Abstract
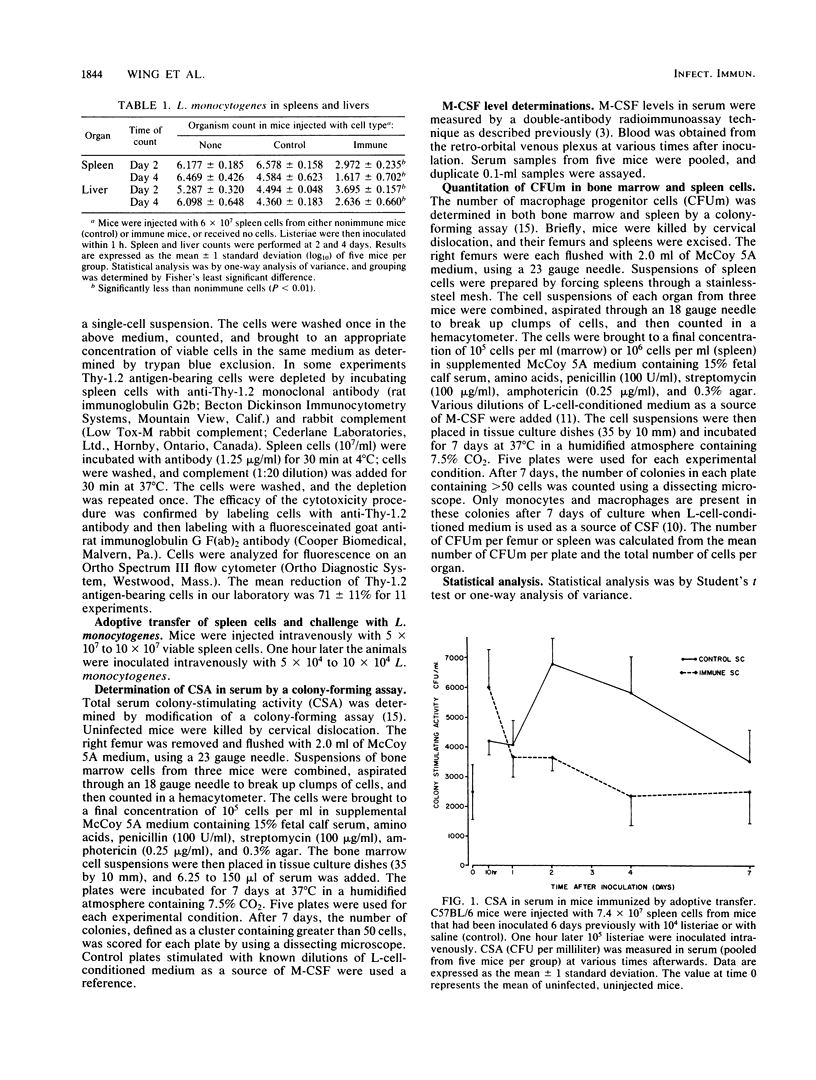
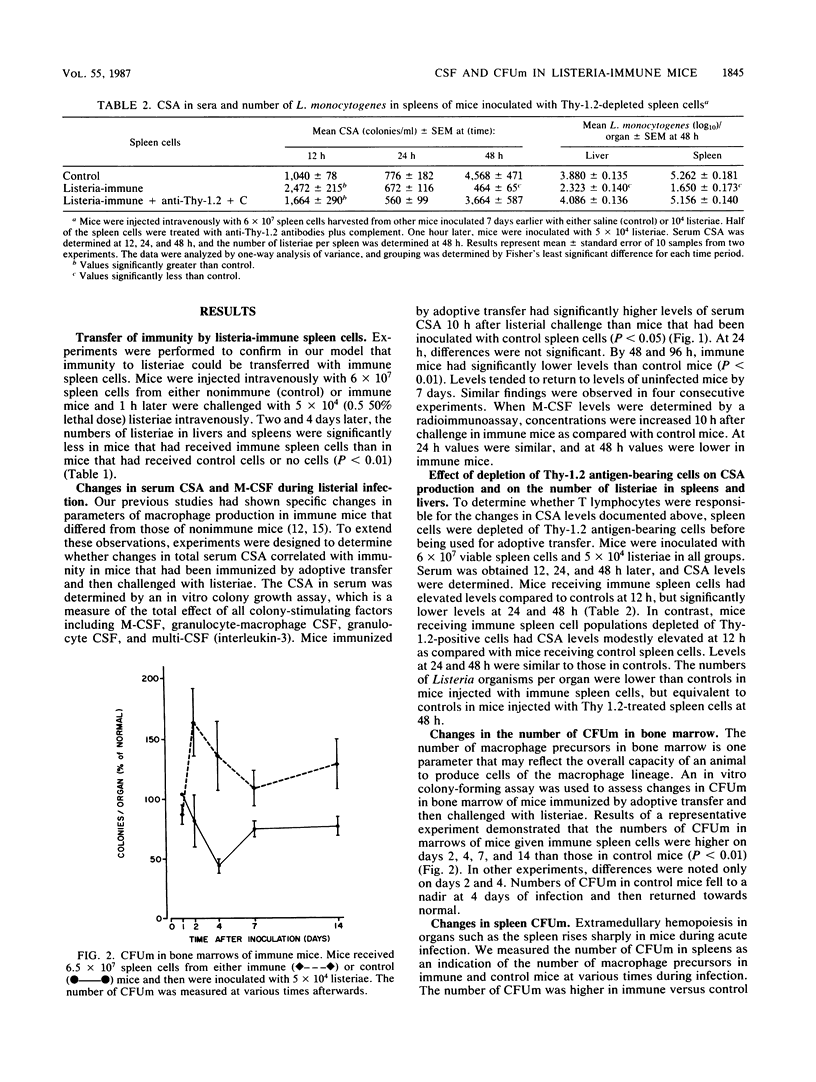
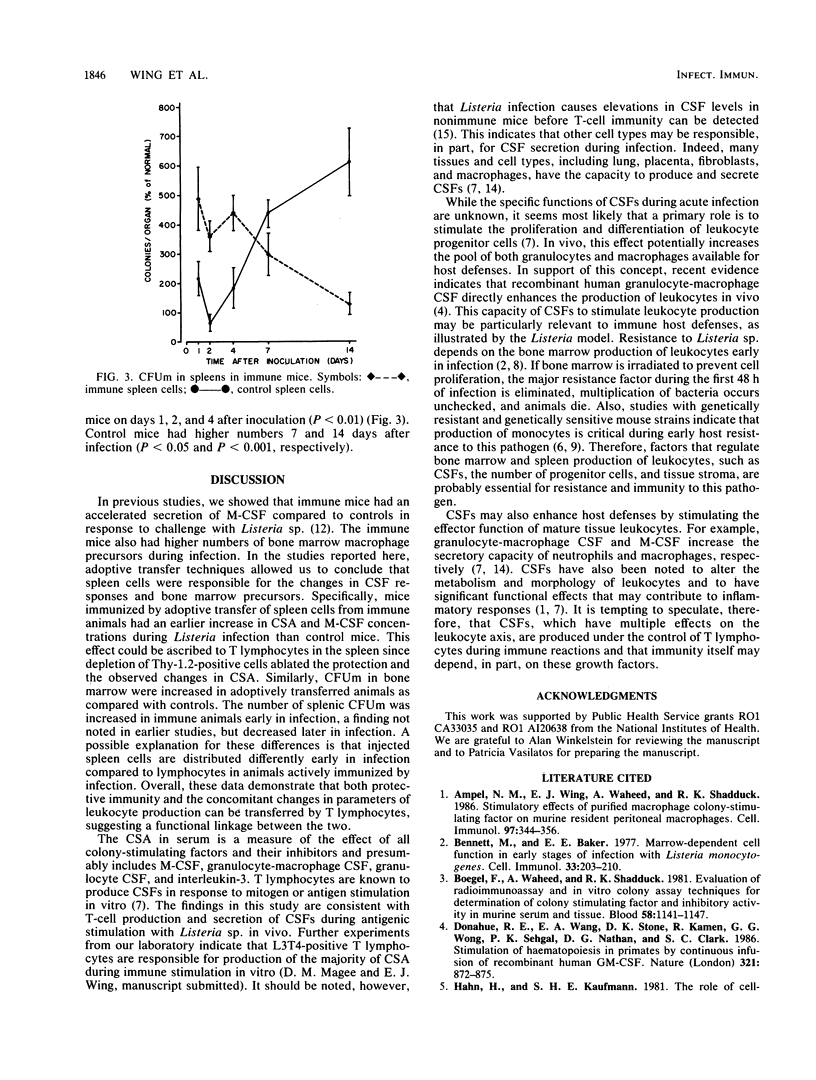
Experiments were performed to elucidate the role of colony-stimulating factors in host defenses to the intracellular pathogen Listeria monocytogenes. Mice were protected against Listeria sp. by adoptive transfer of immune spleen cells and were then challenged with listeriae intravenously. Control mice were injected with spleen cells from uninfected mice. Adoptively immunized (immune) mice had significantly fewer listeriae in spleens and livers 2 and 4 days after Listeria challenge than did control mice. During acute infection, colony-stimulating activity in serum was increased earlier (10 h) in immune mice than in controls. Concentrations of colony-stimulating activity were equal at 24 h. By 48 h, values were decreased in immune mice, but were elevated in control mice. Similar changes were noted when a specific colony-stimulating factor, macrophage colony-stimulating factor, was measured in serum by using a radioimmunoassay. The changes in serum colony-stimulating activity in mice adoptively immunized with immune spleen cells were eliminated if spleen cells were first treated with anti-Thy-1.2 monoclonal antibodies. The number of macrophage progenitor cells in bone marrow and spleen were also determined as measures of the hemopoietic potential in these organs. The number of macrophage progenitor cells in bone marrow was higher in immune animals than control animals at 1, 2, and 4 days of infection. Similarly, the number of these cells in spleens was higher during the early stages of infection in immune mice. These results indicate that both the regulation of leukocyte production and the transfer of specific cellular immunity by spleen cells are associated, and they therefore suggest that hemopoietic regulatory factors play a role in immune host defenses.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ampel N. M., Wing E. J., Waheed A., Shadduck R. K. Stimulatory effects of purified macrophage colony-stimulating factor on murine resident peritoneal macrophages. Cell Immunol. 1986 Feb;97(2):344–356. doi: 10.1016/0008-8749(86)90405-3. [DOI] [PubMed] [Google Scholar]
- Bennett M., Baker E. E. Marrow-dependent cell function in early stages of infection with Listeria monocytogenes. Cell Immunol. 1977 Sep;33(1):203–210. doi: 10.1016/0008-8749(77)90147-2. [DOI] [PubMed] [Google Scholar]
- Boegel F., Waheed A., Shadduck R. K. Evaluation of radioimmunoassay and in vitro colony assay techniques for determination of colony-stimulating factor and inhibitory activity in murine serum and tissue. Blood. 1981 Dec;58(6):1141–1147. [PubMed] [Google Scholar]
- Donahue R. E., Wang E. A., Stone D. K., Kamen R., Wong G. G., Sehgal P. K., Nathan D. G., Clark S. C. Stimulation of haematopoiesis in primates by continuous infusion of recombinant human GM-CSF. 1986 Jun 26-Jul 2Nature. 321(6073):872–875. doi: 10.1038/321872a0. [DOI] [PubMed] [Google Scholar]
- Hahn H., Kaufmann S. H. The role of cell-mediated immunity in bacterial infections. Rev Infect Dis. 1981 Nov-Dec;3(6):1221–1250. doi: 10.1093/clinids/3.6.1221. [DOI] [PubMed] [Google Scholar]
- Metcalf D. The molecular biology and functions of the granulocyte-macrophage colony-stimulating factors. Blood. 1986 Feb;67(2):257–267. [PubMed] [Google Scholar]
- North R. J. The relative importance of blood monocytes and fixed macrophages to the expression of cell-mediated immunity to infection. J Exp Med. 1970 Sep 1;132(3):521–534. doi: 10.1084/jem.132.3.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadarangani C., Skamene E., Kongshavn P. A. Cellular basis for genetically determined enhanced resistance of certain mouse strains to listeriosis. Infect Immun. 1980 May;28(2):381–386. doi: 10.1128/iai.28.2.381-386.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shadduck R. K., Waheed A., Pigoli G., Boegel F., Higgins L. Fractionation of antibodies to L-cell colony-stimulating factor by affinity chromatography. Blood. 1979 Jun;53(6):1182–1190. [PubMed] [Google Scholar]
- Waheed A., Shadduck R. K. Purification and properties of L cell-derived colony-stimulating factor. J Lab Clin Med. 1979 Jul;94(1):180–193. [PubMed] [Google Scholar]
- Wing E. J., Barczynski L. C., Waheed A., Shadduck R. K. Effect of Listeria monocytogenes infection on serum levels of colony-stimulating factor and number of progenitor cells in immune and nonimmune mice. Infect Immun. 1985 Aug;49(2):325–328. doi: 10.1128/iai.49.2.325-328.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wing E. J., Kresefsky-Friedman D. Y. Decreased resistance to Listeria monocytogenes in mice injected with killed corynebacterium parvum: association with suppression of cell-mediated immunity. J Infect Dis. 1980 Feb;141(2):203–211. doi: 10.1093/infdis/141.2.203. [DOI] [PubMed] [Google Scholar]
- Wing E. J., Waheed A., Shadduck R. K. Changes in serum colony-stimulating factor and monocytic progenitor cells during Listeria monocytogenes infection in mice. Infect Immun. 1984 Jul;45(1):180–184. doi: 10.1128/iai.45.1.180-184.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]


