Abstract
Bone marrow-derived mesenchymal stromal cells (MSCs) localize to solid tumors. Defining the signaling mechanisms that regulate this process is important to understanding the role of MSCs in tumor growth. Using a combination of chromatography and electrospray tandem mass spectrometry we have identified novel soluble signaling molecules that induce MSC chemotaxis present in conditioned medium of the breast carcinoma cell line MDA-MB231. Previous work has employed survey strategies using ELISA assay to identify known chemokines that promote MSC chemotaxis. While these studies provide valuable insights into the intercellular signals that impact MSC behavior, many less well-described, but potentially important soluble signaling molecules could be overlooked using these methods. Through the less directed method of column chromatography we have identified novel candidate MSC chemotactic peptides. Two proteins, cyclophilin B and hepatoma-derived growth factor were then further characterized and shown to promote MSC chemotaxis.
Keywords: Mesenchymal Stromal Cell, Tumor microenvironment, chemotaxis
Introduction
Multipotent mesenchymal stromal cells (MSCs) are bone marrow-derived plastic-adherent cells that were initially described by Friedenstein and colleagues [1, 2]. They have a fibroblast-like phenotype[1, 2] and can be differentiated along osteoblastic, chondrocytic, adipogenic, and myofibroblastic lineages[3, 4]. MSCs within the bone marrow support hematopoiesis and the recent identification of MSCs in the peripheral blood[5–8] as well as tissues such as fat[9] and placenta[10] support the hypothesis that MSCs have important functions outside of the bone marrow. Systemically delivered MSCs localize to areas of active inflammation including sites of bone fracture[11], cutaneous incisional wounds[12], myocardial infarction[13] and solid tumors[14–16] indicating that these cells are involved in tissue repair and growth.
While the propensity of MSCs to migrate to tumors and areas of tissue damage has been well-documented, the molecular signals guiding this movement are not completely defined. MSCs are known to respond to a number of chemotactic factors including stromal-derived factor-1 (SDF-1)[17, 18], vascular endothelial growth factor A (VEGF-A)[18, 19], platelet derived growth factor (PDGF)[20], Monocyte chemoattractant protein-1 (MCP-1), macrophage inflammatory protein 1-alpha (MIP-1α, and interleukin 8 (IL-8) [21]. Tumor cells are a potential source of MSC chemotactic factors and we have previously shown that tumor cell line conditioned medium promotes MSC chemotaxis [22].
Proteoglycans are found on cell surfaces and within the extracellular matrix and have been implicated in several aspects of cell-to-cell communication including the interaction of chemokines with cell surface receptors, protection of signaling peptides from degradation, and facilitating the formation of chemokine gradients[23–25]. We therefore hypothesized that factors that induce MSC chemotaxis may bind proteoglycans and used heparin sulfate column chromatography as the basis for enrichment of pro-chemotactic molecules from tumor-conditioned medium. Peptides within the fraction of conditioned medium with chemotactic activity were identified using electrospray tandem mass spectometry protein sequencing. Of these, cyclophilin B and hepatoma-derived growth factor were further investigated and found to promote MSC chemotaxis.
Using proteomic techniques we have identified candidate chemotactic molecules for MSCs that have not been previously recognized using molecular genetic or ELISA-based identification strategies.
Materials and Methods
Antibodies
The monoclonal antibody against CypB (4E11G1) was obtained from Invitrogen (Carlsbad, CA). The anti-CD147 antibody (HIM6) was purchased from BD Biosciences (Franklin Lakes, NJ). The HDGF-blocking antibody (C-14) and anti-osteocalcin antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA).
Isolation and culture of Mesenchymal Stem Cells
Rat MSCs were isolated as previously described[22]. Briefly, rats were euthanized by CO2 inhalation and the bilateral femora and tibias were dissected under aseptic conditions and washed in phosphate-buffered saline (PBS) containing 2% fetal bovine serum (FBS) (Invitrogen, Carlsbad, CA). Bone marrow cells were extruded by flushing media through the bone, and cells filtered through a 70 μm nylon mesh. The cells were then plated in T150 or T 75 cm2 flasks with Minimum Essential Medium alpha medium (α MEM) (Invitrogen, Carlsbad, CA) containing 10% fetal bovine serum (FBS) and penicillin/streptomycin. The cultures were incubated at 37°C in a humidified atmosphere containing 5% CO2. Nonadherent cells were removed after 24 h, and the medium was changed every other day. Adherent cells were detached from the flasks by treatment with 0.05% trypsin and ethylenediaminetetraacetic acid (EDTA) and subcultured every 4 to 5 days and aliquots from passage 3 to 5 were frozen in liquid nitrogen for future use. MSC preparations used in subsequent experiments all demonstrated in vitro myogenic, osteogenic, and adipogenic differentiation under appropriate stimuli as we have previously described[26]. Additionally, both the rat and human MSC preparations were tested for the presence of osteogenic committed cells by staining for osteocalcin. Staining for osteocalcin before differentiation was not observed (data not shown).
Two samples of purified MSCs from separate lots (Cambrex Bio Science, East Rutherford, NJ) as well as one sample of MSCs prepared from whole human bone marrow were used in these experiments. For the isolation of human mesenchymal stem cells unprocessed bone marrow (36×106 cells/ml) was purchased from Cambrex Bio Sciences (East Rutherford, NJ). Vacutainer CPT cell preparation tubes (BD Biosciences, Franklin Lakes, NJ) were used to isolate bone marrow mononuclear cells according to the manufacturer’s instructions. Cells were then expanded in T75 cm2 and 6 well plate with MesenCult Basal medium (human) (cat#05401, Stem Cell Technologies, Vancouver, BC) containing MSC stimulatory supplements (Human) (cat#005402, Stem Cell Technologies, Vancouver, BC) and 10 % fetal bovine serum (Invitrogen, Carlsbad, CA). The cultures were incubated at 37°C in a humidified atmosphere containing 5 % CO2. Adherent cells were detached from the flasks by treatment with 0.05% trypsin and ethylenediaminetetraacetic acid (EDTA) and subcultured every 4 to 5 and the medium was changed every other day. Cells were subcultured every 4 to 5 days and aliquots from passage 2 to 5 were frozen in liquid nitrogen for future use. Cell surface markers expressed on these cells include Stro1, CD 105, CD 90, HLA-ABC and CD 44 while they were negative for CD45, HLA-DR and CD11b as determined by flow cytometry using FITC labeled Abs (BD Biosciences, Franklin Lakes, NJ).
Transwell chamber migration assays
A Falcon cell culture insert system along with a companion Falcon tissue culture plate with 24 wells was used for the chemotaxis assay. The insert was removed aseptically with sterile forceps from the package and gently placed in the well with the flanges resting in the notches on the top edge of each well (Becton Dickinson Labware, Franklin Lakes, NJ). The polyethylene terepthalate membrane pore size of 8 μm was selected to allow passage of MSCs. The bottom chamber contained either conditioned medium from tumor cells or control medium (α MEM plus 2% FBS). The top chamber contained 2.5 x 104 MSCs in α MEM and 10% FBS. Migration assays were terminated after 16 h and MSCs that had migrated through the membrane were then stained (after removal of cells remaining on top with a wet Q-tip) using crystal violet. Stained cells in ten fields were counted under high power magnification (x40). For the chemotaxis assays, MSCs were acclimated for 72 h in RPMI + 10% heat inactivated FBS (the growth medium for tumor cell lines) prior to plating in the transwell chambers. Rat MSCs were used for the migration assay for the protein purification experiments. Human MSCs (hMSCs) were then used to validate the results obtained with the rat cells and for additional experiments investigating the chemotactic activity of cyclophilin B and HDGF.
Enrichment of chemotactic factors by chromatography
The breast carcinoma cell line MDA-MB 231 was purchased from American Type Culture Collection (Manassas, VA) and used in these experiments. We have previously demonstrated that MDA-MB231 cells produce soluble factors that induce MSC migration in vitro[22]. We then set out to develop a strategy for the purification and identification of these factors from the conditioned medium of the MDA-MB231 cells. Cells were grown to confluence in tissue culture flasks of 175 cm2 in α MEM + 10% heat inactivated FBS. This was followed by incubation with MEM alpha medium with 2% heat inactivated fetal bovine serum (FBS) in the presence of 1% Penicillin and 1% Streptomycin overnight. The conditioned medium was centrifuged at 200 rcf for 10 min, and the supernatant was then filtered with a 0.22 μm membrane. A number of affinity resins including heparin Sepharose beads (GE Biosciences, Piscataway, NJ), reactive green dye 19, reactive red dye 120, type 100, reactive red dye 120, type 3000, and wheat germ agglutinin crosslinked to agarose beads (Sigma, Saint Louis, MO) were tested for the ability to bind factors present in the conditioned medium of MDA-MB231 cells that promoted migration of rat MSCs. Each affinity resin (0.2ml) was mixed with 2.5ml of conditioned medium and incubated for 1 hour at 4 degrees C. The resin was then centrifuged at 200 rcf for 1 minute and the supernatant was collected and tested for the ability to promote rat MSC migration. Heparin Sepharose CL-6B and Reactive Green Dye 19 resin cross-linked to agarose beads reduced the migration-promoting activity of MDA-MB231 conditioned medium to the basal level and were therefore used in the purification strategy. A Heparin Sepharose CL-6B column with 20 ml resin was loaded with approximately 3000 ml of conditioned medium pooled from multiple culture flasks and washed with 50 mM NaCl and Tris buffer (10 mM Tris-HCl, pH 8). Proteins were eluted in 40ml fractions with a step gradient of 0.2, 0.4, 0.6, and 0.8 M NaCl in Tris buffer. Each fraction was concentrated 10 fold with centrifugal filter devices with a molecular weight cutoff of 10kDa (Ultrafree centrifugal filter device, Millipore, Billerica, MA) and then was brought up to the original volume with Tris buffer. The activity of each fraction was assayed using the transwell chamber migration assay. Fractions eluted with 0.4 and 0.6 M NaCl had the greatest activity and were pooled and then dialyzed against the 10mM Tris PH8.0 buffer using the centrifugal filter device. The pooled fractions were loaded on 10ml of Reactive Green Dye 19 resin cross-linked to agarose beads and washed with 50 ml of 10mM tris pH 8.0 buffer. Proteins were eluted in 20 ml fractions with a step gradient of 0.2, 0.4, 0.6, and 0.8 M NaCl in 10 mM Tris pH 8.0 buffer. Again each fraction was dialyzed against 10mM Tris buffer using the centrifugal filter devices and assayed for activity using the transwell chamber migration assay. MSC migration was stimulated most effectively with column fractions eluted with 0.6M NaCl. The protein profiles of the original conditioned medium as well as the active fractions after each purification step were analyzed by gel electrophoresis.
Protein elution from SDS-gel
The green dye column enriched fractions were concentrated by 10-fold using Ultrafree centrifugal filter devices. The proteins were resolved by 15% SDS gel, followed by incubation with 20% isopropanol and 10 mM Tris-HCl, pH 8.0 for 30 min. After briefly washing with water, the gel was incubated with buffer containing 10 mM Tris-HCl, pH 8.0 overnight. The gel was divided into sections based on molecular size and the gel slices were minced and then incubated with 1 ml α-MEM medium to elute the proteins for use in the migration assay.
Protein identification by electrospray tandem mass spectrometry
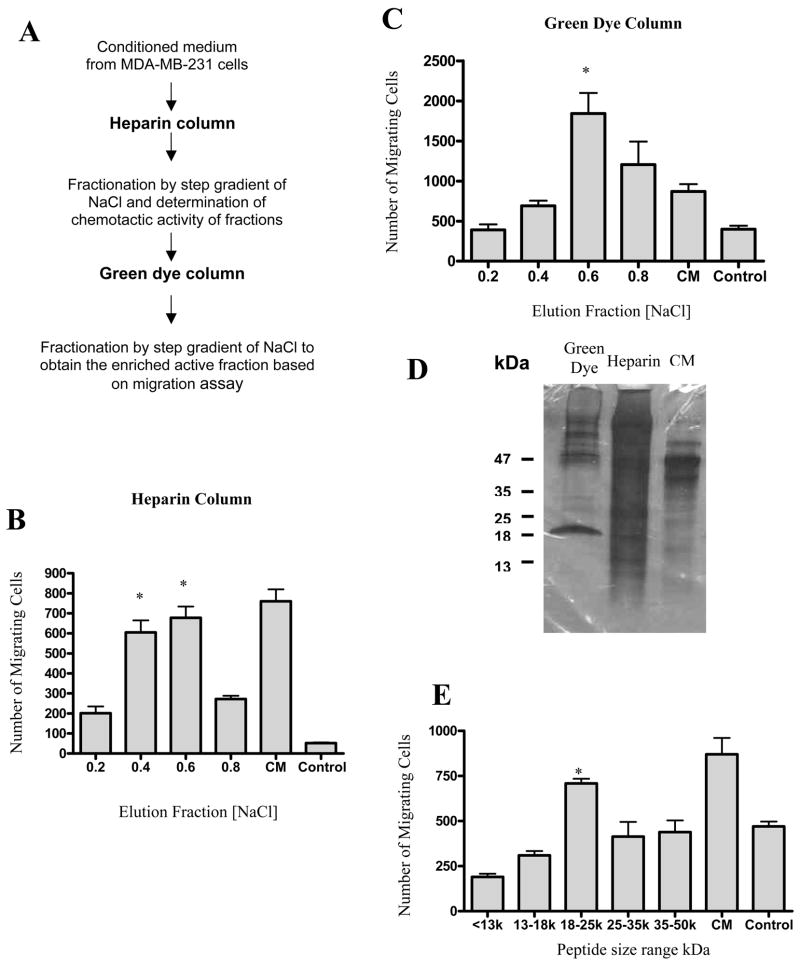
Proteins from the enriched active fraction eluted off of the green dye column were resolved by 12% SDS-PAGE followed by colloidal blue staining according to the protocol suggested by manufacturer (Colloidal Blue Staining Kit, Invitrogen, Carlsbad, CA). A major band was present at a molecular weight of approximately 18 kDa (Figure 1D), corresponding to the peptide size with the greatest activity (Figure 1E). This band was excised from the gel and subjected to sequence analysis that was performed at the Harvard Microchemistry and Proteomics Analysis Facility by microcapillary reverse-phase HPLC nano-electrospray tandem mass spectrometry (μLC/MS/MS) on a Thermo LTQ-Orbitrap mass spectrometer.
Figure 1.
The strategy for enrichment of heparin-sulfate binding pro-migratory factors is shown in A. A heparin column with 10 ml resin was loaded with 3 L conditioned medium (CM) after 18 h incubation with MDA-MB-231 cells. The absorbed proteins were then eluted with 20 ml aliquots of buffer with increasing concentrations of NaCl (0.2, 0.4, 0.6 and 0.8 M). Equal volume (60 μl each) of the eluted fractions were subjected to MSC migration assay. The activity of fractions eluted from the heparin sulfate column are shown in B. The activity is presented as the number of cells migrating in response to each column fraction and compared to the number of cells migrating in response to MDA-MB231 conditioned medium (CM). The control medium (control) is α-MEM with 2% FBS. Active fractions from the heparin sulfate column (0.4 M and 0.6M eluates) were then pooled and loaded on a 10ml column packed with green dye. Again, protein was eluted using increasing concentrations of NaCl. The activity of fractions eluted from the green dye column is shown in C. SDS PAGE was run of column fractions with MSC pro-migratory activity from the green dye and heparin columns as well as MDA-MB-231 conditioned medium and protein bands were visualized using staining as shown in D. The active fraction from the green dye column (0.6M) was subjected to SDS-PAGE and protein was eluted from gel fragments containing various size proteins. The activity of proteins eluted from the gel is shown in E. The response to α-MEM with 2% FBS (control) is shown. Data are representative of one of three experiments with triplicate wells. The chemotactic activity is presented as the total number of migrating cells in response to eluted proteins. Error bars represent SD. Statistical significance: *, p< 0.038.
Recombinant CypB production and purification
GST-tagged human CypB was produced by growing 500 ml of the bacteria (BL21) transfected with the CypB-pGex [27, 28] expression construct. The bacterial pellet was resuspended in 25 ml PBS followed by sonication. Triton X-100 was then added to the lysate to a final concentration of 1%. After incubation at 4 °C for 30 min, the lysate was clarified by centrifugation at 12000 rcf. The supernatant was incubated with 1 ml Glutathione Sepharose 4B resin (GE Biosciences, Piscataway, NJ) for 1 hr at 25 °C, followed by washing with 20 ml PBS. The GST-CypB was eluted with elution buffer containing 20 mM glutathione and 50 mM Tris-HCl, pH 8.0. The purified GST-CypB fusion protein was cleaved by thrombin conjugated with agarose according to manufacture suggested protocol (Sigma, Saint Louis, MO) at room temperature for 6 hours. The purified CypB was obtained by removal of GST peptide from the thrombin-treated protein mixture with glutathione-resin.
Recombinant HDGF production and purification
GST tagged human HDGF (GST-HDGF) [29] was expressed in the BL21 (DE3) strain of E. coli. After overnight culture, 0.1 mM IPTG was added to the culture media and cultured for another 4 hours. The cells were lysed in PBST plus protease cocktail (Sigma, St. Louis, MO) through three cycles of freeze-thaw. The lysate was cleared by centrifugation and GST beads (Amersham Pharmacia Biotech, Uppsala, Sweden) were used for the purification of the GST tagged protein. After three washes by PBS, the purified GST-HDGF was eluted in 20mM Tris-HCL, pH 8.0 with 10mM reduced glutathione. To obtain the purified recombinant HDGF, the same method as described in CypB purification section was used to remove the GST peptide following thrombin cleavage.
Analysis of F Actin organization in response to CypB and HDGF
Human MSCs were placed on glass slides and allowed to adhere for 24 hours. Fresh growth medium was added to gently cover the MSCs. MSCs were exposed to CypB or HDGF. A concentration gradient was created by placing a pipet tip containing the chemotactic stimulus (1 μg in 10μl PBS) at the end of the slide opposite the MSC plating site, so that the proteins could slowly released from the fine opening of the tip to generate concentration gradient. After an additional 18 hours of culture, cells were processed for F-Actin staining. Cells were fixed with 3.7–4.0% paraformaldehyde for 10 minutes, washed three times in PBS, and permeablized with 0.1% Triton for 5 minutes. The cells were then blocked with blocking buffer (PBS + 10% BSA) for 15 minutes. Phalloidin-TRITC (tetramethylrhodamine B isothiocyanate) (Sigma, Saint Louis, MO) was used at a final concentration of 50ng/ml in blocking buffer and cells were stained for 60 minutes. Cells were then washed three times in PBS and mounted using ProLing Gold antifade reagent with DAPI (Invitrogen, Carlsbad, CA). Images were obtained using EX-C1 Spectral Imaging program from Nikon.
Coverglass Chemotaxis Assay
A chambered coverslip system was used (grace bio-labs from gracebio.com). This system consists of a coverslip with a removable silicone gasket which separates two 15mm x 15mm wells at a predetermined distance (1mm). Human mesenchymal stem cells (5X104 cells in 300μl) were plated in α-MEM supplemented with 10%FBS and penicillin/streptomycin in one well of the coverslip and grown for 24 hours in at 37°C and 5% CO2. After 24 hours to allow cells to adhere to the coverslip, MSC were washed in 1XPBS to remove nonadherent cells. Cyclophilin B (1μg) or hepatoma-derived growth factor (1μg) were soaked into a 300μl section of 2% agarose and the gel piece was placed in the opposite well from the MSCs. The gasket from the coverslip was then removed. After the gasket was removed, the coverslips were placed in a 10cm Petri dish and 6–7ml of α-MEM supplemented with 10% FBS and penicillin/streptomycin was added. The cells were incubated for 72 hours at 37°C and 5% CO2. The samples were then fixed in 3.7–4%paraformaldehyde for 15 min. Samples were washed in 1XPBS three times. Cells were permeabilized with 0.1%Triton for 5 min and washed 2–3times in PBS (5 minutes each) then blocked with α-MEM supplemented with 10%FBS for 5–15min. Primary antibody (anti-tubulin antibody, Sigma, clone DM1A) was added and samples were incubated in the dark at room temp for 45 minutes to1hr then washed 2–3X in PBS (5minutes each). Secondary antibody (goat anti-mouse Alexa 568, Molecular Probes) was added and incubated in the dark at room temperature for 45 minutes to 1 hour. Samples were washed 2–3Xin PBS (5 minutes each), mounted and dried overnight and sealed with nail polish. Slides were then viewed and images obtained using an EX-C1 spectral imaging program from Nikon and the number of MSCs more than 0.3 mm outside of the original area of the well both toward the stimulus and away from the stimulus were counted.
Statistical Analysis
At least three independent experiments were performed for each migration assay. Results are presented as means +/− standard deviation. Statistical significance was determined using the Student’s t test and a value of p<0.05 was considered statistically significant. Microsoft Excel software was use for statistical analysis.
Results
Purification and Identification of Pro-migratory Factors from Tumor-conditioned Medium
Using a Boyden chamber migration assay we developed a two-step purification procedure for the enrichment of heparin-binding molecules that induce MSC migration from tumor-conditioned medium from the human breast cancer cell line MDA-MB231 (Fig. 1A). Rat MSCs were used in the migration assay throughout the purification procedure. The tumor-conditioned medium was first loaded onto a heparin column, protein fractions were eluted using a step gradient of 0.2–0.8 M NaCl and fractions were assayed for the induction of MSC migration. The activity was recovered in fractions eluted from 0.4 and 0.6 M NaCl (Fig.1B). These fractions were combined and applied to a green dye column. The proteins were eluted from the green dye column with a step gradient of 0.2–0.8 M NaCl. The major pro-migratory activity was present in fractions eluted at 0.6 M NaCl (Fig. 1C).
Enrichment using a heparin column allowed for an approximately 20-fold purification of the pro-migratory activity based on the ratio of total protein mass from CM and the heparin-eluted fraction. Further enrichment by green dye resin greatly increased the purification to 16,000-fold. The green dye resin-eluted fractions contain major protein bands at approximately 47 and 20 kDa (Figure 1D).
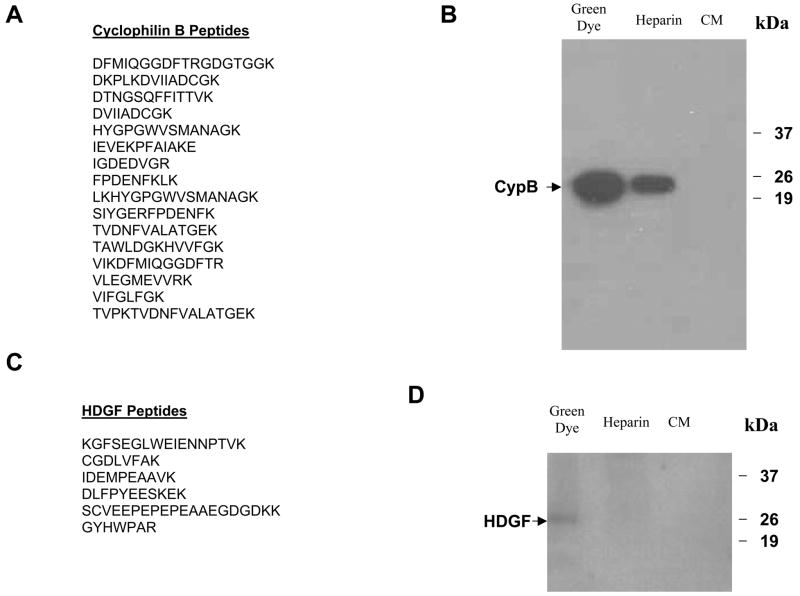
Active fractions from the green dye column were pooled and resolved by the SDS-PAGE. The gel was divided into sections and proteins were eluted from each gel section. The activity of the eluted proteins was then assayed to determine the approximate molecular weight of the chemotactic factors. The results of the migration assay show that the maximum activity is derived from proteins with molecular weight ranging from 18–25 kDa (Fig. 1E). The 18–25 kDa proteins were then subjected to analysis by electrospray tandem mass spectrometry (ES-MS/MS). Thirteen different known human proteins were identified (Table 1). To validate this strategy we further characterized two candidate chemotactic factors. Both cyclophilin B and hepatoma-derived growth factor were represented by multiple peptides in the ES-MS/MS analysis (Fig. 2A and 2C) and are reported to have chemotactic activity in other systems[30–36]. Additionally, both cyclophilin B and HDGF are found in increasing concentrations in the active fractions from the heparin and green dye columns (Fig 2B and 2D). These two proteins were therefore subjected to further analysis.
Table 1.
List of identified known proteins identified by ES-MS/MS analysis
| cyclophilin B (peptidylprolyl isomerase B) |
| hepatoma-derived growth factor |
| tissue inhibitor of metalloproteinase 1 |
| nucleophosmin 1 |
| nascent-polypeptide-associated complex alpha polypeptide |
| translation initiation factor IF2 |
| histidyl-tRNA synthetase |
| apolipoprotein A-I precursor |
| 90 kDa NFAR protein |
| HMGB1 |
| dyskerin |
| insulin-like growth factor binding protein 7 |
| S100 calcium binding protein A13 |
Figure 2.
The fractions from the green dye column with maximum activity were resolved with SDS-PAGE and a gel fragment containing peptides in the 18kDa to 25kDa size range was excised. Proteins were eluted and subject to ES MS / MS. Sixteen peptide fragments corresponding to cyclophilin B were identified (A) and a Western blot of the active column fractions as well as the conditioned medium with an anti-cyclophilin B antibody revealed an increasing concentration of cyclophilin B throughout the enrichment procedure (B). Six peptide fragments corresponding to hepatoma-derived growth factor were identified (C). A Western blot of the active column fractions as well as the conditioned medium with an anti-HDGF antibody only detected in the eluted fraction from green dye column (D).
Recombinant Cyclophilin B induces MSC migration
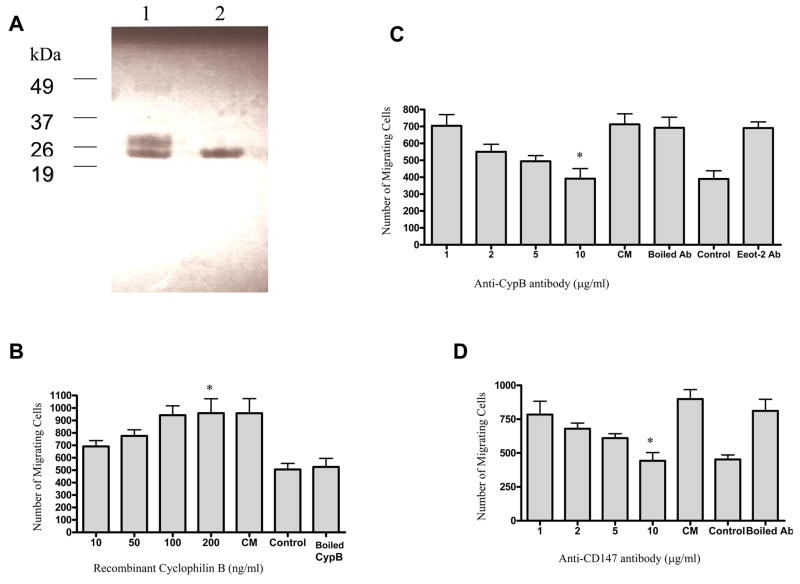
Recombinant bacterially expressed GST-tagged CypB was produced. The protein was purified and the GST peptide removed by thrombin cleavage (Fig. 3A). The ability of recombinant cyclophilin B to induce migration of human MSCs was then determined. Results of a migration assay show that CypB induces human MSC migration in a concentration-dependent fashion: CypB concentration at 50 ng/ml is able to exert levels of pro-migratory activity of approximately fifty percent of the maximal activity obtained using conditioned medium of tumor cells, and maximum migratory effect (about 90% of the effect of conditioned medium) is achieved at concentration of 100 ng/ml (Fig. 3B). Inactivation of CypB by boiling abolished this effect, suggesting that secondary and/or tertiary structures are critical for this function. These data indicate that CypB can induce MSC chemotaxis.
Figure 3.
Recombinant cyclophilin B was produced and resolved by 15% SDS-gel with Coomassie Blue staining (A). Lane 1 shows the purified GST-tagged CypB (0.2 μg) was cleaved by thrombin, and lane 2 shows the purified recombinant CypB (0.2 μg) after removal of the cleaved GST peptide. Recombinant cyclophilin B was able to induce MSC migration in a concentration-dependent manner from 2 to 200 ng/ml. Boiled, recombinant cyclophilin B did not induce MSC migration (B). Statistical comparisons: *, significantly higher than control, p= 0.03. The chemotaxis of MSCs in response to MDA-MB231 conditioned medium (CM) and to medium with 2% FBS (control) are shown. An anti-cyclophilin B antibody blocks the chemotaxis of MSCs in response to MDA-MB-231 conditioned medium (C). Statistical comparisons: *, significantly lower than CM, p= 0.05 The anti-cyclophilin B antibody was added to CM at concentrations from 1 to 10 μg/ml and a concentration-dependent decrease in MSC migration was observed. Neither boiled antibody nor an antibody to an unrelated protein (Eot-2) had this effect. MSC migration in response to 2% FBS (control) is also shown. A specific antibody to a known receptor for cyclophilin B, CD147, was also added to tumor cell CM at concentrations from 1 to 10 μg/ml and also inhibited migration of MSCs. This effect was not observed when the anti-CD147 antibody was boiled (D). comparisons: *, significantly lower than CM, p= 0.01
A specific Cyclophilin B blocking antibody decreases MSC migration in response to tumor-conditioned medium
Increasing concentrations of a specific anti-cyclophilin B antibody were added to tumor-conditioned medium used as a stimulus in the migration assay. Human MSC migration was reduced in a concentration-dependent manner (Figure 3C). Maximum blockade of migration was achieved with an antibody concentration of 10μg/ml. Heat-inactivated antibody and a monoclonal antibody against EOT-2, an unrelated chemokine, did not inhibit MSC migration.
Antibody mediated blockade of a cyclophilin B receptor, CD147, impairs MSC migration in response to tumor-conditioned medium
Cyclophilin B has been reported to induce chemotaxis through its interaction with CD147[37]. Human MSCs were exposed to increasing concentrations of an anti-CD147 antibody prior to assaying their migration in response to tumor-conditioned medium. The results of the migration assay show that CD147 blockade diminishes MSC migration in a concentration-dependent manner (Fig. 3D). The migration is maximally reduced by the antibody at 10 μg/ml. Heat denatured antibody did not effect migration. Inhibition of MSC migration by this antibody suggests that the CD147 is involved in CypB-mediated migration of MSCs.
Hepatoma-derived growth factor induces MSC migration
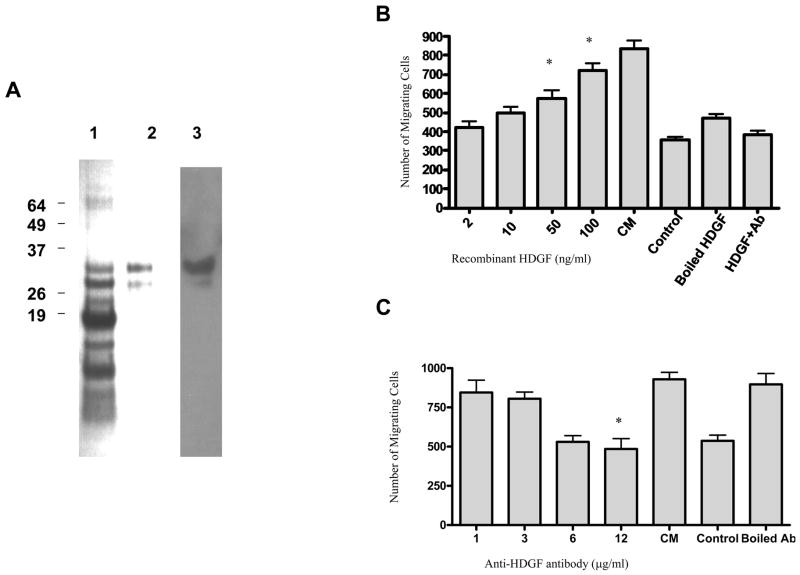
Hepatoma-derived growth factor (HDGF) is a known chemotactic molecule that is present in MDA-MB-231 tumor-conditioned medium. It was represented by six distinct peptides in the ES MS/MS analysis and can be identified in increased amounts in the active column fractions (Figure 2D and 2E). The ability of HDGF to induce human MSC migration was verified through the use of both recombinant HDGF in the Boyden chamber assay and the attenuation of the effect of tumor conditioned medium using a specific antibody to HDGF. Recombinant GST tagged HDGF was produced and purified using GST beads (Figure 4A). Purified HDGF stimulates MSC migration to nearly the same extent as conditioned medium at HDGF concentrations of 50–100ng/ml (Figure 4B). The addition of an anti-HDGF antibody to tumor cell conditioned medium attenuates MSC migration in a concentration-dependent manner (Figure 4C).
Figure 4.
Recombinant GST tagged HDGF was produced. This figure shows Coomassie blue staining of the purified GST-tagged HDGF (1 μg) cleaved by thrombin (A lane 1), purified recombinant HDGF (0.2 μg) after removal of GST peptide (A lane 2) and purified recombinant protein (0.2 μg) analyzed by Western blotting using the antibodies to the proteins. (A lane 3). Recombinant HDGF was able to induce MSC migration in a concentration-dependent manner from 0.4 to 100 ng/ml. Migration of MSCs in response to the medium with 2% FBS (control) is shown. Boiled, recombinant HDGF did not induce MSC migration and addition of an anti-HDGF antibody at a concentration of 12 μg/ml blocks migration in response to 100ng/mL HDGF comparisons: *, significantly higher than control, p= 0.01. (B). An anti-HDGF antibody also blocks the migration of MSCs in response to MDA-MB-231 conditioned medium (C). The anti-HDGF antibody was added to CM at concentrations from 1 to 12 μg/ml and a concentration-dependent decrease in MSC migration was observed. Antibody denatured by boiling did not affect MSC migration. Statistical significance *, significantly lower than CM, p= 0.002.
Cytoskeletal changes in MSCs associated with chemotaxis in response to CypB and HDGF
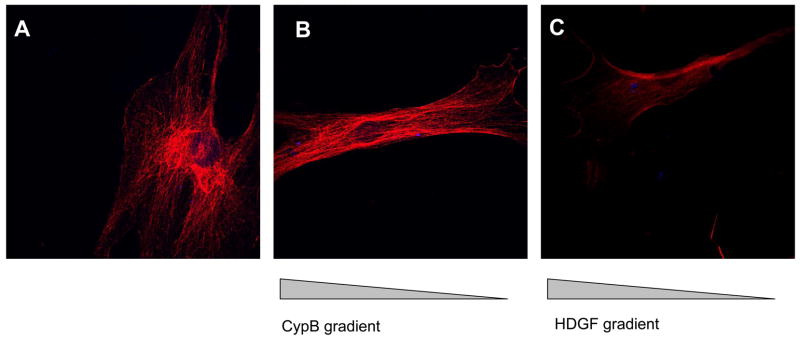
We investigated the changes in human MSC cytoskeletal organization in response to CypB and HDGF. The exposure of MSCs to gradients of CypB or HDGF led to characteristic reorganization of actin filaments as detected by phalloidin staining (Figure 5). In response to these stimuli MSCs were observed to have F actin filaments organized along the length of the cell indicative of cytoskeletal reorganization during chemotaxis.
Figure 5.
Cytoskeletal changes in mesenchymal stromal cells (MSCs) exposed to a CypB or an HDGF gradient. (A): Phalloidin staining shows F actin filament organization of MSCs grown in medium with 2% FBS. (B & C): MSCs were exposed to CypB (B) or HDGF (C). A concentration gradient was created by placing a pipet tip loaded with the stimulus at the opposite edge of the slide from the plated MSCs. After a 24-hour incubation organization of F-actin along the length of the cells can be seen.
CypB and HDGF induce MSC chemotaxis
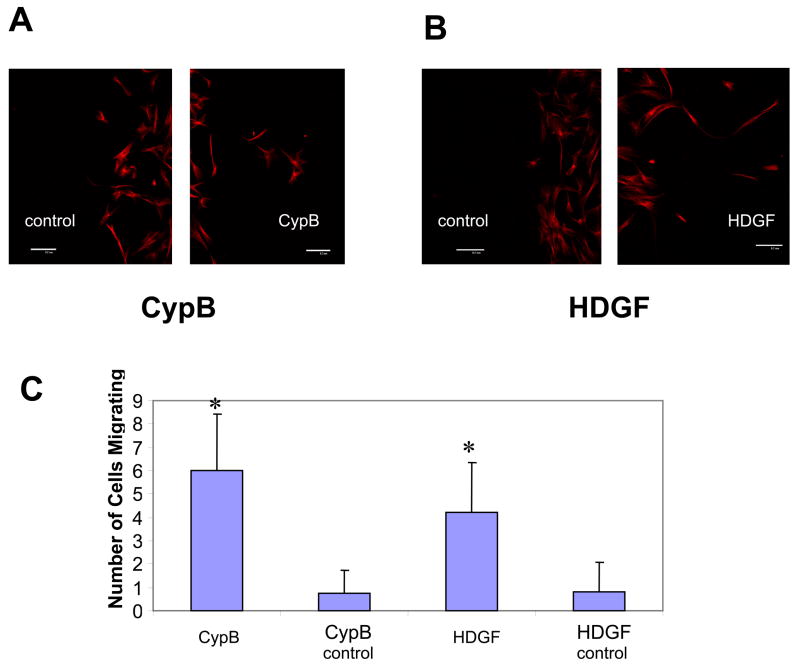
We investigated whether MSCs would display chemotaxis in response to a gradient of HDGF or CypB. Using a chamber coverslip system a gradients of HDGF or CypB were established. MSCs showed chemotaxis toward both the CypB and HDGF stimuli but no migration away from these stimuli (Figure 6). This indicates that MSCs have a directional response to gradients of CypB and HDGF.
Figure 6.
Movement of MSCs in response to gradients of CypB or HDGF. Human MSCs were exposed to a gradient of CypB or HDGF using a chambered coverslip system. After a period of 72 hours of incubation MSCs were stained for αtubulin and cells that had migrated more than 0.3mm from the original plating site both toward the stimulus and away from the stimulus were determined. (A): MSCs are seen migrating from the original plating site toward the CypB source and with minimal migration out of the original plating area away from the CypB stimulus (control). (B): MSCs are observed migrating from the original plating site toward the HDGF source and no cells migrated out of the original plating area away from the HDGF stimulus (control). (C): Graph showing the number of cells greater than 0.3mm from the original plating area in response to CypB and HDGF. Controls are the number of cells migrating greater than 0.3mm from the original plating area in the direction away from the stimulus. Error bars show standard deviation (n=3). Statistical significance *, significantly increased number of cells, p<0.03.
Discussion
Bone marrow-derived MSCs localize to solid tumors and may play an important role within the tumor stroma. In an effort to understand the molecular signals involved in MSC migration, factors that promote MSC chemotaxis have been identified using molecular genetic approaches and ELISA analysis. While important information has been obtained using these methods, less well-described chemotactic stimuli may be overlooked. We have used heparin binding, a specific biochemical property common to multiple known chemotactic factors, as the basis for an enrichment strategy to identify additional molecules that influence MSC localization.
Numerous chemokines bind to proteoglycans and this interaction plays an important role in a variety of biological processes such as cell surface receptor binding, cell adhesion, and the formation of signaling gradients[23, 24, 38–41]. We have used heparin sulfate binding as the basis for the enrichment of factors that promote MSC chemotaxis from tumor conditioned medium. Using electrospray tandem mass spectrometry for protein microsequencing candidate pro-chemotactic molecules were identified. Cyclophilin B and hepatoma-derived growth factor were subjected to further analysis confirming that these two proteins, present in tumor-conditioned medium, promote MSC chemotaxis.
Cyclophilin B is a member of the immunophilin family of proteins. These proteins have peptidyl-prolyl cis-trans isomerase activity and perform roles as chaperones and in cell signaling[42]. Cyclophilins A and B have been reported to induce the chemotaxis of hematopoietic cells such as T cells, eosinophils, and neutrophils [30–33]. Because cyclophilin B was highly represented in our analysis and induces chemotaxis in other systems, we further investigated the ability of cyclophilin B to promote MSC chemotaxis. Recombinant cyclophilin B was able to induce MSC chemotaxis and antibody mediated blockade of both cyclophilin B as well as a known receptor for cyclophilin B, CD147 [37]inhibits MSC migration in response to tumor-conditioned medium. These data indicate that cyclophilin B may be involved in the response of MSCs to tumor cells. However, the concentration of recombinant cyclophilin B needed to stimulate MSC migration was significantly higher than the concentration of cyclophilin B in the tumor CM. As multiple other factors are know to influence MSC chemotaxis it is likely that chemotaxis of MSCs in response to tumor CM is due to a combination of signaling molecules.
Hepatoma-derived growth factor (HDGF) is a known heparin-binding protein that was originally isolated from the conditioned medium of a human hepatoma cell line. It has sequence homology with high mobility group protein 1 (HMG 1). HDGF induces the chemotaxis and growth of smooth muscle cells[34–36]. It also has angiogenic activity[43, 44]. Our data show that HDGF promotes the chemotaxis of MSCs and suggest that HDGF may have a role in the interplay between tumor cells and stromal elements. However, as with cyclophilin B, recombinant HDGF does not promote MSC migration as efficiently as tumor-conditioned medium. It is important to note that identification of the specific role of either Cyp B or HDGF in the homing of MSCs to tumor sites in vivo awaits further study.
Both cyclophilin B and HDGF are know to bind to glycosaminoglycans (GAGs). The important role played by GAGs during inflammation is being increasingly appreciated. GAGs appear on cell surfaces, in the extracellular matrix, and in soluble forms. Interaction with GAGs is important in the function of signaling molecules such as cytokines[45] and has been implicated in leukocyte trafficking through activated endothelium[24], the presentation of chemokines to their cell surface receptors[41, 46, 47], protection from proteolysis[23, 38, 48], and the formation of chemical gradients[40, 49, 50]. In the case of cyclophilin B the interaction with cell surface heparin sulfate proteoglycans may be important in CypB signaling through CD147[31]. Because of the important role of GAG binding in cellular communication this interaction can be exploited for the isolation of signaling molecules.
Our data demonstrate that both HDGF and CypB can influence the behavior of MSCs in a simple in vitro system. While the Boyden chamber assay is a rapid and useful assay for the isolation of pro-migratory stimuli for MSCs, it is unlikely to completely represent the function of these molecules during in vivo MSC biology. However, a number of other signaling molecules that induce MSC chemotaxis in vitro have been shown to play an important role in MSC biology in vivo. Stromal derived factor I stimulates in vitro MSC chemotaxis and has been shown to be important in MSC growth, release from the bone marrow, and resistance to apoptosis. Thus in vitro chemotaxis can serve as an assay for the initial identification and isolation of peptides that may be important in MSC behavior. The influence of HDGF and CypB on other MSC properties such as growth and resistance to apoptosis as well as their importance in different in vivo processes such as wound healing and tumor growth remain an area for additional study.
The tumor stroma is increasingly recognized as a potential therapeutic target for the treatment of solid tumors. A significant portion of the tumor stroma is made up of bone marrow-derived cells and disrupting the localization of these cells to the tumor microenvironment is being investigated in both preclinical settings and early clinical trials. Impairing endothelial progenitor cell mobilization from the bone marrow has been shown to inhibit tumor growth in animal models and is being tested in the clinic[51, 52]. Pharmacological ablation of macrophages, another important component of the tumor microenvironment, has also been demonstrated to impair tumor growth in animal models[53]. Recent evidence suggests that MSCs are also a critical component of the stroma of some solid tumors[54]. A more complete understanding of the role of the signaling pathways that lead to MSC localization to solid tumors may uncover novel strategies for targeting MSCs within the tumor microenvironment.
In summary, we have used an enrichment strategy based on heparin binding to identify novel factors produced by tumor cells that induce MSC chemotaxis. This strategy allows for the identification of candidate molecules that may not be evident through screening for known chemokines using molecular methods or ELISA. A more complete understanding of the crosstalk between tumor cells and MSCs will allow a better understanding of their in solid tumor growth.
Acknowledgments
SYL was supported by NIH grant T32 CA 108455. This work was supported by a collaborative research grant from the Cancer Institute of New Jersey and research grants from The New Jersey Commission on Science and Technology (HESC-06-04-00) and The New Jersey Commission on Cancer Research (05-2406-CCR-EO). Additional support was provided by the AHEPA Cancer Research Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Friedenstein AJ, Chailakhjan RK, Lalykina KS. The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell Tissue Kinet. 1970;3:393–403. doi: 10.1111/j.1365-2184.1970.tb00347.x. [DOI] [PubMed] [Google Scholar]
- 2.Luria EA, Panasyuk AF, Friedenstein AY. Fibroblast colony formation from monolayer cultures of blood cells. Transfusion. 1971;11:345–9. doi: 10.1111/j.1537-2995.1971.tb04426.x. [DOI] [PubMed] [Google Scholar]
- 3.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–7. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 4.Mackay AM, Beck SC, Murphy JM, Barry FP, Chichester CO, Pittenger MF. Chondrogenic differentiation of cultured human mesenchymal stem cells from marrow. Tissue Eng. 1998;4:415–28. doi: 10.1089/ten.1998.4.415. [DOI] [PubMed] [Google Scholar]
- 5.He Q, Wan C, Li G. Concise review: multipotent mesenchymal stromal cells in blood. Stem Cells. 2007;25:69–77. doi: 10.1634/stemcells.2006-0335. [DOI] [PubMed] [Google Scholar]
- 6.Rochefort GY, Delorme B, Lopez A, Herault O, Bonnet P, Charbord P, Eder V, Domenech J. Multipotential mesenchymal stem cells are mobilized into peripheral blood by hypoxia. Stem Cells. 2006;24:2202–8. doi: 10.1634/stemcells.2006-0164. [DOI] [PubMed] [Google Scholar]
- 7.Kassis I, Zangi L, Rivkin R, Levdansky L, Samuel S, Marx G, Gorodetsky R. Isolation of mesenchymal stem cells from G-CSF-mobilized human peripheral blood using fibrin microbeads. Bone Marrow Transplant. 2006;37:967–76. doi: 10.1038/sj.bmt.1705358. [DOI] [PubMed] [Google Scholar]
- 8.Fernandez M, Simon V, Herrera G, Cao C, Del Favero H, Minguell JJ. Detection of stromal cells in peripheral blood progenitor cell collections from breast cancer patients. Bone Marrow Transplant. 1997;20:265–71. doi: 10.1038/sj.bmt.1700890. [DOI] [PubMed] [Google Scholar]
- 9.Kim SJ, Cho HH, Kim YJ, Seo SY, Kim HN, Lee JB, Kim JH, Chung JS, Jung JS. Human adipose stromal cells expanded in human serum promote engraftment of human peripheral blood hematopoietic stem cells in NOD/SCID mice. Biochem Biophys Res Commun. 2005;329:25–31. doi: 10.1016/j.bbrc.2005.01.092. [DOI] [PubMed] [Google Scholar]
- 10.Miao Z, Jin J, Chen L, Zhu J, Huang W, Zhao J, Qian H, Zhang X. Isolation of mesenchymal stem cells from human placenta: comparison with human bone marrow mesenchymal stem cells. Cell Biol Int. 2006;30:681–7. doi: 10.1016/j.cellbi.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 11.Shen FH, Visger JM, Balian G, Hurwitz SR, Diduch DR. Systemically administered mesenchymal stromal cells transduced with insulin-like growth factor-I localize to a fracture site and potentiate healing. J Orthop Trauma. 2002;16:651–9. doi: 10.1097/00005131-200210000-00007. [DOI] [PubMed] [Google Scholar]
- 12.McFarlin K, Gao X, Liu YB, Dulchavsky DS, Kwon D, Arbab AS, Bansal M, Li Y, Chopp M, Dulchavsky SA, Gautam SC. Bone marrow-derived mesenchymal stromal cells accelerate wound healing in the rat. Wound Repair Regen. 2006;14:471–8. doi: 10.1111/j.1743-6109.2006.00153.x. [DOI] [PubMed] [Google Scholar]
- 13.Boomsma RA, Swaminathan PD, Geenen DL. Intravenously injected mesenchymal stem cells home to viable myocardium after coronary occlusion and preserve systolic function without altering infarct size. Int J Cardiol. 2006 doi: 10.1016/j.ijcard.2006.11.022. [DOI] [PubMed] [Google Scholar]
- 14.Studeny M, Marini FC, Champlin RE, Zompetta C, Fidler IJ, Andreeff M. Bone marrow-derived mesenchymal stem cells as vehicles for interferon-beta delivery into tumors. Cancer Res. 2002;62:3603–8. [PubMed] [Google Scholar]
- 15.Nakamizo A, Marini F, Amano T, Khan A, Studeny M, Gumin J, Chen J, Hentschel S, Vecil G, Dembinski J, Andreeff M, Lang FF. Human bone marrow-derived mesenchymal stem cells in the treatment of gliomas. Cancer Res. 2005;65:3307–18. doi: 10.1158/0008-5472.CAN-04-1874. [DOI] [PubMed] [Google Scholar]
- 16.Xin H, Kanehira M, Mizuguchi H, Hayakawa T, Kikuchi T, Nukiwa T, Saijo Y. Targeted-Delivery of CX3CL1 to Multiple Lung Tumors by Mesenchymal Stem Cells. Stem Cells. 2007 doi: 10.1634/stemcells.2006-0461. [DOI] [PubMed] [Google Scholar]
- 17.Bhakta S, Hong P, Koc O. The surface adhesion molecule CXCR4 stimulates mesenchymal stem cell migration to stromal cell-derived factor-1 in vitro but does not decrease apoptosis under serum deprivation. Cardiovasc Revasc Med. 2006;7:19–24. doi: 10.1016/j.carrev.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 18.Wynn RF, Hart CA, Corradi-Perini C, O’Neill L, Evans CA, Wraith JE, Fairbairn LJ, Bellantuono I. A small proportion of mesenchymal stem cells strongly expresses functionally active CXCR4 receptor capable of promoting migration to bone marrow. Blood. 2004;104:2643–5. doi: 10.1182/blood-2004-02-0526. [DOI] [PubMed] [Google Scholar]
- 19.Schichor C, Birnbaum T, Etminan N, Schnell O, Grau S, Miebach S, Aboody K, Padovan C, Straube A, Tonn JC, Goldbrunner R. Vascular endothelial growth factor A contributes to glioma-induced migration of human marrow stromal cells (hMSC) Exp Neurol. 2006;199:301–10. doi: 10.1016/j.expneurol.2005.11.027. [DOI] [PubMed] [Google Scholar]
- 20.Fiedler J, Etzel N, Brenner RE. To go or not to go: Migration of human mesenchymal progenitor cells stimulated by isoforms of PDGF. J Cell Biochem. 2004;93:990–8. doi: 10.1002/jcb.20219. [DOI] [PubMed] [Google Scholar]
- 21.Wang L, Li Y, Chen X, Chen J, Gautam SC, Xu Y, Chopp M. MCP-1, MIP-1, IL-8 and ischemic cerebral tissue enhance human bone marrow stromal cell migration in interface culture. Hematology. 2002;7:113–7. doi: 10.1080/10245330290028588. [DOI] [PubMed] [Google Scholar]
- 22.Menon LG, Picinich S, Koneru R, Gao H, Lin SY, Koneru M, Mayer-Kuckuk P, Glod J, Banerjee D. Differential Gene Expression Associated with Migration of Mesenchymal Stem Cells to Conditioned Medium From Tumor Cells or Bone Marrow Cells. Stem Cells. 2006 doi: 10.1634/stemcells.2006-0257. [DOI] [PubMed] [Google Scholar]
- 23.Webb LM, Ehrengruber MU, Clark-Lewis I, Baggiolini M, Rot A. Binding to heparan sulfate or heparin enhances neutrophil responses to interleukin 8. Proc Natl Acad Sci U S A. 1993;90:7158–62. doi: 10.1073/pnas.90.15.7158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang L, Fuster M, Sriramarao P, Esko JD. Endothelial heparan sulfate deficiency impairs L-selectin- and chemokine-mediated neutrophil trafficking during inflammatory responses. Nat Immunol. 2005;6:902–10. doi: 10.1038/ni1233. [DOI] [PubMed] [Google Scholar]
- 25.Veldkamp CT, Peterson FC, Pelzek AJ, Volkman BF. The monomer-dimer equilibrium of stromal cell-derived factor-1 (CXCL 12) is altered by pH, phosphate, sulfate, and heparin. Protein Sci. 2005;14:1071–81. doi: 10.1110/ps.041219505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Menon LG, Picinich S, Koneru R, Gao H, Lin SY, Koneru M, Mayer-Kuckuk P, Glod J, Banerjee D. Differential gene expression associated with migration of mesenchymal stem cells to conditioned medium from tumor cells or bone marrow cells. Stem Cells. 2007;25:520–8. doi: 10.1634/stemcells.2006-0257. [DOI] [PubMed] [Google Scholar]
- 27.Rycyzyn MA, Clevenger CV. Role of cyclophilins in somatolactogenic action. Ann N Y Acad Sci. 2000;917:514–21. doi: 10.1111/j.1749-6632.2000.tb05416.x. [DOI] [PubMed] [Google Scholar]
- 28.Rycyzyn MA, Reilly SC, O’Malley K, Clevenger CV. Role of cyclophilin B in prolactin signal transduction and nuclear retrotranslocation. Mol Endocrinol. 2000;14:1175–86. doi: 10.1210/mend.14.8.0508. [DOI] [PubMed] [Google Scholar]
- 29.Yang J, Everett AD. Hepatoma-derived growth factor binds DNA through the N-terminal PWWP domain. BMC Mol Biol. 2007;8:101. doi: 10.1186/1471-2199-8-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Allain F, Vanpouille C, Carpentier M, Slomianny MC, Durieux S, Spik G. Interaction with glycosaminoglycans is required for cyclophilin B to trigger integrin-mediated adhesion of peripheral blood T lymphocytes to extracellular matrix. Proc Natl Acad Sci U S A. 2002;99:2714–9. doi: 10.1073/pnas.052284899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pakula R, Melchior A, Denys A, Vanpouille C, Mazurier J, Allain F. Syndecan-1/CD147 association is essential for cyclophilin B-induced activation of p44/42 mitogen-activated protein kinases and promotion of cell adhesion and chemotaxis. Glycobiology. 2007;17:492–503. doi: 10.1093/glycob/cwm009. [DOI] [PubMed] [Google Scholar]
- 32.Sherry B, Yarlett N, Strupp A, Cerami A. Identification of cyclophilin as a proinflammatory secretory product of lipopolysaccharide-activated macrophages. Proc Natl Acad Sci U S A. 1992;89:3511–5. doi: 10.1073/pnas.89.8.3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xu Q, Leiva MC, Fischkoff SA, Handschumacher RE, Lyttle CR. Leukocyte chemotactic activity of cyclophilin. J Biol Chem. 1992;267:11968–71. [PubMed] [Google Scholar]
- 34.Narron JV, Stoops TD, Barringhaus K, Matsumura M, Everett AD. Hepatoma-derived growth factor is expressed after vascular injury in the rat and stimulates smooth muscle cell migration. Pediatr Res. 2006;59:778–83. doi: 10.1203/01.pdr.0000219299.24435.4f. [DOI] [PubMed] [Google Scholar]
- 35.Everett AD, Lobe DR, Matsumura ME, Nakamura H, McNamara CA. Hepatoma-derived growth factor stimulates smooth muscle cell growth and is expressed in vascular development. J Clin Invest. 2000;105:567–75. doi: 10.1172/JCI7497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Everett AD, Stoops T, McNamara CA. Nuclear targeting is required for hepatoma-derived growth factor-stimulated mitogenesis in vascular smooth muscle cells. J Biol Chem. 2001;276:37564–8. doi: 10.1074/jbc.M105109200. [DOI] [PubMed] [Google Scholar]
- 37.Yurchenko V, O’Connor M, Dai WW, Guo H, Toole B, Sherry B, Bukrinsky M. CD147 is a signaling receptor for cyclophilin B. Biochem Biophys Res Commun. 2001;288:786–8. doi: 10.1006/bbrc.2001.5847. [DOI] [PubMed] [Google Scholar]
- 38.Sadir R, Imberty A, Baleux F, Lortat-Jacob H. Heparan sulfate/heparin oligosaccharides protect stromal cell-derived factor-1 (SDF-1)/CXCL12 against proteolysis induced by CD26/dipeptidyl peptidase IV. J Biol Chem. 2004;279:43854–60. doi: 10.1074/jbc.M405392200. [DOI] [PubMed] [Google Scholar]
- 39.Proudfoot AE, Handel TM, Johnson Z, Lau EK, LiWang P, Clark-Lewis I, Borlat F, Wells TN, Kosco-Vilbois MH. Glycosaminoglycan binding and oligomerization are essential for the in vivo activity of certain chemokines. Proc Natl Acad Sci U S A. 2003;100:1885–90. doi: 10.1073/pnas.0334864100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Netelenbos T, Zuijderduijn S, Van Den Born J, Kessler FL, Zweegman S, Huijgens PC, Drager AM. Proteoglycans guide SDF-1-induced migration of hematopoietic progenitor cells. J Leukoc Biol. 2002;72:353–62. [PubMed] [Google Scholar]
- 41.Kuschert GS, Coulin F, Power CA, Proudfoot AE, Hubbard RE, Hoogewerf AJ, Wells TN. Glycosaminoglycans interact selectively with chemokines and modulate receptor binding and cellular responses. Biochemistry. 1999;38:12959–68. doi: 10.1021/bi990711d. [DOI] [PubMed] [Google Scholar]
- 42.Wang P, Heitman J. The cyclophilins. Genome Biol. 2005;6:226. doi: 10.1186/gb-2005-6-7-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Everett AD, Narron JV, Stoops T, Nakamura H, Tucker A. Hepatoma-derived growth factor is a pulmonary endothelial cell-expressed angiogenic factor. Am J Physiol Lung Cell Mol Physiol. 2004;286:L1194–201. doi: 10.1152/ajplung.00427.2003. [DOI] [PubMed] [Google Scholar]
- 44.Okuda Y, Nakamura H, Yoshida K, Enomoto H, Uyama H, Hirotani T, Funamoto M, Ito H, Everett AD, Hada T, Kawase I. Hepatoma-derived growth factor induces tumorigenesis in vivo through both direct angiogenic activity and induction of vascular endothelial growth factor. Cancer Sci. 2003;94:1034–41. doi: 10.1111/j.1349-7006.2003.tb01397.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Handel TM, Johnson Z, Crown SE, Lau EK, Proudfoot AE. Regulation of protein function by glycosaminoglycans--as exemplified by chemokines. Annu Rev Biochem. 2005;74:385–410. doi: 10.1146/annurev.biochem.72.121801.161747. [DOI] [PubMed] [Google Scholar]
- 46.Hoogewerf AJ, Kuschert GS, Proudfoot AE, Borlat F, Clark-Lewis I, Power CA, Wells TN. Glycosaminoglycans mediate cell surface oligomerization of chemokines. Biochemistry. 1997;36:13570–8. doi: 10.1021/bi971125s. [DOI] [PubMed] [Google Scholar]
- 47.Middleton J, Neil S, Wintle J, Clark-Lewis I, Moore H, Lam C, Auer M, Hub E, Rot A. Transcytosis and surface presentation of IL-8 by venular endothelial cells. Cell. 1997;91:385–95. doi: 10.1016/s0092-8674(00)80422-5. [DOI] [PubMed] [Google Scholar]
- 48.Ellyard JI, Simson L, Bezos A, Johnston K, Freeman C, Parish CR. Eotaxin selectively binds heparin. An interaction that protects eotaxin from proteolysis and potentiates chemotactic activity in vivo. J Biol Chem. 2007;282:15238–47. doi: 10.1074/jbc.M608046200. [DOI] [PubMed] [Google Scholar]
- 49.Sweeney EA, Lortat-Jacob H, Priestley GV, Nakamoto B, Papayannopoulou T. Sulfated polysaccharides increase plasma levels of SDF-1 in monkeys and mice: involvement in mobilization of stem/progenitor cells. Blood. 2002;99:44–51. doi: 10.1182/blood.v99.1.44. [DOI] [PubMed] [Google Scholar]
- 50.Dias-Baruffi M, Pereira-da-Silva G, Jamur MC, Roque-Barreira MC. Heparin potentiates in vivo neutrophil migration induced by IL-8. Glycoconj J. 1998;15:523–6. doi: 10.1023/a:1006995222189. [DOI] [PubMed] [Google Scholar]
- 51.Shaked Y, Ciarrocchi A, Franco M, Lee CR, Man S, Cheung AM, Hicklin DJ, Chaplin D, Foster FS, Benezra R, Kerbel RS. Therapy-induced acute recruitment of circulating endothelial progenitor cells to tumors. Science. 2006;313:1785–7. doi: 10.1126/science.1127592. [DOI] [PubMed] [Google Scholar]
- 52.Mancuso P, Colleoni M, Calleri A, Orlando L, Maisonneuve P, Pruneri G, Agliano A, Goldhirsch A, Shaked Y, Kerbel RS, Bertolini F. Circulating endothelial-cell kinetics and viability predict survival in breast cancer patients receiving metronomic chemotherapy. Blood. 2006;108:452–9. doi: 10.1182/blood-2005-11-4570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zeisberger SM, Odermatt B, Marty C, Zehnder-Fjallman AH, Ballmer-Hofer K, Schwendener RA. Clodronate-liposome-mediated depletion of tumour-associated macrophages: a new and highly effective antiangiogenic therapy approach. Br J Cancer. 2006;95:272–81. doi: 10.1038/sj.bjc.6603240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Karnoub AE, Dash AB, Vo AP, Sullivan A, Brooks MW, Bell GW, Richardson AL, Polyak K, Tubo R, Weinberg RA. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature. 2007;449:557–63. doi: 10.1038/nature06188. [DOI] [PubMed] [Google Scholar]