Abstract
It has been reported that blocking Notch signaling in tumor-bearing mice results in abortive angiogenesis and tumor regression. However, given that Notch signaling influences numerous cellular processes in vivo, a comprehensive evaluation of the effect of Notch inactivation on tumor growth would be favorable. In this study, we inoculated four cancer cell lines in mice with the conditional inactivation of recombination signal-binding protein-Jκ (RBP-J), which mediates signaling from all four mammalian Notch receptors. We found that whereas three tumors including hepatocarcinoma, lung cancer, and osteogenic sarcoma grew slower in the RBP-J-deficient mice, at least a melanoma, B16, grew significantly faster in the RBP-J-deficient mice than in the controls, suggesting that the RBP-J-deficient hosts could provide permissive cues for tumor growth. All these tumors showed increased microvessels and up-regulated hypoxia-inducible factor 1α, suggesting that whereas defective angiogenesis resulted in hypoxia, different tumors might grow differentially in the RBP-J-deleted mice. Similarly, increased infiltration of Gr1+/Mac1+ cells were noticed in tumors grown in the RBP-J-inactivated mice. Moreover, we found that when inoculated in the RBP-J knockout hosts, the H22 hepatoma cells had a high frequency of metastasis and lethality, suggesting that at least for H22, deficiency of environmental Notch signaling favored tumor metastasis. Our findings suggested that the general blockade of Notch signaling in tumor-bearing mice could lead to defective angiogenesis in tumors, but depending on tumor cell types, general inhibition of Notch signaling might result in tumor regression, progression, or metastasis.
Introduction
Interactions between tumor cells and host environments are critical for tumor growth and metastasis. Whereas the immune system of host intends to exclude tumors, tumor cells recruit vessels and growth factors from host environment to support their own expanding and metastasis. These environmental elements have been targets for novel therapies of cancers. More than 30 years ago, Folkman [1] proposed that solid tumor growth depends on vascular network formation. Since then, endeavors to intervene tumor angiogenesis have attracted worldwide attentions and exciting progresses have been made [2,3]. The vascular endothelial growth factor (VEGF), a major proangiogenic cytokine, was firstly targeted, and anti-VEGF therapies have indeed improved survival of colorectal, lung, and breast cancer patients. However, inhibition of neovascularization by anti-VEGF is not always effective in solid tumor therapy as expected [4]. Thus, new targets on tumor vascular network formation are badly required.
Notch signaling, one evolutionarily conserved pathway, is instrumental in many developmental processes by participating in cell fate determination during embryonic and postnatal stages. Hitherto, four Notch receptors (Notch1–4) and five ligands (Jagged1, Jagged2, and Delta-like ligands (Dll) 1, 3, and 4) have been identified in mammals. On ligand-receptor binding, Notch intracellular domain is cleaved by consecutive enzymatic reactions, and Notch intracellular domain subsequently translocates into the nucleus, where it interacts with the transcription factor C promoter-binding factor 1/recombination signal-binding protein J/κ (RBP-J). After that, target genes such as Hes family basic helix-loop-helix members are activated to transcribe [5].
Notch signaling is involved in the regulation of tumor behavior in multiple dimensions. Notch signaling can directly function as an oncogene or a tumor suppressor and influence tumor cell proliferation, differentiation, apoptosis, and genome instability [6,7]. On the host side, bulk evidence has shown that Notch signaling is a key regulator of vascular development in tumors. Notch signaling is critical in angiogenesis and endothelial cell fate determination both in embryonic and postnatal development [8,9]. Gene mutations in the Notch pathway give rise to embryonic lethality caused by defects in vascular remodeling [10–14]. Supported by conditional knockout strategy, we have shown that in adult mice, Notch signaling plays a critical role in the maintenance of homeostasis of normal vasculature by repressing endothelial cell proliferation [15]. It was reported recently that in tumor-bearing mice, down-regulation of Dll4, one ligand of Notch receptors, led to retarded tumor growth due to more but functionally impaired neovascularization, which imposed more severe hypoxia on tumor cells [16–19]. On the basis of these findings, the Notch signaling has been chosen as a potential intervening target for vessel-based tumor therapies.
In addition to endothelial cells, Notch signaling regulates cell fate commitment, proliferation, and apoptosis in the hematopoietic system. Therefore, universal intervening of Notch signaling influences multiple steps in cell differentiation in the immune system and immune responses [20], which is also critical for tumor behaviors. It has been demonstrated that Notch signaling determines cell fate decisions at the developmental checkpoints of T cells versus B cells [21], αβ-T cells versus γδ-T cells [22], follicular B cells versus marginal zone B cells [23], and so on. Notch signaling may also regulate immune responses by modulating dendritic cells [24], nature killer cells, and T cell proliferation [25]. More importantly, it has been proved that Dll4, which is believed to be specifically involved in the vascular system, also regulates cell fate determinations in the immune system [20]. Therefore, it would be advantageous to know the comprehensive effects of universal Notch signal inhibition in host environment for tumor growth and metastasis.
In this study, we used a mouse model in which RBP-J [23], the common transcription factor of all four Notch receptors, was conditionally inactivated. We found that tumors inoculated in the RBP-J-inactivated mice showed differential features: hepatocarcinoma, lung cancer, and osteosarcoma grew slower but melanoma grew significantly faster than inoculated in normal environment, in contrast with previous reports. All inoculated tumors had increased neovascularization and decreased perfusion as shown by up-regulated hypoxia-inducible factor (HIF) 1α expression and by increased infiltration of immunorepressive Gr1+/Mac1+ immature monocytes. Moreover, hepatocarcinoma showed increased metastasis. These results strongly suggested that the output of Notch-targeted cancer therapy might depend on cancer cell types.
Materials and Methods
Mice
Mice were maintained in a specific pathogen-free condition. The RBP-J-floxed (RBP-Jf) mice were as described [26] and were crossed with the Mx-Cre transgenic mice to get the RBP-Jf/f-MxCre and RBP-J+/f-MxCre (as controls) mice (hence, referred as RBP-J-/- and RBP-J+/-, respectively). Mice were genotyped by polymerase chain reaction [24]. Four-week-old mice were injected intraperitoneally with 300 µg/100 µl poly(I)-poly(C) (Sigma, St. Louis, MO) for four times at 2-day intervals and were then injected with the same dosage of poly (I)-poly(C) for another set of four times at 1-week intervals (eight injections in total). All animal experiments were approved by the Fourth Military Medical University.
Cell Culture
Lewis lung carcinoma (LLC), mouse S180 sarcoma (S180), mouse H22 hepatocarcinoma (H22), and mouse melanoma (B16) cell lines were gifts from Dr. C.H. Shi. All the tumor cell lines were maintained in RPMI 1640 medium supplemented with 10% fetal bovine serum, 2 mM l-glutamine, 50 IU/ml penicillin, and 50 µg/ml streptomycin sulfate.
Tumor-Bearing Mouse Models
Tumor cells (5 x 106) were injected subcutaneously into the RBP-J knockout and the control mice. Seven days after the initial inoculation, tumor growth was monitored every 2 days by measuring tumor length (L) and short (S) with a sliding caliper. Tumor size was calculated as L x S2 x 0.51.
Two weeks after the initial inoculation, weight of tumor-bearing mice was recorded before being killed. Tumors were excised and the weight was measured. Tumor weight index was calculated as the ratio of tumor weight to body weight.
FACS Analysis
Cells were collected from minced tumor tissues. After filtrating through a nylon filter, cells were resuspended with phosphate-buffered saline containing 2% fetal bovine serum and 0.05% NaN3 and were counted. Cells (3–5 x 105) were stained with PE-anti-Mac1 (M/10) and fluorescein isothiocyanate-anti-Gr1 (M815214) antibodies (both from Pharmingen, San Diego, CA) at 4°C for 30 minutes before being analyzed using a FACSCalibur (BD Immunocytometry System, San Jose, CA). Dead cells were excluded by propidium iodide gating. Data were analyzed using the CellQuest software.
Histology and Immunohistochemistry
Tissues were fixed in 4% paraformaldehyde, embedded in paraffin, sectioned at 10 µm thickness, and stained with hematoxylin and eosin by standard methods. Immunohistochemistry was performed by standard procedures, with rat antimouse CD31 (1:500 dilution; Chemicon International) or rabbit antimouse HIF1α antibody (1:200 dilution; Chemicon International, Temecula, CA) as the primary antibody. Secondary antibodies included horseradish peroxidase-conjugated goat antirat IgG or antirabbit IgG (Boster BioTec, Wuhan, China). Samples were developed using standard DAB reagents and were observed under a microscope. To evaluate the density of microvessels, microvessels were counted by different technicians based on “hot field,” which showed the most concentrated vessel regions. For the quantification, pictures were captured and then pixels were determined by Image-Pro Plus 5.1 software (MediaCybernetics Inc., Bethesda, MD).
Dorsal Air Sac Assay
Dorsal air sac assay was carried out as described [27]. In brief, subcutaneous dorsal air sac was created in mice by injecting 2.5 ml of air. Chambers were produced by covering both sides of a Millipore ring with Millipore filters (0.45 µm) and were filled with a suspension of 1 x 107 tumor cells in 0.15 ml of phosphate-buffered saline. Three days later, the chambers were implanted into the dorsal air sac. Five days after the implantation, the local skin was cut open, and tumor angiogenesis was assessed under a microscope.
Statistics
The significance of the difference between groups was statistically analyzed by SPSS 11.0 program (SPSS, Inc., Chicago, IL) using Student's t test.
Results
Differential Tumor Cell Growth in RBP-J Knockout Mice
Although it has been reported that blocking Notch signaling in tumor-bearing mice induces tumor regression owing to abortive angiogenesis, the whole scenario of the effects of Notch signal inactivation on tumor growth has not been fully investigated. To explore this, we used the RBP-J conditional deletion mouse model [26]. In the adult RBP-Jf/f-MxCre mice, injection of the α-interferon inducer poly(I)-poly(C) induced deletion of the DNA-binding domain of RBP-J. The deletion was almost complete in hematopoietic cells and endothelial cells [15,26].
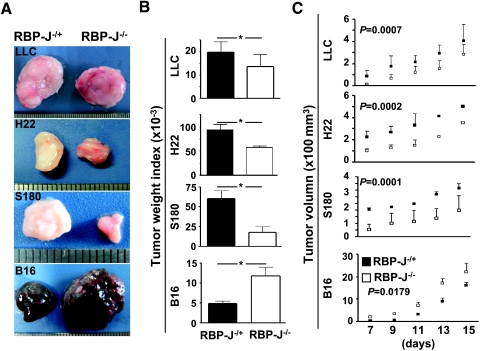
We inoculated subcutaneously four mouse tumor cell lines, including LLC, H22, S180, and B16, into the RBP-J-inactivated and the control mice. Both S180 and H22 expressed Notch4 and Dll4, whereas LLC expressed Notch2 and B16 expressed Notch1, as detected by reverse transcription-polymerase chain reaction (data not shown). On day 15 after the inoculation of 5 x 106 tumor cells, compared with tumors inoculated in the control mice, LLC, H22, and S180 tumors in the RBP-J-/- mice were significantly smaller (Figure 1A). Tumor weight index also indicated that these three tumors were significantly smaller in the RBP-J-/- mice than in the controls (Figure 1B). We monitored the dynamic increase of tumor volumes from the seventh day after the injection of tumor cells. In the case of LLC, H22, and S180, tumor volumes in the RBP-J-/- mice were significantly smaller than those in the control mice, and the differences remained even when the tumors grew larger (Figure 1C). This was in agreement with previous reports showing that interruption of Notch signaling in mice retarded tumor growth, most likely attributed to defective angiogenesis [16–19].
Figure 1.
Tumor growth in the RBP-J-/- and control mice. (A) LLC, H22, S180, and B16 tumor cells (5 x 106) were injected subcutaneously into the RBP-J-/- and RBP-J+/- mice. Tumors were dissected 15 days after the inoculation and were photographed; representative tumors were shown. (B) Tumor weight index (ratio of tumor weight-body weight) was compared on day 15 after the tumor inoculation. (C) Tumor volume. Tumor volume was monitored every 2 days from the seventh day after the inoculation, by measuring tumor length (L) and short (S) with a sliding caliper. Tumor volume = L x S2 x 0.51. Bars, means ± SD. *P < .05. n = 3 in LLC; n = 3 in S180; n = 5 in H22; and n = 5 in B16.
In contrary to LLC, H22, and S180 tumors, the size of B16 melanoma grown in the RBP-J-deleted mice was significantly larger than in the control mice (Figure 1A, lowest panel). Comparison of tumor weight index on day 15 after inoculation also indicated that the B16 tumors in the RBP-J-/- mice were significantly larger than those in the control mice (Figure 1B, lowest panel). Tumor growth curve showed that the B16 grew significantly faster in the RBP-J-/- mice than in the control mice (Figure 1C, lowest panel). This suggested that in a Notch signal-deficient host, the growth of tumors might be different, depending on tumor cell types.
Defective Angiogenesis in Tumors Grown in the RBP-J-Inactivated Mice Did Not Necessarily Lead to Tumor Regression
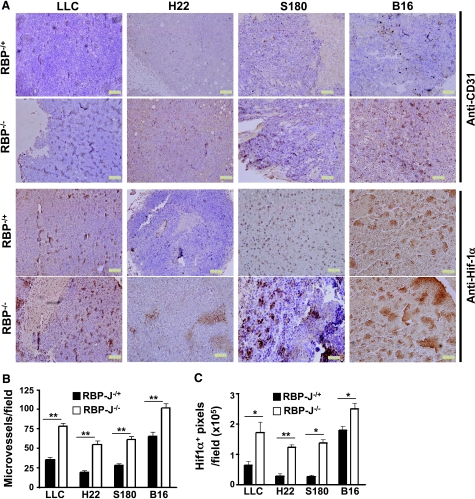
Abortive formation of neovasculature and consequent poor perfusion in solid tumors were considered as the reasons of tumor regression in the absence of Notch signaling [16–19]. We therefore examined microvessels in tumors inoculated in the RBP-J knockout and the control mice. Immunohistochemical staining of vasculature with anti-CD31 antibody showed that the microvessel density of tumors grown in the RBP-J-deleted mice increased significantly in all four types of tumors compared with the controls (Figure 2A, upper panel). Quantification of microvessel counts also indicated that the amount of microvessels in tumors grown in the RBP-J-deficient mice were significantly higher than those in tumors in the control mice (Figure 2B).
Figure 2.
Density of microvessels and hypoxia in tumor tissues. (A) Tumors from the RBP-J-/- and control mice were sectioned at 10 µm and were stained for CD31 and HIF1α by immunohistochemistry. (B) Microvessels in (A; upper panel) were counted under a microscope, and microvessel densities (microvessels per field) were compared. (C) HIF1α-positive signals in (A; lower panel) were quantified using Image-Pro Plus 5.1 and were compared for statistical significance. Bars, means ± SD. *P < .05, **P < .01; n = 5.
We next evaluated tissue hypoxia in tumors by the examination of HIF1α expression using immunohistochemistry. The expression of HIF1α was up-regulated in tumors grown in the RBP-J-deficient mice (Figure 2A, lower panel). Quantification analysis also indicated that the hypoxia areas of tumors grown in the RBP-J-deficient mice were significantly larger than those in tumors in the control mice (Figure 2C), suggesting augmented hypoxia in all four types of tumors, although they showed different growth (Figure 1). These data indicated that in the RBP-J-deleted mice, defective vascular network formation might lead to poor perfusion and tissue hypoxia, but this did not have to result in tumor regression.
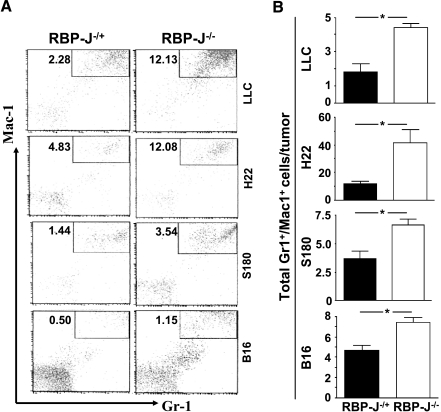
Increased Infiltration of Gr1+/Mac1+ Cells in Tumors Grown in the RBP-J-/- Mice
Notch signaling controls multiple steps of hematopoiesis in addition to angiogenesis [20]. Recent reports have shown that Gr1+/Mac1+ cells are immunorepressive, promoting solid tumor growth [28], and mediating resistance to anti-VEGF therapy [29], so we examined this population of cells in tumors inoculated in the RBP-J-mutated and the control mice. FACS analysis showed that Gr1+/Mac1+ cell infiltration was significantly higher in tumors grown in the RBP-J-deleted mice than those grown in the control mice, both in cell proportion (Figure 3A) and in absolute cell number (Figure 3B) per tumor. T cells, B cells, natural killer cells, and dendritic cells infiltrated equally in tumors grown in the RBP-J knockout and the control mice (data not shown). These results suggested that increased Gr1+/Mac1+ cell infiltration in tumors in the RBP-J-disrupted mice might not account for differential growth of tumors inoculated in these mice.
Figure 3.
Infiltration of Gr1+/Mac1+ cells in tumors grown in the RBP-J knockout and control mice. (A) FACS analysis. Tumors grown in the RBP-J-/- and control mice were dissected and were minced to obtain single-cell suspensions. FACS was carried out using PE-anti-Mac1 and fluorescein isothiocyanate-anti-Gr1. Pictures represent one set of three to five independent experiments. (B) The number of Gr1+/Mac1+ cells in tumors was calculated based on FACS shown in (A). Bars, means ± SD. *P < .05. n = 3 in LLC; n = 3 in S180; n = 5 in H22; and n = 5 in B16, respectively.
RBP-J Deletion in Hosts Increased Tumor Metastasis of H22 Hepatocarcinoma
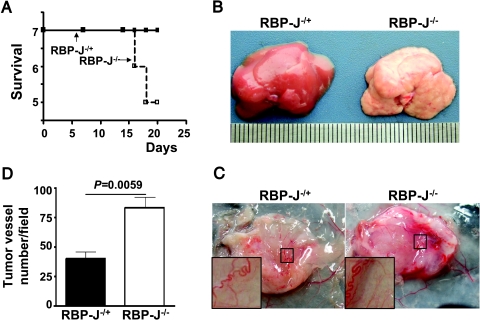
In our tumor-bearing experiments, we found that H22 hepatocarcinoma had a higher lethality when inoculated in the RBP-J-deficient mice (Figure 4A). Mouse autopsy showed that there was a higher frequency of metastases (3/7) in the liver and abdominal cavity of the RBP-J-deficient mice inoculated with H22 cells (Figure 4B). Because the density of microvessels in H22 tumors grown in the RBP-J-deficient mice was higher than that of tumors grown in the controls and abnormal microvessel structure could be a risk factor of increased tumor metastasis, we further examined typical general tumor angiogenesis in H22 tumors using the dorsal air sac analysis. As shown in Figure 4, C and D, more typical general tumor vessels formed in H22 hepatocarcinoma grown in the RBP-J-/- mice, compared with tumors grown in the control mice. These data suggested that the systemic inhibition of the Notch pathway could facilitate metastasis in some cancers.
Figure 4.
RBP-J-/- environment promoted metastasis of H22 hepatocarcinoma. (A) Survival of mice subcutaneously inoculated with H22 hepatocarcinoma cells. (B) Liver metastasis in the RBP-J-/- mice subcutaneously inoculated with H22 cells. Seven pairs of the RBP-/- and RBP+/- mice were inoculated with H22 tumor cells. Liver metastasis was examined 15 days later after tumor injection. (C) Dorsal air sac assays of tumor angiogenesis. Typical tumor vasculatures were shown in the insets. (D) Quantification of newly formed tumor vessels. Microvessels with the characteristics of tumor vasculature were counted under a microscope and were compared. Bars, means ± SD. *P < .05, n = 3.
Discussion
Antiangiogenesis has been considered as a novel strategy for the therapy for malignant solid tumors, because solid tumor growth depends on vascular network formation, as proposed by Folkman [1] more than 30 years ago. Indeed, colorectal cancers, lung cancers, and breast cancers are sensitive to Avastin [30], a monoclonal antibody against VEGF. However, other solid tumors, such as lymphoma, appear refractory to the same therapy [29]. Thus, new targets for antiangiogenic therapy for tumors are desired. Notch signaling is essential for normal angiogenesis and the maintenance of vascular homeostasis [8,9,15]. Recently, Notch signaling has been chosen as a therapeutic target for tumors because the blockade of Dll4, one of the Notch ligands, induces defective angiogenesis leading to tumor regression in many tumor cell types, including human colon cancer (HM7), human colon adenocarcinoma (Colo205), human non-small cell lung carcinoma (Calu6), human breast carcinoma (MDA-MB-435), human lung adenocarcinoma (MV-522), human fibrosarcoma (HT1080), and mouse myelomonocytic tumor (WEHI3) and rat glioma (C6) [17,18]. However, although the consequence of the Dll4 intervention is mainly restricted to the vascular system [18], Dll4 has been shown expressed in a large spectrum of cell types and functions in other systems than only in vasculature [20–26]. It is therefore essential to investigate the comprehensive effects of Notch signal blockade on tumor growth before those Notch-targeted therapies could be adopted in clinics. Because of the functional redundancy among different ligands or receptors in the Notch pathway [20], in this study, we used the mouse model that bears a conditional inactivation of RBP-J [26], the common transcription factor of all four Notch receptors in mammals.
We found that not all types of tumors adapted in this study regressed when inoculated in the RBP-J knockout mice. Indeed, several types of tumors, such as those tested in previous studies [17,18] and LLC, H22, and S180 in this study, were retarded in an environment deprived of Notch signaling. However, the B16 melanoma grew faster when inoculated in the RBP-J knockout mice. Abortive formation of neovasculature and, consequently, poor perfusion in solid tumors has been considered as reasons of tumor regression in the absence of Notch signaling [16–19]. In this study, increased angiogenesis (anti-CD31 staining) and decreased tissue perfusion (anti-HIF1α staining) were prominent in all tumors grown in the RBP-J knockout mice, suggesting that defective angiogenesis resulting from Notch signal intervention might not have to result in tumor regression in all tumor cell types.
Antitumor immune responses might be another issue that must be considered because the Notch pathway has been demonstrated as one of the major players in controlling the development and function of the immune system. In tumors grown in the RBP-J knockout mice, the most significant change was the higher amount of infiltrated Gr1+/Mac1+ cells in tumor tissues. Infiltration of Gr1+/Mac1+ cells in solid tumors was reported more than 20 years ago [31]. Recently, it was found that Gr1+/Mac1+ cells play a role to promote tumor growth and mediate refractoriness to anti-VEGF therapy [29], possibly by resulting in the down-regulated expression of MHC II [28]. In the RBP-J-/- mice, Gr1+/Mac1+ cells in peripheral lymphoid organs increased (unpublished results). However, we did not find significant difference in Gr-1+/Mac-1+ cell infiltration among different tumors inoculated in the RBP-J knockout mice, suggesting that this might not be the reason of differential growth of these tumors. However, the fact that systemically blockade of Notch pathway gives rise to a significantly increase of Gr1+/Mac1+ cells might still be an important consideration for the development of Notch-based tumor therapies.
In addition to the vascular and immune systems, tumor-bearing host might provide other signals influencing tumor growth. For example, endothelial cells could promote liver development and regeneration in addition to functions in vascular formation. This is achieved by VEGF signaling through VEGFR1, which ultimately leads to the secretion of interleukin 6 and hepatocyte growth factor by endothelial cells to support the proliferation of hepatocytes [32]. Endothelial cells invading into solid tumors might also produce cytokines and other factors that favor tumor growth. Inactivation of Notch signaling might influence this process and lead to differential growth of tumors in the RBP-J deficient mice. In this study, it is possible that these are the reasons why tumors grow slower in LLC, H22, and S180, as suggested in prior studies, and that additional, specific factors are induced only in melanomas causing increased growth. Similarly, specific factors induced only in hepatocarcinomas could induce metastasis.
Although Notch signaling was reported to be involved in metastasis of prostate cancer cell [33] and pancreas cancer cells [34] in vitro, little information in detail was obtained about tumor metastasis once the Notch pathway was blocked in tumor-bearing hosts in vivo. Tumor metastasis through blood vessels involves the disruption of local microvessels, entering the blood stream, and homing of cancer cells in the target tissues [35]. We have found that newly formed vascular networks in the absence of Notch signaling are leaky [15]. Our dorsal air sac assay showed that more general typical tumor vessels formed in H22 tumors grown in the RBP-J-/- mice, which might increase the possibility of metastasis.
Solid tumor growth and metastasis are complicated processes involving local growth environment and immune responses in addition to tumor-intrinsic factors. The Notch signaling pathway may influence all these aspects. Our findings suggested that the general blockade of Notch signaling in tumor-bearing mice could lead to defective angiogenesis in tumors, but depending on tumor cell types, general inhibition of Notch signaling might result in tumor repression, progression, or metastasis.
Acknowledgments
The authors thank Hui Wang for her critical correction of English of the manuscript.
Abbreviations
- RBP-J
recombination signal-binding protein-Jκ
- VEGF
vascular endothelial growth factor
- HIF
hypoxia-inducible factor
Footnotes
This work was supported by grants from the Natural Science Foundation (30830361, 30800454, 30425015, and 30700415) and from the Ministry of Science and Technology of China (2009CB521706).
References
- 1.Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285:1182–1186. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- 2.Folkman J. Fundamental concepts of the angiogenic process. Curr Mol Med. 2003;3:643–651. doi: 10.2174/1566524033479465. [DOI] [PubMed] [Google Scholar]
- 3.Neri D, Bicknell R. Tumour vascular targeting. Nat Rev Cancer. 2005;5:436–446. doi: 10.1038/nrc1627. [DOI] [PubMed] [Google Scholar]
- 4.Casanovas O, Hicklin DJ, Bergers G, Hanahan D. Drug resistance by evasion of antiangiogenic targeting of VEGF signaling in late-stage pancreatic islet tumors. Cancer Cell. 2005;8:299–309. doi: 10.1016/j.ccr.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 5.Kato H, Taniguchi Y, Kurooka H, Minoguchi S, Sakai T, Nomura-Okazaki S, Tamura K, Honjo T. Involvement of RBP-J in biological functions of mouse Notch1 and its derivatives. Development. 1997;124:4133–4141. doi: 10.1242/dev.124.20.4133. [DOI] [PubMed] [Google Scholar]
- 6.Alimirah F, Panchanathany R, Davisy FJ, Cheny J, Choubey D. Restoration of p53 expression in human cancer cell lines upregulates the expression of Notch1: implications for cancer cell fate determination after genotoxic stress. Neoplasia. 2007;9:427–434. doi: 10.1593/neo.07211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baia GS, Stifani S, Kimura ET, McDermott MW, Pieper RO, Lal A. Notch activation is associated with tetraploidy and enhanced chromosomal instability in meningiomas. Neoplasia. 2008;10:604–612. doi: 10.1593/neo.08356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hofmann JJ, Iruela-Arispe ML. Notch signaling in blood vessels: who is talking to whom about what? Circ Res. 2007;100:1556–1568. doi: 10.1161/01.RES.0000266408.42939.e4. [DOI] [PubMed] [Google Scholar]
- 9.Gridley T. Notch signaling in vascular development and physiology. Development. 2007;134:2709–2718. doi: 10.1242/dev.004184. [DOI] [PubMed] [Google Scholar]
- 10.Krebs LT, Xue Y, Norton CR, Shutter JR, Maguire M, Sundberg JP, Gallahan D, Closson V, Kitajewski J, Callahan R, et al. Notch signaling is essential for vascular morphogenesis in mice. Genes Dev. 2000;14:1343–1352. [PMC free article] [PubMed] [Google Scholar]
- 11.Leong KG, Hu X, Li L, Noseda M, Larrivee B, Hull C, Hood L, Wong F, Karsan A. Activated Notch4 inhibits angiogenesis: role of beta 1-integrin activation. Mol Cell Biol. 2002;22:2830–2841. doi: 10.1128/MCB.22.8.2830-2841.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Uyttendaele H, Ho J, Rossant J, Kitajewski J. Vascular patterning defects associated with expression of activated Notch4 in embryonic endothelium. Proc Natl Acad Sci USA. 2001;98:5643–5648. doi: 10.1073/pnas.091584598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fischer A, Schumacher N, Maier M, Sendtner M, Gessler M. The Notch target genes Hey1 and Hey2 are required for embryonic vascular development. Genes Dev. 2004;18:901–911. doi: 10.1101/gad.291004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krebs LT, Shutter JR, Tanigaki K, Honjo T, Stark KL, Gridley T. Haploinsufficient lethality and formation of arteriovenous malformations in Notch pathway mutants. Genes Dev. 2004;18:2469–2473. doi: 10.1101/gad.1239204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dou GR, Wang YC, Hu XB, Hou LH, Wang CM, Xu JF, Wang YS, Liang YM, Yao LB, Yang AG, et al. RBP-J, the transcription factor downstream of Notch receptors, is essential for the maintenance of vascular homeostasis in adult mice. Faseb J. 2008;22:1606–1617. doi: 10.1096/fj.07-9998com. [DOI] [PubMed] [Google Scholar]
- 16.Li JL, Sainson RC, Shi W, Leek R, Harrington LS, Preusser M, Biswas S, Turley H, Heikamp E, Hainfellner JA, et al. Delta-like 4 Notch ligand regulates tumor angiogenesis, improves tumor vascular function, and promotes tumor growth in vivo. Cancer Res. 2007;67:11244–11253. doi: 10.1158/0008-5472.CAN-07-0969. [DOI] [PubMed] [Google Scholar]
- 17.Noguera-Troise I, Daly C, Papadopoulos NJ, Coetzee S, Boland P, Gale NW, Lin HC, Yancopoulos GD, Thurston G. Blockade of Dll4 inhibits tumour growth by promoting non-productive angiogenesis. Nature. 2006;444:1032–1037. doi: 10.1038/nature05355. [DOI] [PubMed] [Google Scholar]
- 18.Ridgway J, Zhang G, Wu Y, Stawicki S, Liang WC, Chanthery Y, Kowalski J, Watts RJ, Callahan C, Kasman I, et al. Inhibition of Dll4 signalling inhibits tumour growth by deregulating angiogenesis. Nature. 2006;444:1083–1087. doi: 10.1038/nature05313. [DOI] [PubMed] [Google Scholar]
- 19.Scehnet JS, Jiang W, Kumar SR, Krasnoperov V, Trindade A, Benedito R, Djokovic D, Borges C, Ley EJ, Duarte A, et al. Inhibition of Dll4-mediated signaling induces proliferation of immature vessels and results in poor tissue perfusion. Blood. 2007;109:4753–4760. doi: 10.1182/blood-2006-12-063933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maillard I, Fang T, Pear WS. Regulation of lymphoid development, differentiation, and function by the Notch pathway. Annu Rev Immunol. 2005;23:945–974. doi: 10.1146/annurev.immunol.23.021704.115747. [DOI] [PubMed] [Google Scholar]
- 21.Radtke F, Wilson A, Stark G, Bauer M, van Meerwijk J, MacDonald HR, Aguet M. Deficient T cell fate specification in mice with an induced inactivation of Notch1. Immunity. 1999;10:547–558. doi: 10.1016/s1074-7613(00)80054-0. [DOI] [PubMed] [Google Scholar]
- 22.Tanigaki K, Tsuji M, Yamamoto N, Han H, Tsukada J, Inoue H, Kubo M, Honjo T. Regulation of alphabeta/gammadelta T cell lineage commitment and peripheral T cell responses byNotch/RBP-J signaling. Immunity. 2004;20:611–622. doi: 10.1016/s1074-7613(04)00109-8. [DOI] [PubMed] [Google Scholar]
- 23.Tanigaki K, Han H, Yamamoto N, Tashiro K, Ikegawa M, Kuroda K, Suzuki A, Nakano T, Honjo T. Notch-RBP-J signaling is involved in cell fate determination of marginal zone B cells. Nat Immunol. 2002;3:443–450. doi: 10.1038/ni793. [DOI] [PubMed] [Google Scholar]
- 24.Caton ML, Smith-Raska MR, Reizis B. Notch-RBP-J signaling controls the homeostasis of CD8- dendritic cells in the spleen. J Exp Med. 2007;204:1653–1664. doi: 10.1084/jem.20062648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rolink AG, Balciunaite G, Demoliere C, Ceredig R. The potential involvement of Notch signaling in NK cell development. Immunol Lett. 2006;107:50–57. doi: 10.1016/j.imlet.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 26.Han H, Tanigaki K, Yamamoto N, Kuroda K, Yoshimoto M, Nakahata T, Ikuta K, Honjo T. Inducible gene knockout of transcription factor recombination signal binding protein-J reveals its essential role in T versus B lineage decision. Int Immunol. 2002;14:637–645. doi: 10.1093/intimm/dxf030. [DOI] [PubMed] [Google Scholar]
- 27.Nishikawa T, Akiyama N, Kunimasa K, Oikawa T, Ishizuka M, Tsujimoto M, Natori S. Inhibition of in vivo angiogenesis by N-beta-alanyl-5-S-glutathionyl-3,4-dihydroxyphenylalanine. Eur J Pharmacol. 2006;539:151–157. doi: 10.1016/j.ejphar.2006.03.084. [DOI] [PubMed] [Google Scholar]
- 28.Yang L, DeBusk LM, Fukuda K, Fingleton B, Green-Jarvis B, Shyr Y, Matrisian LM, Carbone DP, Lin PC. Expansion of myeloid immune suppressor Gr+CD11b+ cells in tumor-bearing host directly promotes tumor angiogenesis. Cancer Cell. 2004;6:409–421. doi: 10.1016/j.ccr.2004.08.031. [DOI] [PubMed] [Google Scholar]
- 29.Shojaei F, Wu X, Malik AK, Zhong C, Baldwin ME, Schanz S, Fuh G, Gerber HP, Ferrara N. Tumor refractoriness to anti-VEGF treatment is mediated by CD11b+Gr1+ myeloid cells. Nat Biotechnol. 2007;25:911–920. doi: 10.1038/nbt1323. [DOI] [PubMed] [Google Scholar]
- 30.Jain RK, Duda DG, Clark JW, Loeffler JS. Lessons from phase III clinical trials on anti-VEGF therapy for cancer. Nat Clin Pract Oncol. 2006;3:24–40. doi: 10.1038/ncponc0403. [DOI] [PubMed] [Google Scholar]
- 31.Schmid MC, Varner JA. Myeloid cell trafficking and tumor angiogenesis. Cancer Lett. 2007;250:1–8. doi: 10.1016/j.canlet.2006.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.LeCouter J, Moritz DR, Li B, Phillips GL, Liang XH, Gerber HP, Hillan KJ, Ferrara N. Angiogenesis-independent endothelial protection of liver: role of VEGFR-1. Science. 2003;299:890–893. doi: 10.1126/science.1079562. [DOI] [PubMed] [Google Scholar]
- 33.Santagata S, Demichelis F, Riva A, Varambally S, Hofer MD, Kutok JL, Kim R, Tang J, Montie JE, Chinnaiyan AM, et al. JAGGED1 expression is associated with prostate cancer metastasis and recurrence. Cancer Res. 2004;64:6854–6857. doi: 10.1158/0008-5472.CAN-04-2500. [DOI] [PubMed] [Google Scholar]
- 34.Wang Z, Banerjee S, Li Y, Rahman KM, Zhang Y, Sarkar FH. Down-regulation of notch-1 inhibits invasion by inactivation of nuclear factor-kappaB, vascular endothelial growth factor, and matrix metalloproteinase-9 in pancreatic cancer cells. Cancer Res. 2006;66:2778–2784. doi: 10.1158/0008-5472.CAN-05-4281. [DOI] [PubMed] [Google Scholar]
- 35.Steeg PS. Tumor metastasis: mechanistic insights and clinical challenges. Nat Med. 2006;12:895–904. doi: 10.1038/nm1469. [DOI] [PubMed] [Google Scholar]