Summary
Hypochlorous acid (HOCl), the active ingredient of household bleach, is an effective antimicrobial produced by the mammalian host defense to kill invading microorganisms. Despite the widespread use of HOCl, surprisingly little is known about its mode of action. In this study we demonstrate that low molar ratios of HOCl to protein cause oxidative protein unfolding in vitro and target thermolabile proteins for irreversible aggregation in vivo. As a defense mechanism, bacteria employ the redox-regulated chaperone Hsp33, which responds to bleach treatment with the reversible oxidative unfolding of its C-terminal redox switch domain. HOCl-mediated unfolding turns inactive Hsp33 into a highly active chaperone holdase, which protects essential E. coli proteins against HOCl-induced aggregation and increases bacterial HOCl-resistance. Our results substantially improve our molecular understanding about HOCl’s functional mechanism. They suggest that the antimicrobial effects of bleach are largely based on HOCl’s ability to cause aggregation of essential bacterial proteins.
Introduction
The accumulation of reactive oxygen species (ROS), a condition termed oxidative stress, is associated with a variety of different human diseases (Aliev et al., 2002). ROS also serve beneficial roles for mammals during host defense, when macrophages and neutrophils produce high concentrations of hydrogen peroxide (H2O2), superoxide (O2−) and hypochlorous acid (HOCl) to kill invading microorganisms (Miller and Britigan, 1997).
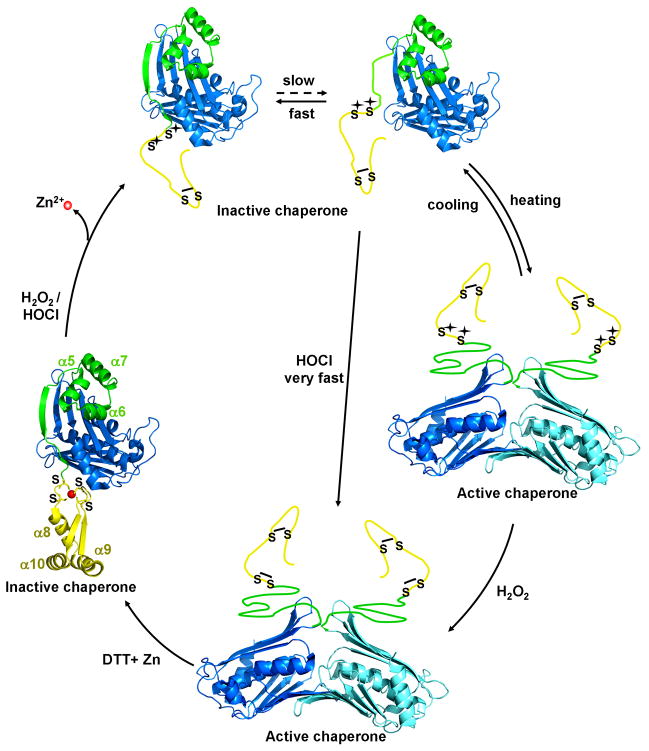
The heat shock protein Hsp33 is a member of a highly conserved family of molecular chaperones, which specifically protects bacteria against the lethal consequences of oxidative stress at elevated temperatures (i.e., oxidative heat stress) (Winter et al., 2005). Recent in vitro studies confirmed that Hsp33 utilizes a dual stress sensing mechanism, which responds to the simultaneous presence of oxidants such as H2O2 and mild protein unfolding conditions (e.g., elevated temperatures, 1 M guanidinium-hydrochloride) with the activation of its chaperone function (Ilbert et al., 2007). H2O2-sensing occurs through a four-cysteine zinc center while elevated temperatures are sensed by an adjacent linker region, whose conformation appears to control the reactivity or accessibility of two of Hsp33’s four redox-active cysteines, Cys232 and Cys234 (Ilbert et al., 2007; Leichert et al., 2008). Exposure of Hsp33 to oxidative heat stress leads to the consecutive formation of both intramolecular disulfide bonds and zinc(II) release (Graumann et al., 2001). These posttranslational modifications cause Hsp33’s linker region and zinc binding domain to adopt a natively unfolded structure (Graf et al., 2004; Ilbert et al., 2007). Large hydrophobic surfaces, which are the likely binding sites for unfolded proteins, become exposed and Hsp33 assembles into the dimeric, fully active chaperone (Graf et al., 2004; Ilbert et al., 2007).
Hsp33’s activation requires both peroxide stress and heat stress while neither peroxide treatment alone nor high temperatures alone cause the activation of Hsp33 in vitro or in vivo (Ilbert et al., 2007; Winter et al., 2005). This makes good physiological sense; peroxide stress itself does not cause protein unfolding in vivo and therefore does not require additional chaperones (Ilbert et al., 2007) while protein unfolding induced by elevated temperatures is effectively prevented by ATP-dependent chaperones such as the DnaK-system (Mayer et al., 2001). Oxidative heat stress, however, causes protein unfolding and simultaneously decreases cellular ATP-levels, which incapacitates ATP-dependent chaperones (Winter et al., 2005). Activation of Hsp33’s ATP-independent chaperone function under these specific stress conditions appears therefore to compensate for the functional loss of ATP-dependent chaperones.
These findings helped explain the cytoprotective effects of Hsp33 under oxidative heat stress conditions. However, these results also raised the questions as to when and how often organisms encounter conditions that cause oxidative stress conditions that lead to protein unfolding in nature. In this study we identified HOCl, the active ingredient of household bleach, as a reagent that rapidly induces oxidative protein unfolding in vitro and causes irreversible protein aggregation in vivo. This capacity likely contributes to the highly bactericidal effect of HOCl. Hsp33 exploits these features and becomes rapidly activated by the oxidative unfolding of its redox switch domain. It can then effectively protect numerous essential bacterial proteins against HOCl-mediated aggregation and E. coli and Vibrio cholerae cells against HOCl-induced cell death.
Results
Bleach – A potent activator of Hsp33’s chaperone function
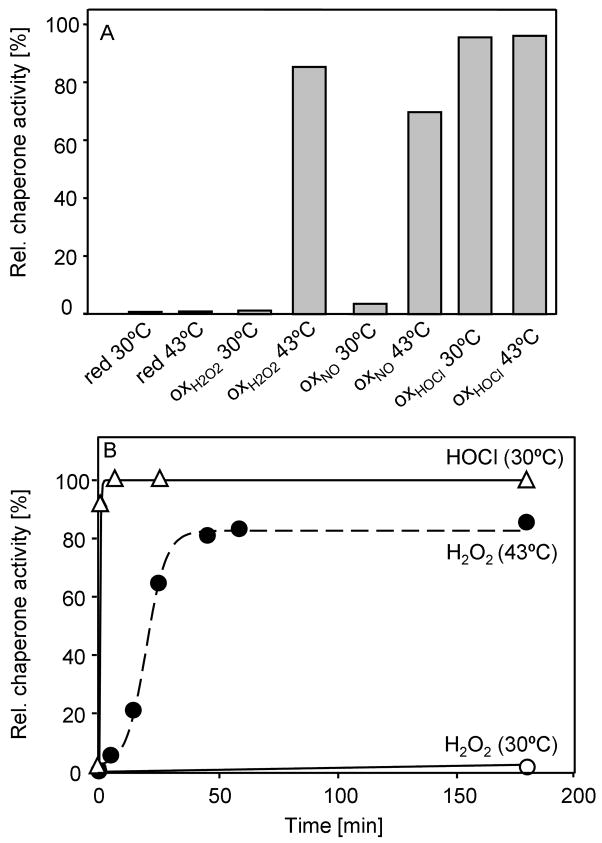
The predominant source of exogenous oxidative and nitrosative stress for pathogenic bacteria is generated by cells of the innate immune system, which release high concentrations of ROS into the phagosome to kill the engulfed bacteria (Miller and Britigan, 1997). One protein that has been shown to protect microorganisms against H2O2-mediated oxidative stress is the highly conserved redox-regulated chaperone Hsp33 (Winter et al., 2005). This protection is, however, restricted to peroxide stress at heat shock temperatures. To investigate what physiologically relevant reactive oxygen or nitrogen species might cause the activation of Hsp33 also at non-stress temperatures, we incubated reduced, inactive Hsp33 (Hsp33red) with either H2O2, hypochlorous acid (HOCl) or the nitric oxide-donor DEANO at both 30°C or 43°C and analyzed Hsp33’s functional activity as a molecular chaperone. As shown previously, H2O2 only significantly activated Hsp33 when the treatment was performed at elevated temperatures (Fig. 1A) (Ilbert et al., 2007). Simultaneous presence of oxidants and mild unfolding conditions appear to be required for the formation of Hsp33’s second disulfide bond between Cys232 and Cys234, which has been suggested to be the rate-determining step in Hsp33’s activation process (Leichert et al., 2008). Similar results were obtained upon incubation of Hsp33red with DEANO, which caused significant activation at 43°C but not at 30°C (Fig. 1A). This result indicated that DEANO is, like H2O2, unable to activate Hsp33 unless when combined with protein unfolding conditions. In contrast, however, treatment of Hsp33 with HOCl fully activated Hsp33 at both 30°C and 43°C. These results suggested that HOCl is an oxidant that might alleviate Hsp33’s need for elevated temperatures during its activation process.
Figure 1. HOCl – A Potent Activator of Hsp33’s Chaperone Function.
A. 50 μM Hsp33red was incubated in the presence of 2 mM H2O2, 2 mM DEANO or 500 μM HOCl at either 30°C or 43°C. After 20 min (HOCl) or 3 hours (DEANO, H2O2) of incubation, the oxidants were removed and the influence of Hsp33 (0.3 μM) on the aggregation of 75 nM chemically denatured citrate synthase (CS) was determined at 30°C. The light scattering signal of CS in the absence of Hsp33 was defined as 0% while complete suppression of CS aggregation was set to 100%.
B. 50 μM Hsp33red was incubated with 2 mM H2O2 (open circles) or 500 μM HOCl (open triangles) at 30°C or with 2 mM H2O2 at 43°C (filled circles). At various time points, aliquots were removed and the influence of Hsp33 on the aggregation of CS was determined as described above. Activation of Hsp33red with 500 μM HOCl at 43°C was completed within the mixing time of the experiment.
To determine the minimal HOCl concentration that can effectively activate Hsp33 in vitro and possibly in vivo, we incubated various concentrations of Hsp33red (5 – 50 μM) with increasing concentrations of HOCl and tested its chaperone activity (Supplemental Fig. 1A, data not shown). We found that independent of the absolute HOCl concentration, a 10:1 molar ratio of HOCl to Hsp33 was sufficient to activate Hsp33. To investigate how rapidly HOCl activates Hsp33 in vitro, we then incubated Hsp33red with a 10-fold molar excess of HOCl at various temperatures and determined the activation kinetics of Hsp33 (Fig. 1B). At both 43°C and 30°C, the HOCl-mediated activation of Hsp33 was completed within the mixing time of the experiment (T1/2 <1 min). This result was in stark contrast to the activation of Hsp33 in H2O2, which proceeded with an apparent half time of more than 20 min at 43°C and reached less than 10% final activity upon incubation at 30°C. Moreover, while equally fast activation kinetics were obtained when 10-fold lower concentrations of both Hsp33 and HOCl were used (i.e., 5 μM Hsp33 and 50 μM HOCl) (data not shown), the rate of H2O2–mediated activation at 43°C was strictly dependent on the absolute H2O2 concentration (Supplemental Figure 1B). Further reduction of the incubation temperature decreased HOCl-mediated activation kinetics but even at 5°C, Hsp33 was still found to be fully activated within 60 min of incubation (data not shown). These results revealed that HOCl is a very potent activator of Hsp33 that rapidly converts reduced, inactive Hsp33 into its oxidized, active conformation without the additional need of elevated temperatures or chemical denaturants.
HOCl causes oxidative unfolding of Hsp33’s redox switch domain
To begin to understand how HOCl-induced activation of Hsp33 can occur so rapidly even under non-stress temperatures, we monitored the redox status of the two proximal cysteines Cys232 and Cys234, whose formation appears to be rate-limiting for Hsp33’s activation. We used our recently developed OxICAT technique to precisely determine the kinetics of oxidative thiol modifications at distinct cysteines in proteins (Leichert et al., 2008). OxICAT is based on a differential thiol modification principle, in which reduced cysteines in a protein are labeled with the isotopically light 12C-ICAT reagent while oxidized cysteines are, upon their reduction, labelled with the 9 Da heavier, isotopically heavy 13C-ICAT reagent. Upon peptide digestion, MS and MS/MS analysis are conducted. Peptides that contained originally oxidized cysteines will be labelled with 13C-ICAT and will reveal mass peaks that are exactly 9 Da or multiples of 9 Da higher than the corresponding peptides that harbored originally reduced cysteines. Because the peptides are chemically identical, the precise ratios of reduced and oxidized peptide species can then be determined from the height of the respective mass peaks (Leichert et al., 2008).
We compared the mass spectra of Hsp33’s tryptic peptide aa232-236 containing Cys232 and Cys234 in OxICAT-treated Hsp33red and Hsp33 that was treated with a 10-fold molar excess of HOCl for either 2 min (Fig. 2A) or 20 min (data not shown) at 30°C. In Hsp33red, the majority of peptide aa232-236 was labelled with two light 12C-ICAT molecules (m/zobs = 1023.46), confirming that both thiol groups are reduced (Fig. 2A). Within 2 min of incubation in HOCl at 30°C, however, we observed a near complete shift of this peptide to the 18 Da heavier form (m/zobs = 1041.55) (Fig. 2A). In contrast, an approximately 60 min incubation in 2 mM H2O2 at 43°C was necessary to achieve a similar shift (Leichert et al., 2008). These results demonstrated that HOCl is indeed capable of rapidly oxidizing the two crucial cysteines in Hsp33 even under non-heat shock conditions. Because the ICAT-labelled peptide containing the second pair of cysteines (Cys265/Cys268) is not detectable in the MS-analysis (Leichert et al., 2008), we performed thiol trapping experiments using iodoacetamide combined with tryptic digest and MS/MS analysis to analyze their thiol status. As expected, we found that the cysteines are fully oxidized in both H2O2 and HOCl-treated Hsp33 (data not shown). In agreement with these results, electrospray mass spectrometry (ESI-MS) of full-length non-labelled Hsp33HOCl showed a predominant mass peak at 32,774.6 +/− 3 Da, which is consistent with the expected molecular mass of 32,774.8 for Hsp33 containing two intramolecular disulfide bonds (Fig. 2B). These results confirmed that HOCl-treatment of Hsp33 at non-stress temperatures induces the formation of both disulfide bonds in Hsp33 and activates the chaperone.
Figure 2. HOCl - Activation by Disulfide Bond Formation.
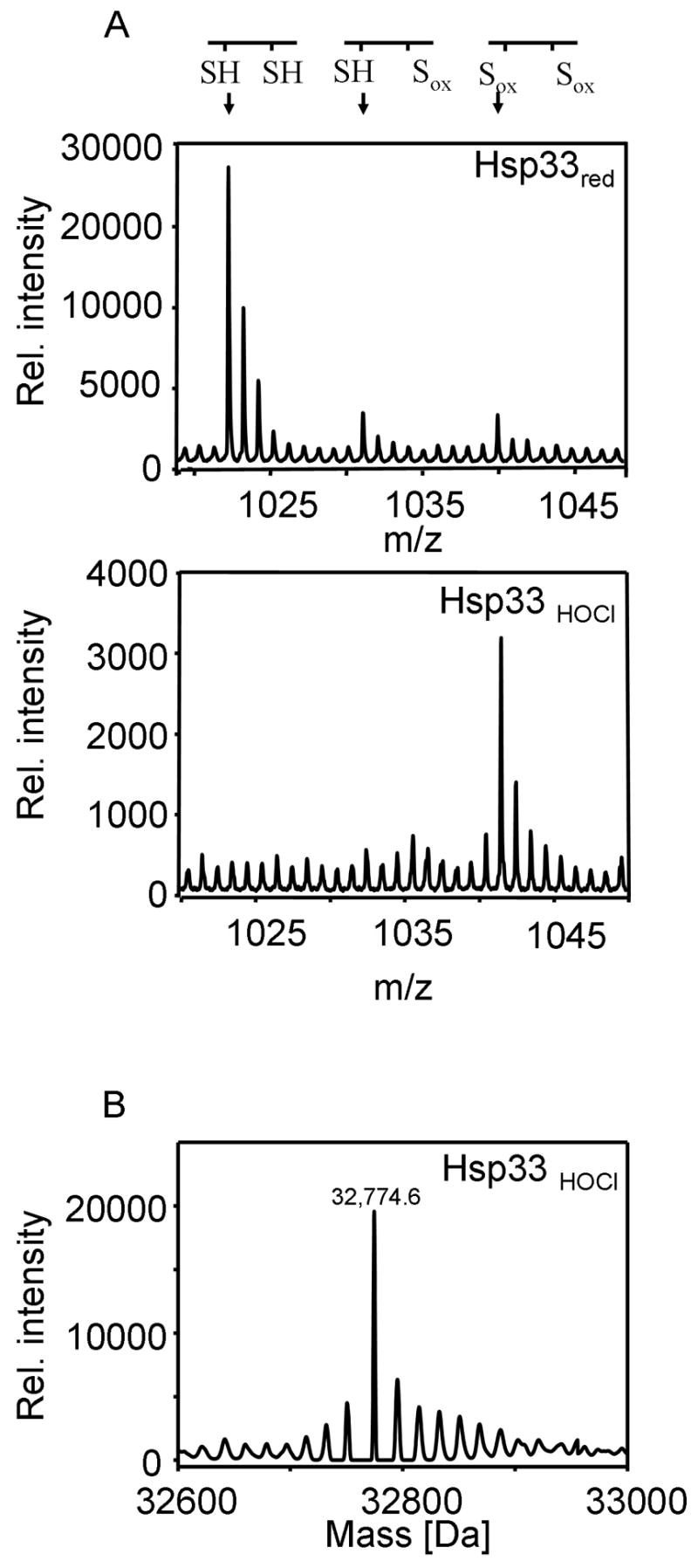
A. The thiol status of tryptic peptide aa232-236 of Hsp33red or Hsp33HOCl that was oxidized for 2 min with a 10-fold molar excess of HOCl was analyzed using OxICAT. The aa232-236 peptide has an expected m/z-value of 1023.46 in the reduced form, a 9 Da higher m/z-value of 1032.50 in the partially oxidized and an 18 Da higher m/z-value of 1041.55 in the fully oxidized form. B. Nano-ESI-MS of Hsp33HOCl after 20 min of oxidation using a 10-fold molar excess of HOCl. The deconvoluted mass spectrum reveals a predominant mass peak at 32,774.6 +/− 2 Da. Very similar mass spectra were obtained for Hsp33HOCl that was oxidized for 2 min instead.
To assess changes in the secondary structure and hydrophobicity of Hsp33HOCl, circular dichroism (CD) spectroscopy and bis-ANS binding studies were performed. We found that Hsp33HOCl showed major structural rearrangements and exposure of hydrophobic surfaces, which are very similar to Hsp33 that was activated by H2O2 at 43°C (Hsp33 H2O2−43°C) (Supplemental Figure 2A, B). Analytical ultracentrifugation sedimentation velocity experiments further agreed with these findings and revealed that Hsp33HOCl sediments in a monomer-dimer equilibrium with a Kd for dimerization of 0.57 μM (data not shown). This is nearly identical to the previously determined Kd of Hsp33H2O2−43°C (Kd = 0.59 μM) (Graumann et al., 2001). These studies contradicted our previous model, which proposed that Hsp33’s dimerization process might be the basis for Hsp33’s unusual temperature dependence (Graumann et al., 2001). Instead they demonstrated that once disulfide bond formation occurred, native unfolding of Hsp33’s C-terminus, dimerization and activation of Hsp33 can take place also at low temperatures.
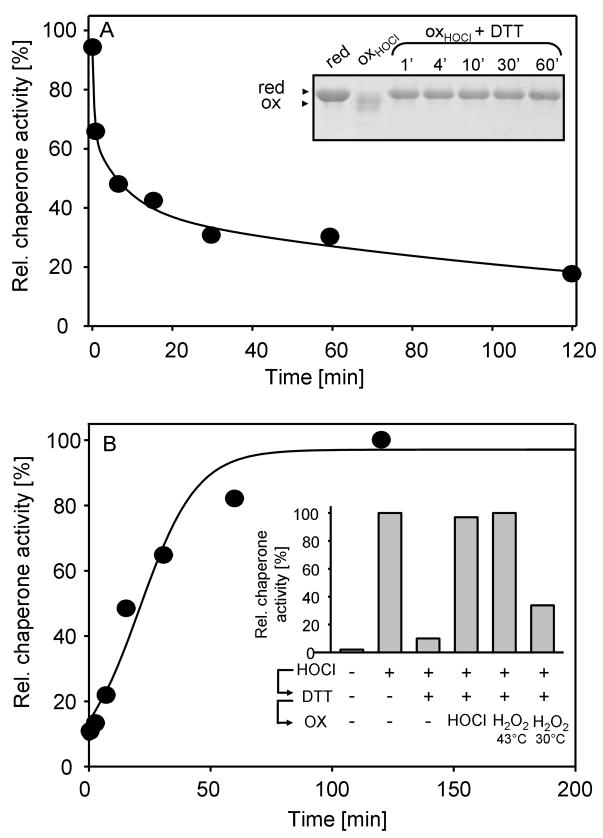
HOCl-induced activation of Hsp33 is reversible by thiol reductants in vitro
In vitro activation of Hsp33 has so far only been successful for Hsp33 preparations that have been simultaneously treated with thiol oxidants and protein unfolding conditions (Ilbert et al., 2007). To investigate whether HOCl-mediated activation of Hsp33 proceeds via thiol oxidation that is directly thermodynamically linked to protein unfolding or whether it involves additional side chain modifications that lower Hsp33’s overall stability, we tested the DTT-reversibility of Hsp33’s activation. In contrast to oxidative modifications of non-thiol containing amino acids, which are either irreversible (i.e., dityrosine) or require specialized reductants (i.e., methionine sulfoxide) (Boschi-Muller et al., 2008), most oxidative thiol modifications such as disulfide bonds are fully reversible upon treatment with thiol reductants like dithiothreitol (DTT). We, therefore, incubated Hsp33HOCl with DTT at 30°C and tested its thiol status and chaperone activity over time. Incubation with DTT rapidly restored the reduced thiol status of Hsp33 (Fig. 3A, inset), and caused the time-dependent inactivation of Hsp33’s chaperone function (Fig. 3A). That thiol reduction precedes the rather slow inactivation of Hsp33’s chaperone function has previously also been observed with H2O2-activated Hsp33 and is therefore not specific for HOCl-activated Hsp33 (Hoffmann et al., 2004). Although it is known that inactivation of Hsp33 involves the formation of reduced kinetically stable Hsp33 dimers with high chaperone activity, the precise mechanism of Hsp33’s inactivation remains still to be elucidated. In either case, our finding that thiol-specific reductants inactivate Hsp33HOCl suggested that thiol modifications are indeed integral to the HOCl-mediated rapid activation of Hsp33. This result also agreed well with our ESI-analysis of activated Hsp33HOCl, which revealed only a minor fraction of oxidized Hsp33HOCl molecules with stable non-thiol modifications even after 20 min of incubation in HOCl (Fig. 2B). To further exclude, however, that non-thiol modifications that are potentially unstable in ESI-MS are involved in the rapid HOCl-mediated activation of Hsp33, we then analyzed the re-activation of Hsp33 upon its HOCl-mediated oxidation and subsequent reduction (Hsp33HOCL⇒DTT). The rationale was that any relevant non-thiol modification should be maintained during the DTT-treatment and would be expected to influence the re-activation kinetics of Hsp33HOCL⇒DTT. Incubation of inactivated Hsp33HOCL⇒DTT with HOCl caused full re-activation of Hsp33 within the mixing time of the experiment (data not shown). Re-activation with H2O2, on the other hand, was again strongly dependent on the environmental temperature (Fig. 3B, inset) and proceeded with kinetics that were very similar to the activation kinetics observed with freshly purified and reduced Hsp33red at 43°C (compare Fig. 3B with Fig. 1B). Reactivation at 30°C was again very slow and incomplete although slightly higher reactivation yields were obtained after 3 hours of incubation when Hsp33HOCL⇒DTT was used instead of freshly reduced Hsp33. These differences in yield most likely correspond to the minor fraction of Hsp33HOCl molecules that were irreversibly modified by HOCl. These results ruled out the possibility that HOCl-mediated oxidative modification of critical side chains other than the four active site thiols are involved in the HOCl-mediated activation mechanism of Hsp33. We therefore concluded that HOCl functions primarily as a thiol oxidant in Hsp33’s activation process in vitro and that disulfide bond formation and domain unfolding are thermodynamically linked.
Figure 3. HOCl-Mediated Activation of Hsp33 is Reversible.
A. 5 μM Hsp33HOCl was incubated with 5 mM DTT and 5 μM zinc at 30°C. At defined time points, aliquots of the Hsp33HOCl ⇒DTT preparation were removed and tested for either chaperone activity (filled circles) as described in Fig. 1 or (inset) TCA-precipitated to conduct AMS thiol trapping experiments. AMS is a 490 Da thiol-specific molecule, which covalently modifies free thiol groups. Differences in the thiol oxidation status are reflected by mobility differences of the proteins on 14% SDS-PAGE.
B. 50 μM inactivated Hsp33HOCl⇒DTT was incubated in the presence of 2 mM H2O2 at 43°C. Aliquots were removed at various time points and the influence of Hsp33 on the aggregation of CS was determined as described in the legend of Fig. 1. Inset: 50 μM inactive Hsp33red was activated with HOCl, subsequently inactivated with DTT and re-oxidized with either 500 μM HOCl at 30°C or 2 mM H2O2 at 43°C or 30°C. Activity measurements were performed after 3 hours incubation as described above.
HOCl functions as general protein unfolding reagent in vitro
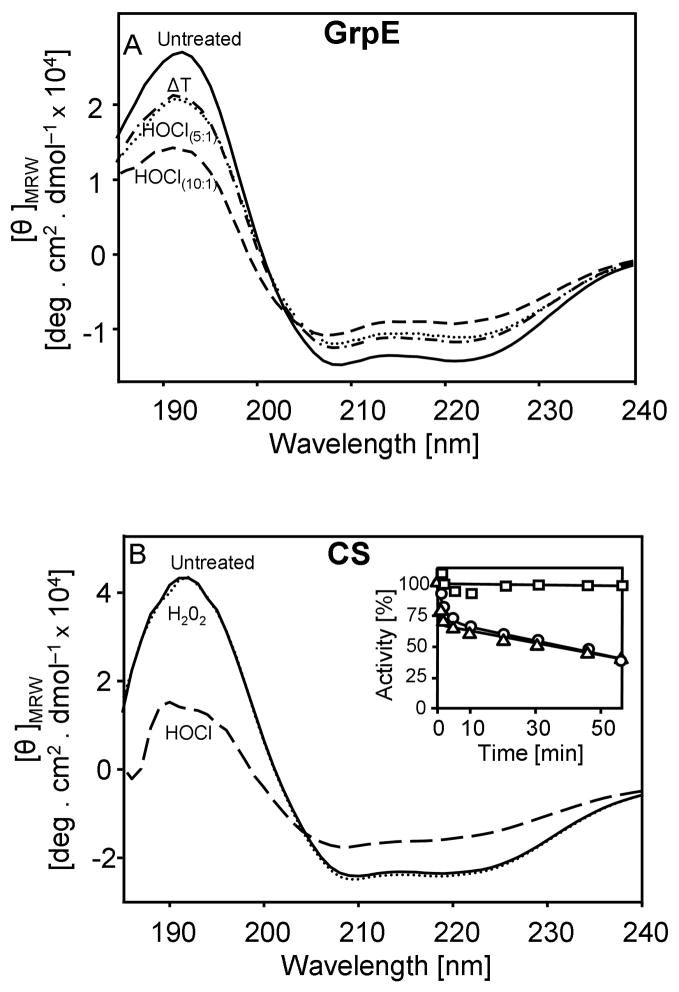
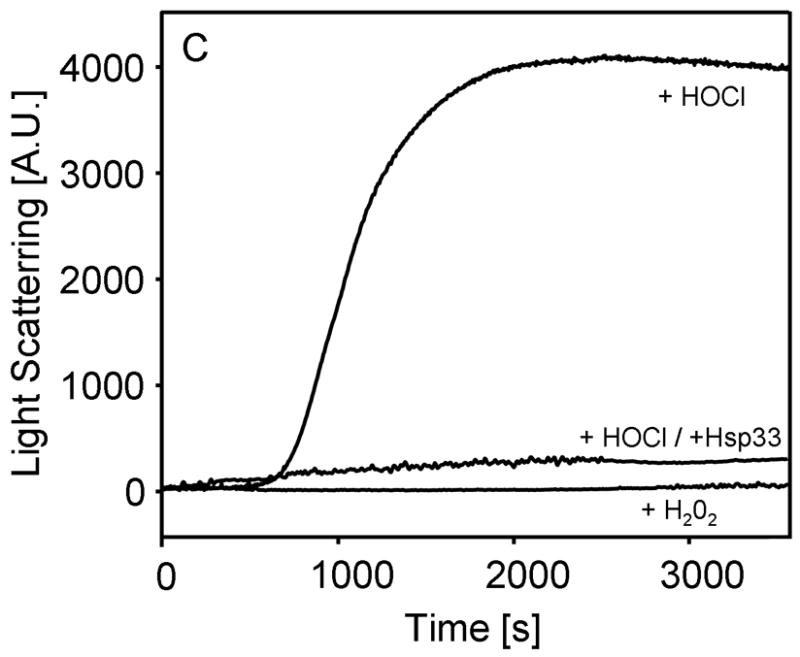
Although the effect of HOCl on purified proteins has been previously studied, most research focused on the nature of HOCl-mediated side chain modifications in vitro (Hawkins et al., 2003; Winterbourn and Hampton, 2008). Few studies investigated the influence of HOCl-treatment on protein activity, and only a very select number of investigations dealt with the potential influence of low concentrations of HOCl on the folding status of proteins (Hawkins and Davies, 2005; Jayaraman et al., 2008). Our results indicated that low HOCl to protein ratios cause the unfolding of Hsp33 in vitro. To evaluate whether this is Hsp33 specific or might represent a general effect of HOCl, we tested the influence of HOCl on the secondary structure of three unrelated proteins; the heat shock protein GrpE, the disulfide oxidoreductase DsbA and the chaperone substrate protein citrate synthase (CS). Short-term incubation (5–10 min) of both GrpE and DsbA in a 10-fold molar excess of HOCl caused a partial loss in the secondary structure of both proteins (Fig. 4A, data not shown). Similar results were observed with CS albeit higher HOCl to protein ratios (>50:1) were required to induce protein unfolding (Fig. 4B). This, however, is probably due to residual amounts of the Tris-containing storage buffer of CS, which, as tertiary amine, will rapidly quench HOCl. Mass spectrometric analysis of HOCl-treated proteins revealed oxidative side chain modifications including methionine oxidation. Therefore not surprisingly, incubation with thiol reductants was insufficient to restore the original protein conformation, suggesting that the oxidative modifications are either irreversible or require specialized reducing systems such as methionine sulfoxide reductases.
Figure 4. HOCl Induces Protein Unfolding and Aggregation in vitro.
A. Far UV-CD spectra of 10 μM untreated GrpE (solid line), GrpE pre-treated with a 5–fold molar excess of HOCl (dotted line) or pre-treated with a 10–fold molar excess of HOCl (dashed line) were recorded at 30°C. To monitor heat shock-induced structural changes, spectra of untreated GrpE were recorded at 45°C (ΔT) (dashed and dotted line).
B. Far-UV CD spectra of CS (3 μM) in the absence of oxidants (solid line) or after 30 min incubation in the presence of 450 μM H2O2 (dotted line) or 450 μM HOCl (dashed line) were recorded at 30°C. Inset: To test the influence of oxidants on CS activity, 3 μM CS was incubated with 450 μM H2O2 (square) or 450 μM HOCl either in the absence (triangles) or presence (circle) of 3 μM Hsp33HOCl. Aliquots were taken and CS activity was monitored.
C. Light scattering measurements of CS (3 μM) were performed with 450 μM H2O2 or 450 μM HOCl in the absence of presence of 3 μM Hsp33HOCl.
Interestingly, incubation of GrpE with low ratios of HOCl to protein (5:1) resulted in conformational changes that resembled those observed in heat shock treated GrpE (Fig. 4A). At elevated temperatures, GrpE’s thermolabile sensor region unfolds and enables GrpE to maintain the DnaK-DnaJ-GrpE complex in a high affinity binding state (Grimshaw et al., 2003). Our results suggested that low concentrations of HOCl are sufficient to target amino acids, whose side chain modifications like in the case of Hsp33 will shift the equilibrium of thermolabile regions towards the unfolded state. We concluded from these results that HOCl has indeed general protein-unfolding properties, presumably by oxidatively modifying critical side chains in proteins.
Incubation of thermolabile CS with HOCl led to the irreversible unfolding and the gradual inactivation of the protein (Fig. 4B, inset). Light scattering measurements of HOCl-treated CS revealed that this unfolding leads to the massive aggregation of the protein (Fig. 4C). In sharp contrast, incubation of CS with other physiological oxidants such as H2O2 did not change the secondary structure of CS or its activity (Fig. 4B) and did not cause any detectable protein aggregation (Fig. 4C). This result agreed well with in vivo studies, which revealed no detectable protein aggregation in response to H2O2 treatment (Ilbert et al., 2007).
Because the major function of chaperone holdases such as Hsp33 is to prevent irreversible protein aggregation, we tested whether Hsp33 protects CS against HOCl-mediated aggregation. As shown in Fig. 4C, presence of equimolar amounts of Hsp33HOCl was sufficient to completely suppress HOCl-mediated CS aggregation. Noteworthy, very similar results were obtained when reduced, inactive Hsp33 was pre-incubated with CS before HOCl addition (data not shown). This result demonstrated that Hsp33’s extremely rapid activation by low concentrations of HOCl makes it ideally suited to protect other proteins against HOCl-mediated aggregation in vitro. Co-precipitation studies using His-tagged Hsp33 confirmed that Hsp33 forms apparently very stable complexes with HOCl-induced folding intermediates of citrate synthase (data not shown). Presence of Hsp33HOCl did not significantly influence the HOCl-mediated inactivation of CS (Fig. 4B, inset), indicating that Hsp33 does not quench the effective HOCl concentration or otherwise delay the inactivation of CS.
Hsp33 null mutants are HOCl sensitive
To investigate whether the observed HOCl-mediated activation of Hsp33’s chaperone function protects E. coli against HOCl-stress, we subjected wild type cells and cells lacking the Hsp33-encoding gene hslO to a 20 min HOCl-stress in phosphate buffer at 30°C and analyzed their viability. As shown in Fig. 5A, the hslO deletion strain was more sensitive towards HOCl treatment than the corresponding wild type strain over a range of different HOCl concentrations. Viability of the hslO deletion strain began to decrease at HOCl concentrations as low as 6 μM. At 8 μM HOCl, a 100-fold decrease in cell viability was observed in the hslO deletion strain relative to wild type E. coli (Fig. 5A). Analysis of the in vivo redox status of Hsp33 in HOCl-treated wild type E. coli cells correlated very well with the observed phenotype (Fig. 5A, inset) and showed that treatment of cells with 6 μM HOCl shifted most of the cellular Hsp33 population into the fully oxidized, presumably active state. Qualitatively very similar results were obtained upon HOCl-treatment of wild type and hslO deletion strains in MOPS minimal medium or LB medium, except that millimolar concentrations of HOCl were required to affect the redox state of Hsp33 and the viability of the strains (Supplemental Fig. 3A, B). This, however, most likely reflects the tendency of HOCl to rapidly react with numerous components of the media, which substantially lowers the effective HOCl concentration. Finally, overexpression of Hsp33 in the chaperone-depleted ΔrpoH strain was found to also significantly increase the viability of the deletion strain (Fig. 5B). This result suggested that Hsp33 is specifically suited to protect E. coli against HOCl-induced cell death.
Figure 5. Hsp33 Increases HOCl-Resistance of E. coli and V. cholerae strains.
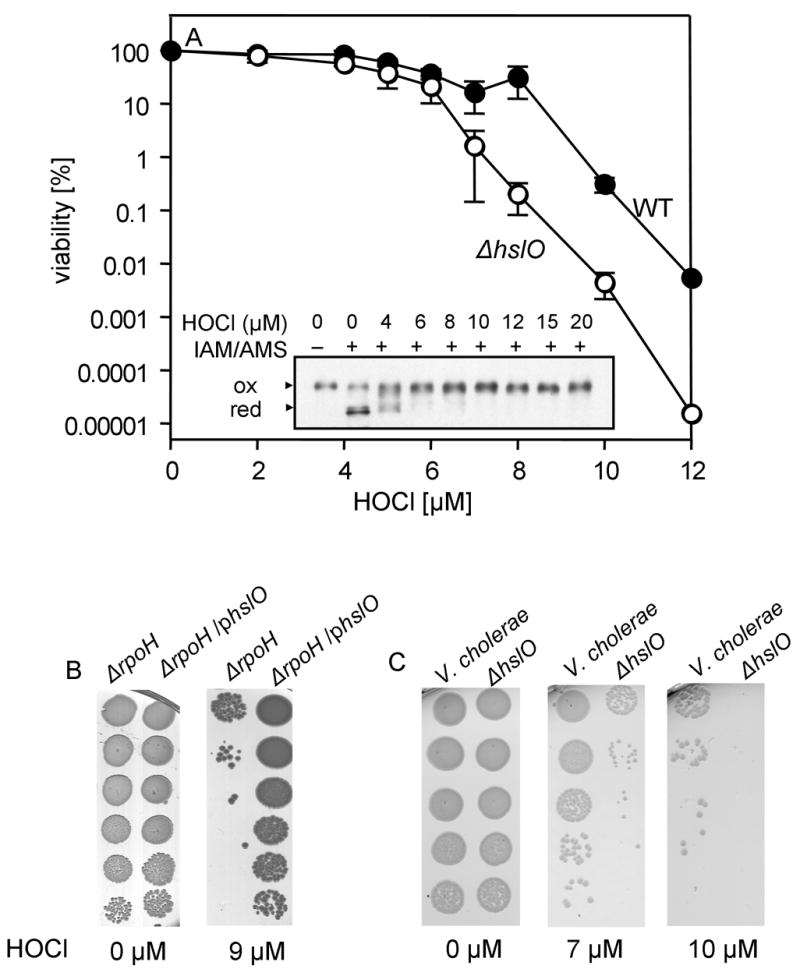
A. E. coli wild type BB7222 (closed circles) and the hslO deletion strain JW176 (open circles) were cultivated in LB medium at 37°C. B. E. coli rpoH deletion strain BB7224 containing an empty pBAD vector and BB7224 overexpressing Hsp33 under pBAD control were cultivated in LB medium containing 0.4% w/v arabinose at 30°C. C. Vibrio cholerae O395 and the corresponding hslO deletion strain were cultivated in LB medium at 37°C. After reaching exponential growth, the individual cultures were washed with phosphate buffer, supplemented with the indicated HOCl concentrations and incubated for 20 min at 30°C. Cell viability was analyzed by preparing serial dilutions of the cultures and spotting them onto LB plates. Inset. In vivo thiol status of Hsp33. BB7222 was treated with the indicated concentrations of HOCl. After 10 min of stress treatment, samples were removed and all in vivo reduced cysteines were modified with iodoacetamide (IAM). After TCA precipitation and DTT reduction, the 490 Da thiol-specific molecule AMS was used to alkylate all in vivo oxidized cysteines. Hsp33 was visualized by western blot analysis.
The Hsp33-encoding gene hslO has been identified in the majority of bacteria that have been sequenced to date. To investigate whether Hsp33 protects other Gram-negative bacteria against HOCl as well, we deleted the hslO (Hsp33) gene in Vibrio cholerae strain O395 and tested the HOCl-sensitivity of both wild type and hslO deletion strains. We observed that the V. cholerae hslO deletion strain was exquisitely sensitive to HOCl stress treatment as compared to wild type (Fig. 5C). At 10 μM HOCl, the viability of the hslO deletion strain was significantly decreased relative to wild type V. cholerae. This result indicated that the highly protective function of Hsp33 towards HOCl-stress is most likely conserved among Hsp33 expressing bacteria.
Hsp33 prevents HOCl-mediated protein aggregation in vivo
Our studies demonstrated that Hsp33, a chaperone specialized in preventing stress-induced protein aggregation, increases the resistance of E. coli and V. cholerae towards HOCl stress. These results were consistent with earlier studies that demonstrated that HOCl-treatment of E. coli triggers the σ32-mediated heat shock response in E. coli (Dukan et al., 1996). Because the E. coli heat shock response is induced by the accumulation of protein unfolding intermediates (Straus et al., 1990), this result suggested that HOCl-treatment might cause protein unfolding and aggregation also in vivo. The transcriptional up-regulation of Hsp33 as a heat shock protein in combination with its rapid posttranslational activation as a redox-sensitive chaperone could provide large reservoirs of a highly effective chaperone holdase that protect E. coli proteins against HOCl-induced aggregation.
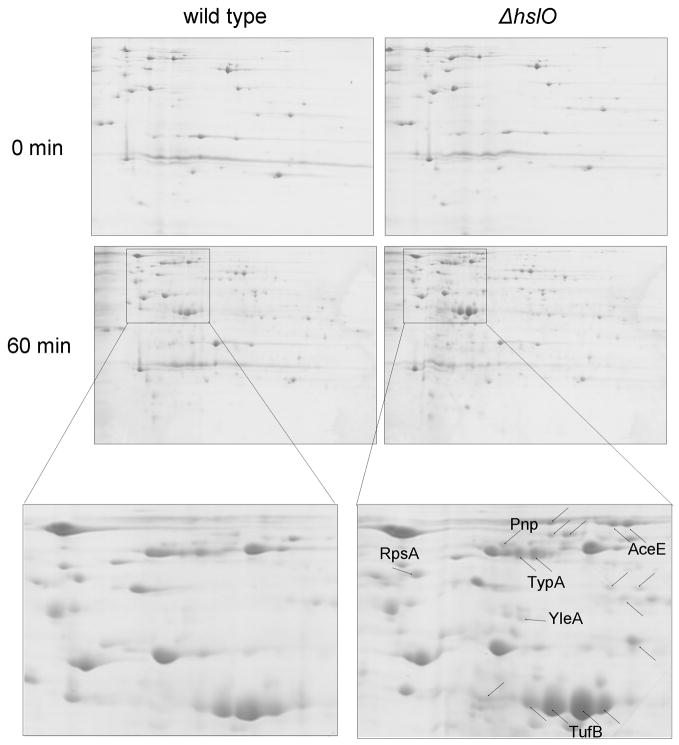
To investigate the effects of HOCl treatment on in vivo protein aggregation, and to evaluate to what extent Hsp33 influences these processes, we compared protein aggregation in HOCl-treated wild type E. coli and hslO deletion strains. We performed these experiments in LB medium instead of phosphate buffer, which allowed us to compare our results with earlier experiments in which the effects of Hsp33 on the aggregation of proteins under oxidative heat stress conditions (4 mM H2O2 at 45°C) were analyzed (Winter et al., 2005). We exposed midlogarithmic cultures of wild type and hslO deletion strains to 6 mM HOCl treatment (Supplemental Figure 3A) and compared protein aggregation before as well as 30 min (data not shown) and 60 min after the HOCl treatment. As shown in Fig. 6, a 60 min exposure of E. coli cells to HOCl-stress causes the accumulation of a significant number of aggregated proteins in E. coli. Importantly, wild type E. coli cells expressing functional Hsp33 revealed less protein aggregation after both 30 min (data not shown) and 60 min as compared to Hsp33 null mutants. This result likely explains the beneficial effect of Hsp33 expression on the HOCl-survival of E. coli. Very similar results were obtained when we treated cell lysates with HOCl in the absence of presence or additional Hsp33 (Supplemental Fig. 4). We found that the majority of proteins, which aggregate in the presence of HOCl, no longer aggregate when the lysates were supplemented with Hsp33. This result clearly showed that Hsp33 is a molecular chaperone that is capable of preventing HOCl-induced protein aggregation.
Figure 6. Hsp33 Protects E. coli against HOCl-mediated protein aggregation.
E. coli wild type (BB7222) and the hslO deletion strain JW176 were cultivated in LB medium at 30°C until OD600 of 0.5 was reached. Then, cells were diluted 1:2 in LB medium and supplemented with 6 mM HOCl. Aliquots were removed before as well as 60 min after the stress treatment. Protein aggregates were prepared as described and analyzed using 2D gels.
Analysis of the in vivo substrate proteins of Hsp33 revealed the identity of 29 individual proteins, which showed significantly reduced aggregation in wild type E. coli cells as compared to Hsp33 null mutants (Supplemental Table 1). These proteins were found to aggregate between 1.5 and up to 17-fold less in the presence of Hsp33 than in its absence. Among the substrate proteins of Hsp33 are enzymes of metabolic pathways (e.g., SucA, AceE, AdhE, SdhA), the ribosomal protein RpsA, trigger factor as well as proteins involved in protein biosynthesis (e.g., TufB, Efp, Tsf). Noteworthy, many of the substrate proteins of Hsp33 are known to be essential for the survival of E. coli (Supplemental Table 1). It remains now to be analyzed whether Hsp33 exerts its cytoprotective effect in HOCl-treated wild type cells through the stabilization of these essential E. coli proteins or through the general reduction of potentially toxic protein aggregates.
HOCl targets thermolabile proteins for irreversible protein aggregation
Noteworthy, analysis of Hsp33’s substrate proteins revealed that more than 50% of the identified proteins have been previously shown to either aggregate in heat shock treated dnaK-deletion strains (Mogk et al., 1999) or turn into Hsp33’s substrate proteins under oxidative heat stress treatment (Winter et al., 2005) (Supplemental Table 1). This result was reminiscent of our in vitro data, which suggested that HOCl-mediated oxidative modifications in thermolabile proteins might induce their unfolding. To investigate whether HOCl-mediated protein aggregation involves indeed mostly thermolabile E. coli proteins, we decided to directly compare HOCl and heat shock-mediated protein aggregation using the chaperone-depleted E. coli strain ΔrpoH (BB7224). This strain, which lacks the heat shock transcription factor 32σ contains substantially reduced levels of most molecular chaperones except GroEL, and is unable to respond to stress conditions with the overexpression of heat shock proteins (Mogk et al., 1999). It is therefore a well-suited strain to study the general aggregation sensitivity of proteins in the absence of their chaperones. To determine the potential overlap between proteins that are sensitive to heat-induced and HOCl-induced protein aggregation, we treated BB7224 with either 6 mM HOCl for 20 min or 45°C for 30 min and compared the aggregated proteins by 2D gel electrophoresis. This analysis revealed that nearly 80% of proteins that aggregate in response to HOCl treatment are thermolabile (Supplemental Table 2). Interestingly, we detected only a few proteins that are specifically sensitive towards HOCl-mediated protein aggregation. One of these proteins is the heat shock protein GroEL, which has recently been shown to be highly HOCl sensitive in vitro (Khor et al., 2004). We concluded from these results that HOCl exerts at least part of its antimicrobial effects in E. coli by causing protein aggregation of predominantly thermolabile proteins.
Discussion
HOCl exerts its bactericidal effects via protein unfolding and aggregation
Hypochlorous acid (HOCl), the active ingredient of household bleach, has numerous industrial applications and plays important roles in host defense as well as in a variety of diseases including chronic inflammation and arteriosclerosis (Heinecke, 1999). It was therefore surprising to learn that little is known about the mechanism by which bleach kills bacteria (Pattison and Davies, 2006). Here we demonstrate i) that HOCl causes the oxidative unfolding of proteins both in vitro and in vivo, ii) that HOCl-stress leads to the irreversible aggregation of essential proteins in bacteria, and most importantly, iii) that a molecular chaperone, whose main function is to prevent protein aggregation during stress conditions in vivo, significantly increases the resistance of E. coli and other bacteria towards HOCl-stress. These results provide powerful arguments that HOCl’s damaging effects to bacteria during host defense and disinfection are likely due to its ability to cause protein unfolding and aggregation of proteins in the cell.
What makes HOCl so much more potent than most other known oxidants? The two-electron oxidant peroxide, which is more oxidizing than HOCl based on its standard redox potential (1.776 V for H2O2 versus 1.482 V for HOCl) does not cause protein aggregation (Fig. 4C, Supplemental Fig. 4) and becomes bactericidal only at very high millimolar concentrations (Britigan et al., 1996). This suggests that the different cellular effects of the two oxidants are not based on their thermodynamic properties but more likely due to differences in their reaction rates with amino acids (Lide, 1994). The reaction rates of peroxide with thiols, one of the very few biomolecules that H2O2 reacts directly with, are apart from few exceptions (i.e., H2O2 sensor proteins) extremely slow (k2 = 2.9 M−1s−1 at pH 7.4–7.6) (Imlay, 2003; Winterbourn and Hampton, 2008). HOCl, on the other hand, reacts with free cysteines about seven orders of magnitudes faster (k2=3.0 ×107 M−1s−1 at pH 7.4) and shows high reaction rates with numerous other amino acid side chains including Met, His, α-amino acids and tryptophans (Pattison and Davies, 2001). These high rates of reaction will enable HOCl to oxidize residues, which are buried and might be only transiently accessible for oxidative modifications. These considerations agree well with our in vitro and in vivo data, which suggest that thermolabile proteins are particularly prone to HOCl-mediated oxidative unfolding and aggregation. Because these proteins are in rapid equilibrium with a partially unfolded conformation, bimolecular oxidation reactions that are fast enough to compete with the refolding reaction will cause protein unfolding and aggregation. Thermostable proteins, on the other hand, might be targeted predominantly on surface exposed residues, which might not affect the activity or thermodynamic stability of the proteins.
HOCl – A specific activator for a highly specialized chaperone
Hsp33, a chaperone that requires oxidative unfolding for its activation serves as excellent paradigm to illustrate the mechanistic differences between HOCl and H2O2. We have developed a working model of Hsp33’s activation process, which is based on the thermodynamic properties of Hsp33’s linker region and the rapid reaction rates of HOCl (Fig. 7). In this model, the linker region of Hsp33 exists in a dynamic equilibrium between the folded and partially unfolded state. Only upon its partial unfolding, the two critical, proximal cysteines (Cys232/Cys234) become accessible for oxidation. Once oxidized, the linker region remains locked in a natively unfolded conformation until reducing conditions are restored. This is essential for dimerization and substrate binding. At low temperatures, the equilibrium favors highly the folded state. Therefore, the bimolecular reactions of kinetically slow oxidants like H2O2 or NO• with cysteines do not effectively compete with the unimolecular refolding reaction, which explains why these oxidants are unable to significantly activate Hsp33 within the time frame of the experiment. Shifting the equilibrium to the partially unfolded state either by incubation at elevated temperatures or low concentrations of denaturants (1 M Gdn*HCl), however, will increase the overall oxidation rate and cause the activation of Hsp33. HOCl, on the other hand, due to its high reaction rates with cysteine thiols, competes with the refolding of Hsp33’s linker region even at non-stress temperatures and rapidly oxidizes the cysteines. Oxidation apparently converts Hsp33’s C-terminus into its natively unfolded conformation and causes the activation of Hsp33 (Fig. 7).
Fig. 7. Hsp33 - A paradigm for HOCl’s oxidative unfolding activity.
Hsp33 consists of an N-terminal domain (blue), a redox sensitive zinc binding domain (yellow) and a flexible linker region (green), which serves as folding sensor in Hsp33. Upon oxidative modification of Hsp33’s distal cysteines (Cys265/Cys268), zinc is released and Hsp33’s zinc binding domain unfolds. This destabilizes the linker region, which is now in a dynamic equilibrium between the folded state, in which no thiol oxidation can occur and an unfolded state, in which oxidative modification can occur with a rate constant determined by the oxidant. Kinetically slow oxidants, such as H2O2 require mild unfolding conditions (e.g., heat) to react with the critical cysteine pair Cys232/Cys234. Kinetically fast oxidants such as HOCl, however, compete with the refolding reaction and allow oxidation even in the absence of unfolding conditions. Disulfide bond formation prevents refolding of Hsp33’s redox switch domain and converts Hsp33 into highly active molecular chaperone.
Hsp33-A Chaperone specialized to protect bacteria against HOCl-stress
ATP-dependent molecular chaperones such as the GroEL/ES system or the DnaK/DnaJ/GrpE system are central chaperone machineries, dedicated to protect proteins against stress-induced protein aggregation (Georgopoulos, 2006; Houry, 2001). This situation changes, however, under oxidative stress treatment when a sudden decrease in cellular ATP levels renders those chaperone machineries devoid of their cofactor that regulates substrate binding and release (Winter et al., 2005). Moreover, both DnaK and GroEL fall victim to oxidative side-chain modifications, which at least transiently inactivate the proteins (Khor et al., 2004; Winter et al., 2005). It is therefore not surprising that bacteria developed Hsp33 as a highly specialized chaperone that protects proteins against aggregation under these specific stress conditions. It is surprising, however, how Hsp33 evolved a mechanism that utilizes as activator what is so highly damaging to most other macromolecules. In fact, to our knowledge, Hsp33 is only the second class of proteins, which is specifically activated by HOCl. Matrix metalloproteinases (MMP-7, MMP-8, MMP-9) were the first proteins discovered to be activated by low concentrations of HOCl (Fu et al., 2001; Weiss et al., 1985). Enzymatically inactive MMP-7 contains a central His3-Cys zinc-binding site. Upon exposure to HOCl, the single cysteine is oxidized to form either sulfinic or sulfonic acid. This oxidation exposes the zinc atom and frees the now catalytically active zinc site to interact with substrates. Increasing HOCl to protein ratios eventually inactivate MMP-7 apparently via the oxidation of non-active site residues (Fu et al., 2004). In Hsp33, however, not even very high HOCl to protein ratios (100:1) diminish the chaperone activity of Hsp33. Quite in contrast, we found that high concentrations of HOCl induce the formation of DTT-resistant Hsp33 oligomers in vitro, which are highly chaperone active (data not shown). Similar high activity Hsp33 oligomers have been observed before when Hsp33 was incubated in 20 mM H2O2 for 3 hours at 43°C (Akhtar et al., 2004). Although it is unclear whether these highly active Hsp33 oligomers play any physiological role, their in vitro formation illustrates the extreme resistance of Hsp33 towards HOCl concentrations that unfold, degrade or otherwise destroy most other cellular proteins.
Experimental Procedures
Strains
The MC4100-derived E. coli strains BB7222, BB7222 hslO::Km (JW176), BB7224 ΔrpoH containing an empty pBAD vector and BB7224 expressing hslO (Hsp33 gene) under pBAD control (JW49) have been previously described (Winter et al., 2005). For our studies with Vibrio cholerae, V. cholerae O395 was used. The hslO deletion strain in Vibrio cholerae O395 (JW371) was constructed as described in Supplemental Materials.
Materials
Sodium hypochlorite (Sigma-Aldrich) when dissolved in water at pH 7.5 forms about equal amounts of hypochlorous acid (HOCl) and its conjugate base hypochlorite (OCl−). We use the term HOCl to describe this mixture. The precise concentration of HOCl was determined by absorbance spectroscopy using an extinction coefficient of 350 M−1cm−1 at 292 nm and pH 10–12 (Morris, 1966). Nitric oxide solutions were freshly prepared by dissolving diethylamine NONOate (DEANO) (Cayman Chemicals, Ann Arbor) in 10 mM KOH. 4-acetamido-4′-maleimidylstilbene-2,2′-disulfonic acid (AMS) and the Isotope Coded Affinity Tag (ICAT) reagents were obtained from MP Biochemicals (Solon, OH) and Applied Biosystems (Foster City, CA), respectively.
Oxidation, reduction and chaperone activity of Hsp33
Reduced, inactive wild type Hsp33 (from hereon referred to as Hsp33red) was purified, reduced and zinc-reconstituted as described (Ilbert et al., 2007). To oxidize Hsp33, 50 μM Hsp33red was treated with either 2 mM DEANO or 2 mM H2O2 for 3 hours at 30°C or 43°C or with 500 μM HOCl for 20 min at 30°C (from hereon called Hsp33HOCl). Then, the oxidants were removed using NAP-5 columns (Amersham Biosciences). To determine activation kinetics, Hsp33 was incubated in the presence of the indicated oxidants. At various time points, aliquots were removed and the influence of Hsp33 on the aggregation of chemically unfolded citrate synthase (CS, Roche Applied Sciences) was analyzed at 30°C (Ilbert et al., 2007). To test the reversibility of Hsp33’s activation, 5 μM of Hsp33HOCl was incubated in 5 mM DTT and 5 μM of zinc at 30°C. At different time points, aliquots were removed and Hsp33’s chaperone activity was determined as before. After 12 hours incubation at 30°C, DTT and zinc were removed by using NAP-5 columns. Hsp33 was concentrated to 50 μM and activation of Hsp33HOCl⇒DTT was tested upon incubation in 500 μM HOCl at 30°C or 2 mM H2O2 at 30°C or 43°C as described above. To investigate the influence of Hsp33 on the aggregation of HOCl-treated CS, 3 μM of CS was incubated in 40 mM potassium phosphate, pH 7.5 at 30°C and treated with 450 μM HOCl in the absence or presence of 3 μM Hsp33HOCl. Light scattering was monitored at λex/λem = 360 nm using a fluorescence spectrophotometer (Hitachi F4500). Detailed protocols for the structural analysis of Hsp33, GrpE and CS by circular dichroism (CD), for monitoring the in vivo and in vitro thiol status of Hsp33 (Graf et al., 2004) and for determining the molecular mass of Hsp33HOCl using ESI-MS can be found in the Supplemental Data.
Citrate synthase (CS) activity
To determine the influence of HOCl and H2O2 on the activity of CS, 3 μM CS in 40 mM potassium phosphate (pH 7.5) was treated with 450 μM HOCl or H2O2 at 30°C. After defined time points, aliquots were diluted 1:1000 into assay buffer supplemented with 0.6 mM L-methionine to quench excess HOCl. Then, CS activity measurements were performed as described (Buchner et al., 1998).
Cultivation of E. coli and V. cholerae strains and stress treatment
To determine the HOCl stress resistance of wild type and Hsp33 deletion strains, the strains were cultivated in LB media at 37°C until OD600 of 0.5–0.6 (BB7222 and JW176) or OD600 of 0.3–0.4 (O395 and JW371) was reached. Then, cells were harvested, washed twice using 83 mM sodium phosphate, pH 7.1 and diluted 1:5 into sodium phosphate buffer supplemented with the indicated concentrations of HOCl (Dukan and Touati, 1996). After 20 min incubation at 30°C in the dark, aliquots were removed, serially diluted in LB medium to quench the remaining HOCl, and spotted onto LB plates. LB plates were incubated at 30°C for 24 hours and the colony forming units were counted.
Analysis of Hsp33’s substrate specificity in vivo
To identify aggregation sensitive proteins in vivo, BB7222, JW176 and the rpoH deletion strain BB7224 were grown at 30°C until midlogarithmic growth. Then, cultures were split and supplemented with an equal volume of LB medium supplemented with HOCl (final concentration 6 mM) for HOCl-stress treatment (BB7222, JW176, BB7224) or shifted to 45°C for 30 min for heat stress treatment (BB7224). At the indicated time points, the cells were harvested. Isolation of the aggregated proteins, 2D gel electrophoresis as well as data analysis and quantification were performed as previously described (Winter et al., 2005) (for details see Supplemental Materials).
Supplementary Material
Acknowledgments
We are very grateful to Dr. Victor DiRita for his advice on generating the Vibrio cholerae null mutant. We thank Dr. Hauke Lilie for conducting the ultracentrifugation experiments and for many invaluable discussions. We are indebted to the Protein Structure Facility of the U of M and the Michigan Proteome Consortium for performing the mass spectrometric analysis. We thank Dr. James Bardwell for critically reading this manuscript. The National Institute of Health Grant GM065318, a Rackham Predoctoral Fellowship to P.C.F.G. and a postdoctoral fellowship from the Leopoldina Gesellschaft Deutscher Naturforscher to J.W. supported this work.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akhtar MW, Srinivas V, Raman B, Ramakrishna T, Inobe T, Maki K, Arai M, Kuwajima K, Rao Ch M. Oligomeric Hsp33 with enhanced chaperone activity: gel filtration, cross-linking, and small angle x-ray scattering (SAXS) analysis. J Biol Chem. 2004;279:55760–55769. doi: 10.1074/jbc.M406333200. [DOI] [PubMed] [Google Scholar]
- Aliev G, Smith MA, Seyidov D, Neal ML, Lamb BT, Nunomura A, Gasimov EK, Vinters HV, Perry G, LaManna JC, Friedland RP. The role of oxidative stress in the pathophysiology of cerebrovascular lesions in Alzheimer’s disease. Brain Pathol. 2002;12:21–35. doi: 10.1111/j.1750-3639.2002.tb00419.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berndt C, Lillig CH, Holmgren A. Thioredoxins and glutaredoxins as facilitators of protein folding. Biochim Biophys Acta. 2008;1783:641–650. doi: 10.1016/j.bbamcr.2008.02.003. [DOI] [PubMed] [Google Scholar]
- Boschi-Muller S, Gand A, Branlant G. The methionine sulfoxide reductases: Catalysis and substrate specificities. Arch Biochem Biophys. 2008 doi: 10.1016/j.abb.2008.02.007. [DOI] [PubMed] [Google Scholar]
- Britigan BE, Ratcliffe HR, Buettner GR, Rosen GM. Binding of myeloperoxidase to bacteria: effect on hydroxyl radical formation and susceptibility to oxidant-mediated killing. Biochim Biophys Acta. 1996;1290:231–240. doi: 10.1016/0304-4165(96)00014-1. [DOI] [PubMed] [Google Scholar]
- Buchner J, Grallert H, Jakob U. Analysis of chaperone function using citrate synthase as nonnative substrate protein. Methods Enzymol. 1998;290:323–338. doi: 10.1016/s0076-6879(98)90029-5. [DOI] [PubMed] [Google Scholar]
- Dukan S, Dadon S, Smulski DR, Belkin S. Hypochlorous acid activates the heat shock and soxRS systems of Escherichia coli. Appl Environ Microbiol. 1996;62:4003–4008. doi: 10.1128/aem.62.11.4003-4008.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dukan S, Touati D. Hypochlorous acid stress in Escherichia coli: resistance, DNA damage, and comparison with hydrogen peroxide stress. J Bacteriol. 1996;178:6145–6150. doi: 10.1128/jb.178.21.6145-6150.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu X, Kao JL, Bergt C, Kassim SY, Huq NP, d’Avignon A, Parks WC, Mecham RP, Heinecke JW. Oxidative cross-linking of tryptophan to glycine restrains matrix metalloproteinase activity: specific structural motifs control protein oxidation. J Biol Chem. 2004;279:6209–6212. doi: 10.1074/jbc.C300506200. [DOI] [PubMed] [Google Scholar]
- Fu X, Kassim SY, Parks WC, Heinecke JW. Hypochlorous acid oxygenates the cysteine switch domain of pro-matrilysin (MMP-7). A mechanism for matrix metalloproteinase activation and atherosclerotic plaque rupture by myeloperoxidase. J Biol Chem. 2001;276:41279–41287. doi: 10.1074/jbc.M106958200. [DOI] [PubMed] [Google Scholar]
- Georgopoulos C. Toothpicks, serendipity and the emergence of the Escherichia coli DnaK (Hsp70) and GroEL (Hsp60) chaperone machines. Genetics. 2006;174:1699–1707. doi: 10.1534/genetics.104.68262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graf PC, Martinez-Yamout M, VanHaerents S, Lilie H, Dyson HJ, Jakob U. Activation of the redox-regulated chaperone Hsp33 by domain unfolding. J Biol Chem. 2004;279:20529–20538. doi: 10.1074/jbc.M401764200. [DOI] [PubMed] [Google Scholar]
- Graumann J, Lilie H, Tang X, Tucker KA, Hoffmann JH, Vijayalakshmi J, Saper M, Bardwell JC, Jakob U. Activation of the redox-regulated molecular chaperone Hsp33--a two-step mechanism. Structure (Camb) 2001;9:377–387. doi: 10.1016/s0969-2126(01)00599-8. [DOI] [PubMed] [Google Scholar]
- Grimshaw JP, Jelesarov I, Siegenthaler RK, Christen P. Thermosensor action of GrpE. The DnaK chaperone system at heat shock temperatures. J Biol Chem. 2003;278:19048–19053. doi: 10.1074/jbc.M300924200. [DOI] [PubMed] [Google Scholar]
- Hawkins CL, Davies MJ. Inactivation of protease inhibitors and lysozyme by hypochlorous acid: role of side-chain oxidation and protein unfolding in loss of biological function. Chem Res Toxicol. 2005;18:1600–1610. doi: 10.1021/tx050207b. [DOI] [PubMed] [Google Scholar]
- Hawkins CL, Pattison DI, Davies MJ. Hypochlorite-induced oxidation of amino acids, peptides and proteins. Amino Acids. 2003;25:259–274. doi: 10.1007/s00726-003-0016-x. [DOI] [PubMed] [Google Scholar]
- Heinecke JW. Mechanisms of oxidative damage by myeloperoxidase in atherosclerosis and other inflammatory disorders. J Lab Clin Med. 1999;133:321–325. doi: 10.1016/s0022-2143(99)90061-6. [DOI] [PubMed] [Google Scholar]
- Hoffmann JH, Linke K, Graf PC, Lilie H, Jakob U. Identification of a redox-regulated chaperone network. Embo J. 2004;23:160–168. doi: 10.1038/sj.emboj.7600016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houry WA. Chaperone-assisted protein folding in the cell cytoplasm. Curr Protein Pept Sci. 2001;2:227–244. doi: 10.2174/1389203013381134. [DOI] [PubMed] [Google Scholar]
- Ilbert M, Horst J, Ahrens S, Winter J, Graf PC, Lilie H, Jakob U. The redox-switch domain of Hsp33 functions as dual stress sensor. Nat Struct Mol Biol. 2007;14:556–563. doi: 10.1038/nsmb1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imlay JA. Pathways of oxidative damage. Annu Rev Microbiol. 2003;57:395–418. doi: 10.1146/annurev.micro.57.030502.090938. [DOI] [PubMed] [Google Scholar]
- Jayaraman S, Gantz DL, Gursky O. Effects of protein oxidation on the structure and stability of model discoidal high-density lipoproteins. Biochemistry. 2008;47:3875–3882. doi: 10.1021/bi7023783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khor HK, Fisher MT, Schoneich C. Potential role of methionine sulfoxide in the inactivation of the chaperone GroEL by hypochlorous acid (HOCl) and peroxynitrite (ONOO−) J Biol Chem. 2004;279:19486–19493. doi: 10.1074/jbc.M310045200. [DOI] [PubMed] [Google Scholar]
- Leichert LI, Gehrke F, Gudiseva HV, Blackwell T, Ilbert M, Walker AK, Strahler JR, Andrews PC, Jakob U. Quantifying changes in the thiol redox proteome upon oxidative stress in vivo. Proc Natl Acad Sci U S A. 2008 doi: 10.1073/pnas.0707723105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leichert LI, Jakob U. Protein thiol modifications visualized in vivo. PLoS Biol. 2004;2:e333. doi: 10.1371/journal.pbio.0020333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lide DR. CRC Handbook of Chemistry and Physics. CRC Press, Inc; 1994. [Google Scholar]
- Mayer MP, Brehmer D, Gassler CS, Bukau B. Hsp70 chaperone machines. Adv Protein Chem. 2001;59:1–44. doi: 10.1016/s0065-3233(01)59001-4. [DOI] [PubMed] [Google Scholar]
- Miller RA, Britigan BE. Role of oxidants in microbial pathophysiology. Clin Microbiol Rev. 1997;10:1–18. doi: 10.1128/cmr.10.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogk A, Tomoyasu T, Goloubinoff P, Rudiger S, Roder D, Langen H, Bukau B. Identification of thermolabile Escherichia coli proteins: prevention and reversion of aggregation by DnaK and ClpB. Embo J. 1999;18:6934–6949. doi: 10.1093/emboj/18.24.6934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JC. The acid ionization constant of HOCl from 5°C to 35°C. Journal of physical Chemistry. 1966;70:3798–3805. [Google Scholar]
- Pattison DI, Davies MJ. Absolute rate constants for the reaction of hypochlorous acid with protein side chains and peptide bonds. Chem Res Toxicol. 2001;14:1453–1464. doi: 10.1021/tx0155451. [DOI] [PubMed] [Google Scholar]
- Pattison DI, Davies MJ. Reactions of myeloperoxidase-derived oxidants with biological substrates: gaining chemical insight into human inflammatory diseases. Curr Med Chem. 2006;13:3271–3290. doi: 10.2174/092986706778773095. [DOI] [PubMed] [Google Scholar]
- Straus D, Walter W, Gross CA. DnaK, DnaJ, and GrpE heat shock proteins negatively regulate heat shock gene expression by controlling the synthesis and stability of sigma 32. Genes Dev. 1990;4:2202–2209. doi: 10.1101/gad.4.12a.2202. [DOI] [PubMed] [Google Scholar]
- Weiss SJ, Peppin G, Ortiz X, Ragsdale C, Test ST. Oxidative autoactivation of latent collagenase by human neutrophils. Science. 1985;227:747–749. doi: 10.1126/science.2982211. [DOI] [PubMed] [Google Scholar]
- Winter J, Linke K, Jatzek A, Jakob U. Severe oxidative stress causes inactivation of DnaK and activation of the redox-regulated chaperone Hsp33. Mol Cell. 2005;17:381–392. doi: 10.1016/j.molcel.2004.12.027. [DOI] [PubMed] [Google Scholar]
- Winterbourn CC, Hampton MB. Free Radic Biol Med. 2008. Thiol chemistry and specificity in redox signaling. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.