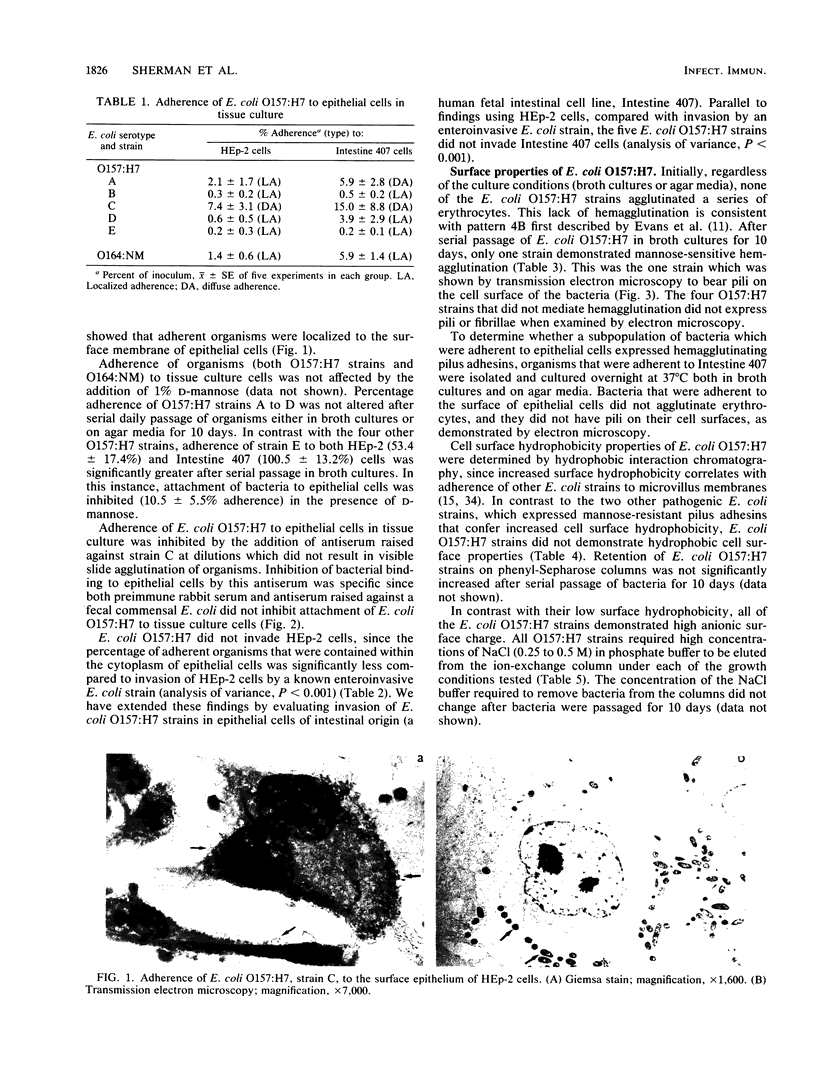
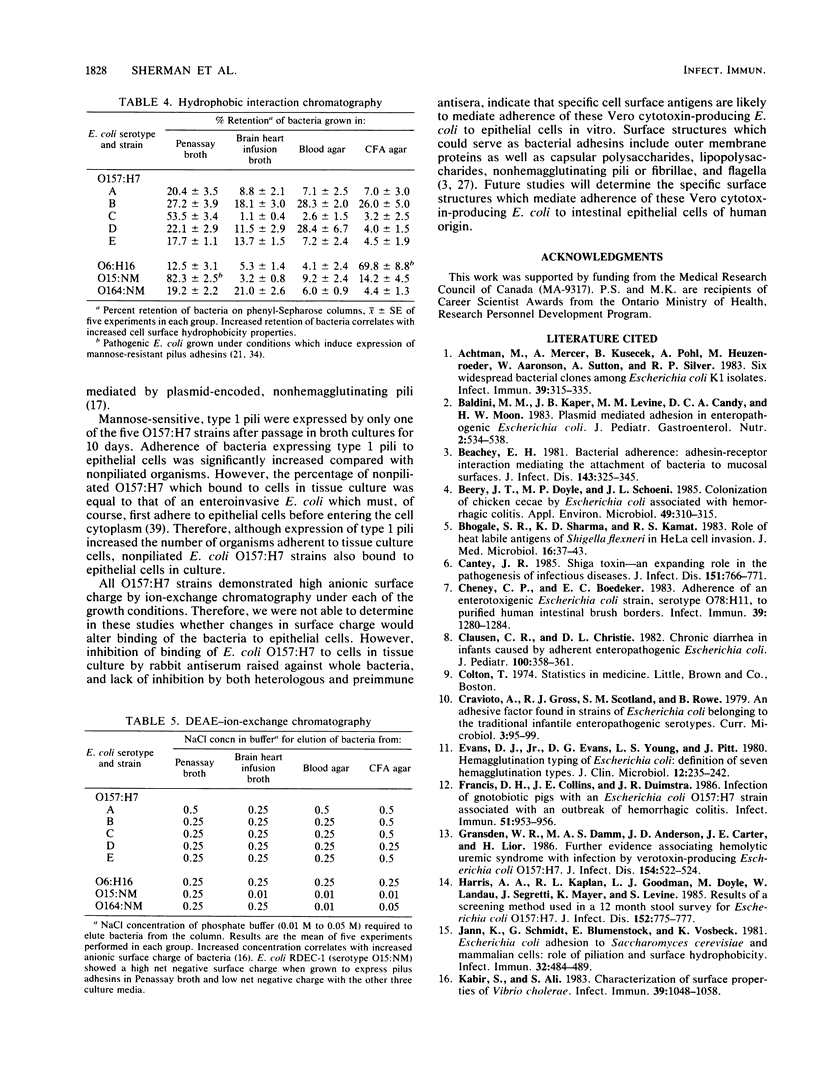
Abstract
Strains of Escherichia coli serotype O157:H7 are Vero cytotoxin-producing enteric pathogens which have been associated with sporadic cases and outbreaks of hemorrhagic colitis and with the hemolytic uremic syndrome in humans. In addition to toxin production, adherence of many pathogenic bacteria to intestinal mucosal surfaces is a critical primary step in the pathogenesis of diarrheal diseases. Although E. coli serotype O157:H7 organisms adhere to intestinal epithelia of orally infected animals in a pattern morphologically identical to that previously described in adherent, effacing E. coli infections, the mechanisms of bacterial adherence are not known. To determine the cell surface adhesins which mediate attachment of E. coli O157:H7 to epithelial surfaces, we evaluated the surface properties of these organisms. Five strains isolated from children with the hemolytic uremic syndrome were grown both in broth cultures and on agar media. Adherence and invasion of E. coli O157:H7 in Intestine 407 and HEp-2 epithelial cell lines was quantitated using an enteroinvasive E. coli strain (serotype O164:NM) as a control. Cell surface properties of E. coli O157:H7 were evaluated by agglutination of a series of erythrocytes, transmission electron microscopy, DEAE-ion-exchange chromatography, and hydrophobic interaction chromatography. E. coli O157:H7 strains adhered to but did not invade either Intestine 407 or HEp-2 cells. Homologous O157:H7 rabbit antiserum blocked attachment of bacteria to tissue culture cells, in contrast to heterologous antiserum and preimmune rabbit serum, which did not inhibit attachment of E. coli O157:H7. None of the five O15:H7 isolates mediated mannose-resistant hemagglutination under any of the in vitro culture conditions. One isolate mediated mannose-sensitive hemagglutination after serial passage in broth cultures. Pili and fibrillae were not visualized by electron microscopy on nonhemagglutinating organisms, but pili were demonstrated on the one isolate which mediated mannose-sensitive hemagglutination. All O157:H7 strains demonstrated high anionic surface charge (DEAE) but low surface hydrophobicity properties (hydrophobic interaction chromatography). The findings suggest that surface structures other than pili can mediate attachment of serotype O157:H7 bacteria to epithelial cells in vitro.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Achtman M., Mercer A., Kusecek B., Pohl A., Heuzenroeder M., Aaronson W., Sutton A., Silver R. P. Six widespread bacterial clones among Escherichia coli K1 isolates. Infect Immun. 1983 Jan;39(1):315–335. doi: 10.1128/iai.39.1.315-335.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldini M. M., Kaper J. B., Levine M. M., Candy D. C., Moon H. W. Plasmid-mediated adhesion in enteropathogenic Escherichia coli. J Pediatr Gastroenterol Nutr. 1983;2(3):534–538. doi: 10.1097/00005176-198302030-00023. [DOI] [PubMed] [Google Scholar]
- Beachey E. H. Bacterial adherence: adhesin-receptor interactions mediating the attachment of bacteria to mucosal surface. J Infect Dis. 1981 Mar;143(3):325–345. doi: 10.1093/infdis/143.3.325. [DOI] [PubMed] [Google Scholar]
- Beery J. T., Doyle M. P., Schoeni J. L. Colonization of chicken cecae by Escherichia coli associated with hemorrhagic colitis. Appl Environ Microbiol. 1985 Feb;49(2):310–315. doi: 10.1128/aem.49.2.310-315.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhogale S. R., Sharma K. D., Kamat R. S. Role of heat labile antigens of Shigella flexneri in HeLa cell invasion. J Med Microbiol. 1983 Feb;16(1):37–43. doi: 10.1099/00222615-16-1-37. [DOI] [PubMed] [Google Scholar]
- Cantey J. R. Shiga toxin--an expanding role in the pathogenesis of infectious diseases. J Infect Dis. 1985 May;151(5):766–771. doi: 10.1093/infdis/151.5.766. [DOI] [PubMed] [Google Scholar]
- Cheney C. P., Boedeker E. C. Adherence of an enterotoxigenic Escherichia coli strain, serotype O78:H11, to purified human intestinal brush borders. Infect Immun. 1983 Mar;39(3):1280–1284. doi: 10.1128/iai.39.3.1280-1284.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clausen C. R., Christie D. L. Chronic diarrhea in infants caused by adherent enteropathogenic Escherichia coli. J Pediatr. 1982 Mar;100(3):358–361. doi: 10.1016/s0022-3476(82)80429-0. [DOI] [PubMed] [Google Scholar]
- Evans D. J., Jr, Evans D. G., Young L. S., Pitt J. Hemagglutination typing of Escherichia coli: definition of seven hemagglutination types. J Clin Microbiol. 1980 Aug;12(2):235–242. doi: 10.1128/jcm.12.2.235-242.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis D. H., Collins J. E., Duimstra J. R. Infection of gnotobiotic pigs with an Escherichia coli O157:H7 strain associated with an outbreak of hemorrhagic colitis. Infect Immun. 1986 Mar;51(3):953–956. doi: 10.1128/iai.51.3.953-956.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gransden W. R., Damm M. A., Anderson J. D., Carter J. E., Lior H. Further evidence associating hemolytic uremic syndrome with infection by Verotoxin-producing Escherichia coli O157:H7. J Infect Dis. 1986 Sep;154(3):522–524. doi: 10.1093/infdis/154.3.522. [DOI] [PubMed] [Google Scholar]
- Harris A. A., Kaplan R. L., Goodman L. J., Doyle M., Landau W., Segreti J., Mayer K., Levin S. Results of a screening method used in a 12-month stool survey for Escherichia coli O157:H7. J Infect Dis. 1985 Oct;152(4):775–777. doi: 10.1093/infdis/152.4.775. [DOI] [PubMed] [Google Scholar]
- Jann K., Schmidt G., Blumenstock E., Vosbeck K. Escherichia coli adhesion to Saccharomyces cerevisiae and mammalian cells: role of piliation and surface hydrophobicity. Infect Immun. 1981 May;32(2):484–489. doi: 10.1128/iai.32.2.484-489.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabir S., Ali S. Characterization of surface properties of Vibrio cholerae. Infect Immun. 1983 Mar;39(3):1048–1058. doi: 10.1128/iai.39.3.1048-1058.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karch H., Heesemann J., Laufs R., O'Brien A. D., Tacket C. O., Levine M. M. A plasmid of enterohemorrhagic Escherichia coli O157:H7 is required for expression of a new fimbrial antigen and for adhesion to epithelial cells. Infect Immun. 1987 Feb;55(2):455–461. doi: 10.1128/iai.55.2.455-461.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karmali M. A., Petric M., Lim C., Fleming P. C., Arbus G. S., Lior H. The association between idiopathic hemolytic uremic syndrome and infection by verotoxin-producing Escherichia coli. J Infect Dis. 1985 May;151(5):775–782. doi: 10.1093/infdis/151.5.775. [DOI] [PubMed] [Google Scholar]
- Klemm P. Fimbrial adhesions of Escherichia coli. Rev Infect Dis. 1985 May-Jun;7(3):321–340. doi: 10.1093/clinids/7.3.321. [DOI] [PubMed] [Google Scholar]
- Levine M. M., Nataro J. P., Karch H., Baldini M. M., Kaper J. B., Black R. E., Clements M. L., O'Brien A. D. The diarrheal response of humans to some classic serotypes of enteropathogenic Escherichia coli is dependent on a plasmid encoding an enteroadhesiveness factor. J Infect Dis. 1985 Sep;152(3):550–559. doi: 10.1093/infdis/152.3.550. [DOI] [PubMed] [Google Scholar]
- Levine M. M., Ristaino P., Marley G., Smyth C., Knutton S., Boedeker E., Black R., Young C., Clements M. L., Cheney C. Coli surface antigens 1 and 3 of colonization factor antigen II-positive enterotoxigenic Escherichia coli: morphology, purification, and immune responses in humans. Infect Immun. 1984 May;44(2):409–420. doi: 10.1128/iai.44.2.409-420.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques L. R., Moore M. A., Wells J. G., Wachsmuth I. K., O'Brien A. D. Production of Shiga-like toxin by Escherichia coli. J Infect Dis. 1986 Aug;154(2):338–341. doi: 10.1093/infdis/154.2.338. [DOI] [PubMed] [Google Scholar]
- Moxley R. A., Francis D. H. Natural and experimental infection with an attaching and effacing strain of Escherichia coli in calves. Infect Immun. 1986 Aug;53(2):339–346. doi: 10.1128/iai.53.2.339-346.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Old D. C., Duguid J. P. Selective outgrowth of fimbriate bacteria in static liquid medium. J Bacteriol. 1970 Aug;103(2):447–456. doi: 10.1128/jb.103.2.447-456.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pai C. H., Gordon R., Sims H. V., Bryan L. E. Sporadic cases of hemorrhagic colitis associated with Escherichia coli O157:H7. Clinical, epidemiologic, and bacteriologic features. Ann Intern Med. 1984 Dec;101(6):738–742. doi: 10.7326/0003-4819-101-6-738. [DOI] [PubMed] [Google Scholar]
- Pai C. H., Kelly J. K., Meyers G. L. Experimental infection of infant rabbits with verotoxin-producing Escherichia coli. Infect Immun. 1986 Jan;51(1):16–23. doi: 10.1128/iai.51.1.16-23.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson P. K., Quie P. G. Bacterial surface components and the pathogenesis of infectious diseases. Annu Rev Med. 1981;32:29–43. doi: 10.1146/annurev.me.32.020181.000333. [DOI] [PubMed] [Google Scholar]
- Potter M. E., Kaufmann A. F., Thomason B. M., Blake P. A., Farmer J. J., 3rd Diarrhea due to Escherichia coli O157:H7 in the infant rabbit. J Infect Dis. 1985 Dec;152(6):1341–1343. doi: 10.1093/infdis/152.6.1341. [DOI] [PubMed] [Google Scholar]
- Remis R. S., MacDonald K. L., Riley L. W., Puhr N. D., Wells J. G., Davis B. R., Blake P. A., Cohen M. L. Sporadic cases of hemorrhagic colitis associated with Escherichia coli O157:H7. Ann Intern Med. 1984 Nov;101(5):624–626. doi: 10.7326/0003-4819-101-5-624. [DOI] [PubMed] [Google Scholar]
- Riley L. W., Remis R. S., Helgerson S. D., McGee H. B., Wells J. G., Davis B. R., Hebert R. J., Olcott E. S., Johnson L. M., Hargrett N. T. Hemorrhagic colitis associated with a rare Escherichia coli serotype. N Engl J Med. 1983 Mar 24;308(12):681–685. doi: 10.1056/NEJM198303243081203. [DOI] [PubMed] [Google Scholar]
- Rothbaum R., McAdams A. J., Giannella R., Partin J. C. A clinicopathologic study of enterocyte-adherent Escherichia coli: a cause of protracted diarrhea in infants. Gastroenterology. 1982 Aug;83(2):441–454. [PubMed] [Google Scholar]
- Scaletsky I. C., Silva M. L., Trabulsi L. R. Distinctive patterns of adherence of enteropathogenic Escherichia coli to HeLa cells. Infect Immun. 1984 Aug;45(2):534–536. doi: 10.1128/iai.45.2.534-536.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman P. M., Houston W. L., Boedeker E. C. Functional heterogeneity of intestinal Escherichia coli strains expressing type 1 somatic pili (fimbriae): assessment of bacterial adherence to intestinal membranes and surface hydrophobicity. Infect Immun. 1985 Sep;49(3):797–804. doi: 10.1128/iai.49.3.797-804.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spika J. S., Parsons J. E., Nordenberg D., Wells J. G., Gunn R. A., Blake P. A. Hemolytic uremic syndrome and diarrhea associated with Escherichia coli O157:H7 in a day care center. J Pediatr. 1986 Aug;109(2):287–291. doi: 10.1016/s0022-3476(86)80386-9. [DOI] [PubMed] [Google Scholar]
- Takeuchi A., Inman L. R., O'Hanley P. D., Cantey J. R., Lushbaugh W. B. Scanning and transmission electron microscopic study of Escherichia coli O15 (RDEC-1) enteric infection in rabbits. Infect Immun. 1978 Feb;19(2):686–694. doi: 10.1128/iai.19.2.686-694.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavendale A., Old D. C. Haemagglutinins and adhesion of Escherichia coli to HEp2 epithelial cells. J Med Microbiol. 1985 Dec;20(3):345–353. doi: 10.1099/00222615-20-3-345. [DOI] [PubMed] [Google Scholar]
- Wells J. G., Davis B. R., Wachsmuth I. K., Riley L. W., Remis R. S., Sokolow R., Morris G. K. Laboratory investigation of hemorrhagic colitis outbreaks associated with a rare Escherichia coli serotype. J Clin Microbiol. 1983 Sep;18(3):512–520. doi: 10.1128/jcm.18.3.512-520.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams P. H., Knutton S., Brown M. G., Candy D. C., McNeish A. S. Characterization of nonfimbrial mannose-resistant protein hemagglutinins of two Escherichia coli strains isolated from infants with enteritis. Infect Immun. 1984 Jun;44(3):592–598. doi: 10.1128/iai.44.3.592-598.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]