Abstract
Conceptualizations of mammalian cochlear mechanics are based on basilar-membrane (BM) traveling waves that scale with frequency along the length of the cochlea, are amplified by outer hair cells (OHCs), and excite inner hair cells and auditory-nerve (AN) fibers in a simple way. However, recent experimental work has shown medial-olivocochlear (MOC) inhibition of AN responses to clicks that do not fit with this picture. To test whether this AN-initial-peak (ANIP) inhibition might result from hitherto unrecognized aspects of the traveling-wave or MOC-evoked inhibition, MOC effects on BM responses to clicks in the basal turns of guinea pig and chinchilla cochleae were measured. MOC stimulation inhibited BM click responses in a time and level dependent manner. Inhibition was not seen during the first half-cycle of the responses, but built up gradually, and ultimately increased the responses’ decay rates. MOC stimulation also produced small phase leads in the response wave forms, but had little effect on the instantaneous frequency or the waxing and waning of the responses. These data, plus recent AN data, support the hypothesis that the MOC-evoked inhibitions of the traveling wave and of the ANIP response are separate phenomena, and indicate that the OHCs can affect at least two separate modes of excitation in the mammalian cochlea.
INTRODUCTION
The sensitivity and frequency selectivity of mammalian hearing are enhanced by the active amplification of hydrodynamic traveling waves that takes place in the cochlea. This amplification results from mechanical feedback between the outer hair cells (OHCs) in the organ of Corti and the basilar membrane (BM), but the details of this mechanism are poorly understood. At the macromechanical level, our knowledge is limited to inferences from model-based mathematical “inversions” of the measured BM motion (e.g., Zweig, 1991; de Boer and Nuttall, 2000). At the micromechanical level, two separate forms of OHC motility are presently being debated as candidates for the cochlear amplifier (for reviews, see Dallos et al., 2006 and Fettiplace, 2006). The present investigation seeks to inform such debates by providing observations as to what OHCs do, and do not do, to the mechanics of the living mammalian cochlea.
According to the simplest models of cochlear mechanics, sound-evoked ossicular motion produces BM traveling waves that evoke a single pattern of vibration at each place along the length of the cochlea. This vibration pattern couples the BM’s motion to the bending of inner-hair-cell (IHC) stereocilia and leads to the excitation of auditory-nerve (AN) fibers with little or no postmechanical filtering (see Narayan et al., 1998). More complex, so-called “micromechanical” models have more than one mechanical degree of freedom within each cochlear cross section, and hence permit larger differences between BM motion and AN excitation (reviewed by Patuzzi, 1996). Such models find support in recent measurements that demonstrate complex, frequency-dependent patterns of vibration in real cochleae, either on the BM itself (e.g., Russell and Nilsen, 1997) or within the organ of Corti and tectorial membrane TM complex (Fridberger et al., 2004; Nowotny and Gummer, 2006; Karavitaki and Mountain, 2007b). However, excitation of the IHCs in an intact cochlea by a mechanical vibration mode that significantly affects AN coding but is not seen in the BM’s traveling wave has not yet been demonstrated.
The present study provides new insights into cochlear amplification by investigating BM responses to clicks with and without electrical stimulation of the medial olivocochlear (MOC) efferent fibers. The MOC fibers innervate the OHCs via large cholinergic synapses, and provide a convenient way to affect the OHCs in a reversible, physiologic way. MOC effects on BM motion have previously been studied only in responses to tones, where their main effect is to turn down the gain of the traveling-wave amplifier (for reviews, see Guinan 1996; Cooper and Guinan, 2006). One reason to study the effects of the MOC fibers on click-evoked motion is to investigate how this gain change is produced. The click stimuli, which have wideband spectra but are punctuate in time, are advantageous because they allow the BM to respond at, and reveal, its own resonant frequencies (in contrast, tones force the BM to follow the externally applied frequency). If, for instance, the MOC-induced changes in the gain of the traveling-wave amplifier were brought about by changes in the effective stiffness of the BM (Dallos et al., 1997), then the frequency content as well as the time course of the click responses would be expected to change. Another advantage of clicks is the spreading out in time (i.e., in response cycles) of the effects of different stages of cochlear amplification, so that the MOC-induced changes in the click responses should give us insight into the buildup and decay of traveling-wave amplification.
A further reason to study MOC effects on BM motion is to search for a mechanical correlate of a newly discovered form of AN inhibition (Guinan et al., 2005). “AN-initial-peak” (ANIP) inhibition is manifested as a reduction in the initial peak of an AN fiber’s response to a click stimulus, and differs substantially from the inhibition produced by reducing the gain of traveling-wave amplification (although traveling-wave inhibition is also apparent in the AN click responses, as shown in Guinan et al., 2005). ANIP inhibition primarily affects responses at moderate-to-high click levels; it inhibits the initial peak of a response more than subsequent peaks; and its effects differ with the polarity of the clicks. Guinan et al. (2005) hypothesized that the ANIP response is due to an OHC-produced motion that is distinct from, and not present in, the classical BM traveling wave. This proposal contrasts strongly with the earlier inferences that only minor signal transformations intervene between BM vibration and AN excitation (e.g., see Narayan et al., 1998). However, there are clear differences between the data on which these two viewpoints were based, the most striking being that they originate at the opposite “ends” of the cochlea: ANIP inhibition has only been demonstrated clearly in the apical half of the cochlea [in cat AN fibers with characteristic frequencies (CFs) of up to ∼6 kHz; see Guinan et al., 2005], while BM motion has only been compared rigorously with AN excitation in the basal half of the cochlea (most notably in the 8–10 kHz CF region of chinchillas; see Narayan et al., 1998). Despite these differences, it is generally assumed that the classic BM traveling wave is present, in much the same form, throughout the cochlea (e.g., see Patuzzi, 1996). Thus, if the traveling wave is little changed throughout the cochlea and there are only minor transformations between BM motion and AN firing, then an inhibition corresponding to the ANIP inhibition should be seen in the early part of basal-turn BM click responses. One goal of the present work is to determine whether such an inhibition is seen in BM motion.
METHODS
Experiments were performed on deeply anesthetized animals in accordance with NIH, UK and US guidelines. Guinea pigs (320–550 g) were anesthetized using either sodium pentobarbitone (25 mg∕kg, I.P.) and Hypnorm (0.6 ml∕kg, I.M.; each milliliter of Hypnorm contains 10 mg fluanisone and 0.315 mg fentanyl citrate), or ketamine (50 mg∕kg, I.M) and xylazine (10 mg∕kg, I.M). Chinchillas (329–393 g) were anesthetized using sodium pentobarbitone alone (65 mg∕kg, I.P.). Maintenance doses of anesthetics were given whenever needed, and the animals were killed humanely without recovery from anesthesia at the end of the experiments. Artificial ventilation was used as required to maintain end-tidal CO2 levels near 4.5%. Core temperatures were maintained near 37.6 °C.
Acoustic stimuli were produced by a reverse-driven Brüel and Kjaer 1∕2 in. microphone and delivered to the ear canal via a closed sound system that produced very little ringing [spectra from a similar acoustic system are shown by Wilson and Johnstone (1975)]. Tone amplitudes are expressed as sound pressure levels (SPLs) in dB with regard to 20 μPa, and were calibrated using a microphone (Brüel and Kjær 4134) with a probe tube placed within 2 mm of the tympanic membrane. Click amplitudes are expressed in peak-equivalent SPLs (pSPL) and were determined from the tone calibration and the spectrum of the electronic pulse that produced the click; the pSPL of a click is the SPL of a tone that produces the same peak sound pressure as the click.
The cochlea was exposed via a dorsolateral bulla opening, and its physiological condition was monitored using AN compound action potential (CAP) thresholds recorded from a fine silver electrode near the round window. CAP thresholds usually deteriorated from their initial values during the opening of the cochlea. Moderate hearing deterioration (e.g., 10–25 dB) decreased the magnitude of the efferent effects but did not appear to change the patterns of the effects that were seen on the BM. Nonetheless, the data that we will consider extensively in this report were selected to show the largest MOC effects, and came from animals with minimal threshold losses (0–10 dB, near CF).
BM responses were monitored in the first turn of the cochlea using a displacement-sensitive interferometer (Cooper, 1999) with the output typically sampled at 200 kHz. The BM was exposed by shaving a small hole into the scala tympani. Gold-coated polystyrene microbeads (PolySciences Inc. 15–25 μm diameter) were dropped through the fluid onto the BM to provide enhanced reflectivity. A small glass cover slip was placed over the cochlear hole to avoid a movable air-to-fluid interface that might create interferometric artifacts (Cooper and Rhode, 1992).
MOC efferents were stimulated via a bipolar electrode at the floor of the fourth ventricle (Guinan and Stankovic, 1996). Shock stimuli were pulse trains with 0.3 ms pulse widths, ac coupled by a transformer, presented at 200–300 pulses∕s, in 100 ms long bursts at 330 ms intervals. Pulse amplitudes were limited to near or just below the threshold for visible muscle twitches so that shock-induced motion did not interfere with the BM measurement. Control measurements of ossicular motion with and without shocks were made to ensure that middle-ear-muscle contractions did not affect the results.
Click stimuli were 20 μs long pulses, alternating in polarity, presented once every 11 ms with 15 clicks per burst, as shown in Fig. 1(a). Click bursts and MOC-shock bursts were presented every 330 ms with the first MOC shock delayed 30 ms after the first click. With this timing, the first four click responses occurred before the effects of the efferent shocks had built up, and were called the “pre-MOC” responses. The four responses that were most affected by the MOC shocks were from clicks 11 to 14, and these were called the “during-MOC” responses [Fig. 1(a)]. Since the responses to the rarefaction clicks mirrored those to the condensation clicks almost perfectly, each set of four responses was averaged (after inverting the rarefaction-click responses) to increase the signal-to-noise ratios of the pre-MOC and during-MOC responses that will be considered throughout this paper. The MOC-induced change of the BM click response was obtained by subtracting the during-MOC response wave form from the pre-MOC response wave form, yielding the “MOC-change” wave form, as illustrated in Fig. 1(b).
Figure 1.
The stimulus paradigm (A), derivation of the MOC change (B), and determining relative phases (C). (A) Schematic showing the timing of shock and click stimuli as well as the sets of four click responses used to get pre-MOC and during-MOC averages. (B) BM click-response wave forms measured prior to (pre-MOC) and during (during-MOC) the effects of the shock train, as well as their difference, the MOC change. (A) and (B) from GP3071, CF=14.5 kHz. The scale bar in (A) also applies to (B). (C) Phase delay calculated from the timing of the MOC-change zero crossings relative to the during-MOC zero crossings.
MOC efferents can inhibit BM motion on two time scales, fast (time constant <100 ms) and slow (time constant ∼10 s) (Sridhar et al., 1995; Cooper and Guinan, 2003). Our MOC-change wave forms are only sensitive to MOC fast effects, because they compared the motion before each shock burst to the motion during that shock burst, as described above (Cooper and Guinan, 2003). Slow effects were also reduced by our data collection paradigm: Data were normally obtained in runs in which the click level was systematically varied (usually from high to low levels), with clicks and shocks paired as in Fig. 1. Slow effects decrease to near zero if MOC stimulation continues for several minutes, and after a run that produced a slow effect, it takes many minutes of recovery before a substantial MOC slow effect can be elicited again (Sridhar et al., 1995). We reduced slow effects by doing the runs close enough in time that there was not sufficient recovery from the previous run to permit a substantial slow effect. To test for the presence of slow effects, occasional runs were done without efferent shocks, and responses from runs with versus without shocks were compared. Generally, this comparison showed little evidence of slow effects, so it seems likely that there was a very little slow effect during the runs reported here.
Analysis of click responses
Click responses were averaged across multiple presentations of a given stimulus before being stored to disk for subsequent analysis. In order to reduce the effects of any artifactual base line position shifts, such as those associated with breathing or shock-evoked animal motion, the first stage of our analysis was to convert the waveforms from displacement to velocity.
To quantify the BM click-response amplitude, we used the amplitude of the largest component of short-term fast Fourier transforms (FFTs) of the response, as calculated by MATLAB software’s standard spectrogram function. To measure the initial part of the response with good time resolution, we used an analysis time of 1.5 CF cycles and a temporal overlap of 75%. A duration longer than a CF period was used to capture below-CF energy at the beginning of the response (using an integral number of CF periods was avoided because it produced a near-CF-period cyclic variation of the amplitude). As the response decayed into the noise in the later parts of the click response, we extended the analysis time to 4.5 and then 8.5 cycles, effectively averaging more cycles to increase the signal-to-noise ratio. Since the frequency content in this part of the response is very close to CF, the filtering aspect of this increase in the analysis window had little effect. However, with a multicycle analysis window and responses that are decaying with time, the initial part of the response is largest and tends to dominate the FFT. The resulting bias is greater for longer windows and faster decays; this prevented us from increasing the signal-to-noise ratio by extending the analysis time even further. In theory, a Hilbert transform method would be less biased by the fast decay of the response, but Hilbert transforms would not produce more accurate results (Lin and Guinan, 2004) because they use a derivative of the response, which is very sensitive to noise.
To characterize the phase changes produced by the MOC stimulation, we used the zero-crossing times of the click responses [Fig. 1(c)]. The time between adjacent (oppositely directed) zero crossings was taken to be one-half period of the instantaneous response frequency. The advance (or delay) of a test wave relative to a reference wave was taken to be the time that its zero crossing came before (or after) the same-direction zero crossing of the reference wave [as shown in Fig. 1(c)]. Positive phase was the advance divided by the period (twice the half-period) of the response. Zero crossings are accurate only when the signal-to-noise ratio is good, so phase was only determined when the response amplitude was significantly larger than the background amplitude. By trial-and-error adjustment to remove aberrant points, we defined a significant amplitude to be 1.3 times larger than the maximum amplitude in the 1 ms period immediately preceding the onset of incus motion (when the incus response reached 10% of its peak value), or in the period between 6 and 11 ms after the click pulse. With the zero-crossing method, the response frequency was taken to be the reciprocal of 2× the time between adjacent zero crossings. The response frequency was also determined from the largest-amplitude component of the short-term FFT, as described above. The FFT and zero-crossing methods produced similar frequency measurements except near a sharp dip in the response. While the short-term FFT method was good for measuring the response frequency as a function of time, it was not satisfactory for comparing the phases of two response wave forms because the frequencies of their maximal responses could be different and phases measured from different frequencies cannot be unambiguously compared. Thus, for phase comparisons, only the zero-crossing method was used.
RESULTS
Click responses without MOC stimulation
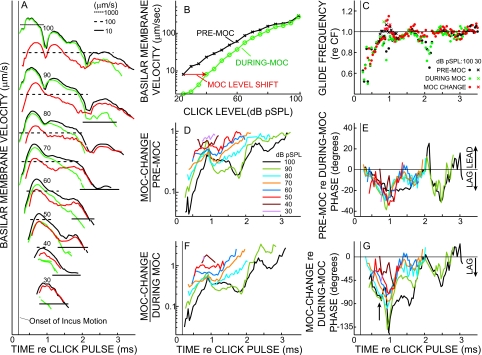
Before MOC stimulation, BM responses to clicks (Figs. 1234) were consistent with those previously reported from the cochlear basal turn of squirrel monkeys, guinea pigs, and chinchillas (Rhode and Robles, 1974; Robles et al., 1976; LePage and Johnstone, 1980; Ruggero and Rich 1990, 1991a, 1991b; Ruggero and Rich, 1991a, 1991b; 1992a, Ruggero, et al., 1992b,1993, 1996; Nuttall and Dolan, 1993; de Boer and Nuttall, 1997; Recio et al. 1998; Recio and Rhode, 2000). The instantaneous frequencies of the responses increased from well below the local CF, to the local CF, over approximately the first millisecond of the BM click response [Fig. 3(c)], in a characteristic pattern called a “glide” (de Boer and Nuttall, 1997; Shera, 2001a). The amplitude of the click response grew quickly, peaked, and then declined. As click level was increased, the initial half-cycle of the response grew approximately linearly with level, while later cycles showed varying degrees of compressive growth, particularly at higher sound levels (Figs. 23), a response pattern that is consistent with previous reports (Robles et al., 1976; Recio et al. 1998). The declining part of the click response (the click “skirt”) often showed waxing and waning [Fig. 3(a)] that were more prominent in animals with good thresholds. The predominance in animals with good thresholds indicates that waxing and waning are not due to pathology (Recio et al., 1998).
Figure 2.
Waveforms showing MOC effects on BM click responses as a function of click level. BM velocity in response to clicks at seven levels, prior to (thin black) and during (dashed green) the effects of MOC stimulation, and the MOC change (thick red; pre-MOC minus during-MOC velocities). Velocities all on the same scale (left) or normalized to the peak of each pre-MOC response (right). GP3096. CF=18 kHz.
Figure 3.
Analyses of MOC effects on BM click responses in one experiment. (A) BM click-response amplitudes from short-term FFTs for pre-MOC (black), during-MOC (green), and MOC-change (red) [same color code applies to (B) and (C)] for eight click levels. The numbers show the click level in dB pSPL. Responses at different levels are shown on the same scale, but are displaced vertically for clarity. The horizontal lines show amplitude registrations (key at top). (B) Pre-MOC (crosses) and during-MOC (circles) click-response amplitudes vs click level, showing the level shift produced by MOC stimulation (arrow). The click-response amplitude is the BM velocity at the frequency with the most energy in the FFT of the entire response [see Fig. 7(a) for full spectra]. (C) Glide frequency as a function of time for the highest and lowest click levels (see key). (D) Relative inhibition as a function of time, as shown by the MOC-change amplitude divided by the pre-MOC amplitude, both derived from short-term FFTs (see See. 2) with click level as a parameter [color key at right also applies to (E)–(G)]. (E) The phase of the pre-MOC response relative to the during-MOC response with click level as a parameter. (F) Relative inhibition shown by the normalized MOC change, which is the MOC-change amplitude divided by during-MOC amplitude (during-MOC chosen instead of pre-MOC because it is closer to the passive response). (G) The phase of the MOC change relative to the during-MOC response, with click level as a parameter. The arrow indicates the time when the phase difference at the highest levels best shows the phase of traveling-wave amplification (see Sec. 4). GP3144. CF=13.5 kHz.
Figure 4.
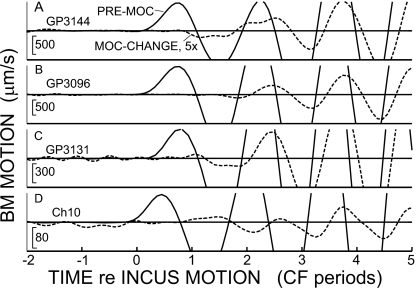
Expanded views of the BM click-response onsets in three guinea pigs and one chinchilla (rows), illustrating the absence of significant MOC changes in the first half-cycle of the response. Each row shows the pre-MOC response (solid line) and the MOC-change (dashed line), which is multiplied by 5. In each row, the amplitude scales at the bottom left are for the pre-MOC responses and the animal is indicated in the top left. The CF’s (top to bottom), are 13.5, 18, 12, and 8.5 kHz.
Click responses with MOC stimulation
To successfully study the effects of MOC stimulation on BM click responses, the preparation had to have both a sensitive BM response and well-positioned MOC electrodes that resulted in a substantial MOC effect. A metric for the quality of the preparation that includes both factors is the MOC-induced level shift in the BM response to low-level clicks [Fig. 3(b)]. The data considered here are from the six guinea pigs with the largest MOC-evoked level shifts of BM click responses (7–15 dB) and the chinchilla with the largest level shift (5 dB). Figures 3, 6, and 7 show data from the guinea pig with the largest level shift (GP3144, shift=15 dB). Data from the two guinea pigs with the next highest level shifts (GP3096, shift=11 dB, and GP3131, shift=7.5 dB) and from the chinchilla are shown in the Supplementary Material (http:∕∕www.aip.org∕pubservs∕epaps.html).
Figure 6.
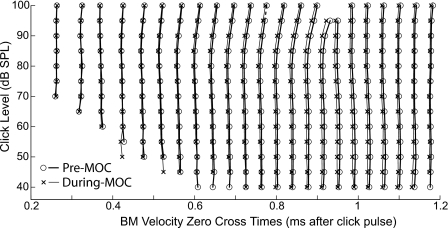
Click-response zero-crossing times before (circles and thick lines) and during (crosses and thin lines) MOC effects for click levels at 5 dB intervals. Note that the pattern of changes produced by MOC stimulation does not correspond to the pattern of changes produced by changing the sound level. Guinea pig 3144.
Figure 7.
The spectra of click responses (top) and of the MOC change (bottom) for guinea pig 3144. The lines are for click levels at 10 dB intervals, alternating solid and dashed, with the numbers indicating the pSPLs of the adjacent solid lines.
The overall features of the MOC effects on BM click responses were similar across all animals, although there were considerable variations in click-response features across animals. MOC stimulation reduced the overall amplitude of the click response, but sometimes briefly increased the instantaneous amplitude near an envelope minimum [Fig. 3(a)]. The biggest percentage reductions (i.e., the MOC change as percentage of the pre-MOC response) occurred towards the declining end of the responses, primarily at low sound levels [Fig. 2 (right), and Figs. 3(d) and 3(f)], but the biggest absolute reductions (i.e., the absolute values of the MOC change) occurred at high click levels near the peaks of the responses [Fig. 2 (left), and Fig. 3(a) red lines]. We did not see any “dc” base line shifts that could be attributed to MOC effects, although we were only measuring MOC “fast effects” so any base line shifts accompanying MOC “slow effects” would not have been seen. Artifactual base line shifts were sometimes induced by shock-evoked animal motion, but these were effectively removed by expressing the motion as BM velocity.
Inhibition near response onsets
Since ANIP inhibition occurs primarily at the onset of AN responses, we looked particularly closely for MOC inhibition near the onset of BM click responses. No significant inhibition was seen during the first half-cycle of the BM responses, and after that the inhibition grew progressively, at least until the first minimum in the envelope of the response (Figs. 234). The MOC inhibition started near zero at the beginning of the response, whether viewed as the MOC change [Fig. 3(a) red lines] or as MOC change normalized to the during-MOC response [Fig. 3(f)] (the during-MOC response was used for normalization because it is closer to the passive response than the pre-MOC response). For comparison, in Fig. 3(d) we also show the MOC change normalized by the pre-MOC response. The values for the first half-cycles are missing in Fig. 3 because none of the MOC-change responses were bigger than our criterion of 1.3 times the base line variation of the responses. The beginning parts of the responses from three guinea pigs and the chinchilla are shown on an expanded scale in Fig. 4. Significant MOC changes do not occur until the third or fourth half-cycle of the responses. The data show that, within the resolution of the experiment (a few percent of the pre-MOC response), there is no change on the first half-cycle of the response. Thus, there is no MOC-induced change in basal-turn BM motion that corresponds to the MOC inhibition of the ANIP response.
Since the effect of MOC stimulation on ANIP responses was much greater for rarefaction than condensation clicks (Guinan et al., 2005), we looked closely throughout the BM click responses for differences in the responses to rarefaction versus condensation clicks. Other than the fact that the responses had opposing polarities, we were unable to find any differences between the responses to condensation and rarefaction clicks.
Inhibition in the declining part of the click response
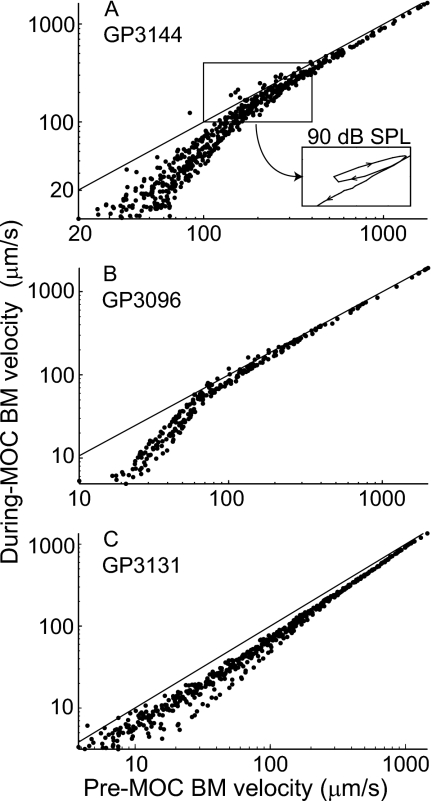
During the declining part of click responses, MOC stimulation increased the overall decay rate of the responses [with greater effects when the responses reached low levels, as shown in Figs. 3(a) and 5] although this pattern was complicated somewhat by the responses’ waxing and waning. The MOC-induced decrease depended primarily on the instantaneous level of the response rather than the click level (Fig. 5). Put another way, no matter whether the response was evoked by a high-level click and monitored several milliseconds after the click, or by a low-level click and monitored near its peak, whenever the instantaneous value of the pre-MOC click-response amplitude reached a given value [e.g., 30 μm∕s in Fig. 5(b)] the value of the during-MOC response measured at the corresponding time and click level was approximately the same [e.g., 10 μm∕s in Fig. 5(b)]. Aberrations from this trend were mostly related to the response waxing and waning [Fig. 5(a), inset]. In some animals, the inhibition appeared to increase when the response decayed to a certain amplitude of BM motion [Fig. 5(b)] whereas in others the inhibition changed only gradually with BM-motion amplitude [Fig. 5(c)].
Figure 5.
BM velocity during MOC stimulation vs before MOC stimulation for responses to clicks in three guinea pigs. Each panel shows data from all click levels superimposed. Note that, in each animal, the points from different click levels fall approximately along the same locus. The inset in (A) shows the data from 90 dB SPL clicks to illustrate the pattern across the period of an envelope minimum. The aberrations induced by such response waning ranged from a loop, as shown in the inset in (A), to a small jump to the side (not illustrated). The diagonal lines illustrate equality, where the during-MOC and pre-MOC velocities are the same.
MOC stimulation had little effect on either the timing or the prominence of waxing and waning in the click responses [Fig. 5(a)]. As shown by the normalized MOC change, the inhibition had regions of growth and regions of relative constancy, especially at moderate-to-high click levels [Fig. 3(f) also see Figs. S1(F), and S2(F)]. Furthermore, similar “plateau” levels of inhibition were reached across a variety of click levels [e.g., see the plateaus at fractional changes of 0.1–0.4 in Figs. 3(F), S1(F), and S2(F)]. These regions of approximately constant (relative) inhibition occurred despite substantial variation in the amplitude of the underlying motion {e.g., almost an order-of-magnitude variation in click-response amplitude at the highest level in GP3131 [Fig. S2(F)], and even greater variations across click levels–compare Figs. 3(a) and 3(f)}. This finding does not seem to fit with the common picture that the gain of the traveling-wave amplifier increases steadily as the motion decreases, for BM response amplitudes that show compressive nonlinearity. However, this view came from BM responses to tones and from the whole of click responses (e.g., Recio et al., 1998), whereas here we are looking at short periods within the decay of click responses, decays which show prominent waxing and waning. Despite the periods of constant versus increasing relative inhibition, if the dispersion introduced by the waxing and waning is ignored, the overall trend is for more inhibition at lower sound levels. Since the inhibitory plateaus are most prominent at high click levels, one might think that constant MOC inhibition is produced when the response amplitude is above some critical level. However, responses to the highest-level clicks in GP3144 and GP3131 [Figs. 3(f) and S2(F)] show a second plateau later in the response, when the response amplitudes were much smaller. What causes waxing and waning and regions of constant versus increasing inhibition remains to be elucidated.
MOC-evoked changes in response phase
The phase changes evoked by MOC stimulation are of considerable interest because they can provide insight into how traveling-wave amplification is accomplished. MOC stimulation produced substantial changes in the phases of click-evoked BM responses [Figs. 3(e) and 3(g); also see 6, S1(E), S1(G), and S2(E), and S2(G)], but these phase changes generally occurred over many cycles so that the response frequencies were little changed. MOC stimulation therefore produced little change in onset glide frequencies or in click-skirt frequencies [Figs. 3(c), S1(C), and S2(C)]; some details of these changes are presented in the Supplementary Material. Figure 3(g) shows the phase of the MOC change relative to the during-MOC response. We used the during-MOC response as the reference, instead of the pre-MOC response, because it is closer to the passive response and normalizing in this way is better for showing the changes in the phase of traveling-wave amplification relative to the passive drive that elicited the amplification (see Sec. 4).
MOC-induced changes in phase varied widely with click level and the time after the click, especially near any minima in the response envelopes [Figs. 3(f) and 3(g)]. The most consistent phase changes occurred during the period from the response onset to just before the first minimum in the response envelope. During this period, the phase of the normalized MOC change grew from near zero to a lag of 70°–90° at the highest sound levels, with the change being less at lower sound levels [Fig. 3(g), arrow]. The finding of variation in the MOC-change phase with level might appear to conflict with the generalization that the click-response zero crossings do not change with level in the onset glide (de Boer and Nuttall, 1997; Recio et al., 1998; Shera, 2001b). However, this generalization is only an approximation (e.g., Fig. 6). Zero crossings at high levels that occur shortly after the start of the response are delayed relative to those at low levels in guinea pigs (Fig. 6), chinchillas, and cats (Recio et al., 1998; Recio and Rhode, 2000; Lin and Guinan, 2000). Note that the patterns of the zero-crossing delays with increasing level versus the advances with MOC stimulation are quite different. The MOC-induced changes are relatively level insensitive whereas the level-induced changes include both small advances as level increases at low-to-moderate levels (up to 80 dB in Fig. 6) and larger delays at high levels (above 80 dB in Fig. 6).
The MOC-induced phase change can be seen in a different way from the comparison of the pre-MOC and during-MOC phases [Fig. 3(e)]. Before the first minimum in the response envelope, the pre-MOC phase consistently lagged the during-MOC phase with the lag starting near zero and growing over time to reach 20–30° at about the time of the first envelope minimum. This pattern changed little with sound level [Figs. 3(e) and 6]. Overall, in both sets of phase data [Figs. 3(e) and 3(g)], the phase change started near zero and increased until about the time of the first minimum. Since there is little traveling-wave amplification at the response onset, where the dominant frequency is far below CF, the lack of a change in the phase near the onset is not surprising. After the onset, the MOC-induced phase changes built up approximately in parallel with the glide frequency approach to CF [Fig. 3(c)]. In the declining part of the response after the first envelope minimum, the phase change decreased and generally approached zero in a pattern that varied widely across level and across animals.
Spectra, and spectral changes in BM click responses
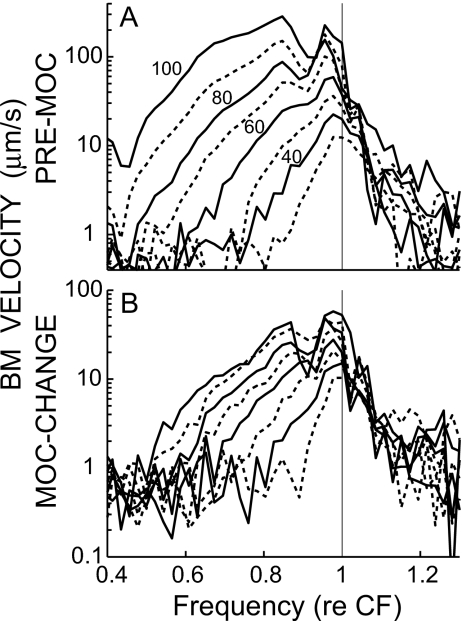
Before MOC stimulation, the spectra of our BM click responses [Fig. 7(a), also see S3(A) and S3(C)] were very similar to those described for the chinchilla by Recio et al. (1998). At low levels, the spectra were centered near CF and were relatively smooth functions of frequency, but at higher levels the energy spread out in the low-frequency direction, the peak energy moved to below CF, and deep dips appeared near and above CF. During MOC stimulation, the overall spectra of BM click responses looked similar to the spectra before MOC stimulation, but there were systematic differences between these as shown by the MOC-change spectra.
The spectra of the MOC changes [Fig. 7(b)] resembled previously described patterns of MOC-induced changes in BM responses to tones (reviewed by Cooper and Guinan, 2006). The largest changes (relative to the pre-MOC responses) were near CF and at low sound levels. The MOC-evoked changes increased in absolute terms, but decreased in a relative sense as sound level increased, with complicated effects at high levels, particularly above CF (Fig. 7). Interestingly, the MOC changes extended to almost an octave below CF [i.e., to near 0.5 CF in Fig. 7(b)], although the changes at these low frequencies were only small fractions of the pre-MOC responses.
DISCUSSION
Our major findings are as follows: (1) MOC stimulation inhibited BM click responses in a time- and intensity-dependent manner (Figs. 234). (2) No inhibition was seen during the first half-cycle of BM click responses (Fig. 4) (in stark contrast to the strong inhibition observed in recent ANIP studies). (3) MOC stimulation caused the click responses to decay faster than normal, particularly at low amplitudes of BM motion (Fig. 5), but had little effect on the timing of waxing and waning [Figs. 2 and 3(a)]. (4) MOC-evoked changes in click-response spectra were largest near CF at low click levels, but spread well below CF at high click levels (Fig. 7). (5) The initial part of during-MOC click responses showed a small (⩽30°) fine-structure phase advance relative to pre-MOC click responses, with the result that the MOC-change phase-lagged the during-MOC response by 70°–90° at the highest levels [Figs. 3(e), 3(g), and 6].
MOC effects in the basal turn
The MOC effects on click responses in the basal turn show exactly what would be expected for MOC inhibition of amplification of the classic traveling wave, but did not show any correlate of MOC inhibition of the ANIP response found by Guinan et al. (2005), which is contrary to what would be expected if the classic traveling wave continued to the apex and produced the ANIP response. As expected for MOC inhibition of the classic traveling wave, the inhibition was greatest (in a relative sense) at low click levels, where the whole response was inhibited. At higher click levels, the pattern of MOC inhibition (none during the first half-cycle, growing over the next few cycles, and waxing and waning later) was similar to the pattern of traveling-wave amplification predicted from the nonlinearities of click responses (Recio et al., 1998). In tone responses, MOC inhibition is greatest at low levels near CF and decreases for frequencies above and below CF (reviewed by Cooper and Guinan, 2006). A similar pattern was seen in the spectra of MOC effects on click responses, when the MOC change is considered relative to the pre-MOC response (Fig. 7). The MOC-change spectra are above the noise floor down to an octave below CF where they are an order-of-magnitude less than the pre-MOC response (Fig. 7, see S3). This click-response MOC change extends to further below CF than has been reported for MOC effects on tone responses (Dolan et al., 1997; Murugasu and Russell, 1996; Cooper and Guinan 2006). In part, this is due to the fact that the MOC change includes MOC effects on both the magnitude and the phase of the responses, whereas only magnitude changes were considered for MOC effects on tone responses However, MOC effects of 1 dB or less on the magnitude of tone responses are not easily distinguished from noise and could have been overlooked in previous studies.
In contrast to the clear MOC inhibition of traveling-wave amplification, there was no inhibition of the first peak of the click response that might correspond to the MOC inhibition of the ANIP response that has been found in the middle and apical cochlea by Guinan et al. (2005). Guinan et al. (2005) could not determine if ANIP inhibition was present in the cochlear base because basal-turn AN responses have inadequate synchrony compared to the local CF. However, basal-turn AN responses show MOC inhibition at certain tail frequencies and this inhibition may be a tone correlate of ANIP inhibition (Guinan et al., 2005). Previous studies of MOC effects on BM tone responses have reported finding no MOC inhibition at tail frequencies (Dolan et al., 1997; Murugasu and Russell, 1996; Cooper and Guinan, 2006); however, these studies did not focus on looking for the BM tail-frequency inhibition that corresponds to the neural tail-frequency inhibition, which is more than an octave below CF, and they could easily have missed small, frequency-specific tail effects. Furthermore, the signal-to-noise ratio of laser-based BM measurements is considerably lower at tail frequencies than at basal-turn CFs. Thus, whether or not there is MOC inhibition of BM motion at tail frequencies more than one octave below CF is still an open question. In summary, in the cochlear base, MOC inhibition of BM motion appears to be due only to inhibition of traveling-wave amplification and a BM-motion correlate of the ANIP motion has not been found.
The origin of waxing and waning
The waxing and waning in the click-response envelope appear to be due to beats between vibrations at two near-CF frequencies (Recio et al., 1998; Lin and Guinan, 2000), but little is known about these vibrations. The waxing and waning period was not changed by MOC stimulation, which suggests that the frequencies involved were not changed. Note that two near-CF resonances were found in the BM impedance calculated from an inverse solution based on BM responses to tones (Zweig, 1991). If one presumes that there are two resonances that are independent and are excited with the same phase at response onset, then the two resonances should become out of phase and cancel at times: Tc=(2n+1)π∕ΔF, where n is an integer, and ΔF is the difference in frequency between the two resonances. Thus, the first cancellation should be after one unit of time, with subsequent cancellations at intervals of twice this. This is not the pattern of BM click responses (Figs. 23, S1, and S2). Hypotheses that fit the data better are that the resonances are excited in opposite phases or that they are strongly coupled and exchange energy.
Interference of two motion components has also been seen in high-level tone data as response dips in BM motion (Rhode and Recio, 2000; Guinan and Cooper, 2003; Rhode, 2007). MOC stimulation inhibits one of these components more than the other (Guinan and Cooper, 2003) in a way that accounts for the MOC-induced increase in BM motion first noted by Dolan et al. (1997). It might seem that the two factors that produce the tone-response dips also produce the click-response waxing and waning. Arguing against this possibility are the following: (1) The tone-response dips are seen only at high levels whereas the click-response waxing and waning can also be found at low levels. (2) Tone-response dips are seen each time the traveling-wave phase crosses the same value (plus or minus an integer number of cycles), indicating that the non-traveling-wave component has a very high velocity and may be an evanescent or compression wave (Cooper and Rhode, 1996; Dong and Cooper, 2006;Rhode, 2007). Since evanescent waves are not generally “tuned” to a particular CF, they are highly unlikely to produce the waxing and waning in the click response. A more likely solution is that other forms of tuned motion exist within the organ of Corti and TM complex, perhaps motions similar to those that have been observed in various in vitro preparations (e.g., Nowotny and Gummer, 2006; Karavitaki and Mountain, 2007a). The most general conclusion that can be drawn from this is that both tone and click data do not fit with the idea that a single traveling wave excites a single mode of BM and organ of Corti vibration, even in the base of the cochlea.
Phase changes and traveling-wave amplification
The phases of BM click responses were changed in complex ways by MOC stimulation, but at least some aspects of these changes can be understood. For instance, it might seem surprising that MOC stimulation decreased the phase (i.e., decreased the latency of the waveform fine structure) of the BM click response, considering that MOC stimulation increases the latency of AN CAPs (Gifford and Guinan, 1987). However, the BM latency change can be understood as being consistent with the hypotheses that (1) traveling-wave amplification produces a delay in the BM click-response wave form and (2) the MOC reduction of this amplification reduces this delay, i.e., produces a phase advance. It seems likely that the decreased delays of human transient-evoked otoacoustic emissions produced by MOC activity evoked by contralateral sound (Ryan et al., 1991; Giraud et al., 1996) were due to decreases in BM response delays similar to those found here.
One motivation for looking at MOC-induced changes in BM response phase is that they may give clues to the processes of cochlear amplification. The best estimates of the phase of traveling-wave amplification come from mathematical “inversion” of BM measurements (e.g., de Boer and Nuttall, 2000), which show that traveling-wave amplification for a tone is due to a cochlear-partition impedance that has negative damping (i.e., energy injection in phase with the motion) just basal to the BM CF place. In contrast, the impedance of the cochlear partition at the CF place has positive damping (energy absorption). In a simplified vector explanation, in response to pressure, the negative damping impedance would produce a response phase of −180°, while the stiffness-dominated passive response would have a phase of −90°. Thus, in this simplified view, the response component from cochlear amplification would lag the passive response by 90°.
How do we compare the results of this mathematical inversion based on tone responses with our click data? In an attempt to glean what we can about cochlear amplification phase from our click responses, we restrict our attention to the most telling part of the response. First, we consider only high-level responses where the during-MOC response is most heavily influenced by the passive response, but the MOC change still represents removal of the contribution from the cochlear amplifier. Second, we restrict consideration to times when the glide frequency is at or near CF, but avoid comparisons of phases during the decaying portion of the click responses because these are complicated by the waxing and waning of the response [the phase of the main Fourier component of the response almost reverses at a deep minimum, as illustrated by Recio et al., 1998]. Under these conditions [shown by the arrow in Fig. 3(g)], the phase of the normalized MOC change was a lag of 70°–90°, which is similar to the 90° predicted by our simplified analysis that presumed cochlear amplification is produced by negative damping. Although it focuses on only one part of the click response, this analysis of the MOC change provides an independent measure consistent with the hypothesis that traveling-wave amplification is accomplished by negative damping of the cochlear partition.
Implications for cochlear mechanics in the middle and apical cochlea
Our finding of almost no MOC inhibition of the earliest cycle of the basal-turn BM click response contrasts with the strong inhibition found in the ANIP responses in the middle and apex of the cochlea (Guinan et al., 2005). We presume that this BM-AN difference is not due to the species difference (guinea pig versus cat), but this must be checked in future work. Another possible difference is that the BM data were from somewhat damaged preparations whereas the AN data were from undamaged preparations. This seems unlikely to explain the BM-AN difference because we saw no change in the pattern of BM effects with different degrees of damage, and because there was less than a 10 dB threshold deterioration in some animals (e.g., guinea-pig 3144 and chinchilla 10) so the condition of these animals was similar to the condition for much of the AN data.
Several other factors can be ruled out as candidates for producing the ANIP inhibition. A small (∼1 dB) inhibition at tail frequencies is produced by the MOC-induced decrease in endocochlear potential (EP), but this change in EP cannot account for the ANIP inhibition because it is too small and cannot act only on the first peak (similarly, it cannot account for the 10 dB inhibition of AN responses at certain tail frequencies because it is too small and not frequency selective; Stankovic and Guinan, 1999). Alternately, it has been suggested that MOC activation produces a stiffness change in OHCs (He and Dallos, 2000; but see Hallworth, 2007) and this stiffness change might be thought to produce the ANIP inhibition. However, the putative OHC stiffness change occurred slowly and appeared to be associated with MOC slow effects, which our methods exclude. Furthermore, any fast stiffness change would be expected to alter the first cycle of the BM response, which we did not see. In addition, any MOC-evoked stiffness change would be present throughout the click response and should affect much more than just the first cycle of the responses: in particular, the characteristic frequency of the click response would be affected strongly, which is inconsistent with our data.
There are two important differences in the AN versus BM data: (1) the CF difference, i.e., the ANIP response was found in the apical half of the cochlea (CFs<6 kHz) whereas the BM click responses were measured in the basal turn, and (2) the measurement location, i.e., AN versus BM. We note that nothing corresponding to the ANIP inhibition was seen in BM responses at the chinchilla 8.5 kHz place [Fig. 4(d)], a frequency only slightly above 6 kHz where ANIP inhibition was found in the cat. Unfortunately, there are no data available for MOC effects on BM motion in the middle or apical part of the cochlea. Furthermore, for the middle or apical part of the cochlea, there are no BM data from preparations that have been demonstrated to have normal thresholds (the lowest CF with good BM data is 6 kHz—Rhode, 2007).
The classic traveling-wave view is not sufficient to account for the ANIP data
The common conception of cochlear mechanics throughout the cochlea is an extrapolation of BM motion in the basal turn where it can be measured and the normality of the preparation can be checked with AN CAPs. In models, the classic traveling wave is the motion of the cochlear partition produced by a slow, apically moving, sound-frequency pressure difference across the cochlear partition acting on the impedance of the cochlear partition (e.g., see Patuzzi, 1996). Based on the gradual change in cochlear anatomy from base to apex, classic cochlear models assume that the traveling wave exists, and scales with the local CF, throughout the cochlea. In this conception, the traveling wave produces all of the excitation of the AN, with little or no room for any intervening filtering or active processes (e.g., Patuzzi, 1996; Narayan et al., 1998). However, this conception is not compatible with the finding that there is no MOC inhibition of the first peak of basal-turn BM motion.
If a frequency-scaled version of the traveling wave shown by basal-turn BM motion contained all of the motion that produces AN firing throughout the cochlea, then the first peak of the classic traveling wave must be the drive for the ANIP response, and the MOC inhibition of the ANIP response would be present throughout the cochlea. This follows because with the classic traveling-wave hypothesis, the form of BM motion in the base is the form of the traveling wave that must exist throughout the cochlea. Thus, if there is no MOC inhibition of the first peak of the traveling wave in the base, then the same is true throughout the cochlea. However, since we see MOC inhibition of the first peak of the AN response in the middle and apex of the cochlea, then the classic traveling wave cannot be directly responsible for the ANIP response. We infer that some other motion must produce the ANIP response.
Note that with the classic-traveling-wave hypothesis, the earliest peak of the neural click response must come from the first peak of the traveling-wave click response, because the amplitude of the traveling-wave response grows gradually from one peak to the next. If the second or a later traveling-wave peak excites a neural response, then the first peak of the traveling wave will excite a response simply by using a higher click level. Similarly, if one supposes that the ANIP response is not produced by motion that forms a part of the traveling wave, then this means it must come from some other motion. Either way, we conclude that some motion other than the traveling wave must produce the ANIP response.
Could the traveling-wave change from base to apex enough to account for the data?
One might argue that the traveling wave changes in form from base to apex and this change in form is sufficient for the traveling wave in the middle and apex to account for the ANIP inhibition. For this to be true, the first peak of the traveling wave must change enough to be inhibited by MOC stimulation, but the rest of the traveling wave must stay sufficiently the same to account for the other aspects of the apical AN responses, which show the expected properties of the classic traveling wave. Traditional cochlear models show that the first peak of the traveling wave is from below-CF energy and is passive (e.g., Shera, 2001a). Such cochlear models would need serious revision to accommodate a “traveling wave” that shows MOC inhibition of the first peak. While it cannot be said that this “proves” that the traveling wave could not change enough from the classic traveling wave to make ANIP inhibition possible, the necessity of a drastic change in the first peak (or first two half-cycles) but little or no change in the rest of the traveling wave poses a great difficulty for this hypothesis. The alternative, that there is a non-traveling-wave motion that produces the ANIP response, is far more attractive. The conceptual framework for this conclusion is that cochlear motion is the sum of vibrational modes: The traditional traveling wave is one vibrational mode and the inferred ANIP motion is another. Since there is little evidence for the ANIP motion in the base, we assume that in the base the traditional traveling wave is the dominant motion, but as one moves apically, the role of the ANIP motion gradually increases. Thus, although not compelling, the most parsimonious explanation of the data is that in addition to the classic traveling wave (which exists in scaled forms throughout the cochlea—Patuzzi, 1996), there is another motion, the ANIP motion, that is negligible (or not discernable) in the base and grows in prominence towards the apex.
With this conceptual framework, the BM motion in the apex may, or may not, show the ANIP motion, depending on whether the ANIP motion vibrational mode includes significant movement of the BM. Mechanical measurements of click responses in the cochlear apex show weakly nonlinear growth of the first peak (Cooper and Rhode, 1996), which may have the same origin as the ANIP motion, a conjecture that awaits experimental verification.
If both the traveling wave and the inferred ANIP motion contribute to BM motion in the apical half of the cochlea, then the nonlinearity seen in BM motion will depend on both motions. Strong MOC inhibition of traveling-wave amplification occurs when there is strong nonlinearity in the BM input-output function. If this is also true for the ANIP motion, then the strong ANIP inhibition may indicate that there is strong nonlinearity in the growth of the ANIP motion. If the first peak of the apical BM motion is a combination of a linear first-peak response to the classic traveling wave and a highly nonlinear ANIP motion vibrational mode, then the strong ANIP motion nonlinearity might be considerably watered down by the linear first peak of the traveling wave, when seen in BM motion.
Two cochlear amplifiers?
The MOC inhibitions of the classic traveling wave and the inferred ANIP motion appear to be separate phenomena, and raise the question of whether there are actually two mechanical amplifiers in the mammalian cochlea. Traveling-wave amplification is shown by basal-turn BM motion and AN responses throughout the cochlea, and all aspects of this amplification appear susceptible to MOC inhibition. The ANIP motion, inferred from AN responses in the apical half of the cochlea, is also strongly inhibited by MOC stimulation, which indicates that it comes from, or is strongly influenced by, OHCs. Since these are due to two separate motions (as argued above), they reveal the presence of two separate ways in which OHCs influence cochlear motions that drive IHC stereocilia and excite auditory nerve fibers. If the inferred ANIP motion is produced or enhanced by the action of OHCs, it could be called a second cochlear amplifier. The MOC inhibition of this motion is consistent with such a hypothesis. However, whether OHCs amplify, or simply influence, the inferred ANIP motion remains to be determined.
With two modes of OHC-influenced motion that excite AN responses, and two known forms of OHC motility, a natural question is whether each form of motility primarily affects one type of motion. One possibility is that somatic motility produces traveling-wave amplification, and hair-bundle motility amplifies or influences the inferred ANIP motion. Somatic motility extends well beyond 50 kHz (Scherer and Gummer, 2004), high enough in frequency to provide basal-turn amplification. Hair-bundle motility shows asymmetry with the direction of motion, at least in nonmammalian vertebrates (Jaramillo and Hudspeth, 1993; Ricci et al., 2000), potentially making it able to amplify or influence the inferred ANIP motion and produce more inhibition for rarefaction clicks than for condensation clicks. However, the ANIP asymmetry may have other origins (e.g., in IHC mechanoelectrical transduction, or in the IHC-AN synapse) and may not require an asymmetric mechanical drive. Alternately, it may be that the two forms of OHC motility may interact (Kennedy et al., 2006). More evidence is needed to determine the relationship between the forms of OHC motility and the traveling wave and ANIP motions.
What is the ANIP motion?
There are several possibilities for what the inferred ANIP motion might correspond to physically, but no data that reveal its identity. Two possibilities for the ANIP motion that involve OHC somatic motility are fluid flow past IHC stereocilia due to reticular-lamina tilting (Nowotny and Gummer, 2006), and∕or motion from fluid flow along the tunnel of Corti due to OHCs squeezing the organ of Corti (Karavitaki and Mountain, 2007a). Another possibility for the ANIP motion is radial motion of the TM (Hemmert et al., 2000), which has recently been shown to propagate longitudinally along the TM (Ghaffari et al., 2007). TM radial motion might originate from, or be influenced by, stereocilia motility. The ANIP motion may arrive at a given cochlear place before the classical traveling wave (Guinan et al., 2005) but this does not help us distinguish the origin of the ANIP response because either fluid flow along the tunnel of Corti or fast propagation of a radial shear wave along the TM might account for this. In summary, work in excised preparations shows several motions that may correspond to the ANIP motion, but there are no data tying any of these to the ANIP motion, so the exact identity of the ANIP motion, and whether a component of it is apparent in BM motion, are unknown.
What have we learned?
Previous data show cochlear motions other than the classic traveling wave and indicate that cochlear scaling breaks down in the apex (e.g., Nowotny and Gummer, 2006; Karavitaki and Mountain, 2007a,b; Shera and Guinan, 2003), so one might ask the following: “What is new here?” The breakdown of scaling in the apex is usually thought of as indicating that the apical traveling wave loses its basal-turn form, not, as indicated here, that a second motion becomes important enough to drive AN firing. Further, the breakdown of scaling is usually thought to be restricted to the extreme apex (in the cat, a 1 kHz transition point is typically given), whereas the data of Guinan et al. (2005) indicate that the ANIP motion extends at least up to 6 kHz, which includes the middle and apex of the cochlea. Although work in excised preparations has shown that the cochlea can support non-traveling-wave cochlear motions (at least when driven electrically), the importance of these motions in the intact cochlea is unknown. This paper, plus the data of Guinan et al. (2005), provides the first credible arguments from intact, normally functioning, acoustically stimulated cochleae, that a non-traveling-wave motion that excites AN fibers is produced or amplified by the action of OHCs. In summary, the present manuscript (1) rules out that the classic traveling wave can fully explain cochlear mechanics in the middle and apex of the cochlea, (2) puts strong constraints on models that attempt to explain both basal and apical cochlear mechanics, and (3) provides evidence from normal cochleae that strongly points to there being two modes of cochlear excitation affected by active processes, thus raising the possibility that there is another “cochlear amplifier” in addition to the classic traveling-wave amplifier.
ACKNOWLEDGEMENTS
We thank Dr. M.C. Liberman and Dr. C. Shera for comments on an earlier version of the manuscript. Supported by NIDCD RO1DC00235, the Royal Society, and Deafness Research UK.
NOMENCLATURE
- AN
Auditory nerve
- ANIP
Auditory nerve initial peak
- BM
Basilar membrane
- CAP
Compound action potential
- CF
Characteristic frequency
- during-MOC
During the MOC effect
- EP
Endocochlear potential
- FFT
Fast Fourier transform
- IHC
Inner hair cell
- MOC
Medial olivocochlear
- MOC-change
Pre-MOC minus during MOC
- OHC
Outer hair cell
- pre-MOC
Before MOC effect
- pSPL
Peak equivalent SPL
- SPL
Sound pressure level
- TM
Tectorial membrane
References
- Cooper, N. P. (1999). “An improved heterodyne laser interferometer for use in studies of cochlear mechanics,” J. Neurosci. Methods 10.1016/S0165-0270(99)00017-5 88, 93–102. [DOI] [PubMed] [Google Scholar]
- Cooper, N. P., and Guinan, J. J., Jr. (2003). “Separate mechanical processes underlie fast and slow effects of medial olivocochlear efferent activity,” J. Physiol. (London) 10.1113/jphysiol.2003.039081 548, 307–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper, N. P., and Guinan, J. J., Jr. (2006). “Efferent-mediated control of basilar membrane motion,” J. Physiol. (London) 576, 49–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper, N. P., and Rhode, W. S. (1992). “Basilar membrane mechanics in the hook region of cat and guinea-pig cochleae: Sharp tuning and nonlinearity in the absence of baseline position shifts,” Hear. Res. 10.1016/0378-5955(92)90083-Y 63, 163–190. [DOI] [PubMed] [Google Scholar]
- Cooper, N. P., and Rhode, W. S. (1996). “Fast travelling waves, slow travelling waves and their interactions in experimental studies of apical cochlear mechanics,” Aud. Neurosci. 2, 289–299. [Google Scholar]
- Dallos, P., He, D. Z., Lin, X., Sziklai, I., Mehta, S., and Evans, B. N. (1997). “Acetylcholine, outer hair cell electromotility, and the cochlear amplifier,” J. Neurosci. 17, 2212–2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallos, P., Zheng, J., and Cheatham, M. A. (2006). “Prestin and the cochlear amplifier,” J. Physiol. (London) 10.1113/jphysiol.2006.114652 576, 37–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Boer, E., and Nuttall, A. L. (1997). “The mechanical waveform of the basilar membrane. I. Frequency modulations (“glides”) in impulse responses and cross-correlation functions,” J. Acoust. Soc. Am. 10.1121/1.418319 101, 3583–3592. [DOI] [PubMed] [Google Scholar]
- de Boer, E., and Nuttall, A. L. (2000). “The mechanical waveform of the basilar membrane. II. From data to models—and back,” J. Acoust. Soc. Am. 10.1121/1.428435 107, 1487–1496. [DOI] [PubMed] [Google Scholar]
- Dolan, D. F., Guo, M. H., and Nuttall, A. L. (1997). “Frequency-dependent enhancement of basilar membrane velocity during olivocochlear bundle stimulation,” J. Acoust. Soc. Am. 10.1121/1.421008 102, 3587–3596. [DOI] [PubMed] [Google Scholar]
- Dong, W., and Cooper, N. P. (2006). “An experimental study into the acousto-mechanical effects of invading the cochlea,” J. R. Soc., Interface 3, 561–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fettiplace, R. (2006). “Active hair bundle movements in auditory hair cells,” J. Physiol. (London) 576, 29–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridberger, A., Widengren, J., and Boutet de Monvel, J. (2004). “Measuring hearing organ vibration patterns with confocal microscopy and optical flow,” Biophys. J. 86, 535–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghaffari, R., Aranyosi, A. J., and Freeman, D. M. (2007). “Longitudinally propagating traveling waves of the mammalian tectorial membrane,” Proc. Natl. Acad. Sci. U.S.A. 10.1073/pnas.0703665104 104, 16510–16515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gifford, M. L., and Guinan, J. J., Jr. (1987). “Effects of electrical stimulation of medial olivocochlear neurons on ipsilateral and contralateral cochlear responses,” Hear. Res. 10.1016/0378-5955(87)90166-3 29, 179–194. [DOI] [PubMed] [Google Scholar]
- Giraud, A. L., Perrin, E., Chery Croze, S., Chays, A., and Collet, L. (1996). “Contralateral acoustic stimulation induces a phase advance in evoked otoacoustic emissions in humans,” Hear. Res. 10.1016/0378-5955(96)00002-0 94, 54–62. [DOI] [PubMed] [Google Scholar]
- Guinan, J. J., Jr. (1996). “The physiology of olivocochlear efferents,” in The Cochlea, edited by Dallos P. J., Popper A. N., and Fay R. R. (Springer-Verlag, New York: ), pp. 435–502. [Google Scholar]
- Guinan, J. J., Jr., and Cooper, N. P. (2003). “Fast effects of efferent stimulation on basilar membrane motion,” in The Biophysics of the Cochlea: Molecules to Models, edited by Gummer A. W., Dalhoff E., Nowotny M., and Scherer M. P. (World Scientific, Singapore: ), pp. 245–251. [Google Scholar]
- Guinan, J. J., Jr., and Stankovic, K. M. (1996). “Medial efferent inhibition produces the largest equivalent attenuations at moderate to high sound levels in cat auditory-nerve fibers,” J. Acoust. Soc. Am. 10.1121/1.416066 100, 1680–1690. [DOI] [PubMed] [Google Scholar]
- Guinan, J. J., Jr., Lin, T., and Cheng, H. (2005). “Medial-olivocochlear-efferent inhibition of the first peak of auditory-nerve responses: Evidence for a new motion within the cochlea,” J. Acoust. Soc. Am. 10.1121/1.2017899 118, 2421–2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallworth, R. (2007). “Absence of voltage-dependent compliance in high-frequency cochlear outer hair cells,” J. Assoc. Res. Otolaryngol. 8, 464–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He, D. Z., and Dallos, P. (2000). “Properties of voltage-dependent somatic stiffness of cochlear outer hair cells,” J. Assoc. Res. Otolaryngol. 1, 64–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemmert, W., Zenner, H. P., and Gummer, A. W. (2000). “Three-dimensional motion of the organ of Corti,” Biophys. J. 78, 2285–2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaramillo, F., and Hudspeth, A. J. (1993). “Displacement-clamp measurement of the forces exerted by gating springs in the hair bundle,” Proc. Natl. Acad. Sci. U.S.A. 10.1073/pnas.90.4.1330 90, 1330–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karavitaki, K. D., and Mountain, D. C. (2007a). “Evidence for outer hair cell driven oscillatory fluid flow in the tunnel of corti,” Biophys. J. 10.1529/biophysj.106.084087 92, 3284–3293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karavitaki, K. D., and Mountain, D. C. (2007b). “Imaging electrically evoked micromechanical motion within the organ of Corti of the excised gerbil cochlea,” Biophys. J. 92, 3294–3316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy, H. J., Evans, M. G., Crawford, A. C., and Fettiplace, R. (2006). “Depolarization of cochlear outer hair cells evokes active hair bundle motion by two mechanisms,” J. Neurosci. 10.1523/JNEUROSCI.3808-05.2006 26, 2757–2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LePage, E. L., and Johnstone, B. M. (1980). “Nonlinear mechanical behavior of the basilar membrane in the basal turn of the guinea pig cochlea,” Hear. Res. 10.1016/0378-5955(80)90056-8 2, 183–189. [DOI] [PubMed] [Google Scholar]
- Lin, T., and Guinan, J. J., Jr. (2000). “Auditory-nerve-fiber responses to high-level clicks: Interference patterns indicate that excitation is due to the combination of multiple drives,” J. Acoust. Soc. Am. 10.1121/1.428648 107, 2615–2630. [DOI] [PubMed] [Google Scholar]
- Lin, T., and Guinan, J. J., Jr. (2004). “Time-frequency analysis of auditory-nerve-fiber and basilar-membrane click responses reveal glide irregularities and non-characteristic-frequency skirts,” J. Acoust. Soc. Am. 10.1121/1.1753294 116, 405–416. [DOI] [PubMed] [Google Scholar]
- Murugasu, E., and Russell, I. J. (1996). “The effect of efferent stimulation on basilar membrane displacement in the basal turn of the guinea pig cochlea,” J. Neurosci. 16, 325–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayan, S. S., Temchin, A. N., Recio, A., and Ruggero, M. A. (1998). “Frequency tuning of basilar membrane and auditory nerve fibers in the same cochleae,” Science 10.1126/science.282.5395.1882 282, 1882–1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowotny, M., and Gummer, A. W. (2006). “Nanomechanics of the subtectorial space caused by electromechanics of cochlear outer hair cells,” Proc. Natl. Acad. Sci. U.S.A. 10.1073/pnas.0511125103 103, 2120–2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuttall, A. L., and Dolan, D. F. (1993). “Basilar membrane velocity responses to acoustic and intracochlear electric stimuli,” in Biophysics of Hair-Cell Sensory Systems, edited by Duifhuis H., Horst J. W., van Dijk P., and van Netten S. M. (World Scientific, Singapore: ), pp. 288–294. [Google Scholar]
- Patuzzi, R. (1996). “Cochlear micromechanics and macromechanics,” in The Cochlea, edited by Dallos P. J., Popper A. N., and Fay R. R. (Springer-Verlag, New York: ), pp. 186–257. [Google Scholar]
- Recio, A., Rich, N. C., Narayan, S. S., and Ruggero, M. A. (1998). “Basilar-membrane responses to clicks at the base of the chinchilla cochlea,” J. Acoust. Soc. Am. 10.1121/1.421377 103, 1972–1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recio, A., and Rhode, W. S. (2000). “Basilar membrane responses to broadband stimuli,” J. Acoust. Soc. Am. 10.1121/1.1318898 108, 2281–2298. [DOI] [PubMed] [Google Scholar]
- Rhode, W. S. (2007). “Basilar membrane mechanics in the 6–9 kHz region of sensitive chinchilla cochleae,” J. Acoust. Soc. Am. 10.1121/1.2718397 121, 2792–2804. [DOI] [PubMed] [Google Scholar]
- Rhode, W. S., and Recio, A. (2000). “Study of mechanical motions in the basal region of the chinchilla cochlea,” J. Acoust. Soc. Am. 10.1121/1.429404 107, 3317–3332. [DOI] [PubMed] [Google Scholar]
- Rhode, W. S., and Robles, L. (1974). “Evidence from Mössbauer experiments for nonlinear vibration in the cochlea,” J. Acoust. Soc. Am. 10.1121/1.1914569 55, 588–597. [DOI] [PubMed] [Google Scholar]
- Ricci, A. J., Crawford, A. C., and Fettiplace, R. (2000). “Active hair bundle motion linked to fast transducer adaptation in auditory hair cells,” J. Neurosci. 20, 7131–7142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robles, L., Rhode, W. S., and Geisler, C. D. (1976). “Transient response of the basilar membrane measured in squirrel monkeys using the Mössbauer effect,” J. Acoust. Soc. Am. 10.1121/1.380953 59, 926–939. [DOI] [PubMed] [Google Scholar]
- Ruggero, M. P., and Rich, N. C. (1990). “Systemic injection of furosemide alters the mechanical response to sound of the basilar membrane,” in The Mechanics and Biophysics of Hearing, edited by Dallos P., Geisler C. D., Matthews D. B., Ruggero M., and Steele C. R.(Springer-Verlag, Berlin: ). [Google Scholar]
- Ruggero, M. A., and Rich, N. C. (1991a). “Furosemide alters organ of Corti mechanics: Evidence for feedback of outer hair cells upon the basilar membrane,” J. Neurosci. 11, 1057–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruggero, M. A., and Rich, N. C. (1991b). “Application of a commercially-manufactured Doppler-shift laser velocimeter to the measurement of basilar-membrane vibration,” Hear. Res. 10.1016/0378-5955(91)90038-B 51, 215–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruggero, M. A., Robles, L., Rich, N. C., and Recio, A. (1992a). “Basilar membrane responses to two-tone and broadband stimuli,” Philos. Trans. R. Soc. London, Ser. B 336, 307–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruggero, M. A., Robles, L., and Rich, N. C. (1992b). “Two-tone suppression in the basilar membrane of the cochlea: Mechanical basis of auditory-nerve rate suppression,” J. Neurophysiol. 68, 1087–1099. [DOI] [PubMed] [Google Scholar]
- Ruggero, M. A., Rich, N. C., and Recio, A. (1993). “Alteration of basilar membrane responses to sound by acoustic overstimulation,” in Biophysics of Hair-Cell Sensory Systems, edited by Duifhuis H., Horst J. W., van Dijk P., and van Netten S. M. (World Scientific, Singapore: ), pp. 258–265. [Google Scholar]
- Ruggero, M. A., Rich, N. C., and Recio, A. (1996). “The effect of intense acoustic stimulation on basilar-membrane vibrations,” Aud. Neurosci. 2, 329–345. [Google Scholar]
- Russell, I. J., and Nilsen, K. E. (1997). “The location of the cochlear amplifier: spatial representation of a single tone on the guinea pig basilar membrane,” Proc. Natl. Acad. Sci. U.S.A. 10.1073/pnas.94.6.2660 94, 2660–2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan, S., Kemp, D. T., and Hinchcliffe, R. (1991). “The influence of contralateral acoustic stimulation on click-evoked otoacoustic emission in humans,” Br. J. Audiol. 25, 391–397. [DOI] [PubMed] [Google Scholar]
- Shera, C. A. (2001a). “Frequency glides in click responses of the basilar membrane and auditory nerve: Their scaling behavior and origin in traveling-wave dispersion,” J. Acoust. Soc. Am. 10.1121/1.1366372 109, 2023–2034. [DOI] [PubMed] [Google Scholar]
- Shera, C. A. (2001b). “Intensity-invariance of fine time structure in basilar-membrane click responses: Implications for cochlear mechanics,” J. Acoust. Soc. Am. 10.1121/1.1378349 110, 332–348. [DOI] [PubMed] [Google Scholar]
- Shera, C. A., and Guinan, J. J., Jr. (2003). “Stimulus-frequency-emission group delay: A test of coherent reflection filtering and a window on cochlear tuning,” J. Acoust. Soc. Am. 10.1121/1.1557211 113, 2762–2772. [DOI] [PubMed] [Google Scholar]
- Scherer, M. P., and Gummer, A. W. (2004). “Vibration pattern of the organ of Corti up to 50 kHz: Evidence for resonant electromechanical force,” Proc. Natl. Acad. Sci. U.S.A. 10.1073/pnas.0408232101 101, 17652–17657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sridhar, T. S., Liberman, M. C., Brown, M. C., and Sewell, W. F. (1995). “A novel cholinergic “slow effect” of olivocochlear stimulation on cochlear potentials in the guinea pig,” J. Neurosci. 15, 3667–3678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stankovic, K. M., and Guinan, J. J., Jr. (1999). “Medial efferent effects on auditory-nerve responses to tail-frequency tones I: Rate reduction,” J. Acoust. Soc. Am. 10.1121/1.427102 106, 857–869. [DOI] [PubMed] [Google Scholar]
- Wilson, J. P., and Johnstone, J. R. (1975). “Basilar membrane and middle-ear vibration in guinea pig measured by capacitive probe,” J. Acoust. Soc. Am. 10.1121/1.380472 57, 705–723. [DOI] [PubMed] [Google Scholar]
- Zweig, G. (1991). “Finding the impedance of the organ of Corti,” J. Acoust. Soc. Am. 10.1121/1.400653 89, 1229–1254. [DOI] [PubMed] [Google Scholar]