Abstract
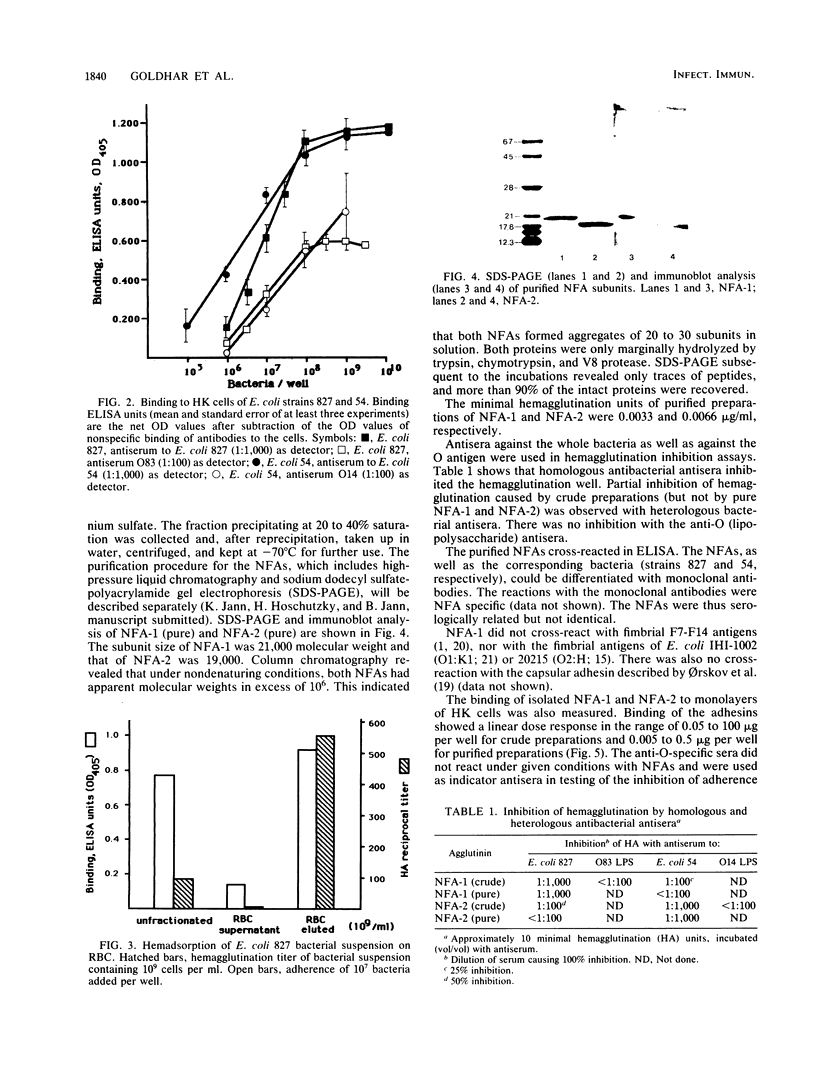
Nonfimbrial, mannose-resistant hemagglutinins (nonfimbrial adhesions [NFA] NFA-1 and NFA-2) were extracted from two agar-grown urinary isolates of Escherichia coli strains 827 (O83:K1:H4) and 54 (O14:K?:H11). The proteins were purified to homogeneity by ammonium sulfate precipitation and column chromatography. Nonfimbrial adhesins are soluble proteins, which tend to form aggregates of molecular weight above 10(6). NFA-1 and NFA-2 consist of subunits of 21,000 and 19,000 molecular weight, respectively. Both hemagglutinins caused hemagglutination of human erythrocytes and bound to human kidney cell monolayers. The binding of bacteria and hemagglutinins was assessed by using suitable antisera as detectors in an enzyme-linked immunosorbent assay. NFA-1 and NFA-2 inhibited the adherence of their respective strains to human kidney cells in a linear dose response. NFA-2 also inhibited heterologous strain adherence, but NFA-1 did not. Hemabsorption of bacterial suspension with erythrocytes at 4 degrees C, followed by differential centrifugation, enabled us to obtain a bacterial suspension lacking nonfimbrial adhesins in the supernatant and an adhesin-enriched bacterial suspension that was eluted from erythrocytes at 40 degrees C. Bacteria eluted from erythrocytes exhibited a higher adherence capacity than unfractionated cells. Bacteria of the fraction lacking adhesins did not adhere to human kidney cells. Electron microscope examinations showed the presence of an extracellular capsule-like layer in adhering E. coli 827, but not in nonadhering bacteria. E. coli 54 did not express the adhesin as a capsule. We conclude that E. coli 827 and 54 produce extracellular adhesins consisting of soluble proteins which are differently expressed and antigenically distinct. The adhesins seem to share a common receptor and mediate the adherence of two uropathogenic E. coli strains to epithelial cells.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe C., Schmitz S., Moser I., Boulnois G., High N. J., Orskov I., Orskov F., Jann B., Jann K. Monoclonal antibodies with fimbrial F1C, F12, F13, and F14 specificities obtained with fimbriae from E. coli 04:K12:H-. Microb Pathog. 1987 Jan;2(1):71–77. doi: 10.1016/0882-4010(87)90116-1. [DOI] [PubMed] [Google Scholar]
- Bayer M. E., Thurow H. Polysaccharide capsule of Escherichia coli: microscope study of its size, structure, and sites of synthesis. J Bacteriol. 1977 May;130(2):911–936. doi: 10.1128/jb.130.2.911-936.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duguid J. P., Clegg S., Wilson M. I. The fimbrial and non-fimbrial haemagglutinins of Escherichia coli. J Med Microbiol. 1979 May;12(2):213–227. doi: 10.1099/00222615-12-2-213. [DOI] [PubMed] [Google Scholar]
- Evans D. G., Evans D. J., Jr, Clegg S., Pauley J. A. Purification and characterization of the CFA/I antigen of enterotoxigenic Escherichia coli. Infect Immun. 1979 Aug;25(2):738–748. doi: 10.1128/iai.25.2.738-748.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hacker J., Schmidt G., Hughes C., Knapp S., Marget M., Goebel W. Cloning and characterization of genes involved in production of mannose-resistant, neuraminidase-susceptible (X) fimbriae from a uropathogenic O6:K15:H31 Escherichia coli strain. Infect Immun. 1985 Feb;47(2):434–440. doi: 10.1128/iai.47.2.434-440.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ip S. M., Crichton P. B., Old D. C., Duguid J. P. Mannose-resistant and eluting haemagglutinins and fimbriae in Escherichia coli. J Med Microbiol. 1981 May;14(2):223–226. doi: 10.1099/00222615-14-2-223. [DOI] [PubMed] [Google Scholar]
- Korhonen T. K., Väisänen V., Saxén H., Hultberg H., Svenson S. B. P-antigen-recognizing fimbriae from human uropathogenic Escherichia coli strains. Infect Immun. 1982 Jul;37(1):286–291. doi: 10.1128/iai.37.1.286-291.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labigne-Roussel A. F., Lark D., Schoolnik G., Falkow S. Cloning and expression of an afimbrial adhesin (AFA-I) responsible for P blood group-independent, mannose-resistant hemagglutination from a pyelonephritic Escherichia coli strain. Infect Immun. 1984 Oct;46(1):251–259. doi: 10.1128/iai.46.1.251-259.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labigne-Roussel A., Schmidt M. A., Walz W., Falkow S. Genetic organization of the afimbrial adhesin operon and nucleotide sequence from a uropathogenic Escherichia coli gene encoding an afimbrial adhesin. J Bacteriol. 1985 Jun;162(3):1285–1292. doi: 10.1128/jb.162.3.1285-1292.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mett H., Kloetzlen L., Vosbeck K. Properties of pili from Escherichia coli SS142 that mediate mannose-resistant adhesion to mammalian cells. J Bacteriol. 1983 Feb;153(2):1038–1044. doi: 10.1128/jb.153.2.1038-1044.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Normark S., Lark D., Hull R., Norgren M., Båga M., O'Hanley P., Schoolnik G., Falkow S. Genetics of digalactoside-binding adhesin from a uropathogenic Escherichia coli strain. Infect Immun. 1983 Sep;41(3):942–949. doi: 10.1128/iai.41.3.942-949.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orskov I., Birch-Andersen A., Duguid J. P., Stenderup J., Orskov F. An adhesive protein capsule of Escherichia coli. Infect Immun. 1985 Jan;47(1):191–200. doi: 10.1128/iai.47.1.191-200.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orskov I., Orskov F. Serology of Escherichia coli fimbriae. Prog Allergy. 1983;33:80–105. [PubMed] [Google Scholar]
- Parkkinen J., Finne J., Achtman M., Väisänen V., Korhonen T. K. Escherichia coli strains binding neuraminyl alpha 2-3 galactosides. Biochem Biophys Res Commun. 1983 Mar 16;111(2):456–461. doi: 10.1016/0006-291x(83)90328-5. [DOI] [PubMed] [Google Scholar]
- Schmidt G., Jann B., Jann K. Genetic and immunochemical studies on Escherichia coli O14:K7:H-. Eur J Biochem. 1974 Feb 15;42(1):303–309. doi: 10.1111/j.1432-1033.1974.tb03340.x. [DOI] [PubMed] [Google Scholar]
- Sheladia V. L., Chambers J. P., Guevara J., Jr, Evans D. J. Isolation, purification, and partial characterization of type V-A hemagglutinin from Escherichia coli GV-12, O1:H-. J Bacteriol. 1982 Nov;152(2):757–761. doi: 10.1128/jb.152.2.757-761.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanislawski L., Simpson W. A., Hasty D., Sharon N., Beachey E. H., Ofek I. Role of fibronectin in attachment of Streptococcus pyogenes and Escherichia coli to human cell lines and isolated oral epithelial cells. Infect Immun. 1985 Apr;48(1):257–259. doi: 10.1128/iai.48.1.257-259.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svenson S. B., Källenius G., Möllby R., Hultberg H., Winberg J. Rapid identification of P-fimbriated Escherichia coli by a receptor-specific particle agglutination test. Infection. 1982;10(4):209–214. doi: 10.1007/BF01666912. [DOI] [PubMed] [Google Scholar]
- Walz W., Schmidt M. A., Labigne-Roussel A. F., Falkow S., Schoolnik G. AFA-I, a cloned afimbrial X-type adhesin from a human pyelonephritic Escherichia coli strain. Purification and chemical, functional and serologic characterization. Eur J Biochem. 1985 Oct 15;152(2):315–321. doi: 10.1111/j.1432-1033.1985.tb09200.x. [DOI] [PubMed] [Google Scholar]
- Williams P. H., Knutton S., Brown M. G., Candy D. C., McNeish A. S. Characterization of nonfimbrial mannose-resistant protein hemagglutinins of two Escherichia coli strains isolated from infants with enteritis. Infect Immun. 1984 Jun;44(3):592–598. doi: 10.1128/iai.44.3.592-598.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]




