Abstract
Activation of the RET gene by chromosomal rearrangements generating RET/PTC oncogenes is a frequent, early, and causative event in papillary thyroid carcinoma (PTC). We have previously shown that, in human primary thyrocytes, RET/PTC1 induces a transcriptional program including the MET proto-oncogene. In PTCs, β-catenin is frequently mislocated to the cytoplasm nucleus. We investigated the interplay between Ret/ptc1 signaling and Met in regulating the proinvasive phenotype and β-catenin localization in cellular models of human PTC. Here, we show that Met protein is expressed and is constitutively active in human thyrocytes exogenously expressing RET/PTC1 as well as a mutant (Y451F) devoid of the main Ret/ptc1 multidocking site. Both in transformed thyrocytes and in the human PTC cell line TPC-1, Ret/ptc1-Y451-dependent signaling and Met cooperated to promote a proinvasive phenotype. Accordingly, gene/functional silencing of either RET/PTC1 or MET abrogated early branching morphogenesis in TPC-1 cells. The same effect was obtained by blocking the common downstream effector Akt. Y451 of Ret/ptc1 was required to promote proliferation and nuclear translocation of β-catenin, suggesting that these oncogene-driven effects are Met-independent. Pharmacologic inhibition of Ret/ptc1 and Met tyrosine kinases by the multitarget small molecule RPI-1 blocked cell proliferation and invasive ability and dislocated β-catenin from the nucleus. Altogether, these results support that Ret/ptc1 cross talks with Met at transcriptional and signaling levels and promotes β-catenin transcriptional activity to drive thyrocyte neoplastic transformation. Such molecular network, promoting disease initiation and acquisition of a proinvasive phenotype, highlights new options to design multitarget therapeutic strategies for PTCs.
Introduction
Papillary thyroid carcinoma (PTC), the most prevalent neoplastic disease of the thyroid gland, presents several morphologic variants, usually characterized by slow growth and clinical indolence, although aggressive forms associated with local and distant invasion can occur [1]. Four alternative genetic lesions have been identified as driving oncogenic alterations in PTCs: rearrangements of RET or TRK genes and activating mutations of BRAF or RAS [2].
The RET proto-oncogene, encoding the receptor tyrosine kinase for the glial cell line-derived neurotrophic factor family of peptides, plays a crucial role in transducing growth and differentiation signals in tissues derived from the neural crest [3]. RET oncogenic activation by somatic chromosomal rearrangement is a specific event in PTC tumorigenesis. The resulting RET/PTC oncogenes are among the most frequent genetic alterations in this pathology. Twelve different fusion partner genes have been identified so far with the most prevalent variant being RET/PTC1 (60–70%) derived from the fusion of RET with the H4 (D10S170) gene. The products of the resulting rearranged genes are constitutively active oncoproteins that have lost the transmembrane domain [1,4]. Phosphorylation of the residue corresponding to tyrosine 1062 in proto-RET is essential for the recruitment of several adaptor and signaling proteins, thus playing a crucial role in the transforming effects of Ret oncoproteins including increased cell proliferation, migration, and altered adhesion [2]. Experimental evidence supports RET/PTC rearrangements as causative factors in the pathogenesis of PTC. Exogenous expression of RET/PTCs in human thyrocytes has been shown to stimulate their proliferation [5,6] and to induce typical changes in nuclear envelope and chromatin, which are diagnostic for PTC [7]. The ability of RET/PTCs to initiate carcinogenesis has been confirmed in transgenic mice [8]. Nevertheless, other alterations of signaling through growth factors and their receptors, cell cycle regulators, and adhesion molecules seem to contribute to thyroid neoplasia progression [1].
Owing to the specialized structure of the thyroid, the epithelial mechanisms of cell-cell adhesion play an important role in tissue integrity. In the normal thyroid, the E-cadherin/catenins system constitutes the main epithelial adhesion complex [9]. It has been suggested that loss of E-cadherin and altered expression/localization of β-catenin, which have been described in subsets of thyroid carcinomas [10–12], may represent tumor progression factors. Indeed, deregulation of this system, which promotes the transcriptional function of β-catenin, has been involved in the development and progression of several malignancies [13]. Phosphorylation is a major mechanism regulating the dual function of β-catenin. In particular, tyrosine phosphorylation by different protein kinases switches the function of β-catenin from adhesion to transcription [14].
The tyrosine kinase receptor for hepatocyte growth factor (HGF) Met is overexpressed in most PTCs, whereas it is not present in the normal thyroid follicle [15]. Experimental and clinical data point to Met deregulation as a key event in tumor invasive growth and metastatic spreading [16]. In particular, in thyroid cancer, HGF-Met signaling modulates cell motility and invasiveness and promotes angiogenesis [17,18]. Met transcription in thyroid carcinomas is thought to be regulated as an effect secondary to the activation of driving oncogenes such as RET, HRAS, and BRAF [19,20].
We previously demonstrated that exogenous expression of RET/PTC1 in human primary thyrocytes activates a complex transcriptional program leading to the up-regulation of several genes involved in inflammation, invasion, and matrix remodeling, including MET [6]. In the present study, we investigated the respective contribution of Ret/ptc1 and Met in the proinvasive phenotype and β-catenin dysregulation in PTC. We addressed this issue by using the previously described in vitro model of human thyroid carcinogenesis [6] and the human PTC cell line, TPC-1, endogenously expressing the RET/PTC1 oncogene. Genotypic/phenotypic and biochemical/biological correlations were investigated either by selective silencing of RET/PTC1 and MET or by pharmacological inhibition of both tyrosine kinases using the multitarget inhibitor RPI-1 [21,22]. The results indicated that RET/PTC1 induces Met protein expression and β-catenin nuclear translocation in human follicular thyroid cells, the first effect being independent from the Ret/ptc1 crucial multidocking site Y451. In addition, a functional cooperation between Ret/ptc1 andMet signaling in the promotion of cell invasive ability is shown. These findings support a direct causative relationship between a specific oncogene, RET/PTC1, and two other alterations, Met expression and β-catenin mislocation, implicated in the pathogenesis of PTC.
Materials and Methods
Chemicals and Antibodies
The synthesis and the chemical structure of RPI-1 (1,3-dihydro-5,6-dimethoxy-3-[(4-hydroxyphenyl) methylene]-H-indol-2-one) were reported previously (Cpd1) [23]. The drug dissolved in dimethylsulfoxide was further diluted in cell culture medium (final solvent concentration, 0.5%). Only where indicated, HGF (Sigma, St. Louis, MO) was added to the medium at 20 ng/ml.
Mouse monoclonal antibodies used were as follows: antiphosphotyrosine, clone 4G10, from Upstate Biotechnology (Lake Placid, NY); anti-PKBα/Akt from Transduction Laboratories (Lexington, KY); anti-β-tubulin from Sigma; anti-β-catenin from Abcam (Cambridge, UK); and anti-cyclin D1 (R-124) and anti-p-β-catenin (1B11) from Santa Cruz Biotechnology (Santa Cruz, CA).
Rabbit polyclonal antibodies used were as follows: anti-phospho-Ret (Y1062), anti-Met (C-12), and anti-Ret (C-19) from Santa Cruz Biotechnology; anti-phospho-Met (Y1234/Y1235) from Upstate Biotechnology; anti-phospho-Akt (S473) and anti-phospho-Ret (Y905) from Cell Signaling (Beverly, MA); anti-β-catenin and antiactin from Sigma; anti-Ret recognizing a C-terminal sequence (aa 1000–1014) [24]; and anti-phospho β-catenin (Y142) from Abcam.
Cell Culture and Proliferation Assay
The human PTC cell line TPC-1 and the Nthy-ori 3-1 cell line derived from human thyroid follicular epithelium were maintained in Dulbecco's modified Eagle's medium (DMEM) and RPMI (Cambrex Bio Science, Walkerville, MD), respectively, supplemented with 10% fetal bovine serum. Establishment of human primary thyrocytes and infection with retroviral vectors containing RET/PTC1 or RET/PTC1-Y451F were previously described [6]. Parental (normal human thyrocytes, NTs) and infected thyrocytes (RTPC and RTPC-YF) were cultured in medium containing DMEM, F12, and MDCB 104 (at a ratio of 2:1:1; Invitrogen, Carlsbad, CA) and supplemented with 10% fetal bovine serum. Mouse fibroblasts NIH3T3 transfected with RET/PTC1 (NIH3T3PTC1) and parental NIH3T3 cells were maintained in DMEM containing 5% and 10%calf serum(Colorado Serum Company, Denver, CO), respectively, in a 10% CO2 atmosphere.
For cell growth assays, cells were plated in 96-well plates and, the day after seeding, were exposed to solvent or different concentrations of RPI-1 for 72 or 144 hours as indicated. Then, cells were subjected to the sulforhodamine B (SRB) colorimetric assay as previously described [21]. Drug concentrations able to inhibit cell proliferation by 50% (IC50) were calculated from dose-response curves.
Biochemical Analyses
When indicated, cells were treated with different concentrations of RPI-1 the day after seeding. To study HGF-induced effects in the presence of RPI-1, TPC-1 cells were serum-starved for 24 hours, treated with the drug for 18 hours, and then stimulated with HGF for 10 minutes before lysis. Cells were processed for immunoprecipitation or total protein extraction and were then examined by Western blot analysis as previously described [25].
Early Branching Morphogenesis Assay
Formation of cellular networks was assayed in 48-well plates layered with artificial extracellular matrix (Matrigel; BD Biosciences, San Jose, CA). Cells were suspended in serum-free medium and overlaid on the gelled Matrigel. When indicated, medium was supplemented with 20 ng/ml HGF. In experiments performed in the presence of RPI-1, cells were pretreated with different concentrations of the drug for 24 hours and then transferred in drug-added medium onto the Matrigel layer. After incubating at 37°C for 4 hours, branches were photographed with a digital camera. Quantification of branches was performed by measuring the total length of structures per field by the Image Analysis System software (Delta System, Rome, Italy). Data are reported as percentage of control ± SD.
Cell Migration and Scatter Assay
For the migration assay, cells were seeded in the upper side of Transwell chambers (Costar; Corning Incorporated, Corning, NY) in serum-free medium. To evaluate cell migration in the presence of RPI-1, cells were pretreated with the drug in complete medium for 24 hours. The same drug concentration used for pretreatment was then added in both upper and lower chambers. To assess HGF-induced cell migration, the growth factor was added in the lower chamber. After 5 hours of incubation at 37°C, migrated cells were fixed in 95% ethanol, stained with a solution of 2% crystal violet in 70% ethanol and counted under an inverted microscope.
For the scatter assay, cells were seeded at a low density in complete medium to allow growing as islets. The day after, cells were serum-starved for 24 hours. Then, cells were treated with RPI-1 in the presence or absence of HGF for 18 hours before observation under a phase-contrast microscope equipped with photo camera.
Antibody Intracellular Transfer
Cells were subjected to protein delivery assay using PULSin (PolyPlus Transfection, Illkirch, France) as a delivery reagent for specific antibodies or aspecific rabbit IgG (Sigma) according to the manufacturer's instructions. After 4 hours of incubation with the PULSin/antibody mixture at 37°C, the antibody transfer solution was removed and replaced with fresh complete medium. Cells were used for the tubulogenesis assay 24 hours later, as previously described.
RNA Interference
To silence RET/PTC1, the Hs-RET-9-HP-validated siRNA (100 nM) from Qiagen (Santa Clarita, CA) was used. MET silencing was induced using the MET2 and MET5 specific 21 nucleotides (200 nM) previously described [26] (Ambion, Austin, TX). Negative control siRNA (AllStars) were from Qiagen. The oligonucleotides were transfected into the cells using the SiImporter reagent (Upstate Biotechnology) in serum-free medium according to the manufacturer's instructions. Cells were incubated for 6 hours before serum addition and were then further incubated for 3 or 5 days to downmodulate Met or Ret/ptc1 protein, respectively. Cells were then lysed or subjected to biological assays.
Indirect Immunofluorescence Staining of β-Catenin
Cells were fixed with 3% paraformaldehyde in PBS for 15 minutes, then permeabilized in 100% cold methanol for 1 minute, and washed in PBS. After blocking in 1% BSA in PBS for 1 hour, cells were incubated with anti-β-catenin antibody (1:2000; Sigma) for 1 hour. Alexa 488-conjugated goat antirabbit IgG-FITC (Molecular Probes, Inc, Eugene, OR) was used as a secondary antibody. After washing, nuclei were counterstained with Hoechst 33341 (Sigma), and slides were examined by a fluorescent microscope equipped with a digital camera.
Statistical Analysis
Statistical analyses were performed using Student's 2-tailed t test. A value of P < .05 was considered statistically significant.
Results
RET/PTC1 Oncogene Specifically Induces Met Protein Expression in Human Thyrocytes Independently from Its Crucial Multidocking Site Y451
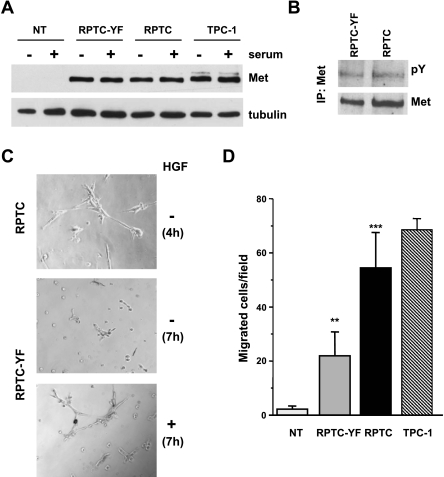
We used human NTs and thyrocytes infected with retroviral vector expressing RET/PTC1 (RPTC), or the RET/PTC1-Y451F mutant (RPTC-YF), as a model for PTC [6] to investigate the cross talk between Ret/ptc1 and Met kinases and their role in thyroid carcinogenesis. We confirmed the expression of Met in Ret/ptc1-expressing thyrocytes at the protein level (Figure 1A). Met expression in both infected thyrocytes was similar to that detected in the Ret/ptc1-positive PTC cell line TPC-1, whereas it was absent in NT cells. Moreover, Western blot analysis indicated that Met protein expression was independent from the Ret/ptc1 Y451-mediated signaling because both RPTC and RPTC-YF cells showed similar levels of the protein (Figure 1A). The presence of serum in the medium did not affect Met expression. The analysis of the phosphorylation status of Met immunoprecipitated from serum-starved cells revealed that the receptor tyrosine kinase was constitutively active in RPTC and RPTC-YF thyrocytes (Figure 1B) and in TPC-1 cells (Figure 3A). At variance, stable expression of RET/PTC1 oncogene neither induced Met expression in NIH3T3 murine fibroblasts nor modulated Met endogenous levels in HeLa human cervical cancer cells (not shown), thus suggesting a cellular-specific RET/PTC1 effect in thyrocytes.
Figure 1.
Met expression/activation and morphogenic/motogenic phenotype induced by ectopic expression of RET/PTC1 oncogene in human thyrocytes. (A) Western blot analysis of Met protein expression in thyrocytes uninfected (NT), infected with RET/PTC1 (RPTC), or the RET/PTC1-Y451F mutant (RPTC-YF) and in the RET/PTC1-positive PTC cell line TPC-1. Whole cell lysates were obtained after 24 hours in complete (+) or in serum-free (-) medium. Antitubulin blot is shown as a control for loading. (B) Met tyrosine phosphorylation in RPTC-YF and RPTC cells. Met protein was immunoprecipitated from cells serum-starved for 24 hours, and its phosphorylation was analyzed by Western blot analysis using a pan anti-ptyrosine antibody (pY). The filter was reprobed with anti-Met antibody after stripping. (C) Early tubulogenesis assay. Cells were seeded in Matrigel-coated wells in the absence of serum, with (+) or without (-) HGF. Four hours after plating, extensive tubule networks were formed by RPTC thyrocytes. RPTC-YF cells formed branched cellular cords after 7 hours of incubation only in the presence of HGF. Original magnification, x400. (D) Spontaneous cell migration. Cells were subjected to a migration assay in serum-free medium. Migrating cells were counted under a light microscope and reported as cell number per field. Columns represent mean values ± SD of two independent experiments. **P < .005, ***P < .0005 versus NT.
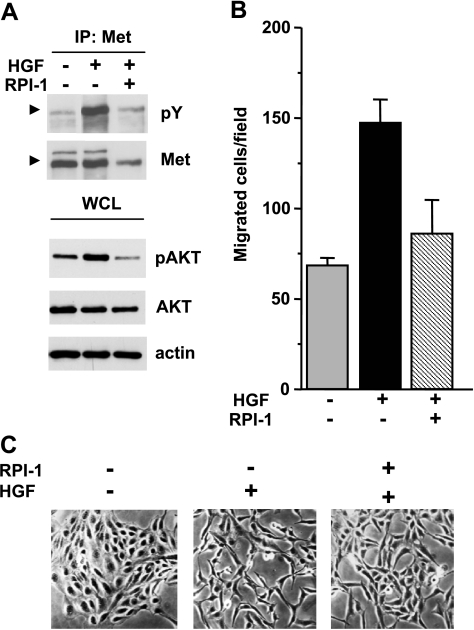
Figure 3.
Biochemical and motogenic effects of HGF in TPC-1 cells. (A) Activation of Met and Akt. Serum-starved cells were treated for 18 hours with solvent (-) or 15 µM RPI-1 (+) and then stimulated with HGF for 10 minutes. Met was immunoprecipitated (IP), and its tyrosine phosphorylation was examined by Western blot analysis using a pan anti-ptyrosine antibody (pY). Arrows indicate the Met mature form. Western blot analysis of Akt activation was performed on whole cell lysates using an antibody recognizing Akt phosphorylated at S473 (pAkt). Filters were stripped and reprobed with anti-Akt and anti-actin antibodies. (B) Cell migration. Cells were exposed to vehicle or to 15 µM RPI-1 for 24 hours and then transferred in Transwell chambers in serum-free medium with or without HGF. Migrated cells are reported as number of cells per field. One experiment representative of three is shown. (C) Cell scattering. Cells were seeded at a low density to allow growing as islets and, 24 hours later, serum-starved and treated with vehicle or 15 µM RPI-1, in the presence or absence of HGF, for 18 hours. Representative images are shown. Original magnification, x100.
Met Cooperates with Ret/ptc1 to Confer Invasive But Not Proliferative Ability to NTs
We examined Ret/ptc1-induced biological effects including invasive-related cell abilities, such as migration and early branching morphogenesis, and the proliferative capability, in human thyrocytes.
Morphogenic properties of thyrocytes were evaluated by an early tubulogenesis assay testing the cells' ability to aggregate and form branches in a few hours when cultured in Matrigel in the absence of serum [27]. RPTC cells, endowed with fully functional Ret/ptc1 oncoprotein, spontaneously formed complex branched structures, already evident 4 hours after seeding (Figure 1C). In contrast, NT (not shown) and RPTC-YF cells (Figure 1C) did not form branched structures even after a more prolonged period. After 7 hours of incubation, morphogenesis of RPTC-YF cells was stimulated in the presence of HGF. These results indicated that in RET/PTC1-transformed thyrocytes Y451-mediated signaling is required in the events leading to branching morphogenesis and suggested that Met, in a hyperactivated state, may contribute to the morphogenic phenotype of transformed thyrocytes.
In a migration assay, NT displayed only marginal spontaneous cell motility, whereas RPTC thyrocytes showed a significant increase in motile capacity similar to that of TPC-1 cells (Figure 1D). Y451F mutant thyrocytes showed a low intermediate motility that could be further stimulated by exogenous HGF by nearly 20% (not shown). These findings suggested that both Ret/ptc1 and Met signaling pathways contributed to confer motile capacity to human thyrocytes. In addition, such property seemed not exclusively dependent on the Ret oncoprotein Y451 multidocking site.
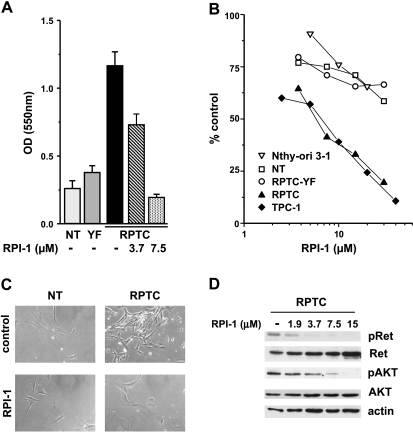
We previously showed that the exogenous expression of RET/PTC1 conferred a proliferative advantage to human thyroid follicular cells requiring a functional Y451 multidocking site [6]. Because Met protein was induced in both RPTC and RPTC-YF thyrocytes (Figure 1A), our present data suggested that Met did not influence the growth of human thyrocytes. In agreement, HGF did not stimulate proliferation of TPC-1 cells (not shown) and it was reported not to affect the survival of Met-positive cell lines established from thyroid carcinomas [28]. Consistently, treatment with the tyrosine kinase inhibitor RPI-1, targeting both Ret/ptc1 and Met [21,22], abrogated the proliferative advantage of RPTC thyrocytes (Figure 2A), whereas it only marginally affected the low proliferative capacity of parental NT and RPTC-YF cells (not shown). The growth of either RPTC thyrocytes or established tumor cells (TPC-1) harboring fully functional RET/PTC1 was inhibited by a 72-hour treatment with RPI-1 in a similar dose-dependent way (Figure 2B). In fact, RPI-1 showed a comparable IC50 in RPTC and TPC-1 cells (5.4 ± 0.4 and 6.3 ± 0.2 µM, respectively), whereas more than a six-fold increase in IC50 was observed in parental NT and RPTC-YF thyrocytes and in the thyroid follicular cell line Nthy-ori 3-1 (IC50 > 30 µM). The transformed morphology of RPTC thyrocytes, characterized by increased refraction and loss of cell contact inhibition, was reverted by RPI-1 treatment with the restoration of the flat contact-inhibited morphology of the parental cells (Figure 2C). In RPTC thyrocytes, Ret/ptc1 inhibition by RPI-1 in the range of concentrations affecting both cell proliferation and morphology was confirmed by the abrogation of Y451 phosphorylation (Figure 2D). Activation of Akt, previously shown to be induced by RET/PTC1 expression in thyrocytes [6], was also inhibited by RPI-1 treatment in a dose-dependent way.
Figure 2.
Effects of RPI-1 on human primary thyrocytes and thyroid cell lines. (A) Abrogation of RET/PTC1 growth-promoting effect. Uninfected thyrocytes (NT) or thyrocytes infected with RET/PTC1 (RPTC) or RET/PTC1-Y451F (YF) were treated with solvent (-) or with the indicated concentrations of RPI-1 for 6 days. Cell density was measured by the SRB colorimetric assay and was expressed as optical density (OD) at 550 nm (mean ± SD). One experiment representative of three is shown. (B) Cell growth inhibition. Primary thyrocytes and thyroid cell lines (follicular epithelial Nthy-ori 3-1 and PTC TPC-1) were exposed to solvent or to different concentrations of RPI-1 for 72 hours. The drug antiproliferative effect was evaluated by the SRB assay. Representative dose-response curves are shown. (C) Reversion of RET/PTC1-transformed morphologic phenotype. Normal human thyrocytes and RPTC thyrocytes were treated with 10 µM RPI-1 for 48 hours and were then photographed under phase-contrast microscope. Original magnification, x100. (D) Inhibition of Ret/ptc1-Y451 and Akt-S473 phosphorylation. RPTC cells were exposed to solvent (-) or to the indicated concentrations of RPI-1 for 24 hours. Western blot analysis of total protein extracts was performed with phospho-specific antibodies recognizing phosphorylated Y451 (pRet) and S473 (pAkt) of Ret/ptc1 and Akt, respectively. The filter was then stripped and reprobed with the respective antiprotein antibodies. Antiactin blot is shown as protein loading control.
TPC-1 Cell Morphogenic Phenotype Depends on Both Met and Ret/ptc1 Signaling
We investigated the role of Met and Ret/ptc1 and the effects of kinase inhibition in the regulation of the motile phenotype in the PTC cellular context.
In serum-starved TPC-1 cells, tyrosine phosphorylation of Met was indicative of the receptor constitutive activation (Figure 3A). On HGF addition, Met and Akt phosphorylation were markedly enhanced, indicating that the receptor tyrosine kinase retains biochemical responsiveness to its physiological ligand. Cell pretreatment with RPI-1 prevented HGF-induced activation of both Met and Akt.
The biological responsiveness of TPC-1 cells to HGF was examined by functional assays. In a migration assay, cells stimulated with HGF doubled their basal migratory ability (Figure 3B). Such ligand-induced cell motility was prevented by 24 hours of pretreatment with 15 µM RPI-1, whereas higher doses of the drug also inhibited the spontaneous cell motility (not shown).
The HGF-dependent motogenic properties of TPC-1 cells were further evaluated in a scatter assay in which cell response to the growth factor comprises an initial cell dissociation, involving cytoskeletal reorganization and loss of intercellular junctions, followed by active migration [27]. Whereas control unstimulated cells appeared packed in islands with tight cell-to-cell junctions (cell clusters), HGF addition to the culture medium caused loss of cell junctions and cell dispersal throughout the culture dish (Figure 3C). In addition, cells acquired a spindle-shaped morphology resembling a typical epithelial-to-mesenchymal transition (EMT) [29]. Pretreatment with RPI-1 impaired this process, preserving most cell-to-cell contacts.
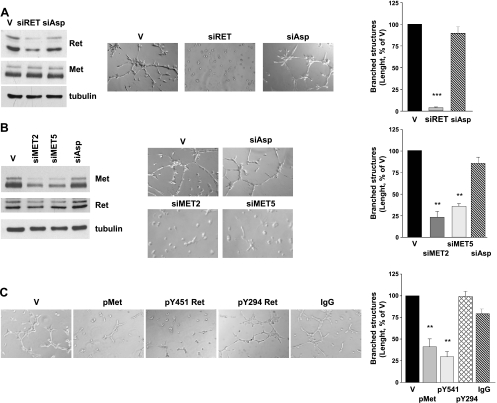
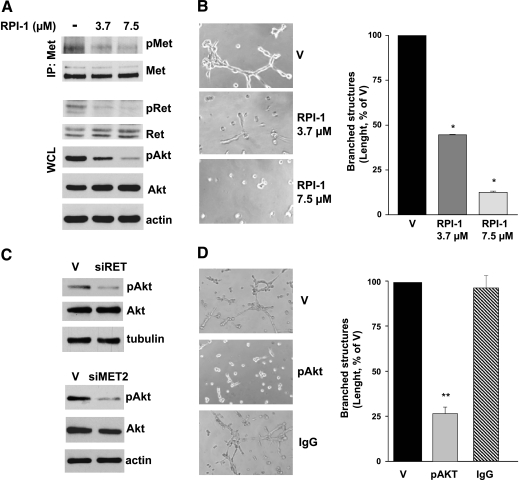
Because the previously described results suggested that both Ret/ptc1 and Met play a role in the regulation of the motogenic phenotype of human transformed thyrocytes, we investigated the contribution of the single protein kinase on the morphogenic phenotype of TPC-1 tumor cells using selective knockdown approaches. The RNA interference technology was used to elucidate gene function in the branching morphogenesis assay. Transfection with a specific RET siRNA caused the down-regulation of the two Ret/ptc1 isoform proteins (Figure 4A), which was reflected in a high reduction of their overall tyrosine phosphorylation (not shown). No downmodulation of Met levels was observed. RET/PTC1 knockdown heavily impaired the morphogenic activity of TPC-1 cells (Figure 4A). In a complementary experiment, transfection with two different MET siRNA caused a marked reduction of both the precursor and the mature form of the receptor without affecting Ret/ptc1 expression (Figure 4B). Again, the spontaneous ability of TPC-1 cells to form branched structures seemed significantly impaired (Figure 4B).
Figure 4.
Effects of selective expression or signaling knockdown of Ret/ptc1 and Met on TPC-1 cell morphogenic phenotype. (A) RET/PTC1 silencing by siRNA. Cells were treated with transfection reagent (V), a specific RET siRNA (siRET) or an aspecific RNA oligonucleotide (siAsp) for 5 days. Left: Ret/ptc1 protein downmodulation. Immunoblot analysis with the indicated antibodies was performed on whole cell lysates. Center: Effect of RET/PTC1 silencing on cell morphogenic properties. Cells were subjected to early branching morphogenesis assay in Matrigel. Right: Total length quantification of branched structures. (B) MET silencing by siRNA. Cells were treated with transfection reagent (V), with one of two different MET-targeting siRNA (siMET2 or siMET5) or with an aspecific oligonucleotide (siAsp) for 3 days. Left: Met protein downmodulation. Total cell lysates were analyzed by Western blot analysis with the indicated antibodies. Center: Effect of MET silencing on cell morphogenic properties. Right: Total length quantification of branched structures. (C) Effects of intracellular delivery of antibodies recognizing specific tyrosine phosphorylations of Ret or Met. Left: Cells loaded with protein delivery reagent alone (V), with anti-p(Y1234/Y1235) Met (pMet), anti-pY451 Ret, anti-pY294 Ret antibody, or aspecific immunoglobulins (IgG) for 24 hours, were subjected to the early branching morphogenesis assay. Right: Total length quantification of branched structures. Representative images are shown. Original magnification, x400. **P < .005, ***P < .0005.
The protein delivery technique was used as an alternative approach for disrupting Met or Ret/ptc1 signaling. TPC-1 cells were loaded with antibodies recognizing the protein kinases phosphorylated at the crucial tyrosine residues Ret/ptc1-Y451 and Met-Y1234/Y1235, which regulate Ret/ptc1 signaling and Met kinase activity [2,30], respectively. Both phospho-specific antibodies caused a significant reduction of the extension of branched structures formed by TPC-1 cells (Figure 4C). In contrast, cells loaded with aspecific IgG or an anti-Ret antibody recognizing phosphorylated Y294 (corresponding to Y905 of proto-Ret) [2] formed a cellular network similar to that of vehicle-treated cells. Furthermore, both approaches, affecting gene expression or kinase functions of Ret/ptc1 or Met, also inhibited TPC-1 cell motility in the migration assay (not shown). Thus, neither Ret/ptc1 nor Met signaling alone seemed sufficient to confer efficient morphogenic ability to thyroid cells.
Ret/ptc1 and Met Signaling Converge on PI3K/Akt Pathway Which Is Critical for the Morphogenic Phenotype of TPC-1 Cells
Because both Ret and Met are known activators of the Akt pathway [2,30], which has been implicated in thyroid cancer invasion [31], we supposed that Akt-dependent signaling could play a central role in the control of PTC morphogenic phenotype. Indeed, pharmacological inhibition of both Ret/ptc1 and Met in TPC-1 cells by RPI-1 was associated with the dose-dependent reduction of Akt activation (Figure 5A) and the loss of cells' ability to form branched structures in Matrigel (Figure 5B). Moreover, the selective knockdown of RET/PTC1 or MET by RNA interference, already shown to counteract the branching morphogenesis (Figure 4, A and B), also reduced Akt activation (Figure 5C).
Figure 5.
Effects of concomitant or selective inhibition of Ret/ptc1, Met, and Akt signaling on TPC-1 cell morphogenic pheynotype. (A) Inhibition of Akt activation by the Ret/Met tyrosine kinase inhibitor RPI-1. Cells were exposed to solvent (-) or to the indicated concentrations of RPI-1 for 24 hours. Whole cell lysates (WCL) were analyzed directly or subjected to Met immunoprecipitation (IP) and then analyzed by Western blot analysis. Activation status of the three kinases was detected using antibodies recognizing phosphorylated Ret/ptc1 Y451 (pRet), Met Y1234/Y1235 (pMet), or Akt S473 (pAkt). Blots were then stripped and reprobed with antibodies against the respective proteins. (B) left: Inhibition of cell ability to form branched structures in Matrigel by a 24-hour treatment with RPI-1. Right: Total length quantification of branched structures. (C) left: Inhibition of AKT activation in RET/PTC1- or MET-silenced cells. Western blot analysis was performed on total cell lysates obtained from cells exposed to RET siRNA (siRET) or MET siRNA (siMET2) for 5 or 3 days, respectively. V indicates transfection reagent. (D) left: Inhibition of branching morphogenesis by anti-pS473 Akt antibody (pAkt) intracellular delivery. V indicates protein delivery reagent; IgG, aspecific immunoglobulins. Right: Total length quantification of branched structures. Representative images are shown. Original magnification, x400. *P < .05, **P < .005.
To specifically address the relevance of Akt, we examined the effect of selective inhibition of its signaling pathway in the branching morphogenesis assay. TPC-1 cells loaded by the protein delivery technique with an antibody recognizing the Akt activating phosphorylation at S473 resulted in significant cell impairment to form branched structures (Figure 5D). These results thus confirmed that Akt may be a critical downstream effector in the regulation of the PTC proinvasive phenotype cooperatively activated by Ret/ptc1 and Met kinases.
Ret/ptc1 Induces Nuclear Translocation of β-Catenin in Thyroid Follicular Cells
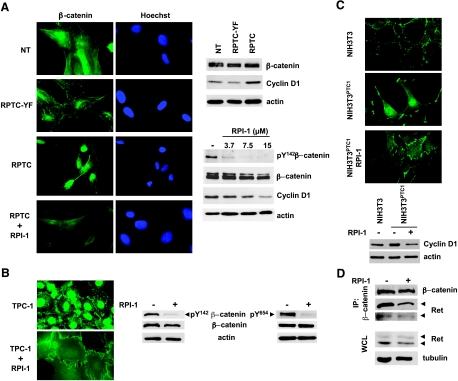
Next, we addressed the involvement of β-catenin in the RET/PTC1-driven transformed phenotype. β-Catenin, a dual-function protein involved in both cell adhesion and transcription, depending on its cellular localization, has been found dysregulated in thyroid cancer [12]. We examined the cellular distribution of this protein in parental and RET/PTC1-infected thyrocytes. NT and RPTC-YF thyrocytes presented a similar perinuclear/cytoplasmic immunostaining of β-catenin (Figure 6A). In contrast, accumulation of β-catenin in the nuclei was observed in RPTC thyrocytes suggesting that a functional Ret/ptc1-Y451-mediated signaling could promote the nuclear translocation of β-catenin. Consistent with a transcriptionally active β-catenin in RPTC cells, cyclin D1, which is known as a β-catenin target gene [13], was up-regulated at both the RNA1 and protein levels (Figure 6A). Treatment with RPI-1 caused β-catenin redistribution in RPTC cells, as evidenced by a diffuse cytoplasmic staining of the protein (Figure 6A). Accordingly, in RPI-1-treated RPTC cells, β-catenin phosphorylation at Y142, described as one of the crucial events switching from the adhesive to the transcriptional role of the protein [32,33], was reduced in a dose-dependent way, and cyclin D1 was downmodulated (Figure 6A). Similar results were obtained in TPC-1 cells, in which RPI-1 treatment caused a marked redistribution of β-catenin with complete disappearance of the protein from nuclei and gain of junctional protein at cell-to-cell surface contacts without altering the overall level of the protein (Figure 6B). Moreover, according to an increased adhesion to the plastic well, RPI-1-treated cells showed a more flattened and enlarged morphology. Furthermore, drug treatment induced a marked inhibition of β-catenin Y142 phosphorylation (Figure 6B). Although Met has been indicated as one of the kinases phosphorylating β-catenin Y142 in other cellular contexts [32,33], in TPC-1 cells, Met stimulation with HGF did not modulate the phosphorylation of this tyrosine residue (not shown). Recently, another β-catenin tyrosine residue, Y654, has been reported as phosphorylated by Ret receptor mutants in medullary thyroid carcinoma models [34]. This tyrosine phosphorylation of β-catenin was also abrogated by RPI-1 treatment (Figure 6B).
Figure 6.
Effects of RET/PTC1 expression and RPI-1 treatment on β-catenin expression, tyrosine phosphorylation, and localization in human follicular thyroid cells and murine NIH3T3 fibroblasts. (A) Left: Immunofluorescence analysis of β-catenin localization in parental thyrocytes (NT) and RET/PTC1- (RPTC) or RET/PTC1-Y451F-infected (RPTC-YF) thyrocytes exposed to solvent or to 15 µM RPI-1 for 72 hours. Nuclei were evidenced with Hoechst 3341 counterstaining. Photos were taken under a florescence microscope equipped with a digital camera. Right top: β-Catenin and cyclin D1 cellular levels. Right bottom: Inhibition of β-catenin Y142 phosphorylation and cyclin D1 expression in RPI-1-treated RPTC thyrocytes. Whole cell lysates were analyzed by Western blot analysis using the indicated antibodies. Antiactin blots are shown as protein loading control. (B) Left: β-Catenin localization examined by indirect immunofluorescence in TPC-1 cells treated with solvent or with 60 µM RPI-1 for 72 hours. Right: β-Catenin expression and phosphorylation at Y142 and Y654 revealed by Western blot analysis on whole cell lysates. (C) Top: β-Catenin localization in RET/PTC1-transformed NIH3T3 cells (NIH3T3PTC-1) exposed to solvent (control) or to RPI-1 for 72 hours evidenced by immunofluorescence. Bottom: Modulation of cyclin D1 expression. Whole cell lysates from parental NIH3T3 or NIH3T3PTC1 cells exposed to solvent (-) or to 30 µM RPI-1 (+) were subjected to Western blot analysis. (D) Inhibition of Ret/ptc1 coimmunoprecipitation with β-catenin in TPC-1 cells treated with RPI-1. Control cells (-) and cells treated with RPI-1 (+) as in B were processed for immunoprecipitation (IP) with a monoclonal anti-β-catenin antibody or used for total protein extraction (WCL). Samples were subjected to Western blot analysis with the indicated antibodies. Arrows indicate the long and short Ret/ptc1 isoforms. Antitubulin or antiactin blots are shown as protein loading controls. Original magnification, x500.
To confirm the ability of Ret/ptc1 to modulate β-catenin localization independently of Met, we investigated β-catenin distribution in a cellular context not expressing Met. To this aim, as most epithelial cell lines express Met protein at variable levels, we used parental and RET/PTC1 stably transfected NIH3T3 fibroblasts, both with undetectable Met protein expression. Contrary to thyrocytes, Met was not up-regulated by RET/PTC1 in murine fibroblasts. RET/PTC1-transformed NIH3T3 cells indeed showed a prevalent nuclear localization of β-catenin (Figure 6C). Treatment with RPI-1 restored the membrane/focal adhesion pattern of β-catenin distribution typical of the NIH3T3 parental cells. Consistently, RPI-1 treatment of RET/PTC1-expressing fibroblasts abrogated the up-regulation of cyclin D1 induced by the oncogene (Figure 6C).
Next, to determine whether Ret/ptc1 could interact with β-catenin in the context of PTC, we performed a coimmunoprecipitation experiment in TPC-1 cells. Ret/ptc1 was indeed present in the β-catenin immunoprecipitate from the PTC cells with the prevalence of the p64 long isoform (Figure 6D). Similarly, Ret/ptc1 and β-catenin were coimmunoprecipitated from NIH3T3 and human embryonic kidney HEK293T cells exogenously expressing genomic RET/PTC1 or the single RET/PTC1 short isoform, respectively (not shown). In RPI-1-treated TPC-1 cells, such association was reduced, whereas overall levels of β-catenin and Ret/ptc1 proteins were unchanged (Figure 6D). These results thus implicate a direct role of Ret/ptc1 in the regulation of β-catenin functions.
Discussion
In the present study, using two different models of PTC, the most frequent thyroid carcinoma histotype, we show that: 1) RET/PTC1, an oncogene specific of this thyroid cancer, induces Met protein expression independently from the Y451 Ret/ptc1 multidocking site; 2) Ret/ptc1 and Met signaling cooperate to confer a proinvasive phenotype to thyroid cells; and 3) independently from Met, Ret/ptc1 is able to induce nuclear translocation of β-catenin. We obtained consistent results in both primary NTs exogenously expressing RET/PTC1 and the PTC cell line TPC-1, endogenously expressing the same oncogene.
Met overexpression is a feature of most PTCs, whereas it is not present in the normal thyroid follicular cells [17]. It is thus conceivable that the driving molecular alterations occurring in PTC might deregulate Met expression [1]. Among these, RET rearrangements have been described by us and others to enhance MET gene transcription [6,19]. Here, we show that Met protein is expressed at comparable levels in TPC-1 cells and in thyrocytes expressing Ret/ptc1 or its mutant Y451F corresponding to the Ret Y1062 residue, known to play a crucial role in the activation of signaling pathways mediating RET-transforming activity. Moreover, Met was constitutively tyrosine-phosphorylated in RET/PTC1- and RET/PTC1-Y451F-expressing thyroid cells, most probably because of overexpression-induced kinase activation [16]. Regulation of Met expression by the RET oncogene seemed specific of the thyroid environment because RET/PTC1 did not affect Met expression in other cellular models such as NIH3T3 or HeLa cells. Regulation of Met expression by its GC-rich TATA-less promoter is complex, involving several transcription factors, including hypoxia-inducible factor 1, and microRNA [35,36]. Our results, showing that RET/PTC1 and the mutant RET/PTC1-Y451F, devoid of Ret multidocking site, elicit comparable expression of MET mRNA and protein, suggest that RET-activated RAS/ERK and PI3K/AKT pathways are dispensable for Met induction [6]. The possible role of other Ret/ptc1-triggered signaling pathways in this event remains to be investigated and would shed light on thyroid environment-specific Met regulation. In transformed thyrocytes, as well as in TPC-1 cells, Met maintained its biochemical and biological responsiveness to HGF. Thus, Met induction by RET/PTC1 seems to confer sensitivity to the motogenic/morphogenic effects of HGF in thyrocytes. Notably, genes up-regulated by fully functional RET/PTC1 in thyrocytes include the urokinase-type plasminogen activator (uPA)/uPA receptor system [6], which catalyze the proteolytic cleavage required by HGF to become an active ligand for Met [37]. HGF/Met, in turn, play a role in the regulation of the uPA/uPA receptor expression and of the proteolytic network important for cell acquisition of invasive potential [38]. These findings therefore indicate the ability of Ret/ptc1 to activate directly, or indirectly through Met, different pathways cooperating in the promotion of thyroid cell transformation and proinvasive phenotype. Further experiments are needed to assess whether Met expression is still dependent on Ret/ptc1 expression in established PTC.
Not only the cross talk between Ret/ptc1 and Met receptor tyrosine kinases was demonstrated at transcriptional level but it was also shown that their signaling converges on the activation of downstream mediators, such as Akt. Our results support a crucial role for Akt-mediated signaling in the acquisition of the invasive phenotype by PTC cells. In fact: 1) either RET/PTC1 or MET silencing lowered Akt phosphorylation; and 2) Ret/ptc1, Met, or Akt functional knockdown abolished the cell morphogenic phenotype. Altogether, these data suggest that hyperactivation of Akt is necessary to promote such phenotype in thyroid cells. Notably, the relative migratory capability displayed by NT, RPTC-YF, and RPTC thyrocytes, as shown in this article, seemed well correlated with the previously reported respective levels of Akt activation [6]. The PI3K/Akt pathway plays a nodal role in the cross talk of different growth factors and oncogenic signaling cascades, integrin receptors, and small GTPases that control cytoskeleton organization [39]. In different cellular contexts, the regulation of cell motility by Met or Ret was shown to require PI3K recruitment to the receptor tyrosine kinase and the subsequent activation of Akt [40,41]. Although Akt activation has been correlated with tumor invasion in thyroid carcinoma [31], its relationship with Ret and Met activation in PTCs was not previously addressed.
In this study, we have also demonstrated that the pleiotropic effects of RET/PTC1 in human follicular thyroid cells include β-catenin nuclear translocation. In fact, differently from parental and RET/PTC1-Y451F mutant thyrocytes, RET/PTC1-expressing thyrocytes showed a prevalent nuclear localization of β-catenin, associated with an increased expression of its transcriptional target gene, cyclin D1. This effect suggested a β-catenin functional switch from adhesive to transcriptional activity. Accordingly, several well-known and putative β-catenin target genes2 [13,42,43] are upmodulated by RET/PTC1, but not by RET/PTC1-Y451F, in human thyrocytes [6]. Such genes include CD44 and its ligand OPN, COX2, LEF1, JUN, EGR-1, CCL2, CXCL8, MMP7, MMP10, and FN1.1 Nuclear mislocation of β-catenin has been found in PTC samples, in particular, in poorly differentiated and micro PTC [10,44]. The relative proliferative potential of NT, RPTC-YF, and RPTC thyrocytes suggested a correlation between β-catenin nuclear localization and proliferation. Indeed, we observed that inhibition of the Wnt/β-catenin pathway in TPC-1 cells by the known inhibitor indomethacin [45] caused disappearance of β-catenin from the nucleus and a marked cell growth inhibition (data not shown), thus supporting such interpretation. Although active Met has been shown able to induce β-catenin translocation to the nucleus in other cellular contexts [46], we have found that in thyrocytes this effect seemed to be driven by Ret/ptc1. β-Catenin nuclear translocation, at variance with Met induction, was indeed dependent on Ret/ptc1-Y451 signaling. In keeping with a Met-independent effect, nuclear translocation of β-catenin was induced by Ret/ptc1 even in NIH3T3 fibroblasts, not expressing detectable Met protein. In fibroblasts, as well as in thyroid cells, inhibition of Ret/ptc1 kinase activity by the small molecule RPI-1 restored β-catenin distribution typical of parental cells. Moreover, an interaction between Ret/ptc1 and β-catenin was supported by coimmunoprecipitation of the two proteins in RET/PTC1-positive cells. Notably, it was recently demonstrated that transmembrane oncogenic forms of Ret associated to multiple endocrine neoplasia activate β-catenin signaling by direct interaction and phosphorylation at Y654 in medullary thyroid carcinoma [34]. Here, we showed that inhibition of Ret/ptc1 kinase activity in PTC cellular models was associated with β-catenin dislocation from the nucleus and dephosphorylation at Y654 as well as Y142, another tyrosine residue implicated in the regulation of β-catenin cellular localization [32,33].
Alterations in cell adhesive properties and gain of a migratory phenotype have been associated with EMT [29,39]. This developmental process whereby embryonic cells switch from a polarized, epithelial phenotype to a highly motile, mesenchymal phenotype has been implicated in carcinoma invasion. Indeed, our data are in keeping with the emerging concept that molecular mechanisms that control cell migration during embryological development, including EMT, may be reactivated in invading thyroid cancer cells [47]. Noteworthy, a cooperative cross talk between Ret and Met has also been suggested during the renal embryonic development [48,49]. The exact role of EMT-related signaling pathways in thyroid cancer cell invasion has not been fully elucidated, although recognized thyroid oncogenes such as RET/PTCs, BRAF, and RAS seem to be involved in the regulation of this process [47]. Molecular markers of EMT, including metalloproteases and the couples of ligand/receptors osteopontin/CD44 and CXCL12/CXCR4 have been associated with RET/PTC1 expression in thyroid cells [6,50]. The present study shows the early induction of additional recognized EMT markers, that is, overexpression of an active Met protein and β-catenin translocation, in association with the acquisition of in vitro phenotypic markers such as increased capacity for migration and extracellular matrix invasion. Furthermore, as discussed, our data support a crucial role for Akt, a well-recognized mediator of EMT [39], in the acquisition of the morphogenic phenotype by PTC cells.
RET/PTC1 has already been shown to activate a proinflammatory program in NTs, thus providing a link between the oncogene and inflammation, a peritumoral reaction frequently observed in PTCs [1,6,51]. The up-regulation of genes implicated in invasion, including several metalloproteases and chemokines, also suggested a direct link with locoregional spread, another pathologic characteristic of PTCs and peculiar of RET/PTC1-positive cases [52]. Our present results, demonstrating an early activation of invasion-related molecules, such as Met, β-catenin, and Akt, in cooperation with the Ret/ptc1 oncoprotein, further contribute to provide a rational molecular basis for understanding clinical distinguishing features of PTCs and may have therapeutic implications. Effective therapies for metastatic thyroid cancers are not available, thus indicating the need for alternative therapies. Preclinical studies by other groups and ours have already demonstrated the possible role of RET oncoproteins as therapeutic targets [4,25], and clinical trials are presently evaluating the efficacy of small molecule Ret inhibitors on thyroid cancers.3 The present study indicates Met tyrosine kinase as an additional target in PTC treatments. The role of Met as a therapeutic target has been extensively documented in preclinical studies [53] and is currently under evaluation in the clinical setting with Met-targeting tyrosine kinase inhibitors.3 Here, we show that concomitant inhibition of PTC cell growth and migration/invasion can be achieved even by a single small molecule inhibitor such as the Ret/Met inhibitor RPI-1. In addition, a marked inhibitory effect on the secretion of chemokines such as CCL2 (unpublished) also suggests an impairment of oncogene-activated inflammatory program by drug treatment.
Altogether, our results suggest that Ret/ptc1 induces and cooperatively interacts with Met signaling and promotes β-catenin transcriptional activity to drive thyrocyte neoplastic transformation. Such molecular network highlights new options to design multitarget therapeutic strategies for PTCs.
Acknowledgments
The authors thank Andrew Fischer and Luisella Alberti for thyrocyte model generation, Manuela Gariboldi for thyrocyte model expression profiles, the Medicinal Chemistry Department of Cell Therapeutics Inc., Europe (Bresso, Italy) for the synthesis of RPI-1, and Laura Zanesi for help in editing the manuscript.
Abbreviations
- EMT
epithelial-to-mesenchymal transition
- HGF
hepatocyte growth factor
- NT
normal human thyrocyte
- PTC
papillary thyroid carcinoma
- RPTC
human thyrocytes expressing RET/PTC1
- RPTC-YF
human thyrocytes expressing the RET/PTC1 Y451F mutant
- SRB
sulforhodamine B
Footnotes
This research was funded by Italian Ministero della Salute, Associazione Italiana per la Ricerca sul Cancro, Alleanza contro il Cancro and, under the 6th Framework Program, by the European Commission.
ArrayExpress database: Accession No. E-MEXP-429.
References
- 1.Kondo T, Ezzat S, Asa SL. Pathogenetic mechanisms in thyroid follicular-cell neoplasia. Nature. 2006;6:292–306. doi: 10.1038/nrc1836. [DOI] [PubMed] [Google Scholar]
- 2.Arighi E, Borrello MG, Sariola H. RET tyrosine kinase signaling in development and cancer. Cytokine Growth Factor Rev. 2005;16:441–467. doi: 10.1016/j.cytogfr.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 3.Takahashi M. The GDNF/RET signaling pathway and human diseases. Cytokine Growth Factor Rev. 2001;12:361–373. doi: 10.1016/s1359-6101(01)00012-0. [DOI] [PubMed] [Google Scholar]
- 4.De Groot JWB, Links TP, Plukker JTM, Lips CJM, Hofstra RMW. RET as a diagnostic and therapeutic target in sporadic and hereditary endocrine tumors. Endocrine Rev. 2006;27:535–560. doi: 10.1210/er.2006-0017. [DOI] [PubMed] [Google Scholar]
- 5.Bond JA, Wyllie FS, Rowson J, Radulescu A, Wynford-Thomas D. In vitro reconstruction of tumour initiation in a human epithelium. Oncogene. 1994;9:281–290. [PubMed] [Google Scholar]
- 6.Borrello MG, Alberti L, Fischer A, Degl'Innocenti D, Ferrario C, Gariboldi M, Marchesi F, Allavena P, Greco A, Collini P, et al. Induction of a proinflammatory program in normal human thyrocytes by the RET/PTC1 oncogene. Proc Natl Acad Sci USA. 2005;102:14825–14830. doi: 10.1073/pnas.0503039102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fischer AH, Bond JA, Taysavang P, Battles OE, Wynford-Thomas D. Papillary thyroid carcinoma oncogene (RET/PTC) alters the nuclear envelope and chromatin structure. Am J Pathol. 1998;153:1443–1450. doi: 10.1016/S0002-9440(10)65731-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Knostman KAB, Jhiang SM, Capen CC. Genetic alterations in thyroid cancer: the role of mouse models. Vet Pathol. 2007;44:1–14. doi: 10.1354/vp.44-1-1. [DOI] [PubMed] [Google Scholar]
- 9.Potter E, Bergwitz C, Brabant G. The cadherin-catenin system: implications for growth and differentiation of endocrine tissues. Endocrine Rev. 2007;20:207–239. doi: 10.1210/edrv.20.2.0362. [DOI] [PubMed] [Google Scholar]
- 10.Rocha AS, Soares P, Seruca R, Maximo V, Matias-Guiu X, Cameselle-Teijeiro J, Sobrinho-Simoes M. Abnormalities of the E-cadherin/catenin adhesion complex in classical papillary thyroid carcinoma and in its diffuse sclerosing variant. J Pathol. 2001;194:358–366. doi: 10.1002/path.905. [DOI] [PubMed] [Google Scholar]
- 11.Ishigaki K, Namba H, Nakashima M, Nakayama T, Mitsutake N, Hayashi T, Maeda S, Ichinose M, Kanematsu T, Yamashita S. Aberrant localization of β-catenin correlates with overexpression of its target gene in human papillary thyroid cancer. J Clin Endocrinol Metab. 2002;87:3433–3440. doi: 10.1210/jcem.87.7.8648. [DOI] [PubMed] [Google Scholar]
- 12.Abbosh PH, Nephew KP. Multiple signaling pathways converge on β-catenin in thyroid cancer. Thyroid. 2005;15:551–561. doi: 10.1089/thy.2005.15.551. [DOI] [PubMed] [Google Scholar]
- 13.Gavert N, Ben-Ze'ev A. beta-Catenin signaling in biological control and cancer. J Cell Biochem. 2007;102:820–828. doi: 10.1002/jcb.21505. [DOI] [PubMed] [Google Scholar]
- 14.Lilien J, Balsamo J. The regulation of cadherin-mediated adhesion by tyrosine phosphorylation/dephosphorylation of β-catenin. Curr Opin Cell Biol. 2005;17:459–465. doi: 10.1016/j.ceb.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 15.Di Renzo MF, Olivero M, Ferro S, Prat M, Bongarzone I, Pilotti S, Belfiore A, Costantino A, Vigneri R, Pierotti MA, et al. Overexpression of the c-MET/HGF receptor gene in human thyroid carcinomas. Oncogene. 1992;7:2549–2553. [PubMed] [Google Scholar]
- 16.Gentile A, Trusolino L, Comoglio PM. The Met tyrosine kinase receptor in development and cancer. Cancer Metastasis Rev. 2008;27:85–94. doi: 10.1007/s10555-007-9107-6. [DOI] [PubMed] [Google Scholar]
- 17.Ruco LP, Stoppacciaro A, Ballarini F, Prat M, Scarpino S. Met protein and hepatocyte growth factor (HGF) in papillary carcinoma of the thyroid: evidence for a pathogenic role in tumourigenesis. J Pathol. 2001;194:4–8. doi: 10.1002/path.847. [DOI] [PubMed] [Google Scholar]
- 18.Scarpino S, Cancellario D'Alena F, Di Napoli A, Ballarini F, Prat M, Ruco LP. Papillary carcinoma of the thyroid: evidence for a role for hepatocyte growth factor (HGF) in promoting tumour angiogenesis. J Pathol. 2003;199:243–250. doi: 10.1002/path.1278. [DOI] [PubMed] [Google Scholar]
- 19.Ivan M, Bond JA, Prat M, Comoglio PM, Wynford-Thomas D. Activated ras and ret oncogenes induce over-expression of c-met (hepatocyte growth factor receptor) in human thyroid epithelial cells. Oncogene. 1997;14:2417–2423. doi: 10.1038/sj.onc.1201083. [DOI] [PubMed] [Google Scholar]
- 20.Giordano TJ, Kuick R, Thomas SG, Misek DE, Vinco M, Sanders D, Zhu Z, Ciampi R, Roh M, Shedden K, et al. Molecular classification of papillary thyroid carcinoma: distinct BRAF, RAS, and RET/PTC mutation-specific gene expression profiles discovered by DNA microarray analysis. Oncogene. 2005;24:6646–6656. doi: 10.1038/sj.onc.1208822. [DOI] [PubMed] [Google Scholar]
- 21.Lanzi C, Cassinelli G, Cuccuru G, Zaffaroni N, Supino R, Vignati S, Zanchi C, Yamamoto M, Zunino F. Inactivation of Ret/Ptc1 oncoprotein and inhibition of papillary thyroid carcinoma cell proliferation by indolinone RPI-1. Cell Mol Life Sci. 2003;60:1449–1459. doi: 10.1007/s00018-003-2381-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cassinelli G, Lanzi C, Petrangolini G, Tortoreto M, Pratesi G, Cuccuru G, Laccabue D, Supino R, Belluco S, Favini E, et al. Inhibition of c-Met and prevention of spontaneous metastatic spreading by the 2-indolinone RPI-1. Mol Cancer Ther. 2006;5:2388–2397. doi: 10.1158/1535-7163.MCT-06-0245. [DOI] [PubMed] [Google Scholar]
- 23.Lanzi C, Cassinelli G, Pensa T, Cassinis M, Gambetta RA, Borrello MG, Menta E, Pierotti MA, Zunino F. Inhibition of transforming activity of the ret/ptc1 oncoprotein by a 2-indolinone derivative. Int J Cancer. 2000;85:384–390. [PubMed] [Google Scholar]
- 24.Borrello MG, Alberti L, Arighi E, Bongarzone I, Battistini C, Bardelli A, Pasini B, Piutti C, Rizzetti MG, Mondellini P, et al. The full oncogenic activity of Ret/ptc2 depends on tyrosine 539, a docking site for phospholipase Cγ. Mol Cell Biol. 1996;16:2151–2163. doi: 10.1128/mcb.16.5.2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cuccuru G, Lanzi C, Cassinelli G, Pratesi G, Tortoreto M, Petrangolini G, Seregni E, Martinetti A, Laccabue D, Zanchi C, et al. Cellular effects and antitumor activity of RET inhibitor RPI-1 on MEN2A-associated medullary thyroid carcinoma. J Natl Cancer Inst. 2004;96:1006–1014. doi: 10.1093/jnci/djh184. [DOI] [PubMed] [Google Scholar]
- 26.Salvi A, Arici B, Portolani N, Giulini SM, De Petro G, Barlati S. In vitro c-met inhibition by antisense RNA and plasmid-based RNAi down-modulates migration and invasion of hepatocellular carcinoma cells. Int J Oncol. 2007;31:451–460. [PubMed] [Google Scholar]
- 27.Tulasne D, Paumelle R, Weidner KM, Vandenbunder B, Fafeur V. The multisubstrate docking site of the MET receptor is dispensable for MET-mediated RAS signaling and cell scattering. Mol Biol Cell. 1999;10:551–565. doi: 10.1091/mbc.10.3.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.De Luca A, Arena N, Sena LM, Medico E. Met overexpression confers HGF-dependent invasive phenotype to human thyroid carcinoma cells in vitro. J Cell Physiol. 1999;180:365–371. doi: 10.1002/(SICI)1097-4652(199909)180:3<365::AID-JCP7>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 29.Turley EA, Veiseh M, Radisky DC, Bissell MJ. Mechanisms of disease: epithelial-mesenchymal transition—does cellular plasticity fuel neoplastic progression? Nat Clin Pract Oncol. 2008;5:280–290. doi: 10.1038/ncponc1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Furge KA, Zhang YW, Vande Woude GF. Met receptor tyrosine kinase: enhanced signaling through adapter proteins. Oncogene. 2000;19:5582–5589. doi: 10.1038/sj.onc.1203859. [DOI] [PubMed] [Google Scholar]
- 31.Vasko V, Saji M, Hardy E, Kruhlak M, Larin A, Savchenko V, Miyakawa M, Isozaki O, Murakami H, Tsushima T, et al. Akt activation and localization correlate with tumour invasion and oncogene expression in thyroid cancer. J Med Genet. 2004;41:161–170. doi: 10.1136/jmg.2003.015339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Piedra J, Miravet S, Castano J, Palmer HG, Heisterkamp N, de Herreros AG, Dunach M. P120 catenin-associated Fer and Fyn tyrosine kinases regulate β-catenin Tyr-142 phosphorylation and β-catenin-α-catenin interaction. Mol Cell Biol. 2003;23:2287–2297. doi: 10.1128/MCB.23.7.2287-2297.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brembeck FH, Schwarz-Romond T, Bakkers J, Wilhelm S, Hammerschmidt M, Birchmeier W. Essential role of BCL9-2 in the switch between beta-catenin's adhesive and transcriptional functions. Genes Dev. 2004;18:2225–2230. doi: 10.1101/gad.317604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gujral TS, van Veelen W, Richardson DS, Myers SM, Meens JA, Acton DS, Dunach M, Elliott BE, Hoppener JWM, Mulligan LM. A novel RET kinase-β-catenin signaling pathway contributes to tumorigenesis in thyroid carcinoma. Cancer Res. 2008;68:1338–1346. doi: 10.1158/0008-5472.CAN-07-6052. [DOI] [PubMed] [Google Scholar]
- 35.Gentile A, Trusolino L, Comoglio PM. The Met tyrosine kinase receptor in development and cancer. Cancer Metastasis Rev. 2008;27:85–94. doi: 10.1007/s10555-007-9107-6. [DOI] [PubMed] [Google Scholar]
- 36.Kim S, Lee MN, Lee E-J, Kim JY, Lee MY, Choung S, Kim YJ, Choi Y-C. MicroRNA miR-199a* regulates the MET proto-oncogene and the downstream extracellular signal-regulated kinase 2 (ERK2) J Biol Chem. 2008;283:18158–18166. doi: 10.1074/jbc.M800186200. [DOI] [PubMed] [Google Scholar]
- 37.Naldini L, Tamagnone L, Vigna E, Sachs M, Hartmann G, Birchmeier W, Daikuhara Y, Tsubouchi H, Blasi F, Comoglio PM. Extracellular proteolytic cleavage by urokinase is required for activation of hepatocyte growth factor/scatter factor. EMBO J. 1992;11:4825–4833. doi: 10.1002/j.1460-2075.1992.tb05588.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pepper MS, Matsumoto K, Nakamura T, Orci L, Montesano R. Hepatocyte growth factor increases urokinase-type plasminogen activator (u-PA) and u-PA receptor expression in Madin-Darby canine kidney epithelial cells. J Biol Chem. 1992;267:20493–20496. [PubMed] [Google Scholar]
- 39.Larue L, Bellacosa A. Epithelial-mesenchymal transition in development and cancer: role of phosphatidylinositol 3′ kinase/AKT pathways. Oncogene. 2005;24:7443–7454. doi: 10.1038/sj.onc.1209091. [DOI] [PubMed] [Google Scholar]
- 40.Natarajan D, Marcos-Gutierrez C, Pachmis V, de Graaff E. Requirement of signaling by receptor tyrosine kinase RET for the directed migration of enteric nervous system progenitor cells during mammalian embryogenesis. Development. 2002;129:5151–5160. doi: 10.1242/dev.129.22.5151. [DOI] [PubMed] [Google Scholar]
- 41.Degl'Innocenti D, Arighi E, Popsueva A, Sangregorio R, Alberti L, Rizzetti MG, Ferrario C, Sariola H, Pierotti MA, Borrello MG. Differential requirement of Tyr1062 multidocking site by RET isoforms to promote neural cell scattering and epithelial cell branching. Oncogene. 2004;23:7297–7309. doi: 10.1038/sj.onc.1207862. [DOI] [PubMed] [Google Scholar]
- 42.El-Tanani MK, Barraclough R, Wilkinson MC, Rudland PS. Regulatory region of metastasis-inducing DNA is the binding site for T cell factor-4. Oncogene. 2001;20:1793–1797. doi: 10.1038/sj.onc.1204358. [DOI] [PubMed] [Google Scholar]
- 43.Mestdagt M, Polette M, Buttice G, Noel A, Ueda A, Foidart JM, Gilles C. Transactivation of MCP-1/CCL2 by beta-catenin/TCF-4 in human breast cancer cells. Int J Cancer. 2006;118:35–42. doi: 10.1002/ijc.21291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lantsov D, Meirmanov S, Nakashima M, Kondo H, Saenko V, Naruke Y, Namba H, Ito M, Abrosimov A, Lushnikov E, et al. Cyclin D1 overexpression in thyroid papillary microcarcinoma: its association with tumour size and aberrant beta-catenin expression. Histopathology. 2005;47:248–256. doi: 10.1111/j.1365-2559.2005.02218.x. [DOI] [PubMed] [Google Scholar]
- 45.Kundu JK, Choi K-Y, Surh Y-J. β-Catenin-mediated signaling: a novel molecular target for chemoprevention with anti-inflammatory substances. Biochim Biophys Acta. 2006;1765:14–24. doi: 10.1016/j.bbcan.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 46.Brembeck FH, Rosario M, Birchmeier W. Balancing cell adhesion and Wnt signaling, the key role of β-catenin. Curr Opin Genet Dev. 2006;16:51–59. doi: 10.1016/j.gde.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 47.Vasko V, Saji M. Molecular mechanisms involved in differentiated thyroid cancer invasion and metastasis. Curr Opin Oncol. 2007;19:11–17. doi: 10.1097/CCO.0b013e328011ab86. [DOI] [PubMed] [Google Scholar]
- 48.O'Rourke DA, Sakurai H, Spokes K, Kjelsberg C, Takahashi M, Nigam S, Cantley L. Expression of c-ret promotes morphogenesis and cell survival in mIMCD-3 cells. Am J Physiol. 1999;276:581–588. doi: 10.1152/ajprenal.1999.276.4.F581. [DOI] [PubMed] [Google Scholar]
- 49.Popsueva A, Poteryaev D, Arighi E, Meng X, Angers-Loustau A, Kaplan D, Saarma M, Sariola H. GDNF promotes tubulogenesis of GFRalpha1-expressing MDCK cells by Src-mediated phosphorylation of Met receptor tyrosine kinase. J Cell Biol. 2003;161:119–129. doi: 10.1083/jcb.200212174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Castellone MD, Celetti A, Guarino V, Cirafici AM, Basolo F, Giannini R, Medico E, Kruhoffer M, Orntoft TF, Curcio F, et al. Autocrine stimulation by osteopontin plays a pivotal role in the expression of the mitogenic and invasive phenotype of RET/PTC-transformed thyroid cells. Oncogene. 2004;23:2188–2196. doi: 10.1038/sj.onc.1207322. [DOI] [PubMed] [Google Scholar]
- 51.Borrello MG, Degl'Innocenti D, Pierotti M. Inflammation and cancer: the oncogene-driven connection. Cancer Lett. 2008;267:262–270. doi: 10.1016/j.canlet.2008.03.060. [DOI] [PubMed] [Google Scholar]
- 52.Nikiforova MN, Nikiforov YE. Molecular genetics of thyroid cancer: implications for diagnosis, treatment and prognosis. Expert Rev Mol Diagn. 2008;8:83–85. doi: 10.1586/14737159.8.1.83. [DOI] [PubMed] [Google Scholar]
- 53.Migliore C, Giordano S. Molecular cancer therapy: can our expectation be MET? Eur J Cancer. 2008;44:641–651. doi: 10.1016/j.ejca.2008.01.022. [DOI] [PubMed] [Google Scholar]