Abstract
Serratia marcescens is an emerging opportunistic pathogen with a remarkably broad host range. The cAMP-regulated catabolite repression system of S. marcescens has recently been identified and demonstrated to regulate biofilm formation through the production of surface adhesions. Here we report that mutations in components of the catabolite repression system (cyaA and crp) eliminate flagellum production and swimming motility. Exogenous cAMP was able to restore flagellum production to adenylate cyclase mutants, as determined by transmission electron microscopy and PAGE analysis. A transposon-generated suppressor mutation of the crp motility defect mapped to upstream of the flhDC operon. This suppressor mutation resulted in an upregulation of flhD expression and flagellum production, indicating that flhDC expression is sufficient to restore flagellum production to crp mutants. Lastly, and contrary to a previous report, we found that flhD expression is controlled by the catabolite repression system using quantitative RT-PCR. Together, these data indicate that flagellum production is regulated by the cAMP-dependent catabolite repression system. Given the role of flagella in bacterial pathogenicity, the regulatory pathway described here may assist us in better understanding the putative role of motility in dissemination and virulence of this opportunistic pathogen.
Keywords: Flagella, Catabolite repression, cAMP, Biofilm, Serratia
1. Introduction
Serratia marcescens is a Gram-negative bacterium that has a significant health and economic impact as an agent of hospital infections and as a plant pathogen [12, 25]. S. marcescens infects a broad range of hosts from insects to coral [8, 26]. Bacterial motility and surface behaviors such as biofilm formation and swarming may play a role in S. marcescens pathogenesis and dissemination [13, 37].
Swimming motility in bacteria is facilitated by formation of an intricate surface organelle called the flagellum, reviewed by Chilcott and Hughes [6]. Flagella-based motility is thought to contribute to the pathogenic capacity of several bacterial species and is often cited as a virulence factor [29]. The regulation of flagellum production is well characterized in Escherichia coli and Salmonella typhimurium [6]. In these organisms there is a flagellum production regulatory hierarchy, with the master regulator operon being flhDC [6]. S. marcescens was shown to have a homologous flhDC operon that codes for transcriptional activators that control swimming, swarming and exoenzyme production [11, 22, 36].
In a previous study, we identified and mutated components of the cAMP-dependent catabolite repression system (CRS) in S. marcescens [16]. Catabolite repression systems inhibit the production of proteins involved in the use of less efficiently metabolized carbon sources when more favorable carbon sources are available [4]. cAMP-dependent CRS uses cyclic nucleotide cAMP as a signaling molecule. Adenylate cyclase (cyaA) generates cAMP in response to environmental carbon sources, reviewed by Botsford and Harman [4]. The cAMP-receptor protein CRP is a transcription factor, coded by the crp gene, which binds to cAMP and can either activate or inhibit expression of multiple genes [4]. In E. coli and S. typhimurium, the reach of the CRS goes well beyond regulation of carbon sources to include virulence, motility, and cell division [4]. In S. marcescens, the CRS functions to control fimbriae production, such that mutations in adenylate cyclase (cyaA) or cAMP-receptor protein (CRP) lead to a dramatic increase in biofilm formation [16]. This trend differs from what is seen in E. coli and Pseudomonas aeruginosa where CRS systems positively regulate biofilm formation [14, 23]. While working with mutants of the S. marcescens CRS, we noticed that swimming motility was defective. In this study, we provide evidence that the CRS is a positive regulator of flagellum production and that flhDC is sufficient to restore swimming motility to CRS mutants. Furthermore, flhDC transcription was significantly reduced in cyaA and crp mutants. A model for the differential regulation of flagellum-based motility versus biofilm formation is presented.
2. Material and methods
2.1 Bacterial strains and growth conditions
All bacteria used in this study are derived from a S. marcescens strain from Presque Isle cultures (Presque Isle, PA), strain number 3611. Construction and analysis of the cyaA-2 and crp-1 mutation have been described previously [16]. The scrp31 mutation was derived from a mariner transposon mutation made with the pBT20 delivery vector [18] using previously reported methods [31]. Bacteria were grown in LB broth in all cases. Swimming medium consisted of LB with a 0.3% (w/v) agar concentration. Swarming medium was identical but with an agar concentration of 0.5–1.2% (w/v), and swarming experiments were performed as previously reported [31]. Kanamycin was supplemented at 100 µg/ml. 3’-5’ cyclic AMP (cAMP, Sigma-Aldrich, St. Louis, MO) was added to LB directly and then filter-sterilized at concentrations up to 10 mM. All experiments were performed at 30°C at least two times with multiple independent biological replicates.
2.2. Surface fractions and PAGE analysis
Surface fractions were performed as Labbate and colleagues [19] with the following exceptions. Bacteria, three independent cultures per strain, were grown in culture for 16 h, washed in PBS and adjusted to five A600 units in 1 ml of PBS. Aliquots were vortexed for two min on a Turbomix attachment on a Genie vortex unit (Scientific Industries, Bohemia, NY), and bacteria were pelleted with a microcentrifuge. The supernatant was filtered with a PVDF 0.22 micron filter (Millipore item number SLGV033RS, Cork Ireland), then proteins were precipitated with TCA (Sigma-Aldrich, St. Louis, MO). One half of the sample was loaded onto an 8–16% polyacrylamide gel (Precise Protein Gel, Pierce, Rockford IL), using a Minigel format (Mini Protean 3, Biorad, CA), and stained with Coomassie brilliant blue. Image J software (NIH) was used to quantify protein levels of scanned gels, using at least three independent samples. Mass spectroscopy and peptide identification were performed by the University of Pittsburgh Genomics and Proteomics core facility.
2.3 Transmission electron microscopy (TEM)
These experiments were performed as previously described [31]. Briefly, bacteria were taken from liquid cultures rotated at high speed on a tissue culture roller (TC-7, New Brunswick Scientific, NJ) for 14–16 h, washed once with PBS, applied to formvar coated copper grids and stained with uranyl acetate (2%). Images were obtained using a JEOL-1011 microscope at the University of Pittsburgh Center for Biological Imaging.
2.4. Genetic manipulations, quantitative-RT-PCR and statistical analysis
Transposon mutagenesis, quantitative RT-PCR (Q-PCR) and arbitrary PCR were performed as previously described [31]. Statistical analysis was performed using Student’s T-tests with Excel software. Plasmids were generated and described previously, and had a medium copy number, pBBR1-based, replicon [16, 30]. The crp-1 mutation was generated by integration of pMQ118 into the crp gene as directed by an internal fragment of crp that disrupts the crp open reading frame [16, 30]. All experiments with the crp-1 mutation were performed with kanamycin to ensure a homogenous crp-1 culture. To restore the wild-type crp gene function in the crp-1 scrp31 double mutant background, we grew the strain in culture to saturation three times in LB medium without antibiotic selection, which maintains pMQ118 integration. Aliquots were then plated on minimal medium with 0.3% glycerol as a sole carbon source to select for bacteria that had experienced a recombination event restoring the crp open reading frame (PCR verified), as crp mutants cannot grow with glycerol as a sole carbon source [16, 30].
3. Results
3.1 Mutation of catabolite repression system genes inhibits swimming motility and flagellum production
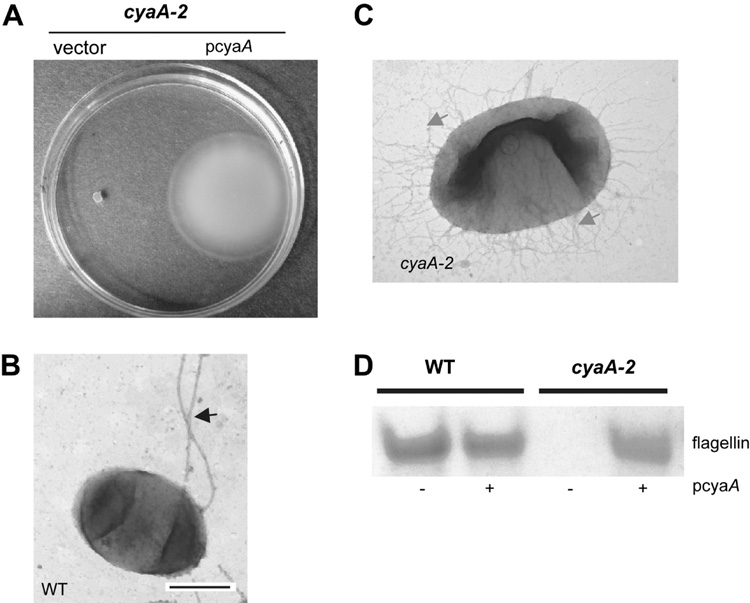
Mutation of the adenylate cyclase gene, cyaA, prevents swimming motility through a semi-solid agar matrix (Fig. 1A). The addition of wild-type cyaA on a plasmid was able to restore swimming to the cyaA-2 mutant (Fig. 1A). In a similar experiment where swim zones were measured at 7.5 h, the cyaA mutant exhibited no detectable zone of swimming compared to the wild type, which exhibited a swim zone of 7.7 ± 1.5 mm. A cyaA-2 + pcyaA had a 6.05 ± 0.8 mm swim zone, with the wild type + pcyaA producing a swim zone of 8.6 ± 1.4 mm swim zone. Mutation of the crp gene which codes for a cAMP-binding transcription factor led to a similar lack of swimming motility (described below).
Fig. 1.
Adenylate cyclase is required for swimming motility and flagellum production by S. marcescens. A. Photograph of swimming zones through 0.3% agar by the cyaA (adenylate cyclase) mutant strain with either an empty vector or the wild-type cyaA gene under control of the Plac promoter on a multicopy plasmid (pcyaA). B–C. TEM micrographs of wild-type (B) and cyaA mutant (C) cells. The black arrow denotes flagella and the gray arrows indicate fimbriae. The bar indicates either 500 nm (B) or 100 nm (C). D. PAGE analysis of surface protein fractions from stationary phase wild-type and cyaA cells with vector control or pcyaA. The flagellin protein was verified by mass spectroscopy.
TEM was used to determine if there was an obvious alteration in surface morphology of the cyaA-2 mutant strain compared to the wild type. We observed that wild-type cells commonly had one or more flagella whereas the cyaA-2 and crp mutants had no flagella (n>400 per strain), yet were covered with fimbriae (Fig. 1B–C, Fig.2A).
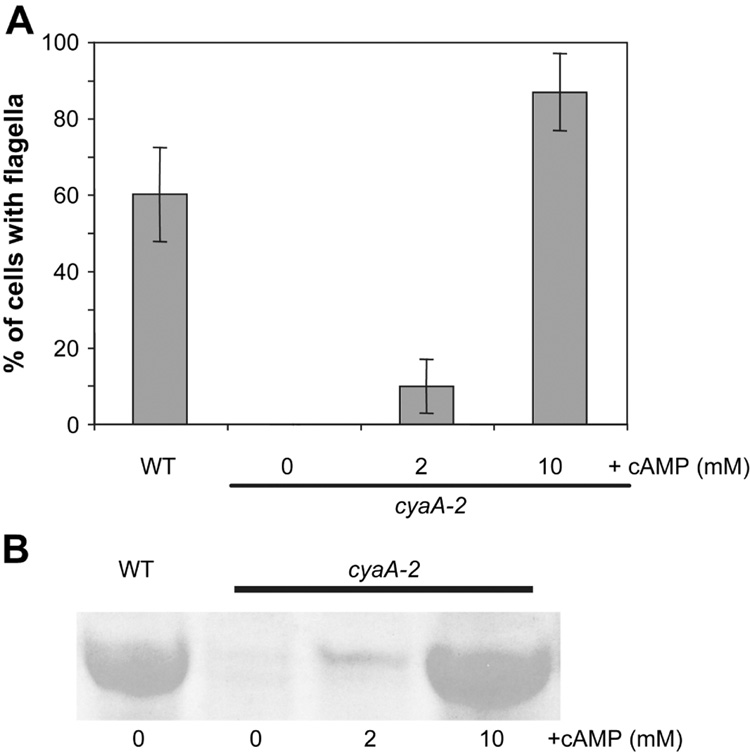
Fig. 2.
Flagellum production can be restored to a cyaA mutant by exogenous cAMP. A. The percent of cells with a flagellum observed by TEM microscopy in response to a cyaA mutation and increasing amounts of exogenous cAMP (n>200 cells per condition). No flagella were observed among cyaA mutant cells without exogenous cAMP (n>400). A statistical difference from the wild type was observed for each condition (p<0.02). B. PAGE analysis of surface protein fractions from stationary phase wild-type and cyaA cultures grown with increasing concentrations of exogenous cAMP.
To provide further evidence that CyaA is a positive regulator of flagella biosynthesis, a biochemical approach was taken. Sheared surface protein fractions were separated on a polyacrylamide gel. A protein was observed to be absent from cyaA-2 mutant fractions that migrated at approximately 40 kD (Fig. 1D). This band was excised and determined by mass spectroscopy to be the flagellin subunit. When the amount of flagellin was determined using Image J software, the wild-type strain with the vector alone had a relative flagellin level of 25851 ± 26, the wild-type with pcyaA had levels of 19593 ± 3861, the cyaA-2 mutant with the vector had only 1177 ± 358, and the cyaA-2 mutant with wild-type cya in trans (pcyaA) exhibited flagellin levels of 16098 ± 3232 (n=3 independent cultures per strain). These data show that flagellin levels were significantly reduced in the cyaA-2 mutant relative to the wild type (p<0.01), and could be complemented by the wild-type cyaA gene added in trans.
3.2. Flagellum production can be restored to the cyaA mutant with exogenous cAMP
The enzymatic product of the CyaA protein of E. coli was demonstrated to be cAMP [4]. To determine whether the lack of flagella was dependent upon cAMP, the effect of exogenous cAMP on flagellum production was assessed. Flagellum production was restored to the cyaA mutant by exogenous cAMP, as determined by TEM analysis (Fig. 2A). WT cells were generally associated with a single flagellum (23% had ≥2 flagella/cell, n=341), and the addition of 10 mM cAMP to the growth medium led to an increased number of flagella relative to the wild type, with 40% of cells associated with 2 or more flagella (n=359). The addition of 2 mM cAMP to the growth medium of cyaA mutants led to only 0.7% of cells exhibiting 2 or more flagella (n=576). Rescue of flagellin production by the cyaA mutant was also observed on polyacrylamide gels (Fig. 2B). Consistent with cAMP stimulating flagellin production, the addition of 10 mM cAMP to wild-type cells led to a significant (p<0.01) 3.3-fold increase in flagellin production compared to the wild-type levels without the addition of 10 mM cAMP.
3.3. High-level expression of flhDC suppressed motility defects of a crp mutant
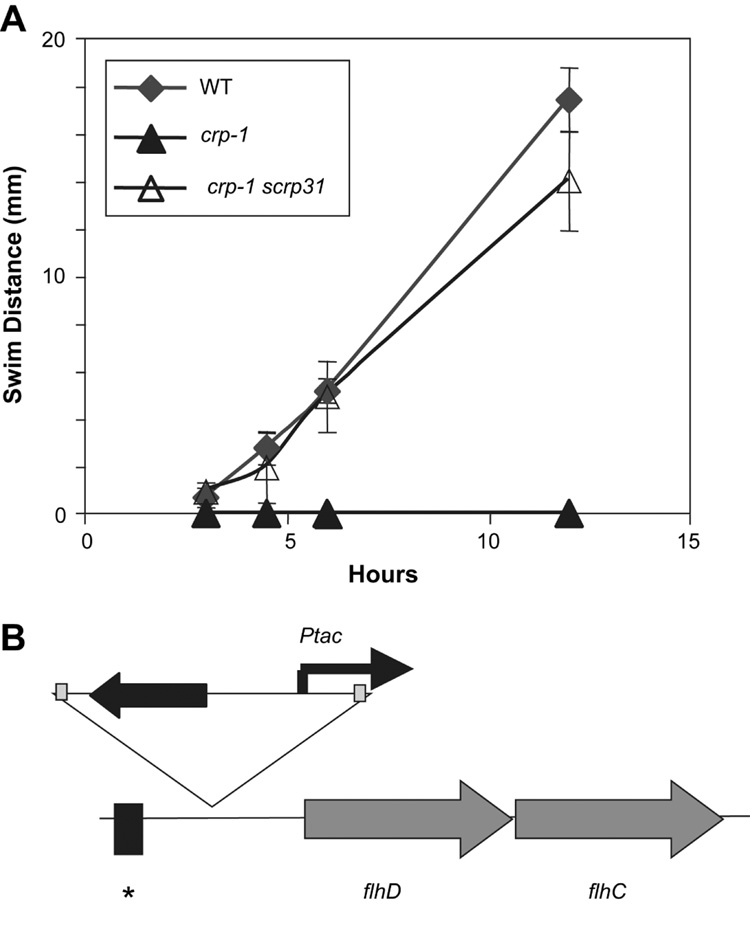
Like cyaA mutants, disruption of the crp gene leads to a defect in swimming motility and flagellum production (Fig. 3A, Fig.4A–B). We identified a transposon-induced mutation that suppressed the swimming deficiency of a crp mutant. This mutation, called scrp31 (for suppressor of crp), restored swimming motility to the crp-1 mutant strain (Fig. 3A). The swimming motility of the wild type was similar to that of the double mutant, suggesting that there are not multiple factors preventing swimming motility in a CRS-deficient strain (Fig. 3A).
Fig. 3.
The scrp31 mutation suppresses the swimming and swarming deficiency phenotypes of a crp mutant strain. A. Swimming motility charted as a function of time. The crp scrp31 double mutant is able to swim, whereas the crp mutant strain is non-motile. B. Genetic map of the flhDC locus with the location of a predicted CRP binding site (black box with asterisk) and the location of a transposon insertion (scrp31), not drawn to scale. The transposon bears a Ptac promoter, which is directed toward the flhDC operon.
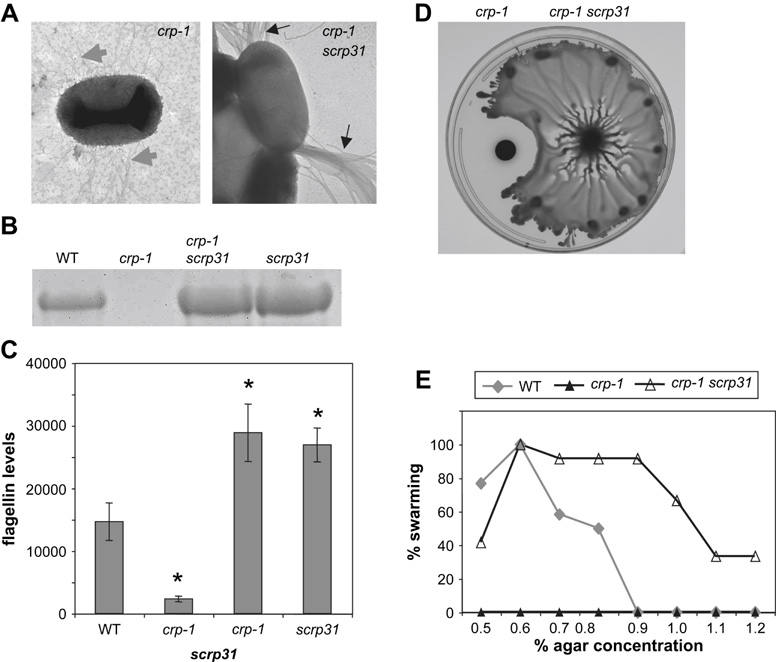
Fig. 4.
The scrp31 mutation restores flagellum production to a crp mutant strain. A. TEM micrographs of a crp-1 mutant with no flagellum, but numerous fimbriae (gray arrows) and crp scrp31 double mutant cells covered with numerous flagella (black arrows). The size bar represents 500 and 100 nm respectively. B. PAGE analysis of surface protein fractions from wild-type culture, crp, crp scrp31 and scrp31 cultures showing flagellin production. C. Quantitation of flagellin levels using Image J analysis software. Surface fractions were taken from stationary phase cultures. Asterisks represent a statistically significant difference from the wild-type (p<0.01). D. Swarming motility on LB with 0.7% agar at 48 h. The crp mutant swarming defect is rescued by the scrp31 mutation. E. Plotting the percentage of positive swarming motility experiments at 48 h as a function of agar concentration shows that the scrp31 mutation confers a hyperswarming phenotype (n≥12 plates for WT and crp-1 scrp31 and n≥8 for the crp-1 mutant, performed on 4 separate occasions with similar results).
The chromosomal locus of the transposon in scrp31 was mapped using arbitrary PCR to an intergenic region 291 base pairs upstream of the predicted flhD start codon (Fig. 3B). The transposon used in this study can upregulate genes near its insertion site via a Ptac promoter at one end of the transposon; in the case of scrp31 the Ptac promoter is directed toward the flhDC operon (Fig. 3B). Q-RT-PCR was used to determine that flhD expression was significantly upregulated 10.96 ± 1.90-fold in the crp-1 scrp31 mutant compared to the wild type (p<0.01). An alternative mechanism for the increase in flhD expression is that a negative regulatory region is disrupted by the transposon mutation, as has been recently reported in Proteus mirabilis [7].
We next sought to characterize the effect of the scrp31 mutation on flagellum production. TEM analysis revealed a hyperflagella phenotype in both the crp-1 scrp31 (Fig. 4A) and scrp31 mutant (not shown), with >99% of the cells exhibiting flagella (n>100 cells per strain) compared to ~60% for the wild type (Fig. 2A) and <0.1% for the crp-1 mutant (n>400). The increase in flagellum production in crp-1 scrp31 and scrp31 mutants was clearly observed in separated surface protein fractions (Fig. 4B). Quantification of this difference using Image J software to analyze PAGE flagellin levels from four independent experiments indicated a significant increase in flagellin production conferred by the scrp31 mutation compared to the wild type (Fig. 4C). There was no statistical difference in flagellin production between the crp-1 scrp31 double mutant and the scrp31 mutant (p=0.499). Nor did addition of cAMP (10 mM) alter flagellin production by either the scrp31 or crp-1 scrp31 double mutant (p= 0.31 and 0.40 respectively, data not shown). These data suggest that the scrp31 mutation renders flagellum production insensitive to catabolite repression.
The multiple flagella associated with the scrp31 mutation were suggestive of the hyperflagellated bacteria induced by growth on surfaces associated with swarming conditions [2]. We tested whether the scrp31 mutation altered swarming by S. marcescens. The wild-type strain swarmed on the surface of LB plates with 0.5–0.8% agar, the crp mutant did not swarm under any condition, and the scrp31 and the crp scrp31 double mutants were able to swarm on plates with 0.5–1.2% agar, suggesting that the scrp31 mutation confers a hyperswarming phenotype (Fig. 4D–E, and data not shown).
3.4. The flagella regulator flhD is controlled by the CRS
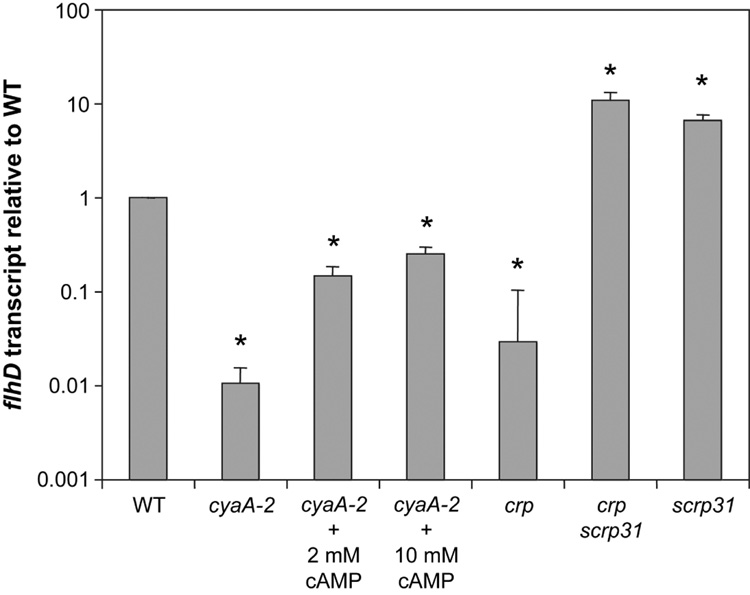
Sequence analysis revealed a predicted CRP binding site (tTGTGActatgTCACAt) 316 base pairs upstream of the flhDC operon in the sequenced Db11 strain (Sanger Center) (Fig. 3B). This suggests that flagella biosynthesis may be regulated by cAMP through transcription of the flagella master regulator operon. The flhDC operon is transcriptionally regulated in a positive manner by CRP in Escherichia coli [34]. The motility defect of S. marcescens cyaA mutants was hypothesized to be a result of a loss of positive regulation of flhDC expression by the CRS. This prediction was tested using Q-RT-PCR. Transcript of the flhD gene was down >10-fold in the cyaA-2 strain compared to the wild type (p<0.01)(Fig. 5A). A consistent prediction would be that exogenous cAMP should restore flhD expression to a cyaA mutant. We found a dose-responsive increase in flhD levels with the addition of exogenous cAMP, which partially complemented the cyaA mutant defect at 10 mM cAMP (Figure 5). Mutation of the cAMP-binding transcription factor crp also led to a significant decrease in flhD transcript levels compared to the wild type (p<0.01). The scrp31 mutation led to elevated flhD levels and was sufficient to restore flhD levels in a crp mutant background.
Fig. 5.
Catabolite repression control of flhD expression. Quantitative RT-PCR analysis of flhD transcript levels relative to wild-type levels shows a significant decrease in transcript produced by the cyaA mutant, which is partially restored by the addition of exogenous cAMP to growth medium. The crp mutant was similarly reduced in the flhD transcript, and was rescued by the scrp31 mutation which caused a significant increase in flhD RNA. RNA was harvested from 3 or more independent cultures at an A600 of 1.0. The asterisk indicates a significant difference versus the wild type (p<0.01).
4. Discussion
The results of this study support a model whereby the cAMP-dependent catabolite repression system of S. marcescens is required for flagella-based motility and flagellum production through control of the master regulator flhDC. The expression of flhDC conferred by the scrp31 mutation was able to restore swimming and swarming motility to the non-motile crp mutant. This suggests that a lack of flhDC expression is the reason for the loss of flagellum production and flagella-based motility conferred by mutations in the CRS. Consistently, flagellin production and flhDC expression defects of the cyaA mutant can be rescued by exogenous cAMP.
The connection between carbon and swimming motility has been established in other Enterobacteria. It was observed that high levels of glucose inhibit swimming motility by E. coli [1]. Later, the catabolite repression machinery was found to be necessary for swimming in both E. coli and S. typhimurium [17, 32]. It was also demonstrated that flhDC is regulated by CRP in E. coli [3, 34]. This has led to the model in which increased cAMP levels caused by the presence of less efficiently metabolized carbon sources leads to an increase in flagellum production, whereas conditions that decrease cAMP levels, namely high glucose, inhibit flagellum production in order to keep the bacterium in this favorable environment. Therefore, the bacteria will become less motile in environments with high levels of glucose and become more motile in environments with less efficient carbon sources. Evidence in support of this model was elegantly shown in a recent report using E. coli as a model organism [40].
We have previously reported that cAMP production inhibits S. marcescens biofilm formation through negative regulation of surface adhesin production [16]. In that report, it was shown that S. marcescens with mutations in genes whose products positively regulate cAMP production (cyaA and crr) and crp, whose gene product responds to cAMP levels, exhibited a major increase in biofilm formation and that this effect was mediated through type 1 fimbriae [16] (Fig. 6). Together, these studies suggest a model by which environments with unfavorable carbon sources lead to a coordinated decrease in attachment factor production and an increase in motility function, and that these changes are mediated by intracellular cAMP levels (Fig. 6). In the opposite setting of environments with favorable carbon sources, biofilm formation is promoted through deregulation of adhesin production and a reduction in positive regulation of motility (Fig. 6). This system of attachment and motility should serve to help bacteria achieve suitable niches to localize and proliferate.
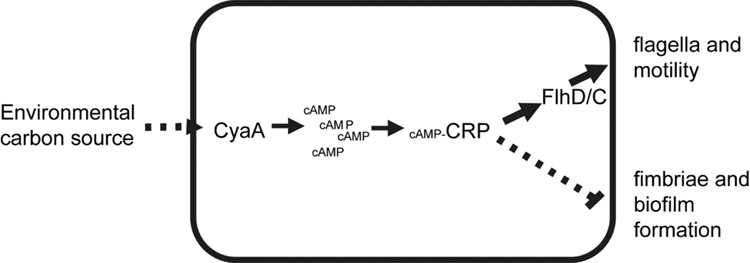
Fig. 6.
Model for coordinated catabolite repression control of attachment and motility processes. The catabolite repression system (CRS) is a signal transduction cascade that responds to environmental carbon. In response to less favorable carbon sources, adenylate cyclase (CyaA) is stimulated to generate cAMP. The activity of the global transcription factor CRP (cAMP receptor protein) is altered through binding with cAMP. The cAMP-CRP complex activates expression of flhDC, which in turn activates flagellum synthesis. At the same time, the cAMP-CRP complex directly or indirectly inhibits fimbriae production. Growth conditions with favorable carbon sources led to decreased adenylate cyclase activity and cAMP levels causing a decrease in production of flagella and derepression of attachment factor expression. The result of growth in an environment with a favorable carbon source(s) is decreased production of the flagellum and increased production of fimbriae steering a bacterium toward attachment and biofilm formation. Growth with less favorable carbon source(s) stimulates production of flagella and inhibits production of fimbriae to generate a motile bacterium that is more able to encounter hospitable environments.
Whereas the flagellum appears to be a major initiator of biofilm formation for Pseudomonas species [24], V. cholerae [38] and other bacteria [5, 27], we did not find a role for flagella in S. marcescens biofilm formation in a genetic screen for biofilm determinants [31]. Given the large target size of the flagellum biosynthetic machinery, it is likely that we screened many motility-defective mutants that were proficient in biofilm formation [31]. Consistently, mutants in the CRS system are unable to swim and form hyperbiofilms [16]. Together, these data suggest but do not prove that flagella are not a major biofilm determinant in S. marcescens.
Given the previously described role of the catabolite repression system in regulating motility in other Gram-negative bacteria, it may not be surprising that S. marcescens uses a similar mechanism [21, 32, 39]; however, this report may be controversial regarding the role of catabolite repression and flagella regulation by S. marcescens. Two previous reports suggest that swimming motility [20] and flhDC expression [22] were not controlled by “glucose catabolite repression”. One reason for the discrepancy between our data and that of Liu et al. [22], could be attributed to strain differences, which are common among Serratia strains. Other differences between the Liu study [22] and this report are that in the former study, a luciferase-reporter system was used to measure flhDC transcription, and glucose-rich (2%) medium was used to elicit the catabolite repression response. In our strain, we have found that, whereas 2% glucose is sufficient to elicit a catabolite repression response, this effect is not as robust as mutation of cyaA or crp [16].
The flhDC master regulator of flagella has also been demonstrated to regulate production of two extracellular enzymes, phospholipase A and nuclease [9, 10, 22]. A role for phospholipases in pathogenesis had been demonstrated in a range of bacteria [28, 33, 35]. Similarly, flagella are a virulence factor for many Gram-negative and -positive bacterial species [15, 38]. The CRS is likely to have an important role in bacterial pathogenesis in response to local carbon sources. Future experiments will be directed to determining the roles of the CRS and flhDC in bacterial virulence.
Acknowledgements
The authors thank the Campbell and Kinchington lab and Stu Powers for help and discussion, Daniel Kadouri and George O’Toole for critical reading of the manuscript, and James Fender for photography. This work was done with support from an NIH core grant for vision research, EY08098, the Eye and Ear Institute of Pittsburgh, Research to Prevent Blindness, and the Charles T. Campbell Laboratory of Ocular Microbiology.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Nicholas A. Stella, Email: stellan@upmc.edu.
Eric J. Kalivoda, Email: ejk9+@pitt.edu.
Dawn M. O’Dee, Email: dmo8@pitt.edu.
Gerard J. Nau, Email: gjnau@mgb.pitt.edu.
Robert M. Q. Shanks, Email: shanksrm@upmc.edu.
References
- 1.Adler J, Templeton B. The effect of environmental conditions on the motility of Escherichia coli. J Gen Microbiol. 1967;46:175–184. doi: 10.1099/00221287-46-2-175. [DOI] [PubMed] [Google Scholar]
- 2.Alberti L, Harshey RM. Differentiation of Serratia marcescens 274 into swimmer and swarmer cells. J Bacteriol. 1990;172:4322–4328. doi: 10.1128/jb.172.8.4322-4328.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bartlett DH, Frantz BB, Matsumura P. Flagellar transcriptional activators FlbB and FlaI: gene sequences and 5′ consensus sequences of operons under FlbB and FlaI control. J Bacteriol. 1988;170:1575–1581. doi: 10.1128/jb.170.4.1575-1581.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Botsford JL, Harman JG. Cyclic AMP in prokaryotes. Microbiol Rev. 1992;56:100–122. doi: 10.1128/mr.56.1.100-122.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chagneau C, Saier MH., Jr Biofilm-defective mutants of Bacillus subtilis. J Mol Microbiol Biotechnol. 2004;8:177–188. doi: 10.1159/000085790. [DOI] [PubMed] [Google Scholar]
- 6.Chilcott GS, Hughes KT. Coupling of flagellar gene expression to flagellar assembly in Salmonella enterica serovar typhimurium and Escherichia coli. Microb Mol Biol Rev. 2000;64:694–708. doi: 10.1128/mmbr.64.4.694-708.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clemmer KM, Rather PN. Regulation of flhDC expression in Proteus mirabilis. Res Microbiol. 2007;158:295–302. doi: 10.1016/j.resmic.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 8.Flyg C, Kenne K, Boman HG. Insect pathogenic properties of Serratia marcescens: phage-resistant mutants with a decreased resistance to Cecropia immunity and a decreased virulence to Drosophila. J Gen Microbiol. 1980;120:173–181. doi: 10.1099/00221287-120-1-173. [DOI] [PubMed] [Google Scholar]
- 9.Givskov M, Eberl L, Christiansen G, Benedik MJ, Molin S. Induction of phospholipase- and flagellar synthesis in Serratia liquefaciens is controlled by expression of the flagellar master operon flhD. Mol Microbiol. 1995;15:445–454. doi: 10.1111/j.1365-2958.1995.tb02258.x. [DOI] [PubMed] [Google Scholar]
- 10.Givskov M, Molin S. Secretion of Serratia liquefaciens phospholipase from Escherichia coli. Mol Microbiol. 1993;8:229–242. doi: 10.1111/j.1365-2958.1993.tb01567.x. [DOI] [PubMed] [Google Scholar]
- 11.Givskov M, Ostling J, Eberl L, Lindum PW, Christensen AB, Christiansen G, Molin S, Kjelleberg S. Two separate regulatory systems participate in control of swarming motility of Serratia liquefaciens MG1. J Bacteriol. 1998;180:742–745. doi: 10.1128/jb.180.3.742-745.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hejazi A, Falkiner FR. Serratia marcescens. J Med Microbiol. 1997;46:903–912. doi: 10.1099/00222615-46-11-903. [DOI] [PubMed] [Google Scholar]
- 13.Hume EB, Willcox MD. Emergence of Serratia marcescens as an ocular surface pathogen. Arch Soc Esp Oftalmol. 2004;79:475–477. [PubMed] [Google Scholar]
- 14.Jackson DW, Simecka JW, Romeo T. Catabolite repression of Escherichia coli biofilm formation. J Bacteriol. 2002;184:3406–3410. doi: 10.1128/JB.184.12.3406-3410.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Josenhans C, Suerbaum S. The role of motility as a virulence factor in bacteria. Int J Med Microbiol. 2002;291:605–614. doi: 10.1078/1438-4221-00173. [DOI] [PubMed] [Google Scholar]
- 16.Kalivoda EJ, Stella NA, O'Dee DM, Nau GJ, Shanks RMQ. The cAMP-dependent catabolite repression system of Serratia marcescens mediates biofilm formation through regulation of type 1 fimbriae. Appl Environ Microbiol. 2008;74:3461–3470. doi: 10.1128/AEM.02733-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Komeda Y, Suzuki H, Ishidsu I, Iino T. The role of cAMP in flagellation of Salmonella typhimurium. Mol Gen Genet. 1975;142:289–298. doi: 10.1007/BF00271253. [DOI] [PubMed] [Google Scholar]
- 18.Kulasekara HD, Ventre I, Kulasekara BR, Lazdunski A, Filloux A, Lory S. A novel two-component system controls the expression of Pseudomonas aeruginosa fimbrial cup genes. Mol Microbiol. 2005;55:368–380. doi: 10.1111/j.1365-2958.2004.04402.x. [DOI] [PubMed] [Google Scholar]
- 19.Labbate M, Zhu H, Thung L, Bandara R, Larsen MR, Willcox MDP, Givskov M, Rice SA, Kjelleberg S. Quorum-sensing regulation of adhesion in Serratia marcescens MG1 is surface dependent. J Bacteriol. 2007;189:2702–2711. doi: 10.1128/JB.01582-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lai HC, Shu JC, Ang S, Lai MJ, Fruta B, Lin S, Lu KT, Ho SW. Effect of glucose concentration on swimming motility in enterobacteria. Biochem Biophys Res Commun. 1997;231:692–695. doi: 10.1006/bbrc.1997.6169. [DOI] [PubMed] [Google Scholar]
- 21.Liang W, Pascual-Montano A, Silva AJ, Benitez JA. The cyclic AMP receptor protein modulates quorum sensing, motility and multiple genes that affect intestinal colonization in Vibrio cholerae. Microbiology. 2007;153:2964–2975. doi: 10.1099/mic.0.2007/006668-0. [DOI] [PubMed] [Google Scholar]
- 22.Liu JH, Lai MJ, Ang S, Shu JC, Soo PC, Horng YT, Yi WC, Lai HC, Luh KT, Ho SW, Swift S. Role of flhDC in the expression of the nuclease gene nucA, cell division and flagellar synthesis in Serratia marcescens. J Biomed Sci. 2000;7:475–483. doi: 10.1007/BF02253363. [DOI] [PubMed] [Google Scholar]
- 23.O'Toole GA, Gibbs KA, Hagar PW, Phibbs PV, Kolter R. The global carbon metabolism regulator Crc is a component of a signal transduction pathway required for biofilm development by Pseudomonas aeruginosa. J Bactiol. 2000;182:425–431. doi: 10.1128/jb.182.2.425-431.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O'Toole GA, Kolter R. Flagellar and twitching motility are necessary for Pseudomonas aeruginosa biofilm development. Mol Microbiol. 1998;30:295–304. doi: 10.1046/j.1365-2958.1998.01062.x. [DOI] [PubMed] [Google Scholar]
- 25.Pair SD, Bruton BD, Mitchell F, Fletcher J, Wayadande A, Melcher U. Overwintering squash bugs harbor and transmit the causal agent of cucurbit yellow vine disease. J Econ Entomol. 2004;97:74–78. doi: 10.1603/0022-0493-97.1.74. [DOI] [PubMed] [Google Scholar]
- 26.Patterson KL, Porter JW, Ritchie KB, Polson SW, Mueller E, Peters EC, Santavy DL, Smith GW. The etiology of white pox, a lethal disease of the Caribbean elkhorn coral, Acropora palmata. Proc Natl Acad Sci USA. 2002;99:8725–8730. doi: 10.1073/pnas.092260099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pratt LA, Kolter R. Genetic analysis of Escherichia coli biofilm formation: roles of flagella, motility, chemotaxis and type I pili. Mol Microbiol. 1998;30:285–293. doi: 10.1046/j.1365-2958.1998.01061.x. [DOI] [PubMed] [Google Scholar]
- 28.Schmiel DH, Young GM, Miller VL. The Yersinia enterocolitica phospholipase gene yplA is part of the flagellar regulon. J Bacteriol. 2000;182:2314–2320. doi: 10.1128/jb.182.8.2314-2320.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schmitt CK, Darnell SC, Tesh VL, Stocker BA, O'Brien AD. Mutation of flgM attenuates virulence of Salmonella typhimurium and mutation of fliA represses the attenuated phenotype. J Bacteriol. 1994;176:368–377. doi: 10.1128/jb.176.2.368-377.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shanks RM, Caiazza NC, Hinsa SM, Toutain CM, O'Toole GA. Saccharomyces cerevisiae-based molecular tool kit for manipulation of genes from Gram-negative bacteria. Appl Environ Microbiol. 2006;72:5027–5036. doi: 10.1128/AEM.00682-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shanks RM, Stella NA, Kalivoda EJ, Doe MR, M ODD, Lathrop KL, Guo FL, Nau GJ. A Serratia marcescens OxyR homolog mediates surface attachment and biofilm formation. J Bacteriol. 2007;189:7262–7272. doi: 10.1128/JB.00859-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Silverman M, Simon M. Characterization of Escherichia coli flagellar mutants that are insensitive to catabolite repression. J Bacteriol. 1974;120:1196–1203. doi: 10.1128/jb.120.3.1196-1203.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sitkiewicz I, Stockbauer KE, Musser JM. Secreted bacterial phospholipase A2 enzymes: better living through phospholipolysis. Trends Microbiol. 2007;15:63–69. doi: 10.1016/j.tim.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 34.Soutourina O, Kolb A, Krin E, Laurent-Winter C, Rimsky S, Danchin A, Bertin P. Multiple control of flagellum biosynthesis in Escherichia coli: role of H-NS protein and the cyclic AMP-catabolite activator protein complex in transcription of the flhDC master operon. J Bacteriol. 1999;181:7500–7508. doi: 10.1128/jb.181.24.7500-7508.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tam C, Lewis SE, Li WY, Lee E, Evans DJ, Fleiszig SM. Mutation of the phospholipase catalytic domain of the Pseudomonas aeruginosa cytotoxin ExoU abolishes colonization promoting activity and reduces corneal disease severity. Exp Eye Res. 2007;85:799–805. doi: 10.1016/j.exer.2007.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tolker-Nielsen T, Christensen AB, Holmstrom K, Eberl L, Rasmussen TB, Sternberg C, Heydorn A, Molin S, Givskov M. Assessment of flhDC mRNA levels in Serratia liquefaciens swarm cells. J Bacteriol. 2000;182:2680–2686. doi: 10.1128/jb.182.10.2680-2686.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Van Houdt R, Givskov M, Michiels CW. Quorum sensing in Serratia. FEMS Microbiol Rev. 2007;31:407–424. doi: 10.1111/j.1574-6976.2007.00071.x. [DOI] [PubMed] [Google Scholar]
- 38.Watnick PI, Lauriano CM, Klose KE, Croal L, Kolter R. The absence of a flagellum leads to altered colony morphology, biofilm development and virulence in Vibrio cholerae O139. Mol Microbiol. 2001;39:223–235. doi: 10.1046/j.1365-2958.2001.02195.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yokota T, Gots JS. Requirement of adenosine 3′,5′-cyclic phosphate for flagellum formation in Escherichia coli and Salmonella typhimurium. J Bacteriol. 1970;103:513–516. doi: 10.1128/jb.103.2.513-516.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhao K, Liu M, Burgess RR. Adaptation in bacterial flagellar and motility systems: from regulon members to 'foraging'-like behavior in E. coli. Nucleic Acids Research. 2007;35:4441–4452. doi: 10.1093/nar/gkm456. [DOI] [PMC free article] [PubMed] [Google Scholar]