) | 1.75 (0.73-4.21) | 0.21 |
| Family history of lung cancer | ||
| Yes (n = 57) | 1.55 (0.81-2.97) | 0.18 |
| No (n = 41) | 1.10 (0.50-2.44) | 0.81 |
Fig. 3.
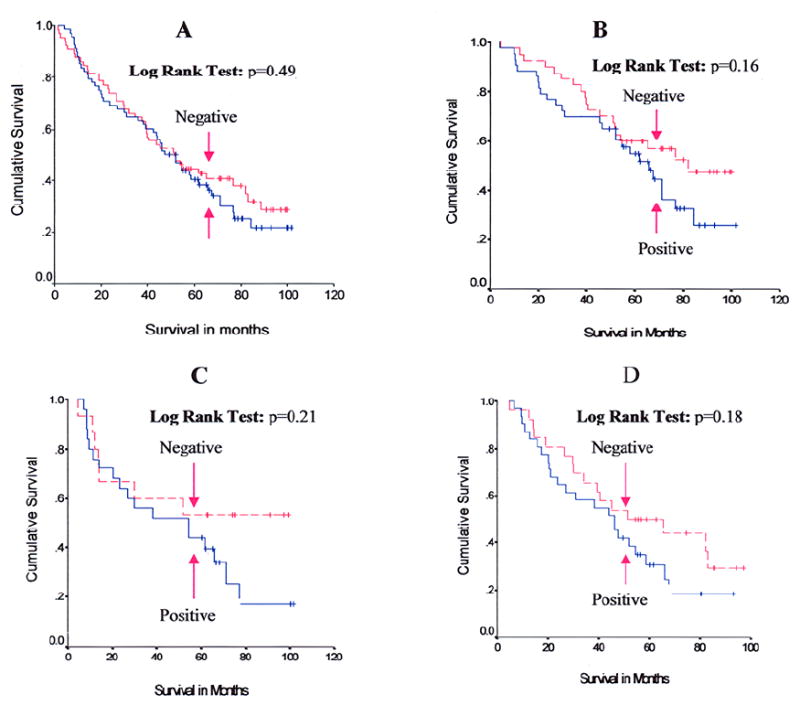
Kaplan-Meier survival curves for hHpr1/p84/Thoc1 expression. A: Survival curves of all 133 patients. B: Survival curves of 83 patients with stage I tumors. C: Survival curves of 40 patients with squamous cell carcinoma. D: Survival curves of 57 patients with family histories of lung cancer. Tick marks represent censored survival times for patients who were still alive at the completion of the study.
Discussion
The present study showed that positive hHpr1/p84/Thoc1 expression was present in approximately half of NSCLC patients, and men and blacks tended to have higher prevalence of positive hHpr1/p84/Thoc1 expression. Although hHpr1/p84/Thoc1 expression was not significantly associated with overall survival in the total sample, it seemed that patients with early stage tumors, squamous cell carcinomas, or family history of lung cancer had higher risk of death if hHpr1/p84/Thoc1 expression was elevated in the tumors.
hHpr1/p84/Thoc1 has been identified as a human nuclear matrix protein for more than a decade [13]. Accumulating in vivo and in vitro data, including those from our group, have shown that hHpr1/p84/Thoc1 is ubiquitously expressed in most human tissues and has important functions pertinent to transcription elongation, RNA processing, and apoptosis [15-17]. Recently, we compared the Thoc1 requirements in proliferation and survival of isogenic normal and oncogene-transformed cells and we found that neoplastic cells rapidly lose viability via apoptotic cell death on depletion of pThoc1. In contrast, the viability of normal cells is largely unaffected by pThoc1 loss. Our in vitro data are consistent with the hypothesis that cancer cells require higher levels of Thoc1 for survival than normal cells and they suggest that Thoc1 may provide a novel molecular target for cancer therapy [19].
The prevalence of hHprl/p84/Thoc1 expression in lung cancer and its clinical significance relative to diagnosis, treatment, and prognosis was hitherto unknown. There exist limited data for human cancers regarding the characteristics of hHprl/p84/Thoc1 expression and its prognostic value. Guo et al [18] conducted a study to assess the prognostic value of hHpr1/p84/Thoc1 expression in breast cancer. They found that breast cancer tissue had high levels of hHpr1/p84/Thoc1, whereas normal breast tissue had low to undetectable levels of hHpr1/p84/Thoc1, and high levels of hHpr1/p84/Thoc1 were significantly associated with lymph node metastasis, large tumor size, and high tumor grade. These results suggest that positive hHpr1/p84/Thoc1 expression might be an adverse factor in relation to breast cancer prognosis [18].
We previously reported that depletion of hHpr1/p84/Thoc1 sensitizes cancer cells to the cytotoxic effects of DNA damage [17], which implies that high levels of hHpr1/p84/Thoc1 expression may increase the resistance of cancer cells to anticancer treatment and thus may confer poorer prognosis. By this token, we postulate that high levels of hHpr1/p84/Thoc1 expression may facilitate tumor progression, leading to poor clinical outcomes in NSCLC. In the present study, hHpr1/p84/Thoc1 expression tended to be associated with poor survival in subgroups of patients at early tumor stage, with squamous cell type of tumor, or with a family history of lung cancer. The real role of hHpr1/p84/Thoc1 in lung cancer has not yet been defined. Further studies with larger sample size are needed to elucidate the biologic and clinical significance of hHpr1/p84/Thoc1 in lung cancer before this marker could be used as a potential prognosis indicator of lung cancer.
Acknowledgments
We thank Ms. Xiaojing Zhang for performing Western blot analyses on the frozen tissue samples. We thank Drs. G. Bepler, F. Hernandez and N. Ramnath for clinical consultations. We thank Ms. Ann Sutton for editing the manuscript. This work was supported by grants from the Roswell Park Alliance Foundation (D.T.), the National Institutes of Health (CA70292 to D.W.G.), and the Ralph Wilson Research Foundation (D.W.G.).
References
- 1.Brunagel G, Vietmeier BN, Bauer AJ, Schoen RE, Getzenberg RH. Identification of nuclear matrix protein alterations associated with human colon cancer. Cancer Res. 2002;62:2437–2442. [PubMed] [Google Scholar]
- 2.Dey P. Chromatin pattern alteration in malignant cells: an enigma. Diagn Cytopathol. 2005;32:25–30. doi: 10.1002/dc.20187. [DOI] [PubMed] [Google Scholar]
- 3.Zink D, Fischer AH, Nickerson JA. Nuclear structure in cancer cells. Nat Rev Cancer. 2004;4:677–687. doi: 10.1038/nrc1430. [DOI] [PubMed] [Google Scholar]
- 4.Nickerson J. Experimental observations of a nuclear matrix. J Cell Sci. 2001;114:463–474. doi: 10.1242/jcs.114.3.463. [DOI] [PubMed] [Google Scholar]
- 5.Ruh MF, Dunn R, 2nd, Ruh TS. Interrelationships between nuclear structure and ligand-activated intracellular receptors. Crit Rev Eukaryot Gene Expr. 1996;6:271–283. doi: 10.1615/critreveukargeneexpr.v6.i2-3.80. [DOI] [PubMed] [Google Scholar]
- 6.Martelli AM, Bareggi R, Bortul R, Grill V, Narducci P, Zweyer M. The nuclear matrix and apoptosis. Histochem Cell Biol. 1997;108:1–10. doi: 10.1007/s004180050140. [DOI] [PubMed] [Google Scholar]
- 7.Leman ES, Getzenberg RH. Nuclear matrix proteins as biomarkers in prostate cancer. J Cell Biochem. 2002;86:213–223. doi: 10.1002/jcb.10218. [DOI] [PubMed] [Google Scholar]
- 8.Getzenberg RH, Konety BR, Oeler TA, Quigley MM, Hakam A, Becich MJ, et al. Bladder cancer-associated nuclear matrix proteins. Cancer Res. 1996;56:1690–1694. [PubMed] [Google Scholar]
- 9.Konety BR, Nangia AK, Nguyen TS, Veitmeier BN, Dhir R, Acierno JS, et al. Identification of nuclear matrix protein alterations associated with renal cell carcinoma. J Urol. 1998;159:1359–1363. [PubMed] [Google Scholar]
- 10.Getzenberg RH, Pienta KJ, Huang EY, Coffey DS. Identification of nuclear matrix proteins in the cancer and normal rat prostate. Cancer Res. 1991;51:6514–6520. [PubMed] [Google Scholar]
- 11.Luftner D, Possinger K. Nuclear matrix proteins as biomarkers for breast cancer. Expert Rev Mol Diagn. 2002;2:23–31. doi: 10.1586/14737159.2.1.23. [DOI] [PubMed] [Google Scholar]
- 12.Partin AW, Briggman JV, Subong EN, Szaro R, Oreper A, Wiesbrock S, et al. Preliminary immunohistochemical characterization of a monoclonal antibody (PRO:4-216) prepared from human prostate cancer nuclear matrix proteins. Urology. 1997;50:800–808. doi: 10.1016/S0090-4295(97)00337-3. [DOI] [PubMed] [Google Scholar]
- 13.Durfee T, Mancini MA, Jones D, Elledge SJ, Lee WH. The amino-terminal region of the retinoblastoma gene product binds a novel nuclear matrix protein that co-localizes to centers for RNA processing. J Cell Biol. 1994;127:609–622. doi: 10.1083/jcb.127.3.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gasparri F, Sola F, Locatelli G, Muzio M. The death domain protein p84N5, but not the short isoform p84N5s, is cell cycle-regulated and shuttles between the nucleus and the cytoplasm. FEBS Lett. 2004;574:13–19. doi: 10.1016/j.febslet.2004.07.074. [DOI] [PubMed] [Google Scholar]
- 15.Doostzadeh-Cizeron J, Evans R, Yin S, Goodrich DW. Apoptosis induced by the nuclear death domain protein p84N5 is inhibited by association with Rb protein. Mol Biol Cell. 1999;10:3251–3261. doi: 10.1091/mbc.10.10.3251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Doostzadeh-Cizeron J, Terry NH, Goodrich DW. The nuclear death domain protein p84N5 activates a G2/M cell cycle checkpoint prior to the onset of apoptosis. J Biol Chem. 2001;276:1127–1132. doi: 10.1074/jbc.M006944200. [DOI] [PubMed] [Google Scholar]
- 17.Li Y, Wang X, Zhang X, Goodrich DW. Human hHpr1/p84/Thoc1 regulates transcriptional elongation and physically links RNA polymerase II and RNA processing factors. Mol Cell Biol. 2005;25:4023–4033. doi: 10.1128/MCB.25.10.4023-4033.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guo S, Hakimi MA, Baillat D, Chen X, Farber MJ, Klein-Szanto AJ, et al. Linking transcriptional elongation and messenger RNA export to metastatic breast cancers. Cancer Res. 2005;65:3011–3016. doi: 10.1158/0008-5472.CAN-04-3624. [DOI] [PubMed] [Google Scholar]
- 19.Li Y, Lin AW, Zhang X, Wang Y, Wang X, Goodrich DW. Cancer cells and normal cells differ in their requirements for Thoc1. Cancer Res. 2007;15:6657–6664. doi: 10.1158/0008-5472.CAN-06-3234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5:649–655. [PubMed] [Google Scholar]
- 21.Travis WD, Brambilla E, Muller-Hermelink HK, Harris CC. WHO Classification of Tumours. IARC Press; Lyon: 2004. Pathology and genetics In: Tumours of the Lung, Pleura, Thymus and Heart; pp. 9–68. [Google Scholar]
- 22.Mountain CF. Revisions in the International System for Staging Lung Cancer. Chest. 1997;111:1710–1717. doi: 10.1378/chest.111.6.1710. [DOI] [PubMed] [Google Scholar]
- 23.Tan D, Li Q, Deeb G, Ramnath N, Slocum HK, Brooks J, et al. Thyroid transcription factor-1 expression prevalence and its clinical implications in non-small cell lung cancer: a high-throughput tissue microarray and immunohistochemistry study. Hum Pathol. 2003;34:597–604. doi: 10.1016/s0046-8177(03)00180-1. [DOI] [PubMed] [Google Scholar]
- 24.Tan D, Deeb G, Wang J, Slocum HK, Winston J, Wiseman S, et al. HER-2/neu protein expression and gene alteration in stage I-IIIA non-small-cell lung cancer: a study of 140 cases using a combination of high throughput tissue microarray, immunohistochemistry, and fluorescent in situ hybridization. Diagn Mol Pathol. 2003;12:201–211. doi: 10.1097/00019606-200312000-00004. [DOI] [PubMed] [Google Scholar]


