Abstract
In recent years, a call for increased research on bipolar disorder has been answered with methodologically diverse studies exploring goal striving, life events, cognitive style, decision-making, and neurobiological abnormalities in bipolar disorder. In order to further this spurt of research and to systematize our understanding of bipolar disorder, an integrative perspective is warranted. The behavioral approach system (BAS) dysregulation theory, proposed by Richard Depue and colleagues, provides such an integrated model for understanding psychosocial and biological aspects of bipolar disorder. In this paper, we review studies on life events, cognitive style and other psychosocial and neurobiological factors to examine whether the BAS dysregulation theory is supported by existing data. Then, we draw on recent advances in the study of emotion and motivation, and propose an expansion of the BAS dysregulation model of bipolar spectrum disorders to foster further biopsychosocial investigations of bipolar disorder. This expanded model provides greater specificity in predictions, especially about the nature of BAS dysregulation, environmental factors and psychological processes (e.g., appraisal processes) featured in a causal chain culminating in bipolar symptoms. Finally, we discuss the implications of the expanded BAS model for the course of bipolar spectrum disorders.
Keywords: Bipolar Disorder, Behavioral Approach System (BAS), Goal Striving, Hypomania, Mania, Depression
…my classwork during these galvanized periods seemed straightforward, and I found examinations, laboratory work, and papers almost absurdly easy during the weeks that the high-flying times would last. I would also become immersed in a variety of political and social causes (….) But then as night inevitably goes after the day, my mood would crash, and my mind again would grind to a halt. I lost all interest in my schoolwork, friends, reading, wandering, and daydreaming.
- Kay Redfield Jamison (1995, p. 43)
In the U.S., approximately 4.4% of the population will experience a form of bipolar disorder during their lifetime (Merikangas et al., 2007). Bipolar disorder is a phenomenon of extreme contrasts both in mood (hypomanic/manic euphoria and irritability vs. depression) and in functioning. As illustrated by certain famous musicians, writers, actors, politicians, etc., suspected to suffer from bipolar disorder (see Goodwin & Jamison, 1990) and a study of prevalence among writers (Andreasen, 1987), bipolar disorder is associated with achievement and artistic creativity. However, it is also coupled with severe impairment in many areas of functioning, like erratic work performance, high rates of divorce, and substance abuse (Goodwin & Jamison, 1990; Harrow et al., 1990; Strakowski, DelBello, Fleck, & Arndt, 2000). Individuals with bipolar disorder also have 12.3 times higher rates of suicide compared to the general population (Angst, Stassen, Clayton, & Angst, 2002). Given that bipolar disorder is the sixth leading cause of disability among physical and psychological disorders worldwide (Murray & Lopez, 1996), it also has significant cost for society. Within bipolar disorder, a group of disorders appears to form a continuum or spectrum of severity from the milder cyclothymia, to bipolar II disorder, to full-blown bipolar I disorder (Akiskal, Djenderedijian, Rosenthal, & Khani, 1977; Akiskal, Khani, & Scott-Strauss, 1979; Cassano et al., 1999; Depue et al., 1981; Goodwin & Jamison, 1990). And, milder forms of bipolar disorder often progress to the more severe forms (e.g., Akiskal et al., 1977; 1979; Shen, Alloy, Abramson, & Grandin, in press). However, not all research supports a bipolar spectrum model idea (e.g., Baldessarini, 2000).
Comprehensive understanding of bipolar disorder requires integration of empirical investigations that often stand on opposite poles of the psychosocial-biological spectrum. For example, genetic work has revealed who is likely to develop a bipolar spectrum disorder. A recent study of monozygotic and dizygotic twins estimated genetic heritability of bipolar disorder at 85% (McGuffin et al., 2003). But, psychosocially oriented research plays a crucial role in exploring the course of bipolar disorder (e.g., exact timing of episodes, a specific episode’s polarity [hypomania/mania vs. depression], predominance of depression vs. hypomania/mania, etc.; see Johnson & Kizer, 2002). In this regard, Akiskal (1986, p. 671) speculated, “…what is transmitted are these affectively disregulated temperaments and that the progression to full-blown bipolar illness is due to environment.” Two decades ago, researchers proposed one such integrative, psychobiological model of bipolar disorder. Specifically, they proposed that weak regulation of the behavioral approach system1 (BAS) might be involved in the rollercoaster of hypomanic/manic highs and depressive lows that characterize bipolar spectrum disorders (Depue, Krauss & Spoont, 1987; Depue & Iacono, 1989; Fowles, 1988, 1993; Gray, 1991).
Briefly, according to the BAS dysregulation model, individuals with bipolar disorder experience extreme fluctuations in activation and deactivation of the BAS, a system involved in approach to reward, which are reflected in bipolar symptoms. The BAS dysregulation model is scientifically compelling because it hints at both specific psychosocial factors and specific neurobiological systems that may be relevant to bipolar disorder. The BAS has been hypothesized as functioning to get an organism in contact with a reward or goal and as triggered by incentive cues in reward paradigms and safety cues (both internal and external) in active avoidance paradigms (e.g., Depue & Collins, 1992; Depue & Iacono, 1989; Gray, 1991; Fowles, 1987). At the behavioral level, locomotor initiation, incentive-reward motivation, positive affect, anger, and complex cognitions are viewed as central components of the activated BAS (e.g., Depue & Collins, 1999; Harmon-Jones & Allen, 1998; Harmon-Jones & Sigelman, 2001; see also Carver, 2004). Fowles’ (1988) hypothesis that obstructed reward is a trigger for BAS-related irritability suggests that environmental cues likely influence whether a high BAS activation state is euphoric or irritable. On the other hand, an individual in a state of low BAS activation should experience anhedonia, decreased energy, and exhibit few, if any, approach behaviors. Although BAS-relevant cognitions are often overlooked, Fowles (e.g., 1988, 1993) related a high outcome expectancy of success to BAS activation and hopelessness to BAS deactivation or shut down.
At the neurobiological level, Depue and colleagues (e.g., Depue & Collins, 1999; Depue & Iacono, 1989) hypothesized that the BAS involves dopaminergic (DA) projections from the A10 nucleus in the ventral tegmental area (VTA) to the frontal cortex, amygdala, nucleus accumbens, ventral pallidum, septum, and hippocampus, with the DA activity in nucleus accumbens playing the central role. In addition, Depue and colleagues (e.g., Depue & Iacono, 1989) implicated specific frontal cortex regions, like dorsomedial prefrontal cortex, anterior cingulate cortex (ACC), orbitofrontal cortex (OFC), and dorsolateral prefrontal cortex (DLPFC) in the neurobiological underpinnings of BAS. Considerable research on BAS functioning in humans has focused on left frontal cortical activity as another neurobiological index of this system (for review see Davidson, 1994, 1999). Studies have found a significant positive relationship between relative left frontal cortical activity, as measured by electroencephalogram (EEG), and self-report measures of sensitivity of the BAS (Harmon-Jones & Allen, 1997; Sutton & Davidson, 1997), experimentally manipulated reward motivational states (Miller & Tomarken, 2001; Sobotka et al., 1992), and a positive dispositional affective style (for review see, Davidson, 1994; Davidson, Jackson, & Kalin, 2000).
Moreover, the model provides a single theme—level of approach motivation—to organize an apparently diverse array of symptoms (e.g., motor, affective, cognitive, vegetative) and account for both poles of bipolar disorder—depression and hypomania/mania. Researchers have debated whether bipolar disorder is better conceptualized as two independent, comorbid illnesses (i.e., depression and mania/hypomania) or as one illness (e.g., Joffe, Young, & MacQueen, 1999). According to the BAS dysregulation model, individuals with bipolar disorder have a single vulnerability, a dysregulated BAS, but polarity-specific triggers for depressive and hypomanic/manic episodes. The one-illness perspective distinguishes the present summary of BAS relevant data and theory from other reviews that focused on one pole only (e.g., for mania see Johnson, 2005; for depression see Mansell, Colom, & Scott, 2005).
Finally, the BAS dysregulation model’s clinical significance can be discerned from bipolar individuals’ descriptions of the extreme “adrenaline rush” as they take on a challenge of obtaining a reward, as well as in their descriptions of the “crashes” in their mood and energy level that sometimes follow. The quotation that began this article provides a potent example.
Early Formulation of the BAS Dysregulation Theory of Bipolar Spectrum Disorders
Depue, Fowles, and others provided groundwork for a BAS dysregulation theory. In this section, we integrate these initial views into a succinct theoretical statement. Early formulations of the BAS dysregulation theory posited that hypomanic/manic and depressive symptoms are reflections of an overly active and inactive BAS, respectively (e.g., Depue et al., 1987; Depue & Iacono, 1989). Vulnerability to bipolar spectrum disorders lies in a dysregulated BAS (e.g., Depue et al., 1987). In brief, individuals with weak BAS regulation are hyperresponsive to relevant environmental stimuli and the symptoms of bipolar disorder reflect extreme manifestations (i.e., affect, thoughts, behaviors) of BAS activity or inactivity.
Thus, once the BAS is (over)activated in individuals with bipolar spectrum disorders, these individuals experience greatly increased energy levels, pressured speech, decreased need for sleep, flight of ideas, increased involvement in goal-directed behaviors, and exaggerated self-confidence in ability to obtain goals, often without regard for negative consequences. In other words, they become hypomanic/manic (DSM-IV-TR, 2000). Furthermore, according to the BAS dysregulation theory, the extreme positive affect (i.e., euphoria, expansiveness) and irritability/anger of hypomania/mania also reflect a highly activated BAS (e.g., Depue et al., 1987; Depue & Iacono, 1989; Fowles, 1988; Gray, 1994). Finally, the theory links bipolar depression to decreased activation of BAS (e.g., Fowles, 1988; Depue et al., 1987; Depue & Iacono, 1989).
According to the BAS dysregulation theory, then, individuals prone to bipolar disorder exhibit trait-like hyperresponsivity of the BAS to relevant stimuli (i.e., the inherited vulnerability or diathesis for bipolar spectrum disorders). This vulnerability, in turn, leads such individuals to experience great variability in their state levels of BAS activation over time and across situations. In other words, due to dysregulation, the BAS of individuals at risk for bipolar spectrum disorders is predisposed to greater peaks and troughs than the BAS of individuals not at risk for these disorders. Of interest, Depue et al. (1987) further suggested that individuals with bipolar spectrum disorders have genetically predetermined mean trait levels of BAS (i.e., average level of BAS activation across time and situations) that predict which type of episodes (hypomanic/manic vs. depressive) will predominate the course of their bipolar illness. For example, the lower the mean, the more likely it is that the course of the disorder will be predominantly depressed. However, due to BAS dysregulation, there will be great variability of BAS activation around the mean.
According to Depue and colleagues (1987; Depue & Iacono, 1989), relevant environmental stimuli—presence or absence of rewards, safety cues, or punishment—exhibit great direct influence on state BAS activation and thereby help predict variability in BAS response among individuals with weak regulation of the BAS (e.g., individuals with bipolar disorder). Thus, environmental influences are suggested to play an important role in determining the exact timing of change in BAS activation and in partially determining the magnitude of this change. Finally, the model suggested that strength of current BAS state activation influences not only response to environmental stimuli but also their evaluation (e.g., more stimuli are pleasurable in a high BAS state; e.g., Depue & Collins, 1992; Depue et al., 1987).
Current State of Affairs in Research on Bipolar Disorder
The BAS dysregulation model of bipolar disorder provides researchers with an integrative model to guide methodologically diverse studies towards more efficient exploration of bipolar disorder phenomena. Next, we review seemingly disparate studies on life events, cognitive style and other psychosocial factors, and neurobiological abnormalities in bipolar disorder. Although the present review is not exhaustive, it is distinctive in its integration of psychosocial and neurobiological data (for review focused on reward system neurobiology data, see Leibenluft, Charney, & Pine, 2003), as well as in its discussion of the data’s significance for the current BAS dysregulation model and their implications for expansion of the model (e.g., causal chain of processes).
Life Events
A seminal paper by Johnson and Roberts (1995) first explicated the implications of psychobiological models of bipolar disorder, like the BAS dysregulation model, for life events research on bipolar spectrum disorders. Still, research on the role of life events in bipolar spectrum disorders has overwhelmingly focused on the effects of the broadly defined category of negative life events (or stress), especially independent and severe negative events (for review, see Alloy et al., 2005; 2006a,b; Johnson & Roberts, 1995). In recent years, a few studies have explored the role of anger-evoking events, positive life events in general, and goal-attainment events more specifically.
Negative life events
Studies found that the broad category of negative life events predicts episode onset (e.g., Ellicott et al., 1990; Hammen & Gitlin, 1997; Hunt, Bruce-Jones, & Silverstone, 1992; Johnson et al., 1999; Swendsen et al., 1995) and up to 3 times longer recovery time from the episode (Johnson et al., 1999; Johnson & Miller, 1997) in bipolar spectrum disorders. Moreover, one study reported that a greater number of negative events preceded the onset of bipolar than unipolar depression (Perris, 1984). Negative life events have predicted episode onset even in bipolar individuals currently on lithium (Kulhara et al., 1999).
Many of these studies combined depressive and manic/hypomanic episodes in their analyses of the effects of negative life events on episode onset and recovery (e.g., Ellicott et al., 1990; Hammen & Gitlin, 1997; Johnson & Miller, 1997; Kulhara et al., 1999; Swendsen et al., 1995). Other studies found no differences in negative events’ relationship to onset of depressive vs. manic/hypomanic episodes (e.g., Hunt, Bruce-Jones, & Silverstone, 1992; McPherson, Herbinson, & Romans, 1993; Reilly-Harrington, Alloy, Fresco, & Whitehouse, 1999). This latter finding requires further specification by the BAS dysregulation model in the prediction of which negative life events trigger depression vs. hypomania/mania.
Anger-evoking events
Bipolar disorder theorists and researchers alike have largely ignored a subclass of negative life events, anger-evoking events. This is an important oversight given that hypomania/mania at times presents with irritable mood. A study by Harmon-Jones and colleagues (2002) was the first to assess the response of individuals prone to bipolar symptoms to an experimentally manipulated anger-evoking event. In this study, undergraduates prone to bipolar symptomatology, who also paid at least 33% of their own tuition, were experimentally presented with a radio editorial proposing a tuition increase. In this experimental context, proneness to depression was related to lower relative left frontal EEG activity in response to the editorial, whereas proneness to hypomania/mania was related to greater left frontal EEG activity in response to the same editorial.
As was reviewed earlier, increased left frontal EEG activation has been related to increased self-reported BAS sensitivity (Harmon-Jones & Allen, 1997; Sutton & Davidson, 1997). Thus, the Harmon-Jones et al. (2002) findings are consistent with the BAS dysregulation perspective of bipolar disorder that emphasizes the hypersensitivity of the BAS in bipolar disorder to environmental cues. However, their findings also emphasize yet again the need for greater specificity and expansion of the BAS dysregulation model of bipolar disorder in order to explain how the same event triggers both increased and decreased BAS activation, as reflected in greater vs. lesser relative left frontal activation.
Goal-attainment events
Johnson and colleagues’ study (2000) was the first to focus on goal-attainment life events and their effect on the course of bipolar disorder. In this study, life events involving high goal-attainment were significantly related to higher levels of subsequent manic, but not depressive, symptoms. Unlike goal-attainment events, positive events in general did not predict subsequent level of mania or depression. When examining goal pursuit in everyday life, similar results emerge. Krumm-Merabet and Meyer (2005) found that adolescents with hypomanic personality report spending more time in pleasurable activities (e.g., socializing, playing sports) than controls.
Nusslock and colleagues (2007) explored the effects of a goal striving event (a pre-goal attainment event) on the course of bipolar spectrum disorders. The goal striving event in this study was college final exams—a challenge to achieve academic success. Thus, bipolar symptoms were assessed during the final exam period and a control period in two groups—a bipolar spectrum group and a control group with no major psychopathology. Each group was further subdivided into individuals currently attending a university and experiencing final exams and individuals not currently attending a university and not experiencing final exams. Individuals in the bipolar group currently enrolled in a university, unlike the bipolar group not currently enrolled in the university and the whole control group, exhibited more onsets of new hypomanic episodes in the final exam period compared to the control period. Indeed, a full 42% of the bipolar individuals attending the university experienced onset of a new hypomanic episode during the final exam period. This pattern of results was not found for depressive episodes.
Other research has revealed that it is particularly challenging and rewarding events that activate part of the neural circuitry of approach motivation in individuals with bipolar II and cyclothymia (Harmon-Jones et al., in press). That is, as compared to control participants, bipolar participants showed similar levels of relative left frontal cortical activation in response to easy and moderately difficult tasks that promised rewards or punishments. However, when faced with very difficult tasks that promised rewards as compared to punishments, bipolar individuals showed greater left frontal activation compared to controls.
In summary, the BAS dysregulation model, with some revisions, shows great promise to be a parsimonious explanation for the research on life events’ influence on bipolar disorder. Presently, the BAS dysregulation model proposes that individuals with bipolar disorder are hypersensitive to environmental cues. Thus, findings of depression onset and prolonged recovery in response to negative life events are consistent with the model. Also, preliminary data linking goal-attainment events to hypomanic/manic episode onset and symptom exacerbation is also consistent with the BAS dysregulation model. However, the model needs greater specificity and revision in order to address: 1) the specific nature of events that trigger depression vs. hypomania; 2) the specific nature of events that trigger hypomania/mania with irritable vs. euphoric mood; and 3) the ability of negative events to predict both hypomanic/manic and depressive episode onset. In short, the model needs to specify which environmental cues are BAS activation-relevant vs. BAS deactivation-relevant and, thus, likely to lead to hypomania/mania vs. depression.
Cognitive Style and Other Psychosocial Factors
Certain psychological processes known to be involved in unipolar depression have been extended to research on bipolar depression and bipolar spectrum disorders in general (e.g., Alloy et al., 1999; 2005; 2006a,b). This approach has provided some support for similar cognitive styles in unipolar and bipolar depression (Alloy et al., 2005; 2006a,b; Johnson & Kizer, 2002). But, some studies have ventured to identify psychosocial factors that might be specific to bipolar disorder, like specific prodromes, cognitive styles, and outcome expectations (e.g., Lam, Wong, & Sham, 2001; Lam, Wright, & Smith, 2004; Meyer, Beevers, & Johnson, 2004). We examine the relevance of this data for the BAS dysregulation model of bipolar disorder and its implication for the model’s expansion.
Cognitive style
Reviews on cognitive style in bipolar disorder concluded that, although negative cognitive styles characterize unipolar depression and bipolar disorder alike (Alloy et al., 2005; 2006a,b; Johnson & Kizer, 2002), cognitive style in bipolar disorder has a distinctive, BAS-relevant aspect to it (Alloy et al., 2005; 2006a,b). Thus, the cognitive style of bipolar individuals is marked by autonomy, perfectionism, and goal-striving, all BAS-relevant characteristics, whereas there is considerably weaker and more inconsistent support for sociotropic or dependent cognitive style in bipolar disorder (e.g., Alloy et al., 2008b; Francis-Raniere, Alloy, & Abramson, 2006; Hammen, Elicott, Gitlin, & Jamison, 1989).
Recently, Francis-Raniere et al. (2006) examined the effect of various cognitive styles on prospective symptoms among individuals with cyclothymia, bipolar II, and bipolar NOS disorder. They found consistent support for the congruency effect (i.e., an interaction of a cognitive style and congruent events predicting prospective symptoms) only for the BAS-relevant cognitive style involving high self-standards, self-criticism and focus on performance. Specifically, the interaction of this BAS-relevant cognitive style and the occurrence of congruent-negative events predicted prospective increases in depressive symptoms, whereas the interaction of this same cognitive style and congruent-positive events predicted prospective increases in hypomanic symptoms (Francis-Raniere et al., 2006).
This is consistent with findings of greater perfectionistic dysfunctional attitudes among individuals with bipolar I disorder compared to controls (Scott, Stanton, Garland, & Ferrier, 2000). Additionally, in a factor analysis of dysfunctional attitudes among a bipolar I sample, Lam and colleagues (2004) obtained three factors—Goal-Attainment (i.e., striving for positive affect and valuing ability to excel at anything), Dependency (i.e., need for approval), and Achievement (i.e., need to achieve). After controlling for current clinical state, only the Goal-Attainment attitudinal scale differentiated between bipolar disorder and unipolar depression patients, with bipolar patients exhibiting greater scores than unipolar patients. In the whole sample, Goal Attainment dysfunctional attitudes were positively related to number of past hospitalizations for bipolar episodes, and mania in particular (Lam et al., 2004). In addition, whereas positive mood induction in unipolar depression led to decrease of Goal Attainment dysfunctional attitudes, individuals with bipolar disorder did not show this change following the same mood induction (Wright, Lam, & Newsom-Davis, 2005). Similarly, Alloy et al. (2008b) found that compared to matched normal controls, bipolar spectrum individuals exhibited greater BAS-relevant cognitive styles of perfectionism, autonomy, self-criticism, and efficacy, but not greater non-BAS-relevant styles of approval-seeking, sociotropy, and dependency. Moreover, the group differences on BAS-relevant cognitive styles were mediated by differences in BAS sensitivity. Finally, Lozano and Johnson (2001) found that, among bipolar I disorder patients, achievement striving and baseline clinical state predicted 30% of the variance in manic, but not depressive, symptoms during a 6-month period.
Bipolar depressed individuals have exhibited more stable, global causal attributions for negative than positive events, as well as lower self-esteem, and endorsed more negative self-descriptors in comparison to controls (Lyon, Startup, & Bentall, 1999). Similarly, internal, stable, and global attributions for negative events—a signature cognitive style of unipolar depression—in interaction with the occurrence of negative life events has been found to predict prospective increases in bipolar depressive symptoms (Alloy et al., 1999; Reilly-Harrington et al., 1999). On the other hand, internal, stable, global causal attributions about positive events interacted with occurrence of positive life events to predict only prospective increases in hypomanic symptoms (Alloy et al., 1999). Negative inferences about negative events in bipolar depression are likely to be accompanied by an extremely low expectancy of being able to change the situation (low efficacy expectancy, or hopelessness). Thus, these findings are consistent with both the hopelessness theory of depression (Abramson, Metalsky, & Alloy, 1989) and some hypotheses of the BAS dysregulation theory of bipolar disorder (e.g., Fowles, 1988).
Some of the early formulations of BAS dysregulation in bipolar disorder have posited that hopelessness and hopefulness are linked to depression and hypomania/mania, respectively (e.g., Fowles, 1988). The current data suggest that goal-striving and perfectionism are also cognitive styles relevant for bipolar disorder. Thus, the BAS dysregulation model needs to be expanded to incorporate these new findings and specify BAS activation-relevant (i.e., hypomania/mania-relevant) and BAS deactivation-relevant (i.e., bipolar depression-relevant) cognitive styles.
Goal appraisal and outcome expectancy
Studies have explored bipolar spectrum individuals’ appraisals of goals, both within experimental tasks and in relation to real life goals. For example, Meyer, Beevers, and Johnson (2004) found that proneness to hypomania among undergraduates was related to more positive appraisal of major personal goals, whereas proneness to depression was related to more negative appraisal of major personal goals. Moreover, positive goal appraisals were positively related to current hypomania and inversely related to current depression. Another study of a large community sample of adolescents found that hypomanic personality was related to overly optimistic (based on their current academic performance) expectations of long-term career and academic success, whereas current depression was related to negative expectations (Meyer & Krumm-Merabet, 2003).
Stern and Berrenberg (1979) examined the relationship between mania and success expectancies for an anticipated task, following experimentally manipulated success on a skill-task or chance-task. In this study, only individuals with manic tendencies had greater success expectancies on an anticipated chance-task following success feedback on the skill-task. In addition, whereas individuals without manic tendencies attributed only manipulated success on skill-tasks to their own agency, individuals with manic tendencies attributed manipulated success both on skill- and chance-tasks to their own agency. In another study, Ruggero and Johnson (2006) also found bipolar I individuals to have higher success expectancies than controls regardless of the type of manipulated feedback.
Murphy and colleagues (2001) explored decision-making abilities in patients currently in a manic episode. The manic patients, in comparison with controls and depressed patients, tended to choose significantly more often the less likely of two possibilities, and consequently, failed on more trials. This tendency to choose the less likely outcome was positively related to the level of current manic symptoms. In another study, proneness to hypomania was related to setting more difficult goals and a trend for greater success expectancy following a success experience in the laboratory (Johnson, Ruggero, & Carver, 2005).
In sum, there is preliminary support for a positive relationship between hypomania/mania and exaggerated positive appraisal of goal importance and high success expectancy, as well as more tentative support for an inverse relationship between these appraisals and depression. The goal appraisal data with its focus on dysregulated expectancies as bipolar individuals approach rewards and goals is significant for the BAS dysregulation model. However, the role of appraisals and outcome expectancies in the causal chain of the BAS dysregulation model of bipolar disorder has been ignored and expansion of the model is necessary to remedy it.
Prodromes
Lam and colleagues (Lam & Wong, 1997; Wong & Lam, 1999) investigated common prodromes, as well as strategies for coping with prodromes, among individuals with bipolar I disorder. They found that individuals with bipolar I disorder most frequently reported reduced sleep (58.3%) and increased goal-directed activity (55.5%) as first signs of mania, whereas anhedonia was the most frequently reported (44.8%) first sign of depression. Furthermore, individuals with good coping strategies for prodromes, as opposed to those with poor coping skills, were more likely to decrease goal-directed activity (e.g., restrain themselves, take extra time for rest) to cope with mania/hypomania and increase their goal-directed activity (e.g., keep busy, become more social) to cope with depression (Lam & Wong, 1997; Wong & Lam, 1999). In other words, individuals with successful coping strategies reported effortfully regulating their BAS activity. Moreover, over an 18-month period, Lam, Wong, and Sham (2001) found that individuals employing these BAS-relevant coping strategies were less likely to relapse (12.5% manic and 8.3% depressive relapses) than those who were not using these strategies (45.5% manic and 46.2% depressive relapses). Accordingly, consistent with the BAS dysregulation theory, Lam and colleagues (2003) modified traditional cognitive therapy to target excessive goal-striving as well as traditional depressogenic dysfunctional attitudes. This modified cognitive therapy significantly reduced relapse and hospitalization rates over a 1-year period in bipolar I disorder (Lam et al., 2003). These findings further support the BAS dysregulation model of bipolar disorder.
BAS Sensitivity
Self-report data
Development of a self-report measure of BAS sensitivity by Carver and White (1994), the BAS scales of the BIS/BAS scales, has aided recent, direct empirical testing of the BAS dysregulation theory. The BAS scales have positively related to a self-report measure of hypomanic personality (Carver & White, 1994) and to proneness to hypomania (Meyer et al., 1999), in undergraduate samples. Similarly, individuals with bipolar spectrum disorder diagnoses exhibited greater BAS sensitivity than individuals without psychopathology (Urosevic et al., 2008). In addition, bipolar spectrum individuals who exhibited a hypomanic personality trait had greater BAS sensitivity compared to the rest of the bipolar sample (Urosevic et al, 2008). Individuals with a bipolar I diagnosis also exhibited greater BAS sensitivity compared to a normative sample and high BAS sensitivity at the time of recovery predicted an increase in manic symptoms over 6 months (Meyer et al., 2001).
Alloy and colleagues (2006c) employed a behavioral high-risk design and selected individuals with high vs. moderate levels of BAS sensitivity and examined their lifetime histories of mood disorders, current symptoms, and personality. High BAS sensitivity individuals were more likely to receive a bipolar spectrum diagnosis and exhibited greater hypomanic personality and current hypomanic, but not depressive, symptoms, than moderate BAS sensitivity individuals. Moreover, using a prospective design, Alloy et al. (2008a) found that high BAS sensitivity predicted prospective onsets of hypomanic and manic episodes over a 2.5-year follow-up among individuals with bipolar spectrum disorders, controlling for initial depressive and hypomanic symptoms.
Using a different self-report measure of BAS sensitivity, the Sensitivity to Reward scale of the Sensitivity to Punishment and Sensitivity to Reward Questionnaire (SPSRQ), Salavert and colleagues (2006) found individuals with bipolar I disorder to exhibit higher BAS sensitivity than controls. Furthermore, in the same study, bipolar individuals who experienced a hypomanic/manic relapse over 18 months had higher BAS sensitivity at the outset compared to controls, whereas bipolar individuals who experienced a depressive relapse had a non-significant trend towards lower BAS sensitivity compared to controls. These self-report findings support the BAS dysregulation model of bipolar spectrum disorders.
Experience-sampling data
In addition to prospective and retrospective behavioral high-risk designs, another powerful method of studying BAS variability in bipolar disorder is experience-sampling. Accordingly, Depue and colleagues (1981) found that individuals with cyclothymia reported more variability in BAS-relevant behaviors in a 28-day-long experience-sampling study. In another experience-sampling study, after exposure to a naturalistic stressor, individuals with cyclothymia took longer to recover to their pre-stressor levels of BAS behaviors and affect than controls (Goplerud & Depue, 1985).
Several experience-sampling studies have explored motor activity changes within day and across days using actigraphy. For example, Jones, Hare, and Evershed (2005) found both greater intradaily and interdaily variability of motor activity, as measured by actigraphy, among individuals with bipolar I disorder compared to controls. Another study of bipolar I individuals found that higher daytime motor activity level during a euthymic period (on lithium) predicted manic relapses following discontinuation of lithium (Klein et al., 1992). Moreover, review of actigraphy studies in bipolar I disorder samples found that currently depressed individuals exhibited reduced activity, whereas currently manic individuals exhibited increased activity (Teicher, 1995). Given that locomotion is theorized to be an integral part of BAS, these actigraphy studies allow for an objective measure of BAS activity and its variability, with high temporal resolution. Thus, actigraphy studies are relevant to and supportive of the BAS dysregulation model of bipolar disorder, especially the hypothesis that individuals with bipolar disorder have more variable BAS activity.
Neurobiology
Next, we turn to studies of neurobiological abnormalities in bipolar disorder. Recent reviewers (e.g., Soares & Mann, 1997a,b; Videbech, 2000) have noted some promising leads for abnormalities in specific brain structures (e.g., DLPFC and prefrontal cortex [PFC] in general) in bipolar spectrum disorders. However, they have warned about the preliminary nature of these findings due to small sample sizes, methodological differences between studies, and a general scarcity of data (e.g. Videbech, 2000). In line with these limitations, we would further add that there is a paucity of theoretically driven neurobiological studies of bipolar disorder. Thus, we focus on findings that are most pertinent to the BAS dysregulation theory and involve PFC, dopaminergic functioning, and certain subcortical structures.
Prefrontal cortex
Left frontal activity, as assessed by EEG, has been related to BAS (see above). Some of the earliest support for the contention that left frontal cortical areas are involved in bipolar disorder came from studies of lesion patients. These studies found that the proximity of left hemisphere lesions to the frontal pole is related to the severity of depressive symptoms (e.g., Narushima, Kosier, & Robinson, 2003), suggesting that deactivation or shut down of left PFC is related to depression. Moreover, right hemisphere lesions, specifically lesions of the structures implicated in the reward system (e.g., orbitofrontal cortex [OFC], basotemporal cortex, thalamus, and caudate), have been related to secondary mania (Robinson, Boston, Starkstein, & Price, 1988). Further support for the idea that left frontal cortical areas are also involved in bipolar disorder comes from EEG studies of frontal asymmetry. Allen, Iacono, Depue, and Arbisi (1993) found lower relative left frontal activity in current bipolar depression with a seasonal pattern, before and after light exposure therapy. Consistently, Kano et al. (1992) found increased right frontal activity in major depressive disorder without melancholia (presumably a mix of unipolar and bipolar depression) and increased left frontal activity in manic episode. Furthermore, in Harmon-Jones et al.’s (2002) study of EEG response to an anger-evoking event, proneness to hypomania was related to greater left frontal activity (controlling for baseline left frontal activity), whereas proneness to depression was related to decreased left frontal activity (again controlling for baseline left frontal activity). Additionally, preliminary data support antidepressant effects of repetitive transcranial magnetic stimulation (rTMS) of the left PFC in a sample of unipolar and bipolar depressed individuals (e.g., George et al., 2000).
An increasing number of studies has also reported neuronal abnormalities in the DLPFC in bipolar disorder (Cotter et al., 2002; Law & Harrison, 2003; Rajkowska, Halaris, & Selemon, 2001; Winsberg et al., 2000). There is some preliminary support for a possibility of structural abnormalities being localized to the left DLPFC (Dickstein et al., 2005), especially in bipolar II disorder (Winsberg et al., 2000). There is also some evidence of reduced gray matter in the left middle and superior PFC (portions of which are part of the DLPFC; Lopez-Larson et al., 2002). Similarly, in currently manic patients, 1H MRS revealed increased glutamine levels in the left DLPFC suggestive of glutaminergic dysfunction, which might be involved in the observed structural abnormalities (Michael et al., 2003). Accordingly, a study with positron emission tomography (PET) of individuals with depression and schizophrenia has related reduced regional cerebral blood flow (rCBF) in the left DLPFC not to any specific diagnosis but to the symptom of psychomotor retardation (Dolan et al., 1993), suggesting that the DLPFC might index the psychomotor activation aspect of BAS activity in bipolar disorder, too.
There is also a number of studies finding structural abnormalities in the subgenual PFC, and in ACC in general, in bipolar disorder (e.g., Benes, Vincent, & Todtenkopf, 2001; Cotter et al., 2002; Drevets et al., 1997; Ongur, Drevets, & Price, 1998). Specifically, reduced volume in the left subgenual PFC (Drevets et al., 1997; Ongur, Drevets, & Price, 1998) was found in individuals with bipolar disorder and familial history of bipolar disorder. However, another study did not replicate this finding (but means were in the same direction; Sharma et al., 2003). Several studies have found structural abnormalities in the ACC in general in bipolar disorder (Benes et al., 2001; Chana et al., 2003; Cotter et al., 2002a), with Kyoon Lyoo et al. (2004) reporting a reduction specifically in the left anterior cingulate gyrus volume.
As far as functional abnormalities in these two regions are concerned, Drevets and colleagues (1997) reported increased metabolism during mania and decreased metabolism during depression in the left subgenual PFC of individuals with bipolar disorder and a familial history of bipolar disorder. Likewise, increased rCBF in the left dorsal ACC, along with increased rCBF in the left caudate, has been detected in mania vs. euthymia in bipolar I disorder (Blumberg et al., 2000). Moreover, in response to a gambling task, currently manic individuals exhibited hyperactivity in the dorsal ACC and decreased activity in regions of ventromedial PFC compared to controls (Rubinsztein et al., 2001).
In addition, recent studies have identified OFC structural and functional abnormalities as characteristic of bipolar disorder. Cotter, Handsau, and Landau (2005) found reduced neuronal size in layer 1 of caudal OFC to differentiate bipolar disorder from schizophrenia and healthy controls. Furthermore, increased glucose metabolism has been noted in left OFC in individuals with bipolar disorder compared to healthy controls (Mah et al., 2007).
In summary, bipolar disorder is associated with structural abnormalities in various PFC regions, notably the DLPFC, OFC, ACC, and subgenual PFC. Moreover, there is a tendency for greater abnormalities in the left hemisphere. The findings of dysfunction in left PFC, coupled with reviewed EEG studies of left frontal cortical activity, are consistent with the BAS dysregulation model, as basic research suggests a link between left PFC and BAS.
Dopaminergic system
Administrations of dopamine agonists have triggered mania in individuals with bipolar disorder (for review see Silverstone, 1985). Similarly, in response to amphetamine challenge, which increases dopamine levels in the nucleus accumbens by blocking dopamine transporter, individuals with bipolar disorder exhibited a greater behavioral response than controls (Anand et al., 2000). Another study of amphetamine challenge in bipolar disorder found increased dopamine release in striatum, which was not affected by antipsychotic drug treatment (Breier et al., 1999). Pimozide, a dopamine receptor blocker, is associated with a decrease in manic symptoms following treatment (Post, Jimerson, Bunney, & Goodwin, 1980). Furthermore, a study examining administration of two forms of clopenthixol, with one form blocking dopamine receptors and the other not having this effect, found only the former to have an antimanic effect (Nolen, 1983). Finally, L-dopa administration has been found to trigger mania in individuals with bipolar disorder (Buki & Goodnick, 1997). Thus, there is some preliminary data implicating abnormal dopaminergic functioning in bipolar disorder, consistent with the BAS dysregulation theories (e.g., Depue & Iacono, 1989).
Other brain structures
The neurobiological dysfunctions in bipolar spectrum disorder are not exclusive to the PFC regions. Several studies have reported enlarged amygdala volume in individuals with bipolar I disorder (e.g., Altshuler et al., 1998, 2000; Brambilla et al., 2003; Strakowski et al., 1999), although this difference has not been reliably replicated (e.g., Rosso et al., 2007), and reduced glial cell numbers in amygdala in the postmortem brains of individuals with bipolar I disorder who were not treated with mood-stabilizers (Bowley, Drevets, Ongur, & Price, 2002). In addition to amygdala, there is support for enlarged thalamus, pallidum, and striatum (e.g., Strakowski et al., 1999). Moreover, pallidotomy has been related to transient manic behaviors (Okun et al., 2003). Thus, subcortical structures proposed to play a role in the reward system and, thus, underlie the BAS, like ventral striatum and amygdala, have been found to be dysfunctional in bipolar spectrum disorders.
Why is expansion of the BAS dysregulation model needed?
As can be seen from the review above, there has been a proliferation of data supporting the BAS dysregulation model of bipolar disorder in recent years. This is an exciting development for the field of bipolar disorder research because the BAS dysregulation model provides an integrative biopsychosocial perspective. However, for this research progress to continue, the BAS dysregulation theory needs to be refined and expanded. Most notably, the model needs a more explicit causal chain for explaining how hypomanic/manic and depressive episodes develop. Furthermore, ambiguities in definitions of environmental triggers for hypomania/mania vs. depression and euphoric vs. irritable hypomania/mania need to be addressed. Also, some implicit ideas in the early formulation of the BAS dysregulation model need to be made explicit, like specifying cognitive appraisals involved in the processes that lead to bipolar episode onset. Moreover, the early formulations of the BAS dysregulation model did not sufficiently conceptualize and operationalize the construct of BAS dysregulation in bipolar disorder. Depue and Iacono (1989) did propose that nucleus accumbens DA activity is core in initiation of goal-directed behavior, and in turn, is modulated by the PFC and serotonergic system. Still, explicit details of how this might be translated into psychological processes are missing. Without clear specification of the nature of BAS dysregulation in bipolar disorder, it has been difficult to empirically test this concept.
Finally, there have been many advances in basic research on emotion and motivation. We highlight two pertinent advances not addressed by the early formulations of the theory:
Anger as a BAS emotion. Using a variety of methodologies, a growing body of work supports a connection among anger, aggression and the BAS. Self-report measures of trait anger and different types of aggression have been related to EEG measures of greater left frontal activity (Harmon-Jones & Allen, 1998), and to self-report measures of BAS (Harmon-Jones, 2003). Additionally, induced anger has been related to BAS activation (Carver, 2004; Harmon-Jones et al., 2004; Harmon-Jones & Sigelman, 2001; Wacker, Heldmann, & Stemmler, 2003). Finally, Harmon-Jones and colleagues (2003) suggested that anger is more likely to be related to approach in adverse situations with perceived control to remedy the situation. These data have clear implications for the BAS dysregulation theory and especially call for further specification of conditions necessary and/or sufficient for dysregulated hypomanic/manic anger.
Motivation. The need for elaboration of cognitive aspects and processes in the BAS dysregulation theory is highlighted by some of the well-established social psychology theories of motivation (e.g., Eccles & Wigfield, 2002; Lazarus, 1991a; Wright & Brehm, 1989). Lazarus (1991b) postulated that certain cognitive processes (i.e., appraisals) are critical in motivation, because “motivation without cognition too would be merely a diffuse, undifferentiated state of activation” (p. 353). Furthermore, expectancy of a positive outcome, or more specifically, efficacy expectancy (i.e., belief in one’s ability to bring about a desired outcome), has been related to increased motivation in many theories of motivation, most prominently in the expectancy-value theory of motivation (e.g., Eccles & Wigfield, 2002). Also, experimentally manipulated expectations of ability to affect a positive outcome are related to a neurobiological index of BAS activation (Harmon-Jones et al., 2003). Thus, there is a pressing need to address the implications of these social psychology theories of motivation for the cognitive processes that may be involved in the BAS dysregulation theory.
Given recent advances and some gaps in the early formulations of this well supported model of bipolar disorder, the time is ripe for expansion of the BAS dysregulation theory.
Expansion of the BAS Dysregulation Theory of Bipolar Spectrum Disorders
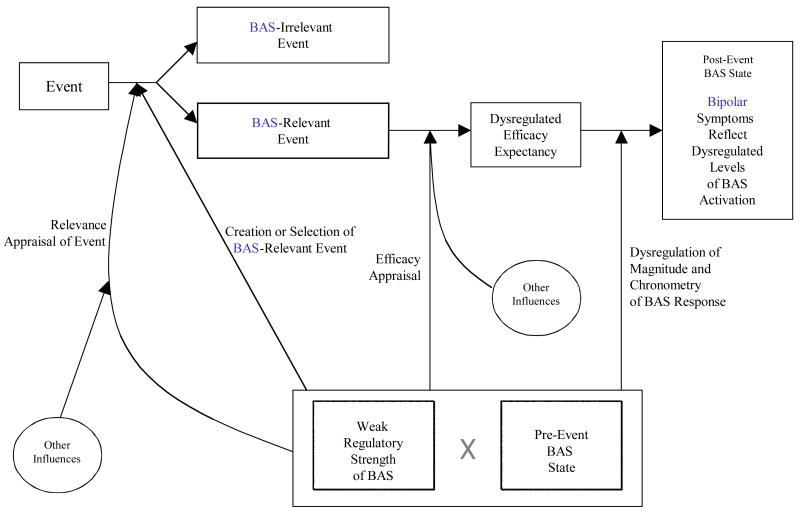
Consequently, the expanded BAS dysregulation theory incorporates contemporary advances in motivation, affect, and bipolar disorder to further specify and build on the pivotal ideas of early formulations. The expanded model retains the core idea that hypomanic/manic and depressive symptoms reflect extreme BAS activity and inactivity states, respectively. However, this core hypothesis is expanded by specifying a causal chain of processes leading to extreme BAS activity and inactivity states (i.e., hypomania/mania and depression, respectively; see Figure 1 for general description, Figure 2 for the specific case of hypomania/mania and Figure 3 for depression). In addition, the expanded model provides specifics of cognitive processes involved in these causal chains. The dysregulated, or hypersensitive, BAS is retained as a vulnerability to bipolar spectrum disorders, while the expanded model extrapolates psychological processes reflective of BAS dysregulation. Like early formulations of the model, the expanded BAS dysregulation theory stresses the importance of environmental cues but goes beyond early formulations by elaborating on the specific nature of BAS-relevant environmental factors.
Figure 1.
Flow diagram of expanded BAS dysregulation model—general.
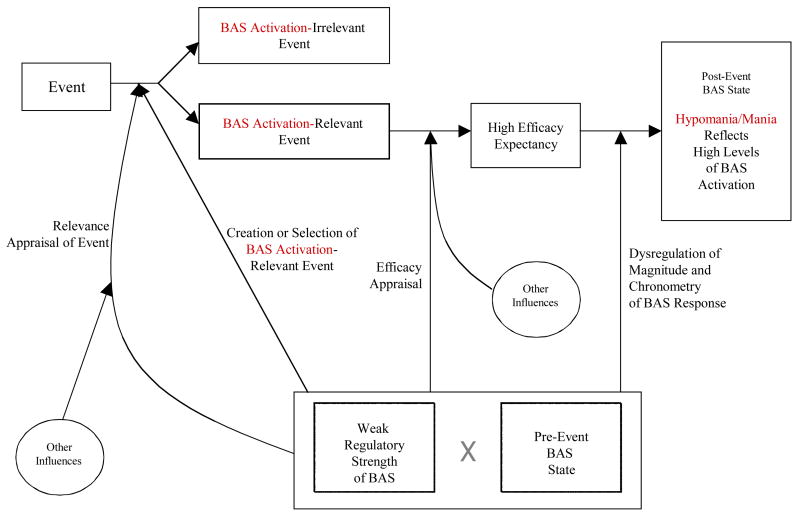
Figure 2.
Flow diagram of expanded BAS dysregulation model—hypomania/mania.
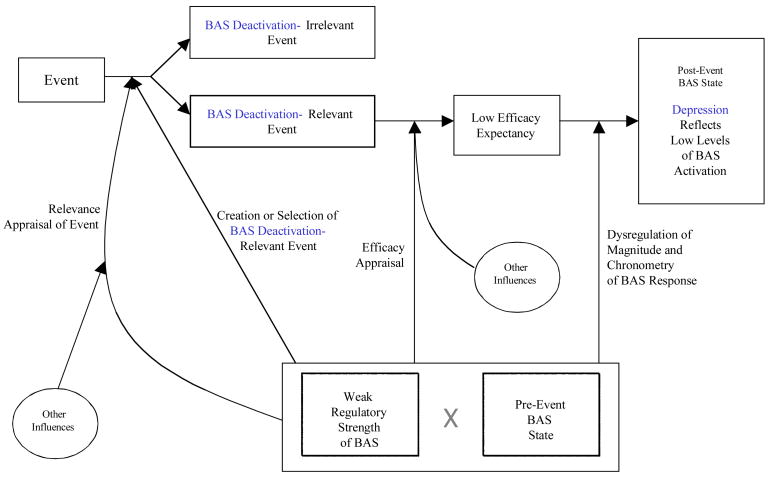
Figure 3.
Flow diagram of expanded BAS dysregulation model—depression.
We first provide an overview of the expanded model, then elaborate the revised factors in the model, followed by the causal chains hypothesized to result in hypomania/mania vs. bipolar depression. Finally, we describe implications of the expanded model for the course of bipolar disorder. Thus, we hope to provide both a unique integration of bipolar disorder empirical findings and propose novel predictions that need additional empirical tests (e.g., causal chain).
Overview of the Expanded BAS Dysregulation Model of Bipolar Disorder
The cornerstone of our expanded model is that individuals with bipolar spectrum disorders have abnormal (e.g., exaggerated, more persistent, quicker) behavioral and neurobiological responses to cues signaling opportunity to gain, or lose, rewards. Figure 1 depicts the causal chain specified by the expanded BAS dysregulation model that leads to bipolar symptomatology. In our expanded model, environmental cues (i.e., events) play the crucial role of perturbing the BAS system. Assuming that the environmental cues are heeded, two appraisal processes follow the occurrence of the event and change activation state of the BAS. Importantly, these appraisals, termed relevance and efficacy appraisals, can be voluntary or automatic, effortful or effortless, with or without awareness2. Relevance appraisal involves construal of an event as BAS-relevant (i.e., relevant to goals/rewards pertinent to the individual) and construal of the event as congruent or incongruent with the individual’s goals, whereas efficacy appraisal involves efficacy expectancy about the future outcome (i.e., how strongly the individual feels that he/she will obtain the pertinent goals/rewards). Although the two appraisals might be experienced as occurring simultaneously, the relevance appraisal precedes the efficacy appraisal.
Our expanded model is also transactional—pre-event BAS state influences these appraisal processes, which, in turn, influence subsequent BAS state. Additionally, our expanded model is transactional because the pre-event BAS state can result in selection and/or creation of environmental cues (i.e., dependent BAS-relevant events) that affect subsequent changes in BAS activation. Finally, the expanded model predicts that individuals with bipolar spectrum disorders exhibit some or all of the following types of dysregulation in BAS response:
Greater number of events appraised as BAS-relevant and/or greater strength of the relevance appraisal of individual event as BAS-relevant (e.g., a bipolar individual notices more opportunities and desires them more);
Creation of a greater number of BAS-relevant events (i.e., dependent events) due to the extreme pre-event BAS states (e.g., mania);
More extreme efficacy appraisal in both directions (e.g., “I am going to conquer the world,” “I can do nothing right”) and a larger perceived domain of efficacy (e.g., “I am great at everything I do”);
Greater magnitude of all aspects of BAS response to BAS-relevant events (e.g., greater locomotor activity or complete lack of this activity); and
Dysregulated chronometry of BAS response (e.g., faster rise to peak, longer sustained response).
See Table 1 for additional novel predictions of the expanded model. The result of all these dysregulations is an individual with bipolar disorder who experiences a more variable or extreme BAS response (i.e., a more sensitive BAS) than one without bipolar disorder. Finally, across different forms of bipolar spectrum disorders, we propose that these dysregulations will be similar in quality but differ in quantity, with severity of dysregulation increasing with severity of the disorder. For example, individuals with bipolar I disorder and individuals with bipolar II disorder may present with qualitatively similar hypersensitivity to goal striving, but the dysregulation of individuals with bipolar I disorder may present with much longer time to recovery following goal striving, greater magnitude of response, etc.
Table 1.
Novel Predictions of the Expanded BAS Dysregulation Model of Bipolar Disorder
|
Components of the Expanded BAS Dysregulation Model of Bipolar Disorder
BAS-relevant events
Vulnerability of weak BAS regulation alone is not sufficient to explain why a vulnerable individual experiences hypomanic/manic vs. depressive symptoms at a given point in time. Based on current data linking goal striving/attainment and hypomania/mania (see above), we propose that BAS activation-relevant events are related to pertinent desires/goals of an individual and involve an opportunity for attainment of those pertinent goals/rewards (i.e., congruent with individual’s desires, biological needs, etc.). Depue and Collins (1999) proposed a variety of rewards to be BAS-relevant—ranging from food, sex, social rewards, to long-term goals. Thus, events like starting to work towards a degree, studying for an exam, meeting an attractive person, being at a party, are all BAS activation-relevant due to the common feature of opportunity for goal/reward attainment. However, in our model, consistent with current research on BAS and anger (Harmon-Jones, 2003), these rewards/goals do not need to be normatively positive (e.g., the goal could be hurting another person).
Based on research on anger (Harmon-Jones, 2003), preliminary findings of anger-evoking events and hypomania (Harmon-Jones et al., 2002), and partially on early formulations of the BAS dysregulation model (e.g., Depue et al., 1987), we propose that the following opportunities for goal attainment (i.e., BAS activation-relevant events) may increase the likelihood of irritable and aggressive BAS activation: 1) opportunity to remove an obstacle in the way of reward; and 2) verbal or physical insult with an opportunity to attain revenge, or “get even.” Further research is needed to expand and validate the list of conditions leading to irritable BAS activation and irritable approach (i.e., aggression).
On the opposite side, our expanded BAS dysregulation model posits that BAS deactivation-relevant events are related to and involve failure to obtain or loss of pertinent rewards/goals (i.e., incongruent with an individual’s goals, biological needs, etc.)3. BAS deactivation-relevant losses/failures encompass the full range of life domains, from being rejected for a job, death of a loved one, break up of a significant relationship, incarceration, failing an exam, failing to remove a bureaucratic obstacle, etc. It is important to emphasize that BAS deactivation-relevant events involve both losses of previously attained rewards/goals (e.g., unwanted divorce) and failures to obtain rewards/goals (e.g., proposing marriage and being rejected). This definition of BAS deactivation-relevant (i.e., depression relevant) events is consistent with the above reviewed findings on the effect of negative life events on onset, recovery, and relapse in bipolar disorder. In addition, BAS deactivation-relevant events may trigger both unipolar and bipolar depression, but research needs to address whether the mechanisms through which they lead to symptoms differs between these two disorders.
Relevance and efficacy appraisals
Relevance appraisal is a process of construing the event as pertinent to an individual’s goals/desired rewards (i.e., determining whether the event is BAS-relevant). This aspect of relevance appraisal might be viewed through the lens of the expectancy-value perspective (e.g., Eccles & Wigfield, 2002) as assigning the event a high value (e.g., high utility, high need). It is important to note that this aspect of relevance appraisal has a strong affective component (e.g., experienced as a strong feeling of wanting). The second aspect of the relevance appraisal entails appraising whether the event is congruent or incongruent with one’s goals and desires. This aspect of relevance appraisal distinguishes BAS activation-relevant events from BAS deactivation-relevant events. For example, if an individual construes an event as signifying the loss of a highly desired boyfriend, the event will become BAS deactivation-relevant; but if an individual construes an event as an opportunity to attain a highly desired boyfriend, the event will become BAS activation-relevant.
The efficacy appraisal entails evaluation of efficacy expectancy for the given situation, which is again consistent with the expectancy-value theoretical perspective on motivation and slightly different from the outcome expectancy proposed by the early formulation (e.g., Fowles, 1987). In our model, efficacy expectancy can be viewed as a special case of outcome expectancy, in which one’s own actions are expected to bring about success, and will result in increase of BAS activation regardless of the relevance appraisal. In other words, if the event has been construed as BAS deactivation-relevant but the individual appraises that she can remedy the situation of failure to obtain the reward, there will be an increase in BAS activation, possibly amelioration of depressive symptoms, or possibly no depressive symptoms at all. Similarly, if the event has been construed as BAS activation-relevant and the individual appraises that she can successfully take advantage of an opportunity to attain a desired goal/reward, there will be an increase in BAS activation, in extreme cases leading to hypomanic/manic symptoms. The efficacy appraisal will be affected by individual differences related to how large the individual perceives his/her domain of competence. Note that the larger the domain of perceived competence and greater the strength of the efficacy appraisal, the more likely the person is to exhibit hypomanic personality traits.
We hypothesize that individuals with bipolar spectrum disorders might exhibit any combination of the following: 1) in relevance appraisal, a greater extent of events might be appraised as pertinent to goals/rewards (e.g., “I want it all”); 2) greater strength of relevance appraisal that the event is pertinent to one’s goals/rewards (e.g., “It’s do or die, I must have it”); and 3) greater strength of belief in extreme efficacy appraisal in a positive direction for BAS activation-relevant events and in a negative direction for BAS deactivation-relevant events. Some of these predictions already have preliminary empirical support, like prediction of extreme expectations of success on skill and chance-based tasks and real life tasks (e.g., Meyer & Krumm-Merabet, 2003; Murphy et al., 2001; Ruggero & Johnson, 2006). Over time, these types of appraisals may lead to perfectionistic, achievement oriented traits that seem to characterize bipolar disorder (e.g., Alloy et al., 2008b; Francis-Raniere et al., 2006). In contrast, we do not postulate that individuals with bipolar spectrum disorders have different relevance appraisals of whether the event is goal-congruent vs. goal-incongruent compared to other individuals.
The nature of BAS dysregulation in bipolar disorder
According to our expanded model, the BAS dysregulation in bipolar disorder may consist of any combination of the following: exaggerated magnitude of BAS response (e.g., high motor activity as one pursues reward), greater frequency of BAS-relevant events (i.e., through greater creation/selection of BAS-relevant events) and dysregulated chronometry of BAS response. Importantly, unlike in unipolar depression, BAS dysregulation characteristic of bipolar disorder reflects hypersensitivity to both BAS activation-relevant and BAS deactivation-relevant events. In addition, severity of BAS dysregulation should reflect the severity of bipolar condition (bipolar I vs. bipolar II disorder). The possibility of dysregulated chronometry of BAS response in bipolar disorder requires further specification. For example, even if individuals with bipolar spectrum disorders were similar to the non-bipolar population in the magnitude of their response to BAS-relevant events, they may exhibit a prolonged BAS response and/or have quicker rise to peak of that response to BAS-relevant events. This postulation suggests new research on temporal aspects of BAS functioning in bipolar spectrum disorders.
Possible neurobiological mechanisms of dysregulated BAS
Early formulations of the BAS dysregulation model implicated the VTA dopaminergic projections and the structures that they innervate, like nucleus accumbens, ventral striatum, septum, pallidum, amygdala, and regions of frontal cortex, such as is consistent with modern theories and data on the neurobiology of motivation, reward, and affect in general (for review, see Berridge, 2003; Davidson & Irwin, 1999; Phillips et al., 2003; Schultz, 1997). Strides have been made towards identifying specific functions of these brain structures, like identifying wanting (not liking) as a psychological process mediated by the dopamine system (see Berridge, 2003; Berridge & Robinson, 1998). In addition, different regions of PFC have been proposed to have specialized functions in affective and motivational processes (for review, see Davidson & Irwin, 1999; Davidson, Pazzigalli, & Nitschke, 2002). For example, initial detection of rewards has been suggested to involve DLPFC, OFC, and ACC (as well as dorsal and ventral striatum; Schultz, 1997). DLPFC also has been implicated in goal representations (Davidson & Irwin, 1999), whereas OFC has been implicated in assigning affective value to environmental cues (Davidson et al., 2002), thus suggesting that both may be involved in relevance appraisal. Efficacy appraisal may involve OFC and ventromedial cortex due to outcome expectancies being suggested to involve these regions (Davidson & Irwin, 1999; Schultz, 1997). Further, abnormalities in left PFC might lead to deficits in BAS-relevant behaviors, while abnormalities in both PFC hemispheres might lead to anhedonia (e.g., Davidson et al., 1999; Harmon-Jones, 2003).
Thus, based on existing data, the expanded BAS dysregulation model proposes that individuals with bipolar spectrum disorders, in comparison to individuals without this disorder, may have: 1) greater variability of left frontal activity over time reflective of dysregulated BAS; 2) greater left frontal activity in response to BAS activation-relevant events and in hypomania/mania; and 3) less left frontal activity in response to BAS deactivation-relevant events and in depression. In addition, the expanded theory would predict that neuronal dysfunction in bipolar disorder may have greater involvement of left (compared to right) PFC regions. However, it is important to note that the BAS is a system with many interrelated components. Thus, predicting that overall left frontal activity, as measured by EEG, will index BAS activity does not imply that every single PFC region will have a positive relationship with BAS activity and it is highly pertinent to explore dysregulation in connectivity of specific brain regions (e.g., PFC and amygdala) in addition to single region activity. Moreover, it will be important to distinguish dysregulated PFC-subcortical regions connectivity that characterize bipolar disorder from connectivity dysregulations more characteristic of schizophrenia and other disorders with implicated PFC malfunction (e.g., OFC dysfunction may differentiate bipolar disorder from schizophrenia [Cotter, Handsau, & Landau, 2005]). Finally, consistent with the formulation by Depue and Iacono (1989), the expanded model predicts that individuals with bipolar spectrum disorders would have dysregulated dopaminergic activity in reward system structures (e.g., striatum or more specifically, nucleus accumbens).
Hypomania/Mania
We now extrapolate from our general model (Figure 1) to show how bipolar individuals develop symptoms of hypomania/mania (Figure 2) at a particular point in time. Figure 2 depicts the temporal flow of processes that lead to hypomania/mania. There are two prerequisites for development of hypomanic/manic symptoms—occurrence of a BAS activation-relevant event and the presence of weak regulation of BAS that interacts with the individual’s pre-event BAS state. According to our model, the weak regulation of BAS is an inherited, or acquired early in life, vulnerability to bipolar disorder. In order for the causal chain to be set in motion, it is crucial that an individual with bipolar disorder interprets an event in the environment (e.g., an assignment of a work presentation for potential clients) as an opportunity to gain a desired reward/goal (e.g., a new client for the company and in the process to impress her superiors),4 or as BAS activation-relevant. Alternatively, the individual may appraise the event as an opportunity to aggress against an important enemy (e.g., a colleague competing for the same promotion), and, in turn, experience a negatively valenced anger, and still trigger the processes depicted in Figure 2 leading to hypomania/mania.
In our model, the pre-event, baseline BAS state (i.e., pre-event symptoms) influences BAS response at all levels, including relevance appraisal. For example, if a bipolar individual is depressed prior to the onset of the event, this would attenuate her/his relevance appraisal of the BAS activation-relevant event (e.g., weak feeling of wanting to gain the new client for the company), just as pre-event hypomania would accentuate this appraisal or possibly lead to greater selection/creation of BAS activation-relevant events. Other potential influences on this relevance appraisal of the event as BAS activation-relevant may stem from the other people present in the situation (e.g., a boss says, “This is an opportunity for you to shine”), or life-long socialization to view certain situations as pre-goal attainment cues.
The same factors affect efficacy appraisal. Thus, the individual with the weakest BAS regulatory system and the highest pre-event BAS state will have the greatest efficacy expectancy for the given BAS activation-relevant event (e.g., the woman expecting to give the best presentation her client has seen). However, as with the relevance appraisal, other influences (e.g., other people in the current situation, socialization, such as being reared to believe that she has an outstanding gift of persuasion) may affect the efficacy appraisal as well. In either case, the higher the efficacy appraisal in a given situation, the higher the BAS response as reflected in severity of hypomanic/manic symptoms (e.g., the woman works through the night and experiences extremely high energy and racing thoughts).
Furthermore, as depicted in Figure 2, an individual with an excessively high pre-event BAS state that is coupled with a weak regulatory system incapable of reining in the BAS response will exhibit greater magnitude, quicker rise to peak, and/or longer duration (i.e., more severe hypomania/mania with sudden onset and long recovery) compared to an individual with a lower pre-event BAS state and/or a stronger regulatory system. In this sense, the weak BAS regulatory system may be viewed as amplifying the dysregulated response to the BAS activation-relevant event and failing to provide any reality checking.
Depression
Figure 3 describes the temporal flow of processes that lead to depression. The causal chain begins with an event being construed as BAS deactivation-relevant in order to trigger the rest of the processes leading to depressive symptoms (e.g., learning that a work presentation failed to attract the new client for the company). Once again, the relevance appraisal of the event as BAS deactivation-relevant is influenced by the interaction of the weak regulatory strength, the pre-event BAS state, and other factors. The lower the pre-event BAS state (e.g., the more depressed a person is already) and the weaker the regulatory system of BAS, the more likely it is that the event is appraised as a failure or loss (i.e., BAS deactivation-relevant). In depression, as in hypomania/mania, other influences on the relevance appraisal may exist, such as cues from other persons involved in the situation, life-long socializations, and cultural norms. Thus, two individuals each can fail to gain a reward, but one of them may construe the event as a challenge to regain the reward and as BAS activation-relevant, whereas the other individual may construe the event only as failure to obtain the reward (i.e., BAS deactivation-relevant) and give up.
Once the event has been appraised as BAS deactivation-relevant, the efficacy appraisal comes into play. The lower the expectations of a positive outcome of one’s actions, or the more hopeless the individual becomes, the greater the subsequent BAS deactivation will be (note the relation to the hopelessness theory here; Abramson et al., 1989; 2002). Moreover, the individual with the weakest BAS regulatory system and the lowest pre-event BAS state will have the lowest efficacy appraisal (hopelessness) for the given BAS deactivation-relevant event. This hopelessness will lead to depression, reflecting the post-event BAS deactivation or shut down. However, as with hypomania/mania, other influences (e.g., other individuals in the situation, socialization, like being reared to believe that one is too stupid to do well at work) may affect the efficacy appraisal here as well.
The pre-event BAS state and weak regulatory system interaction also influences the production of depressive symptoms through dysregulation of magnitude and chronometry of BAS response. In this instance, an individual with an excessively low pre-event BAS state that is coupled with a weak regulatory system incapable of stopping the plummeting of the BAS state level will exhibit greater deactivation of the BAS post-event and more severe depressive symptoms compared to an individual with a higher pre-event BAS state and/or stronger regulatory system. Similarly, the weak regulatory system can lead to dysregulated chronometry in BAS deactivation (e.g., quicker fall to trough, longer recovery)5.
Summary and Implications of the Expanded BAS Dysregulation Model
The expanded BAS dysregulation model of bipolar disorder provides parsimonious explanation for novel data and issues they raise. For example, our expanded model provides a plausible explanation for the seemingly inconsistent findings of negative life events sometimes predicting onset of mania/hypomania and depression. More specifically, our expanded BAS dysregulation model proposes that some negative events (e.g., goal obstruction, insult) will be appraised as BAS activation-relevant. Furthermore, according to the expanded model, when these negative events are construed as incongruent with one’s goals and when this appraisal is coupled with a high efficacy appraisal, the negative event will lead to BAS activation and in turn to hypomania/mania symptoms. Given that pre-event BAS state influences both relevance and efficacy appraisal in the expanded model, currently hypomanic/manic individuals experiencing a negative life event may be especially likely to respond with increase in BAS activation (i.e., exacerbation in hypomanic/manic symptoms).
We propose that BAS-relevant events also play an important role in predicting course and prognosis of bipolar disorder. According to our model, the frequency of BAS activation- vs. BAS deactivation-relevant events will predict concurrent predominance of hypomania/mania vs. depression, respectively. Over time for a subgroup of bipolar individuals, there might be an increase in frequency and severity of BAS-relevant events (e.g., failures and losses) and the clinical picture should progressively become more severe as well. Thus, an individual with cyclothymia experiencing progressively more severe failures due to his/her own choices of unrealistic goals may, in turn, experience a major depressive episode and switch to a bipolar II disorder diagnosis. Another important severity marker, presence of psychoses, may reflect more extreme BAS dysregulation or potentially independent, comorbid pathological processes—a question ripe for empirical exploration.
In sum, early formulations of the BAS dysregulation theory provided a description of who will develop bipolar disorder (i.e., an individual with dysregulated BAS). The expanded BAS dysregulation theory elaborates on this vulnerability to bipolar disorder by specifying behavioral indications of this dysregulation. Moreover, the expanded theory explicates the causal chain of processes that lead to depression vs. hypomania/mania. It also further specifies the nature of events that may trigger BAS activation (i.e., hypomania/mania) vs. BAS deactivation (i.e., depression). Future studies of bipolar spectrum disorders will need to examine temporal parameters (e.g., rise time to peak, duration) of the BAS response (with both behavioral and physiological measures) to experimentally manipulated BAS-relevant stimuli. Also, longitudinal research is needed to examine whether measures of weak regulation of BAS interact with the occurrence of BAS activation- and BAS deactivation-relevant events to predict hypomanic/manic and depressive episodes, respectively, in individuals with bipolar spectrum disorders. Thus, there are many opportunities for future research to address specific predictions of the expanded BAS dysregulation model of bipolar disorder.
Footnotes
Depue and colleagues refer to the behavioral approach system as the behavioral facilitation or engagement system, while Gray (1982; 1991;1994) and Davidson (e.g., 1999) have referred to behavioral activation system and approach system, respectively. These systems are largely conceptually equivalent.
Note, although they have some overlap with appraisals described by Lazarus (1991b), our appraisal processes are not meant to be identical to those of Lazarus.
Debate exists about whether bipolar disorder episodes become more independent of environmental triggers over the course of illness through a process of sensitization (Post, 1992). Thus, over time, bipolar episodes may be triggered by very minor environmental changes usually not captured by standard life events assessments. Furthermore, there is a possibility of bipolar episodes, especially hypomania/mania, occurring without environmental triggers and through other BAS-irrelevant mechanisms. These are some empirical issues that need further testing.
It is less clear whether an event that is construed as an achievement of reward (i.e., a post-attainment cue, like successful completion of the presentation and achievement of the desired approval by the superiors, vs. pre-goal attainment cue as in our example) in itself would set in motion the rest of the processes in the model, because there is no need to approach a reward that already has been received.
According to our expanded BAS dysregulation model, a path to euthymia is through the absence of major BAS activation- or BAS deactivation-relevant events. In other words, even though regulatory strength of the BAS is weak and leads to a prolonged, more extreme response to BAS-relevant events, eventually BAS activation returns to its pre-event level (“wears off”) if no further BAS-relevant events occur to perturb it further. A theory of bipolar disorder needs to be able to explain the switching from hypomania/mania to depression, and vice versa, that often occurs (DSM-IV-TR, 2000). According to our expanded model, individuals in a state of BAS activation or deactivation also can create or select the very events that trigger the opposite BAS activation state. For example, in a state of high BAS activation coupled with weak BAS regulation, a bipolar individual may overestimate her/his abilities, set unrealistic goals, and, in turn, set herself/himself up for a failure. Once she/he fails, a BAS deactivation-relevant event occurs and the processes leading to depression are set in motion.
Snez̆ana Urošević and Lyn Y. Abramson, Department of Psychology, University of Wisconsin-Madison; Eddie Harmon-Jones, Department of Psychology, Texas A&M University; Lauren B. Alloy, Department of Psychology, Temple University. This manuscript was supported by National Institute of Mental Health Grants MH 52662 to Lyn Y. Abramson and MH 52617 to Lauren B. Alloy. Thanks to Richard J. Davidson for comments on earlier versions of this manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abramson LY, Alloy LB, Hankin BL, Haeffel GJ, MacCoon DG, Gibb BE. Cognitive vulnerability-stress models of depression in a self-regulatory and psychobiological context. In: Gotlib IH, Hammen CL, editors. Handbook of depression. New York: Guilford Press; 2002. pp. 268–294. [Google Scholar]
- Abramson LY, Metalsky GI, Alloy LB. Hopelessness depression: A theory-based subtype of depression. Psychological Review. 1989;96:358–372. [Google Scholar]
- Akiskal HS. The clinical significance of the “soft” bipolar spectrum. Psychiatric Annals. 1986;16:667–671. [Google Scholar]
- Akiskal HS, Djenderedjian AH, Rosenthal RH, Khani MK. Cyclothymic disorder: Validating criteria for inclusion in the bipolar affective group. American Journal of Psychiatry. 1977;134:1227–1233. doi: 10.1176/ajp.134.11.1227. [DOI] [PubMed] [Google Scholar]
- Akiskal HS, Khani MK, Scott-Strauss A. Cyclothymic temperamental disorders. Psychiatric Clinics of North America. 1979;2:527–554. [Google Scholar]
- Allen JJ, Iacono WG, Depue RA, Arbisi P. Regional electroencephalographic asymmetries in bipolar seasonal affective disorder before and after exposure to bright light. Biological Psychiatry. 1993;33:642–646. doi: 10.1016/0006-3223(93)90104-l. [DOI] [PubMed] [Google Scholar]
- Alloy LB, Abramson LY, Neeren AM, Walshaw PD, Urošević S, Nusslock R. Psychosocial risk factors for bipolar disorder: Current and early environment and cognitive styles. In: Jones S, Bentall R, editors. The Psychology of bipolar disorder: New developments and research strategies. Oxford, England: Oxford University Press; 2006a. [Google Scholar]
- Alloy LB, Abramson LY, Urošević S, Walshaw PD, Nusslock R, Neeren AM. The psychosocial context of bipolar disorder: Environmental, cognitive, and developmental risk factors. Clinical Psychology Review. 2005;25:1043–1075. doi: 10.1016/j.cpr.2005.06.006. [DOI] [PubMed] [Google Scholar]
- Alloy LB, Abramson LY, Walshaw PD, Cogswell A, Smith J, Hughes M, Iacoviello B, Neeren AM, King R, Keyser J, Urošević S, Nusslock R. Behavioral Approach System (BAS) sensitivity and bipolar spectrum disorders: A retrospective and concurrent behavioral high-risk design. Motivation and Emotion. 2006c;30:143–155. [Google Scholar]
- Alloy LB, Abramson LY, Walshaw PD, Neeren AM. Cognitive vulnerability to unipolar and bipolar mood disorders. Journal of Social and Clinical Psychology. 2006b;25:726–754. [Google Scholar]
- Alloy LB, Abramson LY, Walshaw PD, Cogswell A, Grandin LD, Hughes ME, Iacoviello BM, Whitehouse WG, Urosevic S, Nusslock R, Hogan ME. Behavioral Approach System and Behavioral Inhibition System sensitivities: Prospective prediction of bipolar mood episodes. Bipolar Disorders. 2008a;10:310–322. doi: 10.1111/j.1399-5618.2007.00547.x. [DOI] [PubMed] [Google Scholar]
- Alloy LB, Abramson LY, Walshaw PD, Gerstein RK, Keyser JD, Whitehouse WG, Urosevic S, Nusslock R, Hogan ME, Harmon-Jones E. Behavioral Approach System (BAS) – relevant cognitive styles and bipolar spectrum disorders: Concurrent and prospective associations. 2008b. Manuscript under editorial review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alloy LB, Reilly-Harrington N, Fresco DM, Whitehouse WG, Zechmeister JS. Cognitive styles and life events in subsyndromal unipolar and bipolar disorders: Stability and prospective prediction of depressive and hypomanic mood swings. Journal of Cognitive Psychotherapy: An International Quarterly. 1999;13(1):21–40. [Google Scholar]
- Altshuler LL, Bartzokis G, Grieder T, Curran J, Mintz J. Amygdala enlargement in bipolar disorder and hippocampal reduction in schizophrenia: An MRI study demonstrating neuroanatomic specificity. Archives of General Psychiatry. 1998;55:663–664. doi: 10.1001/archpsyc.55.7.663. [DOI] [PubMed] [Google Scholar]
- Altshuler LL, et al. An MRI study of temporal lobe structures in men with bipolar disorder or schizophrenia. Biological Psychiatry. 2000;48:147–162. doi: 10.1016/s0006-3223(00)00836-2. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual. 4. Washington, D.C: Author; 2000. Text Revised. [Google Scholar]
- Anand A, Verhoeff P, Seneca N, Zoghbi SS, Seibyl JP, Charney DS, Innis RB. Brain SPECT imaging of amphetamine-induced dopamine release in euthymic bipolar disorder patients. American Journal of Psychiatry. 2000;157:1108–1114. doi: 10.1176/appi.ajp.157.7.1108. [DOI] [PubMed] [Google Scholar]
- Andreasen NC. Creativity and mental illness: Prevalence rates in writers and their first-degree relatives. American Journal of Psychiatry. 1987;144:1288–1292. doi: 10.1176/ajp.144.10.1288. [DOI] [PubMed] [Google Scholar]
- Angst F, Stassen HH, Clayton PJ, Angst J. Mortality of patients with mood disorders: Follow-up over 34–38 years. Journal of Affective Disorders. 2002;68:167–181. doi: 10.1016/s0165-0327(01)00377-9. [DOI] [PubMed] [Google Scholar]
- Baldessarini RJ. A plea for integrity of the bipolar disorder concept. Bipolar Disorders. 2000;2:3–7. doi: 10.1034/j.1399-5618.2000.020102.x. [DOI] [PubMed] [Google Scholar]
- Benes FM, Vincent SL, Todtenkopf M. The density of pyramidal and nonpyramidal neurons in anterior cingulate cortex of schizophrenia and bipolar subjects. Biological Psychiatry. 2001;50:395–406. doi: 10.1016/s0006-3223(01)01084-8. [DOI] [PubMed] [Google Scholar]
- Berridge KC. Pleasures of the brain. Brain and Cognition. 2003;52:106–128. doi: 10.1016/s0278-2626(03)00014-9. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Robbins TE. What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Research Review. 1998;28:309–369. doi: 10.1016/s0165-0173(98)00019-8. [DOI] [PubMed] [Google Scholar]
- Blumberg HP, et al. Increased anterior cingulate and caudate activity in bipolar mania. Biological Psychiatry. 2000;48:1045–1052. doi: 10.1016/s0006-3223(00)00962-8. [DOI] [PubMed] [Google Scholar]
- Bowley MP, Drevets WC, Ongur D, Price JL. Low glial numbers in the amygdala of major depressive disorder. Biological Psychiatry. 2002;52:404–412. doi: 10.1016/s0006-3223(02)01404-x. [DOI] [PubMed] [Google Scholar]
- Brambilla P, et al. MRI investigation of temporal lobe structures in bipolar patients. Journal of Psychiatric Research. 2003;37:287–295. doi: 10.1016/s0022-3956(03)00024-4. [DOI] [PubMed] [Google Scholar]
- Breier A, Su T, Malhorta AK, Elman I, Adler CM, Weisenfeld NI, Pickar D. Effects of atypical antipsychotic drug treatment on amphetamine-induced striatal dopamine release in patients with psychotic disorders. Neuropsychopharmacology. 1999;20:340–345. doi: 10.1016/S0893-133X(98)00126-2. [DOI] [PubMed] [Google Scholar]
- Buki VMV, Goodnick PJ. Catecholamines. In: Goodnick PJ, editor. Mania: Clinical and research perspectives. Washington, DC: American Psychiatric Press; 1998. pp. 119–134. [Google Scholar]
- Carver CS. Negative Affect Deriving from the Behavioral Approach System. Emotion. 2004;4:3–22. doi: 10.1037/1528-3542.4.1.3. [DOI] [PubMed] [Google Scholar]
- Carver CS, White TL. Behavioral inhibition, behavioral activation, and affective responses to impending reward and punishment: The BIS/BAS scales. Journal of Personality and Social Psychology. 1994;67:319–333. [Google Scholar]
- Cassano GB, Dell’Osso L, Frank E, Miniati M, Fagiolini A, Shear K, Pini S, Maser J. The bipolar spectrum: A clinical reality in search of diagnostic criteria and an assessment methodology. Journal of Affective Disorders. 1999;54:319–328. doi: 10.1016/s0165-0327(98)00158-x. [DOI] [PubMed] [Google Scholar]
- Chana G, Landau S, Beasley C, Everall IP, Cotter D. Two-dimensional assessment of cytoarchitecture in the anterior cingulate cortex in major depressive disorder, bipolar disorder, and schizophrenia: evidence for deceased neuronal somal size and increased neuronal density. Biological Psychiatry. 2003;53:1086–1098. doi: 10.1016/s0006-3223(03)00114-8. [DOI] [PubMed] [Google Scholar]
- Cotter D, Hudson L, Landau S. Evidence for orbitofrontal pathology in bipolar disorder and major depression, but not in schizophrenia. Bipolar Disorders. 2005;7:358–369. doi: 10.1111/j.1399-5618.2005.00230.x. [DOI] [PubMed] [Google Scholar]
- Cotter D, Landau S, Beasley C, Stevenson R, Chana G, MacMillian L, Everall I. The density and spatial distribution of GABAergic neurons, labelled using calcium binding proteins, in the anterior cingulate cortex in major depressive disorder, bipolar disorder, and schizophrenia. Biological Psychiatry. 2002a;51:377–386. doi: 10.1016/s0006-3223(01)01243-4. [DOI] [PubMed] [Google Scholar]
- Cotter D, Mackay D, Chana G, Beasley C, Landau S, Everall IP. Reduced neuronal size and glial cell density in area 9 of the dorsolateral prefrontal cortex in subjects with major depressive disorder. Cerebral Cortex. 2002b;12:386–394. doi: 10.1093/cercor/12.4.386. [DOI] [PubMed] [Google Scholar]
- Davidson RJ. Cerebral asymmetry, emotion, and affective style. In: Ekman P, Davidson RJ, editors. The nature of emotion: Fundamental questions. New York: Oxford University Press; 1994. pp. 329–331. [Google Scholar]
- Davidson RJ. Neuropsychological perspectives on affective styles and their cognitive consequences. In: Dalgleish T, Power M, editors. Handbook of cognition and emotion. John Wiley & Sons; 1999. pp. 361–387. [Google Scholar]
- Davidson RJ, Abercrombie H, Nitschke JB, Putnam K. Regional brain function, emotion and disorders of emotion. Current Opinion in Neurobiology. 1999;9:228–234. doi: 10.1016/s0959-4388(99)80032-4. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Irwin W. The functional neuroanatomy of emotion and affective style. Trends in Cognitive Sciences. 1999;3:11–21. doi: 10.1016/s1364-6613(98)01265-0. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Jackson DC, Kalin NH. Emotion, plasticity, context, and regulation: Perspectives from affective neuroscience. Psychological Bulletin. 2000;126:890–909. doi: 10.1037/0033-2909.126.6.890. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Pizzagalli D, Nitschke JB. In: Handbook of depression. Gotlib IH, Hammen CL, editors. New York: Guilford Press; 2002. pp. 219–244. [Google Scholar]
- Depue RA, Collins PF. A neurobehavioral system appraoch to developmental psychopathology: Implications for disorders of affect. In: Cicchetti D, Toth SL, editors. Rochester symposium on developmental psychopathology, volume four: Developmental perspectives on depression. Rochester, New York: University of Rochester Press; 1992. pp. 29–101. [Google Scholar]
- Depue RA, Collins PF. Neurobiology of the structure of personality: Dopamine, facilitation of incentive motivation, and extraversion. Behavioral and Brain Sciences. 1999;22:491–569. doi: 10.1017/s0140525x99002046. [DOI] [PubMed] [Google Scholar]
- Depue RA, Slater J, Wolfstetter-Kausch H, Klein D, Goplerud E, Farr D. A behavioral paradigm for identifying persons at risk for bipolar depressive disorder: A conceptual framework and five validation studies (Monograph) Journal of Abnormal Psychology. 1981;90:381–437. doi: 10.1037//0021-843x.90.5.381. [DOI] [PubMed] [Google Scholar]
- Depue RA, Iacono WG. Neurobehavioral aspects of affective disorders. Annual Reviews in Psychology. 1989;40:457–492. doi: 10.1146/annurev.ps.40.020189.002325. [DOI] [PubMed] [Google Scholar]
- Depue RA, Krauss S, Spoont MR. A two-dimensional threshold model of seasonal bipolar affective disorder. In: Magnusson D, Ohman A, editors. Psychopathology: An interactional perspective. New York: Academic Press; 1987. pp. 95–123. [Google Scholar]
- Dickstein DP, Milhalm MP, Nugent AC, Drevets WC, Charney DS, Pine DS, Leibenluft E. Frontotemporal alterations in pediatric bipolar disorder: Results of a voxel-based morphometry study. Archives of General Psychiatry. 2005;62:734–741. doi: 10.1001/archpsyc.62.7.734. [DOI] [PubMed] [Google Scholar]
- Dolan RJ, Bench CJ, Liddle PF, Friston KJ, Frith CD, Grasby PM, Frackowiak RSJ. Dorsolateral prefrontal cortex dysfunction in the major psychoses; symptom or disease specificity? Journal of Neurology, Neurosurgery, and Psychiatry. 1993;56:1290–1294. doi: 10.1136/jnnp.56.12.1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drevets WC, Price JL, Simpson JR, Todd RD, Reich T, Vannier M, Raichie ME. Subgenual prefrontal cortex abnormalities in mood disorders. Nature. 1997;386:824–827. doi: 10.1038/386824a0. [DOI] [PubMed] [Google Scholar]
- Eccles JS, Wigfield A. Motivational beliefs, values, and goals. Annual Review of Psychology. 2002;53:109–132. doi: 10.1146/annurev.psych.53.100901.135153. [DOI] [PubMed] [Google Scholar]
- Ellicott A, Hammen C, Gitlin M, Brown G, Jamison K. Life events and the course of bipolar disorder. American Journal of Psychiatry. 1990;147(9):1194–1198. doi: 10.1176/ajp.147.9.1194. [DOI] [PubMed] [Google Scholar]
- Fowles DC. Application of a behavioral theory of motivation to the concepts of anxiety and impulsivity. Journal of Research in Personality. 1987;21:417–435. [Google Scholar]
- Fowles DC. Presidential Address, 1987. Psychophysiology and psychopathology: A motivational approach. Psychophysiology. 1988;25:373–391. doi: 10.1111/j.1469-8986.1988.tb01873.x. [DOI] [PubMed] [Google Scholar]
- Fowles DC. Biological variables in psychopathology: A psychobiological perspective. In: Sutker PB, Adams HE, editors. Comprehensive handbook of psychopathology. 2. New York: Plenum Press; 1993. pp. 57–82. [Google Scholar]
- Francis-Raniere EL, Alloy LB, Abramson LY. Depressive personality styles and bipolar spectrum disorders: Prospective test of the event congruency hypothesis. Bipolar Disorders. 2006;8:382–399. doi: 10.1111/j.1399-5618.2006.00337.x. [DOI] [PubMed] [Google Scholar]
- George MS, et al. A controlled trial of daily left prefrontal cortex TMS for treating depression. Biological Psychiatry. 2000;48:962–970. doi: 10.1016/s0006-3223(00)01048-9. [DOI] [PubMed] [Google Scholar]
- Goodwin FK, Jamison KR. Manic-depressive illness. New York: Oxford University Press; 1990. [Google Scholar]
- Goplerud E, Depue RA. Behavioral response to naturally occurring stress in cyclothymia and dysthymia. Journal of Abnormal Psychology. 1985;94:128–139. doi: 10.1037//0021-843x.94.2.128. [DOI] [PubMed] [Google Scholar]
- Gray JA. The neuropsychology of anxiety: An enquiry into the functions of the septohippocampal system. Oxford: Oxford University Press; 1982. [Google Scholar]
- Gray JA. Neural systems, emotion and personality. In: Madden J IV, editor. Neurobiology of learning, emotion and affect. New York: Raven Press; 1991. pp. 273–306. [Google Scholar]
- Gray JA. Three fundamental emotion systems. In: Ekman P, Davidson RJ, editors. The nature of emotion: Fundamental questions. New York: Oxford University Press; 1994. pp. 243–247. [Google Scholar]
- Hammen C, Ellicott A, Gitlin M, Jamison KR. Sociotropy/autonomy and vulnerability to specific life events in patients with unipolar depression and bipolar disorders. Journal of Abnormal Psychology. 1989;98(2):154–160. doi: 10.1037//0021-843x.98.2.154. [DOI] [PubMed] [Google Scholar]
- Hammen C, Gitlin M. Stress reactivity in bipolar patients and its relation to prior history of depression. American Journal of Psychiatry. 1997;154(6):856–857. doi: 10.1176/ajp.154.6.856. [DOI] [PubMed] [Google Scholar]
- Harmon-Jones E. Anger and the behavioral approach system. Personality and Individual Differences. 2003;35:995–1005. [Google Scholar]
- Harmon-Jones E, Abramson LY, Sigelman J, Bohlig A, Hogan ME, Harmon-Jones C. Proneness to hypomania/mania symptoms or depression symptoms and asymmetrical frontal cortical responses to an anger-evoking event. Journal of Personality and Social Psychology. 2002;82:610–618. [PubMed] [Google Scholar]
- Harmon-Jones E, Allen JJB. Behavioral activation sensitivity and resting frontal EEG asymmetry: Covariation of putative indicators related to risk for mood disorders. Journal of Abnormal Psychology. 1997;106:159–163. doi: 10.1037//0021-843x.106.1.159. [DOI] [PubMed] [Google Scholar]
- Harmon-Jones E, Allen JJB. Anger and frontal brain activity: EEG asymmetry consistent with approach motivation despite negative affective valence. Journal of Personality and Social Psychology. 1998;74:1310–1316. doi: 10.1037//0022-3514.74.5.1310. [DOI] [PubMed] [Google Scholar]
- Harmon-Jones E, Sigelman JD. State anger and prefrontal brain activity: Evidence that insult related left prefrontal activity is associated with experienced anger and aggression. Journal of Personality and Social Psychology. 2001;80:797–803. [PubMed] [Google Scholar]
- Harmon-Jones E, Sigelman JD, Bohlig A, Harmon-Jones C. Anger, coping, and frontal cortical activity: The effect of coping potential on anger-induced left frontal activity. Cognition and Emotion. 2003;17:1–24. doi: 10.1080/02699930302278. [DOI] [PubMed] [Google Scholar]
- Harmon-Jones E, Vaughn-Scott K, Mohr S, Sigelman J, Harmon-Jones C. The effect of manipulated sympathy and anger on left and right frontal cortical activity. Emotion. 2004;4:95–101. doi: 10.1037/1528-3542.4.1.95. [DOI] [PubMed] [Google Scholar]
- Harrow M, Goldberg JF, Grossman LS, Meltzer HY. Outcome in manic disorders: A naturalistic follow-up study. Archives of General Psychiatry. 1990;47:665–671. doi: 10.1001/archpsyc.1990.01810190065009. [DOI] [PubMed] [Google Scholar]
- Hunt N, Bruce-Jones W, Silverstone T. Life events and relapse in bipolar affective disorder. Journal of Affective Disorders. 1992;25:13–20. doi: 10.1016/0165-0327(92)90088-n. [DOI] [PubMed] [Google Scholar]
- Hyman SE. Goals for research on bipolar disorder: The view from NIMH. Biological Psychiatry. 2000;48:436–441. doi: 10.1016/s0006-3223(00)00894-5. [DOI] [PubMed] [Google Scholar]
- Jamison KR. An unquiet mind: A memoir of moods and madness. New York: Alfred A. Knopf, Inc; 1995. [Google Scholar]
- Joffe RT, Young LT, MacQueen GM. A two-illness model of bipolar disorder. Bipolar Disorders. 1999;1:25–30. doi: 10.1034/j.1399-5618.1999.10107.x. [DOI] [PubMed] [Google Scholar]
- Johnson SL. Mania and dysregulation in goal pursuit: A review. Clinical Psychology Review. 2005;25:241–262. doi: 10.1016/j.cpr.2004.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SL, Kizer A. Bipolar and unipolar depression: A comparison of clinical phenomenology and psychosocial predictors. In: Gotlib IH, Hammen CL, editors. Handbook of depression. New York: Guilford Press; 2002. pp. 141–165. [Google Scholar]
- Johnson SL, Miller I. Negative life events and time to recovery from episodes of bipolar disorder. Journal of Abnormal Psychology. 1997;106(3):449–457. doi: 10.1037//0021-843x.106.3.449. [DOI] [PubMed] [Google Scholar]
- Johnson SL, Roberts JE. Life events and bipolar disorder: Implications from biological theories. Psychological Bulletin. 1995;117(3):434–449. doi: 10.1037/0033-2909.117.3.434. [DOI] [PubMed] [Google Scholar]
- Johnson SL, Ruggero CJ, Carver CS. Cognitive, behavioral, and affective responses to reward: Links with hypomanic symptoms. Journal of Social and Clinical Psychology. 2005;24:894–906. [Google Scholar]
- Johnson SL, Sandrow D, Meyer B, Winters R, Miller I, Solomon D, Keitner G. Increases in manic symptoms after life events involving goal attainment. Journal of Abnormal Psychology. 2000;109:721–727. doi: 10.1037//0021-843x.109.4.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SL, Winett CA, Meyer B, Greenhouse WJ, Miller I. Social support and the course of bipolar disorder. Journal of Abnormal Psychology. 1999;108(4):558–566. doi: 10.1037//0021-843x.108.4.558. [DOI] [PubMed] [Google Scholar]
- Jones SH, Hare DJ, Evershed K. Actigraphic assessment of circadian activity and sleep patterns in bipolar disorder. Bipolar Disorders. 2005;7:1–11. doi: 10.1111/j.1399-5618.2005.00187.x. [DOI] [PubMed] [Google Scholar]
- Kano K, Nakamura M, Matsuoka T, Iida H, Nakajima T. The topographical features of EEGs in patients with affective disorders. Electroencephalography and Clinical Neurophysiology. 1992;83:124–129. doi: 10.1016/0013-4694(92)90025-d. [DOI] [PubMed] [Google Scholar]
- Klein E, Lavie P, Meiraz R, Sadeh A, Lenox RH. Increased motor activity and recurrent manic episodes: Predictors of rapid relapse in remitted bipolar disorder patients after lithium discontinuation. Biological Psychiatry. 1992;31:279–284. doi: 10.1016/0006-3223(92)90051-z. [DOI] [PubMed] [Google Scholar]
- Krumm-Merabet C, Meyer TD. Leisure activities, alcohol, and nicotine consumption in people with a hypomanic/hyperthymic temperament. Personality and Individual Differences. 2005;38:701–712. [Google Scholar]
- Kulhara P, Basu D, Mattoo SK, Sharan P, Chopra R. Lithium prophylaxis of recurrent bipolar affective disorder: Long-term outcome and its psychosocial correlates. Journal of Affective Disorders. 1999;54:87–96. doi: 10.1016/s0165-0327(98)00145-1. [DOI] [PubMed] [Google Scholar]
- Kyoon Lyoo I, et al. Frontal lobe gray matter density decreases in bipolar I disorder. Biological Psychiatry. 2004;55:648–651. doi: 10.1016/j.biopsych.2003.10.017. [DOI] [PubMed] [Google Scholar]
- Lam D, et al. A randomized controlled study of cognitive therapy for relapse prevention for bipolar affective disorder: Outcome of the first year. Archives of General Psychiatry. 2003;60:145–152. doi: 10.1001/archpsyc.60.2.145. [DOI] [PubMed] [Google Scholar]
- Lam D, Wong G. Prodromes, coping strategies, insight and social functioning in bipolar affective disorders. Psychological Medicine. 1997;27:1091–1100. doi: 10.1017/s0033291797005540. [DOI] [PubMed] [Google Scholar]
- Lam D, Wong G, Sham P. Prodromes, coping strategies and course of illness in bipolar affective disorder—a naturalistic study. Psychological Medicine. 2001;31:1397–1402. doi: 10.1017/s003329170100472x. [DOI] [PubMed] [Google Scholar]
- Lam D, Wright K, Smith N. Dysfunctional assumptions in bipolar disorder. Journal of Affective Disorders. 2004;79:193–199. doi: 10.1016/S0165-0327(02)00462-7. [DOI] [PubMed] [Google Scholar]
- Lazarus RS. Cognition and motivation in emotion. American Psychologist. 1991b;46:352–367. doi: 10.1037//0003-066x.46.4.352. [DOI] [PubMed] [Google Scholar]
- Lazarus RS. Progress on a cognitive-motivational-relational theory of emotion. American Psychologist. 1991a;46:819–834. doi: 10.1037//0003-066x.46.8.819. [DOI] [PubMed] [Google Scholar]
- Law AJ, Harrison PJ. The distribution and morphology of prefrontal cortex pyramidal neurons identified using anti-neurofilament antibodies SMI32, N200 and NP7. Normative data and a comparison in subjects with schizophrenia, bipolar disorder, or major depression. Journal of Psychiatric Research. 2003;37:487–499. doi: 10.1016/s0022-3956(03)00075-x. [DOI] [PubMed] [Google Scholar]
- Leibenluft E, Charney DS, Pine DS. Researching the pathophysiology of pediatric bipolar disorder. Biological Psychiatry. 2003;53:1009–1020. doi: 10.1016/s0006-3223(03)00069-6. [DOI] [PubMed] [Google Scholar]
- Lopez-Larson MP, DelBello MP, Zimmerman ME, Schwiers ML, Strakowski SM. Regional prefrontal gray and white matter abnormalities in bipolar disorder. Biological Psychiatry. 2002;52:93–100. doi: 10.1016/s0006-3223(02)01350-1. [DOI] [PubMed] [Google Scholar]
- Lozano BE, Johnson SL. Can personality traits predict increases in manic and depressive symptoms? Journal of Affective Disorders. 2001;63:103–111. doi: 10.1016/s0165-0327(00)00191-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyon HM, Startup M, Bentall RP. Social cognition and the manic defense: Attributions, selective attention, and self-schema in bipolar affective disorder. Journal of Abnormal Psychology. 1999;108:273–282. doi: 10.1037//0021-843x.108.2.273. [DOI] [PubMed] [Google Scholar]
- Mah L, Zarate CA, Singh J, Duan Y, Luckenbaugh DA, Manji HK, Drevets WC. Regional cerebral glucose metabolic abnormalities in bipolar II depression. Biological Psychiatry. 2007;61:765–775. doi: 10.1016/j.biopsych.2006.06.009. [DOI] [PubMed] [Google Scholar]
- Mansell W, Colom F, Scott J. The nature and treatment of depression in bipolar disorder: A review and implications for future psychological interventions. Clinical Psychology Review. 2005;25:1076–1100. doi: 10.1016/j.cpr.2005.06.007. [DOI] [PubMed] [Google Scholar]
- McGuffin P, Rijsdijk F, Andrew M, Sham P, Katz R, Cardno A. The heritability of bipolar affective disorder and the genetic relationship to unipolar depression. Archives of General Psychiatry. 2003;60:497–502. doi: 10.1001/archpsyc.60.5.497. [DOI] [PubMed] [Google Scholar]
- McPherson H, Herbinson P, Romans S. Life events and relapse in established bipolar affective disorder. British Journal of Psychiatry. 1993;163:381–385. doi: 10.1192/bjp.163.3.381. [DOI] [PubMed] [Google Scholar]
- Merikangas KR, Akiskal HS, Angst J, Greenberg PE, Hirschfeld RM, Petukhova M, Kessler RC. Lifetime and 12-month prevalence of bipolar spectrum disorder In the National Comorbidity Survey replication. Archives of General Psychiatry. 2007;64:543–552. doi: 10.1001/archpsyc.64.5.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer B, Beevers CG, Johnson SL. Goal appraisals and vulnerability to bipolar disorder: A personal projects analysis. Cognitive Therapy and Research. 2004;28:173–182. [Google Scholar]
- Meyer B, Johnson SL, Carver CS. Exploring behavioral activation and inhibition sensitivities among college students at risk for bipolar spectrum symptomatology. Journal of Psychopathology and Behavioral Assessment. 1999;21:275–292. doi: 10.1023/A:1022119414440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer B, Johnson SL, Winters R. Responsiveness to threat and incentive in bipolar disorder: Relations of the BIS/BAS scales with symptoms. Journal of Psychopathology and Behavioral Assessment. 2001;23:133–143. doi: 10.1023/A:1010929402770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer TD, Krumm-Merabet C. Academic performance and expectations for the future in relation to a vulnerability marker for bipolar disorders: The hypomanic temperament. Personality and Individual Differences. 2003;35:785–796. [Google Scholar]
- Michael N, Erfurth A, Ohrmann P, Gossling M, Arolt V, Heindel W, Pfleiderer B. Acute mania is accompanied by elevated glutamate/glutamine levels within the left dorsolateral prefrontal cortex. Psychopharmacology. 2003;168:344–346. doi: 10.1007/s00213-003-1440-z. [DOI] [PubMed] [Google Scholar]
- Miller A, Tomarken AJ. Task-dependent changes in frontal brain asymmetry: Effects of incentive cues, outcome expectancies, and motor responses. Psychophysiology. 2001;38:500–511. [PubMed] [Google Scholar]
- Murphy FC, Rubinsztein JS, Michael A, Rogers RD, Robbins TW, Paykel ES, Sahakian BJ. Decision-making cognition in mania and depression. Psychological Medicine. 2001;31:679–693. doi: 10.1017/s0033291701003804. [DOI] [PubMed] [Google Scholar]
- Murray CJL, Lopez AD. The global burden of disease: A comprehensive assessment of mortality and disability from diseases, injuries, and risk factors in 1990 and projected to 2020. Boston: Harvard University Press; 1996. [Google Scholar]
- Narushima K, Kosier JT, Robinson RG. A reappraisal of poststroke depression, intra- and inter-hemispheric lesion location using meta-analysis. The Journal of Neuropsychiatry and Clinical Neurosciences. 2003;15:422–430. doi: 10.1176/jnp.15.4.422. [DOI] [PubMed] [Google Scholar]
- Nolen WE. Dopamine and mania: The effects of trans- and cisclopenthixol in a double-blind pilot study. Journal of Affective Disorders. 1983;5:91–96. doi: 10.1016/0165-0327(83)90001-0. [DOI] [PubMed] [Google Scholar]
- Nusslock R, Abramson LY, Harmon-Jones E, Alloy LB, Hogan ME. Pre-goal attainment life event and the onset of bipolar episodes: Perspective from the behavioral approach system dysregulation theory. Journal of Abnormal Psychology. 2007;116:105–115. doi: 10.1037/0021-843X.116.1.105. [DOI] [PubMed] [Google Scholar]
- Okun MS, Bakay RAE, DeLong MR, Vitek JL. Transient manic behavior after pallidotomy. Brain and Cognition. 2003;52:281–283. doi: 10.1016/s0278-2626(03)00073-3. [DOI] [PubMed] [Google Scholar]
- Ongur D, Drevets WC, Price JL. Glial reductions in the subgenual prefrontal cortex in mood disorders. Proceedings of National Academy of Sciences USA. 1998;95:13290–13295. doi: 10.1073/pnas.95.22.13290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perris H. Life events and depression: Part 2. Results in diagnostic subgroups, and in relation to the recurrence of depression. Journal of Affective Disorders. 1984;7:25–36. doi: 10.1016/0165-0327(84)90061-2. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Drevets WC, Rauch SL, Lane R. Neurobiology of emotion perception I: The neural basis of normal emotion perception. Biological Psychiatry. 2003;54:504–514. doi: 10.1016/s0006-3223(03)00168-9. [DOI] [PubMed] [Google Scholar]
- Post R. Transduction of psychosocial stress into the neurobiology of recurrent affective disorders. American Journal of Psychiatry. 1992;149:999–1010. doi: 10.1176/ajp.149.8.999. [DOI] [PubMed] [Google Scholar]
- Post RM, Jimerson DC, Bunney WE, Goodwin FK. Dopamine and mania: Behavioral and biochemical effects of the dopamine receptor blocker pimozide. Psychopharmacology. 1980;67:297–305. doi: 10.1007/BF00431272. [DOI] [PubMed] [Google Scholar]
- Rajkowska G, Halaris A, Selemon LD. Reductions in neuronal and glial density characterize the dorsolateral prefrontal cortex in bipolar disorder. Biological Psychiatry. 2001;49:741–752. doi: 10.1016/s0006-3223(01)01080-0. [DOI] [PubMed] [Google Scholar]
- Reilly-Harrington NA, Alloy LB, Fresco DM, Whitehouse WG. Cognitive styles and life events interact to predict bipolar and unipolar symptomatology. Journal of Abnormal Psychology. 1999;108(4):567–578. doi: 10.1037//0021-843x.108.4.567. [DOI] [PubMed] [Google Scholar]
- Robinson RG, Boston JD, Starkstein SE, Price TR. Comparison of mania and depression after brain injury: Causal factors. American Journal of Psychiatry. 1988;145:172–178. doi: 10.1176/ajp.145.2.172. [DOI] [PubMed] [Google Scholar]
- Rosso IM, Killgore WDS, Cintron CM, Gruber SA, Tohen M, Yurgelun-Todd DA. Reduced amygdala volumes in first-episode bipolar disorder and correlation with cerebral white matter. Biological Psychiatry. 2007;61:743–749. doi: 10.1016/j.biopsych.2006.07.035. [DOI] [PubMed] [Google Scholar]
- Rubinsztein JS, et al. Decision-making in mania: A PET study. Brain. 2001;124:2550–2563. doi: 10.1093/brain/124.12.2550. [DOI] [PubMed] [Google Scholar]
- Ruggero CJ, Johnson SL. Reactivity to a laboratory stressor among individuals with bipolar I disorder in full or partial remission. Journal of Abnormal Psychology. 2006;15:539–544. doi: 10.1037/0021-843X.115.3.539. [DOI] [PubMed] [Google Scholar]
- Salavert J, Caseras X, Torrubia R, Furest S, Arranz B, Duenas R, San L. The functioning of the Behavioral Activation and Inhibition Systems in bipolar I euthymic patients and its influence in subsequent episodes over an 18-month period. Personality and Individual Differences. 2007;42:1323–1331. [Google Scholar]
- Schultz W. Dopamine neurons and their role in reward mechanisms. Current Opinion in Neurobiology. 1997;7:191–197. doi: 10.1016/s0959-4388(97)80007-4. [DOI] [PubMed] [Google Scholar]
- Scott J, Pope M. Cognitive styles in individuals with bipolar disorders. Psychological Medicine. 2003;33:1081–1088. doi: 10.1017/s0033291703007876. [DOI] [PubMed] [Google Scholar]
- Scott J, Stanton B, Garland A, Ferrier IN. Cognitive vulnerability in patients with bipolar disorder. Psychological Medicine. 2000;30:467–472. doi: 10.1017/s0033291799008879. [DOI] [PubMed] [Google Scholar]
- Sharma V, Menon R, Carr TJ, Densmore M, Mazmanian D, Williamson PC. An MRI study of subgenual prefrontal cortex in patients with familial and non-familial bipolar I disorder. Journal of Affective Disorders. 2003;77:167–171. doi: 10.1016/s0165-0327(02)00109-x. [DOI] [PubMed] [Google Scholar]
- Shen GHC, Alloy LB, Abramson LY, Grandin LD. Social rhythm regularity and the onset of affective episodes in bipolar spectrum individuals. Bipolar Disorders. doi: 10.1111/j.1399-5618.2008.00583.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverstone T. Dopamine in manic depressive illness: A pharmacological synthesis. Journal of Affective Disorders. 1985;8:225–231. doi: 10.1016/0165-0327(85)90020-5. [DOI] [PubMed] [Google Scholar]
- Soares JC, Mann JJ. The anatomy of mood disorders—review of structural neuroimaging studies. Biological Psychiatry. 1997a;41:86–106. doi: 10.1016/s0006-3223(96)00006-6. [DOI] [PubMed] [Google Scholar]
- Soares JC, Mann JJ. The functional neuroanatomy of mood disorders. Journal of Psychiatric Research. 1997b;31:393–432. doi: 10.1016/s0022-3956(97)00016-2. [DOI] [PubMed] [Google Scholar]
- Sobotka SS, Davidson RJ, Senulis JA. Anterior brain asymmetries in response to reward and punishment. Electroencephalography and Clinical Neurophysiology. 1992;83:236–247. doi: 10.1016/0013-4694(92)90117-z. [DOI] [PubMed] [Google Scholar]
- Stern GS, Berrenberg JL. Skill-set, outcome, and mania as determinants of the illusion of control. Journal of Research in Personality. 1979;13:206–220. [Google Scholar]
- Strakowski SM, DelBello MP, Fleck DE, Arndt S. The impact of substance abuse on the course of bipolar disorder. Biological Psychiatry. 2000;48:477–485. doi: 10.1016/s0006-3223(00)00900-8. [DOI] [PubMed] [Google Scholar]
- Strakowski SM, DelBello MP, Sax KW, Zimmerman ME, Shear PK, Hawkins JM, Larson ER. Brain magnetic resonance imaging of structural abnormalities in bipolar disorder. Archives of General Psychiatry. 1999;56:254–260. doi: 10.1001/archpsyc.56.3.254. [DOI] [PubMed] [Google Scholar]
- Sutton SK, Davidson RJ. Prefrontal brain asymmetry: A biological subtrate of the behavioral approach and inhibition systems. Psychological Science. 1997;8:204–210. [Google Scholar]
- Swendsen J, Hammen C, Heller T, Gitlin M. Correlates of stress reactivity in patients with bipolar disorder. American Journal of Psychiatry. 1995;152(5):795–797. doi: 10.1176/ajp.152.5.795. [DOI] [PubMed] [Google Scholar]
- Teicher MH. Actigraphy and motion analysis: New tools for psychiatry. Harvard Review of Psychiatry. 1995;3:18–35. doi: 10.3109/10673229509017161. [DOI] [PubMed] [Google Scholar]
- Urošević S, Abramson LY, Harmon-Jones E, Donovan PM, Guequierre LL, Hogan ME, Alloy LB. The behavioral approach system (BAS) and bipolar spectrum disorders: Relationship of BAS and behavioral inhibition system (BIS) sensitivities to bipolar spectrum diagnoses and hypomanic personality. 2007 Manuscript under review. [Google Scholar]
- Videbech P. PET measurements of brain glucose metabolism and blood flow in major depressive disorder: A critical review. Acta Psychiatrica Scandinavica. 2000;101:11–20. doi: 10.1034/j.1600-0447.2000.101001011.x. [DOI] [PubMed] [Google Scholar]
- Wacker J, Heldman M, Stemmler G. Separating emotion and motivational direction in fear and anger: Effects on frontal asymmetry. Emotion. 2003;3:167–193. doi: 10.1037/1528-3542.3.2.167. [DOI] [PubMed] [Google Scholar]
- Winsberg ME, Sachs N, Tate DL, Adalsteinsson E, Spielman D, Ketter TA. Decreased dorsolateral prefrontal N-acetyl aspartate in bipolar disorder. Biological Psychiatry. 2000;47:475–481. doi: 10.1016/s0006-3223(99)00183-3. [DOI] [PubMed] [Google Scholar]
- Wong G, Lam D. The development and validation of the coping inventory for prodromes of mania. Journal of Affective Disorders. 1999;53:57–65. doi: 10.1016/s0165-0327(98)00096-2. [DOI] [PubMed] [Google Scholar]
- Wright K, Lam D, Newsom-Davis I. Induced mood change and dysfunctional attitudes in remitted bipolar I affective disorder. Journal of Abnormal Psychology. 2005;114:689–696. doi: 10.1037/0021-843X.114.4.689. [DOI] [PubMed] [Google Scholar]
- Wright RA, Brehm JW. Energization and goal attractiveness. In: Pervin LA, editor. Goal concepts in personality and social psychology. Hillsdale, New Jersey: Lawrence Erlbaum Associates, Inc; 1989. pp. 169–210. [Google Scholar]