Abstract
HhAntag691 (GDC-0449), a low-molecular weight inhibitor of the tumor-promoting hedgehog (Hh) signaling pathway, has been used to treat medulloblastoma in animal models and has recently entered clinical trials for a variety of solid tumors. Here, we show that HhAntag691 inhibits multiple ATP-binding cassette (ABC) transporters. ATP-binding cassette transporters are within a family of membrane proteins, the overexpression of which is associated with multidrug resistance, a major impediment to successful cancer treatment. HhAntag691 is a potent inhibitor of two ABC transporters, ABCG2/BCRP and ABCB1/Pgp, and is a mild inhibitor of ABCC1/MRP1. In ABCG2-overexpressing HEK293 cells, HhAntag691 increased retention of the fluorescent ABCG2 substrate BODIPY-prazosin and resensitized these cells to mitoxantrone, an antineoplastic ABCG2 substrate. In Madin-Darby canine kidney II cells engineered to overexpress Pgp or MRP1, HhAntag691 increased the retention of calcein-AM and resensitized them to colchicine. HhAntag691 also resensitized human non-small cell lung carcinoma cells NCI-H460/par and NCI-H460/MX20, which overexpress ABCG2 in response to mitoxantrone, to mitoxantrone, and to topotecan or SN-38. The IC50 values of HhAntag691 for inhibition of ABCG2 and Pgp were ∼1.4 and ∼3.0 µM, respectively. Because ABC transporters are highly expressed at the blood-brain barrier and on many tumor cells, they contribute significantly to treatment failure of many types of cancer, particularly of those within the neuraxis. In addition to its effect on Hh signaling, the ability of HhAntag691 and related compounds to inhibit two key ABC transporters could contribute to their effectiveness in treating malignancies.
Introduction
HhAntag691 (GDC-0449) is a low-molecular weight hedgehog (Hh) pathway inhibitor discovered in a screen searching for compounds targeting the Hh pathway [1]. The Hh pathway is critical for cell growth and differentiation during embryonic development and is also aberrantly activated in a variety of cancers [2]. HhAntag691 is a potent Hh pathway inhibitor that blocks the function of Smoothened and has been used successfully to eliminate medulloblastoma in both Ptc1+/-p53-/- and Cxcr6 mutant mice [1,3]. HhAntag691 is highly effective, with treatment of only 4 days providing complete tumor regression. Thus, HhAntag691 is a promising anticancer drug and has entered phase 1 clinical trials along with other Hh pathway inhibitors such as cyclopamine [2,4]. Cyclopamine, a steroidal alkaloid and less potent Hh inhibitor, also targets Smoothened and has been found effective in treating a variety of cancers in tissue culture and animal models. Cyclopamine enhances the antiproliferative effect of epidermal growth factor receptor (EGFR) inhibitors in pancreatic cancer cells [5], depletes glioblastoma stem-like cancer cells in vitro [6], and inhibits the growth of prostate cancer and medulloblastoma cells [5,7].
The family of ATP-binding cassette (ABC) proteins is another important antitumor target [8]. Overexpression of ABC proteins is associated with multidrug resistance (MDR), a major obstacle for successful treatment. ATP-binding cassette transporters use the energy of ATP hydrolysis to export substrates out of cells, thereby reducing their effective intracellular concentration. The expression of ABC transporters is one mechanism by which cancer cells develop resistance to chemotherapy. Cancer stem-like cells express ABC transporters that may contribute to their resistance to therapy and ability to propagate cancer [9–12]. The Hh pathway has also been found to be up-regulated in cancer stem-like cells [13,14], to regulate the expression of multiple ABC transporters including ABCG2/BCRP and ABCB1/Pgp [14], and to induce ABC transporter-dependent chemoresistance. Agents that simultaneously inhibit Hh signaling and MDR could greatly improve the efficacy of cancer treatment by targeting cancer stem-like cells and increasing the intracellular concentration of chemotherapeutic agents more broadly throughout tumors.
We previously reported that HhAntag691 enhances the bioluminescence imaging (BLI) readout in cells expressing firefly luciferase (fLuc), possibly by inhibiting the export of d-luciferin, a substrate of ABCG2 [15]. In this report, we show that HhAntag691 is indeed a potent inhibitor of both ABCG2 and Pgp and a mild inhibitor of ABCC1/MRP1.
Materials and Methods
Reagents
d-Luciferin sodium salt was obtained from Gold Biotechnology, Inc. (St. Louis, MO). HhAntag691 was a gift from Infinity Pharmaceuticals, Inc. (Cambridge, MA). Verapamil (VP), indomethacin, colchicine, mitoxantrone, topotecan, SN-38, and calcein-AM were purchased from Sigma Chemical Company (St Louis, MO). BODIPY-prazosin was obtained from Invitrogen (Carlsbad, CA). Fumitremorgin C (FTC) was a kind gift of Dr. S. Bates (National Cancer Institute, Frederick, MD). All compounds were prepared in DMSO for in vitro experiments.
Construction of Reporter Plasmid
A CMV promoter-driven fLuc reporter construct carrying a hygromycin B selection marker was generated from pGL4.16[luc2cp/Hygro] from Promega (Madison, WI). The vector was linearized with NheI, and a CMV promoter was inserted. The CMV promoter was first obtained by polymerase chain reaction (PCR) from pCDNA3.1-CMV-trireporter [16], and an additional NheI site was introduced by the 5′ primer. The CMV promoter PCR fragment was then cloned into pCRII-TOPO vector using the TOPO TA cloning kit (Invitrogen) and cut-out with NheI (PCR primers used: 5′: 5′-GCTAGCGAAGAATCTGCTTAGGGTTAGGC-3′, 3′: 5′-CTCGAGGCTAGCCAGCTTGGGTCTC-3′). The DNA fragment was purified with a QIAGEN gel extraction kit (Qiagen, Inc., Valencia, CA) and was then ligated. CMV- luc2CP/Hygro plasmids were prepared from transformants from the ligation product, and the orientation of the promoter inserted was confirmed by NruI and BglII double digestion.
Cell Lines and Transfection
Madin-Darby canine kidney II (MDCKII) cells were cultured in Dulbecco's modified Eagle's medium (Invitrogen) supplemented with 10% FBS, and Pgp, MRP1, or ABCG2/MRP2-overexpressing MDCKII cells [17] were maintained in medium containing 0.8 mg/ml G418. HEK293 cells were cultured in minimum essential medium (Invitrogen) supplemented with 10% FBS, and HEK293 cells were stably transfected with ABCG2-expressing construct, maintained in medium containing 1 mg/ml G418 [18]. Firefly luciferase-expressing HEK293 cells were established by transient transfection with CMV-luc2CP/Hygro, after selection in 50 µg/ml hygromycin B. Transient transfection was performed with FuGENE6 transfection reagent (Roche Pharmaceuticals, Nutly, NJ) according to the manufacturer's instructions. NCI-H460 human non-small cell lung carcinoma cells (National Cancer Institute) and NCI-H460/MX20 with ABCG2 overexpression induced by mitoxantrone were established and characterized as described previously [19] and were maintained in RPMI 1640 medium supplemented with 10% FBS and penicillin and streptomycin. All cultures were maintained at 37°C in a humidified 5% CO2/95% air incubator.
Calcein-AM Uptake Assay
MDCKII cells were plated into 24-well plates at a density of 3 x 105 cells per well and were allowed to attach. Medium was then changed to that containing different drugs (50 µM VP, 50 µM indomethacin, or 20 µM HhAntag691) in DMSO or DMSO alone as control, and nonfluorescent calcein-AM was added to a final concentration of 1.0 µM and incubated at 37°C for 2 hours. Cells were then washed twice with Ca2+, Mg2+-containing Hank's balanced salt solution buffer and lysed by shaking in 0.01% Triton X-100 in PBS buffer for 1 hour at room temperature or overnight at 4°C. The lysate was then transferred into 96-well plates, and the fluorescence signal caused by the cell-derived calcein was quantified spectrophotometrically with a SpectraMax M5 Multi-Detection Reader (Molecular Devices Corp., Sunnyvale, CA) using an excitation wavelength of 495 nm and an emission wavelength of 515 nm [20,21]. All manipulations were performed in the dark. All readings are expressed as mean ± SEM normalized to the control.
BODIPY-Prazosin Uptake Flow Cytometry
ABCG2-overexpressing HEK293 cells were grown in six-well plates until 70% to 80% confluent. Medium was changed to that containing 50 µM VP, 5 µM FTC, and 20 µM HhAntag691 or DMSO only as control. BODIPY-prazosin [18] was added to a final concentration of 0.25 µM. Cells were incubated at 37°C for 2 hours, washed with PBS, and harvested. All cells harvested, including those in the PBS washout, were combined, washed with PBS, and resuspended in ice-cold PBS. Analyses were performed with FACScan (Becton Dickinson, Fullerton, CA) with an excitation wavelength of 488 nm and an emission wavelength of 530 nm. Ten thousand events were counted per sample. The resultant histograms were analyzed with CellQuest software (Becton Dickinson).
Cell Viability Assay
Cell viability was assessed with either an MTT assay [22] or an XTT assay [23]. When MTT assay was performed, an MTT reagent was added to each well to a final concentration of 150 µg/ml, and the cells were incubated for 1 to 2 hours at 37°C. The medium was then replaced with DMSO to dissolve the reaction product. Absorbance at 570 nm was quantified using a spectra MAX 340pc plate reader (Molecular Devices). For the XTT assay, 1 mg/ml XTT (Polysciences, Warrington, PA) was mixed with 0.025 mM PMS (Sigma), and 50 µl of the mixture was added to each well and incubated for 4 hours at 37°C. After the plates were mixed on a plate shaker, absorbance at 450 nm was measured. All results were normalized to a percentage of absorbance obtained in controls.
Bioluminescence Imaging
Cells were imaged in a medium containing 50 µg/ml D-luciferin and specific drugs, as indicated. Bioluminescence imaging was performed with the IVIS 200 small animal imaging system (Xenogen Corp., Alameda, CA). Acquisition times varied depending on signal intensity.
Data Analysis
LivingImage (Xenogen Corp.) and IGOR (Wavemetrics, Lake Oswego, OR) image analysis software were used to superimpose and analyze the corresponding grayscale photographs and false-color BLI images. Light intensities of regions of interest were expressed as total flux (photons/sec). The IC50 values of HhAntag691 as an inhibitor of ABCG2 and Pgp were calculated using GraphPad Prism version 4.0 for Windows (GraphPad Software, San Diego, CA), variable-slope logistic nonlinear regression analysis. Data are presented as mean ± SEM, n = 3.
Results
HhAntag691 Is a Potent Inhibitor of ABCG2
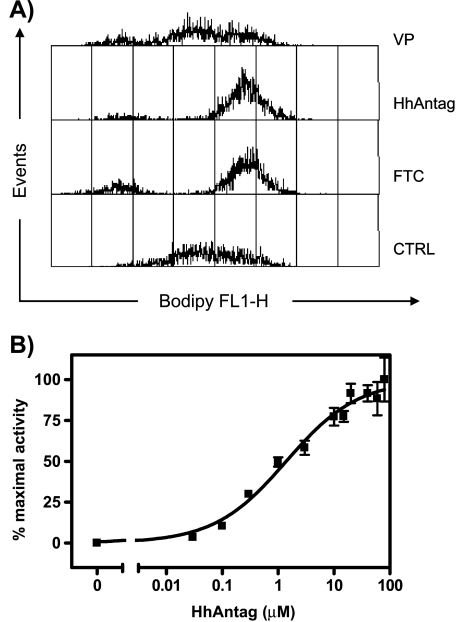
To test the idea that HhAntag691 is an inhibitor of ABCG2, we first used an established fluorescent dye uptake assay using BODIPY-prazosin, a fluorescent ABCG2 substrate. HEK293 cells overexpressing ABCG2 [18] were incubated with a medium containing BODIPY-prazosin with or without HhAntag691 or other ABC transporter inhibitor. Flow cytometry was used to measure the fluorescence retention within the cells. As shown in Figure 1A, compared to controls, HhAntag691 caused a shift of the cell population to higher fluorescence intensity, similar to that induced by FTC, a specific and potent ABCG2 inhibitor [24]. Verapamil, a potent Pgp inhibitor that does not inhibit ABCG2 [8], had no effect. Fumitremorgin C and HhAntag691 caused 80% and 90% of the cell population, respectively, to shift to higher fluorescence intensity, consistent with potent ABCG2 inhibition by HhAntag691.
Figure 1.
(A) BODIPY-prazosin uptake assay indicates that HhAntag691 is a potent ABCG2/BCRP inhibitor. HEK293 cells overexpressing ABCG2 were incubated in medium containing 50 µM VP, 5 µM FTC, 20 µM HhAntag691, or no drug, then BODIPY-prazosin was added to a final concentration of 0.25 µM, and incubated at 37°C for 2 hours. Cells were then harvested, and subjected to flow cytometry (excitation wavelength, 488 nm; emission wavelength, 530 nm). Data were analyzed with CellQuest software. (B) HhAntag691 causes a dose-dependent increase of bioluminescence signal in HEK293/ABCG2 cells expressing fLuc. Cells were imaged in medium containing 50 µg/mL D-luciferin and increasing concentrations of HhAntag691, and bioluminescence signal was quantified. Mean ± SEM, n = 3.
We exploited our recent discovery that D-luciferin, the substrate of fLuc, is also a substrate of ABCG2 [15], and evaluated the effect of HhAntag691 on the BLI signal in ABCG2-expressing cells using D-luciferin as the substrate. FLuc-expressing HEK293/ABCG2 cells were used for this test. The BLI signal was enhanced by HhAntag691 in a dose-dependent manner (Figure 1B). Owing to the dynamic change of BLI signal over time [25], data obtained at 40 minutes after imaging commencement were chosen arbitrarily for analysis, and the IC50 of HhAntag691 as an ABCG2 inhibitor was calculated to be ∼1.4 µM using variable-slope logistic nonlinear regression analysis.
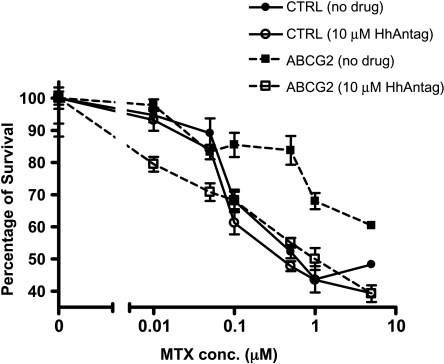
ABCG2 inhibition by HhAntag691 was further tested in a functional assay that examines the ability of compounds to reverse ABCG2-induced drug resistance. Mitoxantrone, an anthracycline antineoplastic agent and ABCG2 substrate, was chosen for this test [26]. Both HEK293 cells engineered to overexpress ABCG2 and control cells carrying an empty vector were incubated for 3 days in medium containing increasing concentrations of mitoxantrone. As expected, the overexpression of ABCG2 protected cells from mitoxantrone, and the ABCG2-overexpressing cells survived better than the control cells at mitoxantrone concentrations ≥0.1 µM. However, the addition of 10 µM HhAntag691 reversed the protection and reduced the survival rate of the ABCG2-expressing cells to about the same level as the control cells (Figure 2). At 5 µM mitoxantrone, 10 µM HhAntag691 decreased the survival rate from ∼60% in control cells to ∼39%. In contrast, HhAntag691 had no effect on the sensitivity of the control parent HEK293 cells to mitoxantrone. These results confirm that HhAntag691 is indeed an ABCG2 inhibitor and can increase the effective intracellular concentration of mitoxantrone, another ABCG2 substrate, through blocking its export.
Figure 2.
HhAntag691 resensitizes ABCG2-overexpressing HEK293 cells to mitoxantrone treatment. Cells were plated at a density of 1 x 105 cells per well in a 24-well plate and were allowed to attach before incubating in medium containing HhAntag691 and/or mitoxantrone for 3 days. Cell viability was assessed with the MTT assay and expressed as percentage of control. Mean ± SEM, n = 3.
HhAntag691 Inhibits Pgp and MRP1
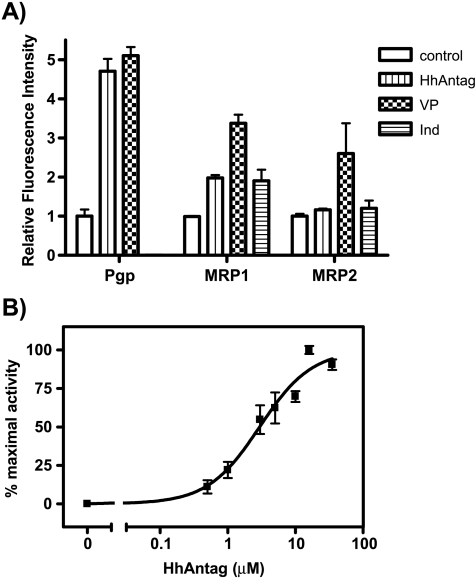
To test if HhAntag691 can also inhibit other ABC transporters (Pgp, MRP1, MRP2) [8], we performed a dye uptake assay using calcein-AM, a general substrate of ABC transporters [20,21]. MDCKII cells overexpressing either Pgp, MRP1, or MRP2 [17] were incubated in medium containing calcein-AM with or without HhAntag691. As shown in Figure 3A, in cells overexpressing Pgp, 20 µM HhAntag691 caused about the same level of fluorescence retention as 50 µM verapamil, a potent inhibitor of Pgp, indicating that HhAntag691 is also a potent inhibitor of Pgp. HhAntag691 also modestly inhibited MRP1 activity and had no effect on cells expressing MRP2. The IC50 of HhAntag691 against Pgp was calculated to be ∼3.0 µM, based on the calcein-AM dye uptake assay using MDCKII cells overexpressing Pgp (Figure 3B).
Figure 3.
(A) Fluorescent dye uptake assay indicates that HhAntag691 inhibits Pgp and MRP1 but not MRP2. MDCKII cells overexpressing Pgp, MRP1, or MRP2 were incubated in medium containing no drug or different ABC transporter inhibitors (50 µM VP, 50 µM indomethacin, or 20 µM HhAntag691), then calcein-AM was added to a final concentration of 1.0 µM, and incubated at 37°C for 1 hour. Cells were then washed and lysed, and calcein fluorescence was determined (excitation wavelength, 495 nm; emission wavelength, 515 nm). All readings are normalized to control when no drug was added. (B) HhAntag691 causes a dose-dependent increase of fluorescent signal retention of calcein-AM in MDCKII/Pgp cells. Cells were incubated at 37°C in medium containing 1 µM calcein-AM and increasing concentrations of HhAntag691 for 2 hours and were then washed and lysed, and fluorescent signal was quantified (excitation wavelength, 495 nm; emission wavelength, 520 nm). Mean ± SEM, n = 3.
HhAntag691 Resensitizes Cells to Colchicine, a Substrate of Pgp and MRP1
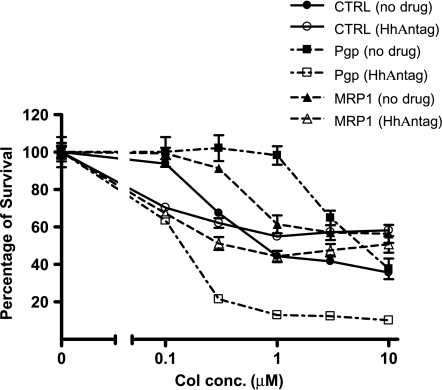
As for the mitoxantrone, a resensitization analysis was performed using colchicine, a tricyclic alkaloid that is a substrate for both Pgp and MRP1 [27–29]. Pgp-overexpressing MDCKII cells and control parent cells were incubated for 3 days in medium containing increasing concentrations of colchicine ± 10 µM HhAntag691. Pgp expression protected MDCKII cells from colchicine, and HhAntag691 (10 µM) resensitized the cells to colchicine as expected for a Pgp inhibitor (Figure 4). HhAntag691 also resensitized MDCKII cells overexpressing MRP1 (Figure 4).
Figure 4.
HhAntag691, 10 µM, resensitizes MDCKII/Pgp cells and MDCKII/MRP1 cells to colchicine treatment. Cells were plated at a density of 2 x 104 cells per well in a 96-well plate and were allowed to attach before incubating in medium containing colchicine in the presence or absence of 10 µM HhAntag691 for 3 days. Cell viability was assessed with the XTT assay and was expressed as percentage of the control cells with no drug added. Mean ± SEM, n = 3.
HhAntag691 Reverses the Resistance of NCI-H460 Human Non-Small Cell Lung Carcinoma Cells to Cytotoxic Agents That Are Substrates of ABCG2
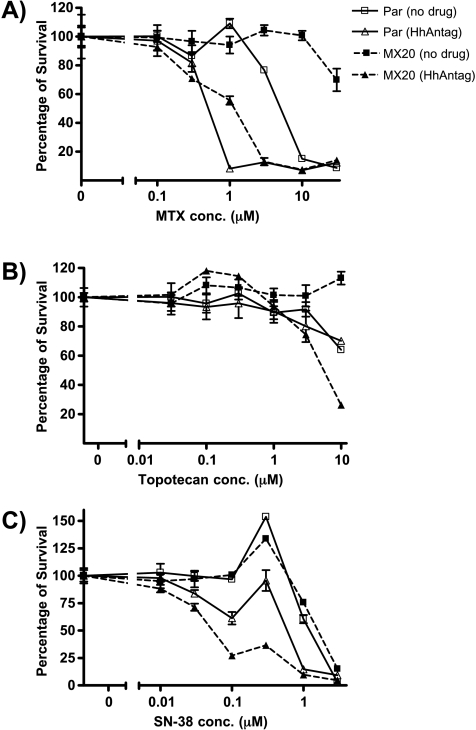
Having proved in cells genetically engineered to overexpress ABC transporters that HhAntag691 is a potent inhibitor of ABCG2 and Pgp, we validated the ability of this compound to reverse the drug resistance in a natural cell line, NCI-H460 human non-small cell lung carcinoma cells [19]. Parent NCI-H460 cells and NCI-H460/MX20 cells with mitoxantrone-induced overexpression of ABCG2 were treated with mitoxantrone and two additional ABCG2 substrates/chemotherapeutic agents, topotecan [30] and SN-38 [31,32], in the presence or absence of 10 µM HhAntag691 for 3 days before their viabilities were assessed using the XTT assay. HhAntag691 reversed the resistance of both the parent NCI-H460 cells and the NCI-H460/MX20 cells to mitoxantrone (Figure 5A) and SN-38 (Figure 5C). HhAntag691 similarly sensitized NCI-H460/MX20 cells to topotecan (Figure 5B). The extent of resensitization in NCI-H460/MX20 cells is more than that in NCI-H460/par cells, consistent with the level of ABCG2 expression in those lines, as characterized previously by immunoblot analysis [19]. For all three drugs tested, NCI-H460/MX20 cells survived better than the parent line owing to the overexpression of ABCG2, but the addition of 10 µM HhAntag691 nullified the protection conferred.
Figure 5.
HhAntag691 reverses the drug resistance of NCI-H460 human non small-lung carcinoma cells to the treatment of ABCG2 substrate anticancer drugs. Mitoxantrone induced ABCG2-overexpressing NCI-H460/MX20, and the parent cells were plated at a density of 1 x 104 cells per well in a 96-well plate and were allowed to attach before being incubated in medium containing mitoxantrone (A), topotecan (B), or SN-38 (C) in the presence or absence of 10 µM HhAntag691 for 3 days. Cell viability was assessed with the XTT assay and was expressed as percentage of control. Mean ± SEM, n = 3.
Discussion
We show that HhAntag691, a promising anticancer drug that targets the tumor-promoting Hh pathway [1], also inhibits ABCG2, Pgp, and MRP1—important ABC transporters associated with MDR. Our findings raise the possibility that the antitumor effects of HhAntag691 might be related, in part, to the ability of this compound to inhibit those transporters. As a potent inhibitor of two ABC transporters that are highly expressed at the blood-brain barrier [33], HhAntag691 should promote passage of certain anticancer drugs across that barrier to ensure effective concentrations within the neuraxis. That HhAntag691 penetrates the blood-brain barrier well was one of the reasons it was selected to treat brain tumors [1].
Besides HhAntag691, there are other effective anticancer agents that are also potent inhibitors of ABC transporters, including the tyrosine kinase inhibitors of the EGFR gefitinib [34–36], imatinib [37], EKI-785, and erlotinib [38]. The possibility has been raised that modulation of MDR may account for an element of the clinical effectiveness of those agents [39,40]. Many antiretroviral drugs, such as lopinavir, nelfinavir, and delavirdine, are also inhibitors of ABCG2, and it is believed that inhibition of this transporter may have a salutary effect on the biodistribution of these compounds [41]. ATP-binding cassette transporters may interact with certain signaling pathways, providing another mechanism to explain why so many effective anticancer drugs are also potent inhibitors of ABCG2. For example, Hh pathway activation induces chemoresistance in an ABC transporter-dependent manner [14]. Targeting the EGFR and Hh pathways concurrently reduced the stem cell-enriched side population, which is characterized by a high expression of ABCG2 [10,13,42]. Several previous studies have found that phosphorylation can regulate the function of ABCG2 through affecting its cell surface expression and that EGF treatment can increase its cell surface expression through the PI3K-Akt signaling pathway or the MAP kinase cascade [43–46]. That may explain, in part, why many tyrosine kinase inhibitors also inhibit ABCG2. HhAntag691 may inhibit ABCG2 function through a similar mechanism, but additional experiments are needed to test if this is indeed the case. HhAntag691 inhibits both the Hh pathway and ABC transporters, providing two mechanisms to account for its effectiveness.
The fact that many effective drugs are also potent ABCG2 inhibitors suggests that ABCG2 inhibition contributes to their efficacy and supports the idea of combining ABCG2 inhibitors with other therapeutics to improve outcome. That is particularly true in the case of antineoplastic agents, so many of which are known substrates of MDR pumps, especially ABCG2 [8]. ABCG2 inhibition has not been evaluated extensively as an adjunct to standard cancer chemotherapy, and previous trials evaluating Pgp inhibitors may have been flawed [47]. HhAntag691, which belongs to a new class of anticancer drugs just entering clinical trials, may act not only through inhibition of Hh but also through inhibition of ABCG2. Others have postulated that targeting Hh might be one way to overcome MDR [14]—and our data strongly support that contention. We believe that recognition of this dual action of HhAntag691 (and likely of other inhibitors of Hh) might make compounds of this class uniquely useful to treat CNS malignancies, where MDR pumps impede access of chemotherapy to tumor.
Footnotes
This work was supported by National Institutes of Health grants CA92871 (M.P.) and NS32148 and NS43987 (J.L.) and by the Maryland Stem Cell Research Fund (M.P. and J.L.).
References
- 1.Romer JT, Kimura H, Magdaleno S, Sasai K, Fuller C, Baines H, Connelly M, Stewart CF, Gould S, Rubin LL, et al. Suppression of the Shh pathway using a small molecule inhibitor eliminates medulloblastoma in Ptc1(+/-)p53(-/-) mice. Cancer Cell. 2004;6:229–240. doi: 10.1016/j.ccr.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 2.Chari NS, McDonnell TJ. The sonic hedgehog signaling network in development and neoplasia. Adv Anat Pathol. 2007;14:344–352. doi: 10.1097/PAP.0b013e3180ca8a1d. [DOI] [PubMed] [Google Scholar]
- 3.Sasai K, Romer JT, Kimura H, Eberhart DE, Rice DS, Curran T. Medulloblastomas derived from Cxcr6 mutant mice respond to treatment with a smoothened inhibitor. Cancer Res. 2007;67:3871–3877. doi: 10.1158/0008-5472.CAN-07-0493. [DOI] [PubMed] [Google Scholar]
- 4.Tabs S, Avci O. Induction of the differentiation and apoptosis of tumor cells in vivo with efficiency and selectivity. Eur J Dermatol. 2004;14:96–102. [PubMed] [Google Scholar]
- 5.Mimeault M, Moore E, Moniaux N, Henichart JP, Depreux P, Lin MF, Batra SK. Cytotoxic effects induced by a combination of cyclopamine and gefitinib, the selective hedgehog and epidermal growth factor receptor signaling inhibitors, in prostate cancer cells. Int J Cancer. 2006;118:1022–1031. doi: 10.1002/ijc.21440. [DOI] [PubMed] [Google Scholar]
- 6.Bar EE, Chaudhry A, Lin A, Fan X, Schreck K, Matsui W, Piccirillo S, Vescovi AL, DiMeco F, Olivi A, et al. Cyclopamine-mediated hedgehog pathway inhibition depletes stem-like cancer cells in glioblastoma. Stem Cells. 2007;25:2524–2533. doi: 10.1634/stemcells.2007-0166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berman DM, Karhadkar SS, Hallahan AR, Pritchard JI, Eberhart CG, Watkins DN, Chen JK, Cooper MK, Taipale J, Olson JM, et al. Medulloblastoma growth inhibition by hedgehog pathway blockade. Science. 2002;297:1559–1561. doi: 10.1126/science.1073733. [DOI] [PubMed] [Google Scholar]
- 8.Szakacs G, Paterson JK, Ludwig JA, Booth-Genthe C, Gottesman MM. Targeting multidrug resistance in cancer. Nat Rev Drug Discov. 2006;5:219–234. doi: 10.1038/nrd1984. [DOI] [PubMed] [Google Scholar]
- 9.Ailles LE, Weissman IL. Cancer stem cells in solid tumors. Curr Opin Biotechnol. 2007;18:460–466. doi: 10.1016/j.copbio.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 10.Zhou S, Schuetz JD, Bunting KD, Colapietro AM, Sampath J, Morris JJ, Lagutina I, Grosveld GC, Osawa M, Nakauchi H, et al. The ABC transporter Bcrp1/ABCG2 is expressed in a wide variety of stem cells and is a molecular determinant of the side-population phenotype. Nat Med. 2001;7:1028–1034. doi: 10.1038/nm0901-1028. [DOI] [PubMed] [Google Scholar]
- 11.Stingl J, Caldas C. Molecular heterogeneity of breast carcinomas and the cancer stem cell hypothesis. Nat Rev Cancer. 2007;7:791–799. doi: 10.1038/nrc2212. [DOI] [PubMed] [Google Scholar]
- 12.Natarajan TG, FitzGerald KT. Markers in normal and cancer stem cells. Cancer Biomark. 2007;3:211–231. doi: 10.3233/cbm-2007-34-506. [DOI] [PubMed] [Google Scholar]
- 13.Lou H, Dean M. Targeted therapy for cancer stem cells: the patched pathway and ABC transporters. Oncogene. 2007;26:1357–1360. doi: 10.1038/sj.onc.1210200. [DOI] [PubMed] [Google Scholar]
- 14.Sims-Mourtada J, Izzo JG, Ajani J, Chao KS. Sonic Hedgehog promotes multiple drug resistance by regulation of drug transport. Oncogene. 2007;26:5674–5679. doi: 10.1038/sj.onc.1210356. [DOI] [PubMed] [Google Scholar]
- 15.Zhang Y, Bressler JP, Neal J, Lal B, Bhang H-E, Laterra J, Pomper MG. ABCG2/BCRP expression modulates D-luciferin based bioluminescence imaging. Cancer Res. 2007;67:9389–9397. doi: 10.1158/0008-5472.CAN-07-0944. [DOI] [PubMed] [Google Scholar]
- 16.Ray P, De A, Min JJ, Tsien RY, Gambhir SS. Imaging tri-fusion multimodality reporter gene expression in living subjects. Cancer Res. 2004;64:1323–1330. doi: 10.1158/0008-5472.can-03-1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Evers R, Kool M, Smith AJ, van Deemter L, de Haas M, Borst P. Inhibitory effect of the reversal agents V-104, GF120918 and Pluronic L61 on MDR1 Pgp-, MRP1- and MRP2-mediated transport. Br J Cancer. 2000;83:366–374. doi: 10.1054/bjoc.2000.1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Robey RW, Honjo Y, Morisaki K, Nadjem TA, Runge S, Risbood M, Poruchynsky MS, Bates SE. Mutations at amino acid 482 in the ABCG2 gene affect substrate and antagonist specificity. Br J Cancer. 2003;89:1971–1978. doi: 10.1038/sj.bjc.6601370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Robey RW, Honjo Y, van de Laar A, Miyake K, Regis JT, Litman T, Bates SE. A functional assay for detection of the mitoxantrone resistance protein, MXR (ABCG2) Biochim Biophys Acta. 2001;1512:171–182. doi: 10.1016/s0005-2736(01)00308-x. [DOI] [PubMed] [Google Scholar]
- 20.Homolya L, Hollo Z, Germann UA, Pastan I, Gottesman MM, Sarkadi B. Fluorescent cellular indicators are extruded by the multidrug resistance protein. J Biol Chem. 1993;268:21493–21496. [PubMed] [Google Scholar]
- 21.Hollo Z, Homolya L, Hegedus T, Sarkadi B. Transport properties of the multidrug resistance-associated protein (MRP) in human tumour cells. FEBS Lett. 1996;383:99–104. doi: 10.1016/0014-5793(96)00237-2. [DOI] [PubMed] [Google Scholar]
- 22.Alley MC, Scudiero DA, Monks A, Hursey ML, Czerwinski MJ, Fine DL, Abbott BJ, Mayo JG, Shoemaker RH, Boyd MR. Feasibility of drug screening with panels of human tumor cell lines using a microculture tetrazolium assay. Cancer Res. 1988;48:589–601. [PubMed] [Google Scholar]
- 23.Scudiero DA, Shoemaker RH, Paull KD, Monks A, Tierney S, Nofziger TH, Currens MJ, Seniff D, Boyd MR. Evaluation of a soluble tetrazolium/formazan assay for cell growth and drug sensitivity in culture using human and other tumor cell lines. Cancer Res. 1988;48:4827–4833. [PubMed] [Google Scholar]
- 24.Rabindran SK, Ross DD, Doyle LA, Yang W, Greenberger LM. Fumitremorgin C reverses multidrug resistance in cells transfected with the breast cancer resistance protein. Cancer Res. 2000;60:47–50. [PubMed] [Google Scholar]
- 25.Gross S, Piwnica-Worms D. Spying on cancer: molecular imaging in vivo with genetically encoded reporters. Cancer Cell. 2005;7:5–15. doi: 10.1016/j.ccr.2004.12.011. [DOI] [PubMed] [Google Scholar]
- 26.Doyle LA, Yang W, Abruzzo LV, Krogmann T, Gao Y, Rishi AK, Ross DD. A multidrug resistance transporter from human MCF-7 breast cancer cells. Proc Natl Acad Sci USA. 1998;95:15665–15670. doi: 10.1073/pnas.95.26.15665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ambudkar SV, Dey S, Hrycyna CA, Ramachandra M, Pastan I, Gottesman MM. Biochemical, cellular, and pharmacological aspects of the multidrug transporter. Annu Rev Pharmacol Toxicol. 1999;39:361–398. doi: 10.1146/annurev.pharmtox.39.1.361. [DOI] [PubMed] [Google Scholar]
- 28.Shen DW, Cardarelli C, Hwang J, Cornwell M, Richert N, Ishii S, Pastan I, Gottesman MM. Multiple drug-resistant human KB carcinoma cells independently selected for high-level resistance to colchicine, adriamycin, or vinblastine show changes in expression of specific proteins. J Biol Chem. 1986;261:7762–7770. [PubMed] [Google Scholar]
- 29.Klappe K, Hinrichs JW, Kroesen BJ, Sietsma H, Kok JW. MRP1 and glucosylceramide are coordinately over expressed and enriched in rafts during multidrug resistance acquisition in colon cancer cells. Int J Cancer. 2004;110:511–522. doi: 10.1002/ijc.20140. [DOI] [PubMed] [Google Scholar]
- 30.Litman T, Brangi M, Hudson E, Fetsch P, Abati A, Ross DD, Miyake K, Resau JH, Bates SE. The multidrug-resistant phenotype associated with overexpression of the new ABC half-transporter, MXR (ABCG2) J Cell Sci. 2000;113(Pt 11):2011–2021. doi: 10.1242/jcs.113.11.2011. [DOI] [PubMed] [Google Scholar]
- 31.Kawabata S, Oka M, Shiozawa K, Tsukamoto K, Nakatomi K, Soda H, Fukuda M, Ikegami Y, Sugahara K, Yamada Y, et al. Breast cancer resistance protein directly confers SN-38 resistance of lung cancer cells. Biochem Biophys Res Commun. 2001;280:1216–1223. doi: 10.1006/bbrc.2001.4267. [DOI] [PubMed] [Google Scholar]
- 32.Brangi M, Litman T, Ciotti M, Nishiyama K, Kohlhagen G, Takimoto C, Robey R, Pommier Y, Fojo T, Bates SE. Camptothecin resistance: role of the ATP-binding cassette (ABC), mitoxantrone-resistance half-transporter (MXR), and potential for glucuronidation in MXR-expressing cells. Cancer Res. 1999;59:5938–5946. [PubMed] [Google Scholar]
- 33.Begley DJ. ABC transporters and the blood-brain barrier. Curr Pharm Des. 2004;10:1295–1312. doi: 10.2174/1381612043384844. [DOI] [PubMed] [Google Scholar]
- 34.Baselga J, Averbuch SD. ZD1839 (“Iressa”) as an anticancer agent. Drugs. 2000;60(Suppl 1):33–40. doi: 10.2165/00003495-200060001-00004. discussion 41–42. [DOI] [PubMed] [Google Scholar]
- 35.Nakamura Y, Oka M, Soda H, Shiozawa K, Yoshikawa M, Itoh A, Ikegami Y, Tsurutani J, Nakatomi K, Kitazaki T, et al. Gefitinib (“Iressa”, ZD1839), an epidermal growth factor receptor tyrosine kinase inhibitor, reverses breast cancer resistance protein/ABCG2-mediated drug resistance. Cancer Res. 2005;65:1541–1546. doi: 10.1158/0008-5472.CAN-03-2417. [DOI] [PubMed] [Google Scholar]
- 36.Elkind NB, Szentpetery Z, Apati A, Ozvegy-Laczka C, Varady G, Ujhelly O, Szabo K, Homolya L, Varadi A, Buday L, et al. Multidrug transporter ABCG2 prevents tumor cell death induced by the epidermal growth factor receptor inhibitor Iressa (ZD1839, gefitinib) Cancer Res. 2005;65:1770–1777. doi: 10.1158/0008-5472.CAN-04-3303. [DOI] [PubMed] [Google Scholar]
- 37.Houghton PJ, Germain GS, Harwood FC, Schuetz JD, Stewart CF, Buchdunger E, Traxler P. Imatinib mesylate is a potent inhibitor of the ABCG2 (BCRP) transporter and reverses resistance to topotecan and SN-38 in vitro. Cancer Res. 2004;64:2333–2337. doi: 10.1158/0008-5472.can-03-3344. [DOI] [PubMed] [Google Scholar]
- 38.Shi Z, Peng XX, Kim IW, Shukla S, Si QS, Robey RW, Bates SE, Shen T, Ashby CR, Jr, Fu LW, et al. Erlotinib (Tarceva, OSI-774) antagonizes ATP-binding cassette subfamily B member 1 and ATP-binding cassette subfamily G member 2-mediated drug resistance. Cancer Res. 2007;67:11012–11020. doi: 10.1158/0008-5472.CAN-07-2686. [DOI] [PubMed] [Google Scholar]
- 39.Ozvegy-Laczka C, Hegedus T, Varady G, Ujhelly O, Schuetz JD, Varadi A, Keri G, Orfi L, Nemet K, Sarkadi B. High-affinity interaction of tyrosine kinase inhibitors with the ABCG2 multidrug transporter. Mol Pharmacol. 2004;65:1485–1495. doi: 10.1124/mol.65.6.1485. [DOI] [PubMed] [Google Scholar]
- 40.Dai H, Marbach P, Lemaire M, Hayes M, Elmquist WF. Distribution of STI-571 to the brain is limited by P-glycoprotein-mediated efflux. J Pharmacol Exp Ther. 2003;304:1085–1092. doi: 10.1124/jpet.102.045260. [DOI] [PubMed] [Google Scholar]
- 41.Weiss J, Rose J, Storch CH, Ketabi-Kiyanvash N, Sauer A, Haefeli WE, Efferth T. Modulation of human BCRP (ABCG2) activity by anti-HIV drugs. J Antimicrob Chemother. 2007;59:238–245. doi: 10.1093/jac/dkl474. [DOI] [PubMed] [Google Scholar]
- 42.Chen JS, Pardo FS, Wang-Rodriguez J, Chu TS, Lopez JP, Aguilera J, Altuna X, Weisman RA, Ongkeko WM. EGFR regulates the side population in head and neck squamous cell carcinoma. Laryngoscope. 2006;116:401–406. doi: 10.1097/01.mlg.0000195075.14093.fb. [DOI] [PubMed] [Google Scholar]
- 43.Takada T, Suzuki H, Gotoh Y, Sugiyama Y. Regulation of the cell surface expression of human BCRP/ABCG2 by the phosphorylation state of Akt in polarized cells. Drug Metab Dispos. 2005;33:905–909. doi: 10.1124/dmd.104.003228. [DOI] [PubMed] [Google Scholar]
- 44.Meyer zu Schwabedissen HE, Grube M, Dreisbach A, Jedlitschky G, Meissner K, Linnemann K, Fusch C, Ritter CA, Volker U, Kroemer HK. Epidermal growth factor-mediated activation of the map kinase cascade results in altered expression and function of ABCG2 (BCRP) Drug Metab Dispos. 2006;34:524–533. doi: 10.1124/dmd.105.007591. [DOI] [PubMed] [Google Scholar]
- 45.Nakanishi T, Shiozawa K, Hassel BA, Ross DD. Complex interaction of BCRP/ABCG2 and imatinib in BCR-ABL-expressing cells: BCRP-mediated resistance to imatinib is attenuated by imatinib-induced reduction of BCRP expression. Blood. 2006;108:678–684. doi: 10.1182/blood-2005-10-4020. [DOI] [PubMed] [Google Scholar]
- 46.Xie Y, Xu K, Linn DE, Yang X, Guo Z, Shimelis H, Nakanishi T, Ross DD, Chen H, Fazli L, et al. The 44-kDa Pim-1 kinase phosphorylates BCRP/ABCG2 and thereby promotes its multimerization and drug-resistant activity in human prostate cancer cells. J Biol Chem. 2008;283:3349–3356. doi: 10.1074/jbc.M707773200. [DOI] [PubMed] [Google Scholar]
- 47.Dean M, Fojo T, Bates S. Tumour stem cells and drug resistance. Nat Rev Cancer. 2005;5:275–284. doi: 10.1038/nrc1590. [DOI] [PubMed] [Google Scholar]