Abstract
Type II heat-labile enterotoxin (LT-II) from Escherichia coli causes characteristic morphological changes and accumulation of cyclic AMP in Y-1 adrenal cells, but it is not neutralized by antisera against choleragen (CT) or the classical type I heat-labile enterotoxin (LT-1) from E. coli. The action of purified LT-II on CT- and LT-I-responsive human fibroblasts was investigated and compared with that of CT. Fibroblasts incubated with LT-II or CT had an increased cyclic AMP content as well as a fourfold elevation of membrane adenylate cyclase activity. In membranes, activation of cyclase by toxin was enhanced by NAD, GTP, and dithiothreitol. The effect of LT-II on intact fibroblasts or membranes was increased by trypsin treatment of toxin. Since activation of adenylate cyclase by LT-II was stimulated by NAD, the ability of LT-II to catalyze the [32P]ADP-ribosylation of membrane proteins in the presence of [32P]NAD from control and LT-II- and CT-treated fibroblasts was investigated. Similar proteins were [32P]ADP-ribosylated in membranes exposed to LT-II or CT; LT-II- and CT-specific labeling was significantly decreased in membranes prepared from cells preincubated with either LT-II or CT. These studies are consistent with the hypothesis that LT-II, similar to CT and LT-I, increases cyclic AMP by activating adenylate cyclase through the GTP-dependent ADP-ribosylation of specific membrane proteins.
Full text
PDF
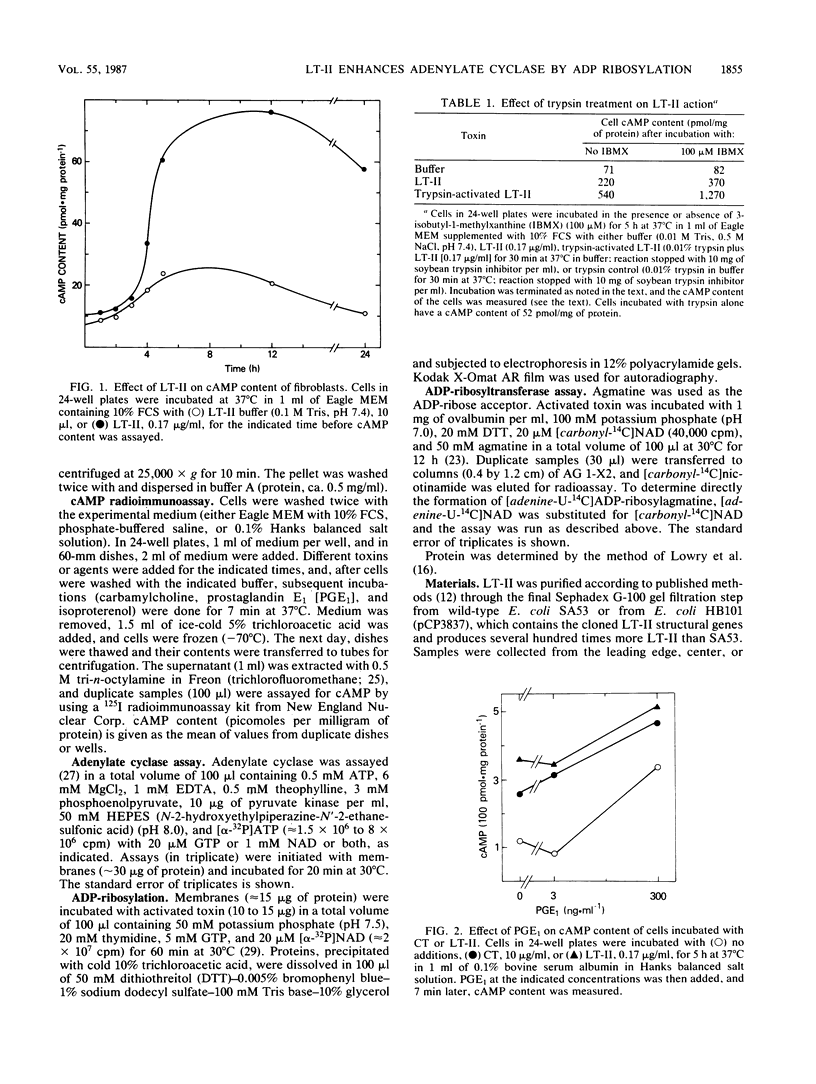
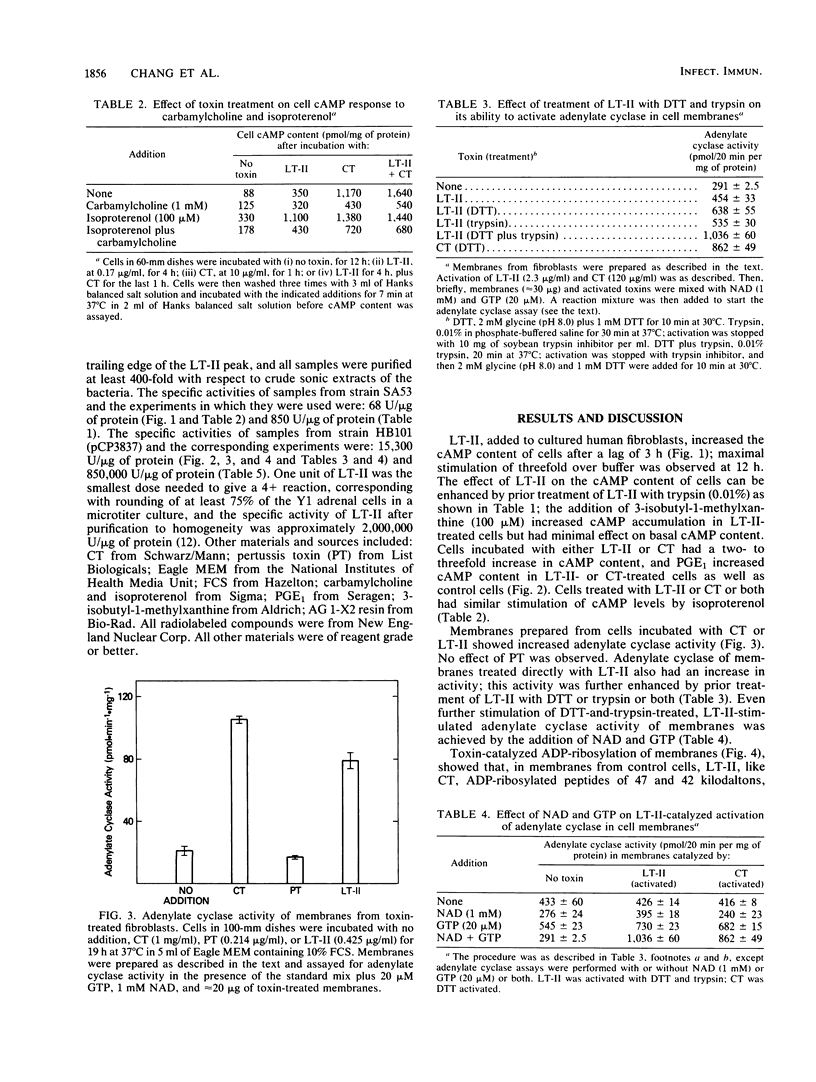


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Clements J. D., Yancey R. J., Finkelstein R. A. Properties of homogeneous heat-labile enterotoxin from Escherichia coli. Infect Immun. 1980 Jul;29(1):91–97. doi: 10.1128/iai.29.1.91-97.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans D. J., Jr, Chen L. C., Curlin G. T., Evans D. G. Stimulation of adenyl cyclase by Escherichia coli enterotoxin. Nat New Biol. 1972 Apr 5;236(66):137–138. doi: 10.1038/newbio236137a0. [DOI] [PubMed] [Google Scholar]
- Field M. Mechanisms of action of cholera and Escherichia coli enterotoxins. Am J Clin Nutr. 1979 Jan;32(1):189–196. doi: 10.1093/ajcn/32.1.189. [DOI] [PubMed] [Google Scholar]
- Gill D. M. Mechanism of action of cholera toxin. Adv Cyclic Nucleotide Res. 1977;8:85–118. [PubMed] [Google Scholar]
- Gill D. M., Richardson S. H. Adenosine diphosphate-ribosylation of adenylate cyclase catalyzed by heat-labile enterotoxin of Escherichia coli: comparison with cholera toxin. J Infect Dis. 1980 Jan;141(1):64–70. doi: 10.1093/infdis/141.1.64. [DOI] [PubMed] [Google Scholar]
- Green B. A., Neill R. J., Ruyechan W. T., Holmes R. K. Evidence that a new enterotoxin of Escherichia coli which activates adenylate cyclase in eucaryotic target cells is not plasmid mediated. Infect Immun. 1983 Jul;41(1):383–390. doi: 10.1128/iai.41.1.383-390.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guth B. E., Pickett C. L., Twiddy E. M., Holmes R. K., Gomes T. A., Lima A. A., Guerrant R. L., Franco B. D., Trabulsi L. R. Production of type II heat-labile enterotoxin by Escherichia coli isolated from food and human feces. Infect Immun. 1986 Nov;54(2):587–589. doi: 10.1128/iai.54.2.587-589.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guth B. E., Twiddy E. M., Trabulsi L. R., Holmes R. K. Variation in chemical properties and antigenic determinants among type II heat-labile enterotoxins of Escherichia coli. Infect Immun. 1986 Nov;54(2):529–536. doi: 10.1128/iai.54.2.529-536.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewlett E. L., Guerrant R. L., Evans D. J., Jr, Greenough W. B., 3rd Toxins of Vibrio cholerae and Escherichia coli stimulate adenyl cyclase in rat fat cells. Nature. 1974 May 24;249(455):371–373. doi: 10.1038/249371a0. [DOI] [PubMed] [Google Scholar]
- Holmes R. K., Twiddy E. M., Pickett C. L. Purification and characterization of type II heat-labile enterotoxin of Escherichia coli. Infect Immun. 1986 Sep;53(3):464–473. doi: 10.1128/iai.53.3.464-473.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmgren J. Comparison of the tissue receptors for Vibrio cholerae and Escherichia coli enterotoxins by means of gangliosides and natural cholera toxoid. Infect Immun. 1973 Dec;8(6):851–859. doi: 10.1128/iai.8.6.851-859.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynie S., Rasková H., Sechser T., Vanecek J., Matejovská D., Matejovská V., Treu M., Polák L. Stimulation of intestinal and liver adenyl cyclase by enterotoxin from strains of Escherichia coli enterpathogenic for calves. Toxicon. 1974 Mar;12(2):173–179. doi: 10.1016/0041-0101(74)90242-6. [DOI] [PubMed] [Google Scholar]
- Kantor H. S., Tao P., Gorbach S. L. Stimulation of intestinal adenyl cyclase by Escherichia coli enterotoxin: comparison of strains from an infant and an adult with diarrhea. J Infect Dis. 1974 Jan;129(1):1–9. doi: 10.1093/infdis/129.1.1. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Manganiello V. C., Breslow J. Effects of prostaglandin E1 and isoproterenol on cyclic AMP content of human fibroblasts modified by time and cell density in subculture. Biochim Biophys Acta. 1974 Oct 8;362(3):509–520. doi: 10.1016/0304-4165(74)90146-9. [DOI] [PubMed] [Google Scholar]
- Mashiter K., Mashiter G. D., Hauger R. L., Field J. B. Effects of cholera and E. coli enterotoxins on cyclic adenosine 3',5'-monophosphate levels and intermediary metabolism in the thyroid. Endocrinology. 1973 Feb;92(2):541–549. doi: 10.1210/endo-92-2-541. [DOI] [PubMed] [Google Scholar]
- Mekalanos J. J., Collier R. J., Romig W. R. Enzymic activity of cholera toxin. II. Relationships to proteolytic processing, disulfide bond reduction, and subunit composition. J Biol Chem. 1979 Jul 10;254(13):5855–5861. [PubMed] [Google Scholar]
- Moss J., Burns D. L., Hsia J. A., Hewlett E. L., Guerrant R. L., Vaughan M. NIH conference. Cyclic nucleotides: mediators of bacterial toxin action in disease. Ann Intern Med. 1984 Nov;101(5):653–666. doi: 10.7326/0003-4819-101-5-653. [DOI] [PubMed] [Google Scholar]
- Moss J., Garrison S., Fishman P. H., Richardson S. H. Gangliosides sensitize unresponsive fibroblasts to Escherichia coli heat-labile enterotoxin. J Clin Invest. 1979 Aug;64(2):381–384. doi: 10.1172/JCI109472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss J., Osborne J. C., Jr, Fishman P. H., Nakaya S., Robertson D. C. Escherichia coli heat-labile enterotoxin. Ganglioside specificity and ADP-ribosyltransferase activity. J Biol Chem. 1981 Dec 25;256(24):12861–12865. [PubMed] [Google Scholar]
- Moss J., Vaughan M. Mechanism of action of choleragen. Evidence for ADP-ribosyltransferase activity with arginine as an acceptor. J Biol Chem. 1977 Apr 10;252(7):2455–2457. [PubMed] [Google Scholar]
- Pickett C. L., Twiddy E. M., Belisle B. W., Holmes R. K. Cloning of genes that encode a new heat-labile enterotoxin of Escherichia coli. J Bacteriol. 1986 Feb;165(2):348–352. doi: 10.1128/jb.165.2.348-352.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomon Y., Londos C., Rodbell M. A highly sensitive adenylate cyclase assay. Anal Biochem. 1974 Apr;58(2):541–548. doi: 10.1016/0003-2697(74)90222-x. [DOI] [PubMed] [Google Scholar]
- Watkins P. A., Moss J., Vaughan M. ADP ribosylation of membrane proteins from human fibroblasts. Effect of prior exposure of cells to choleragen. J Biol Chem. 1981 May 25;256(10):4895–4899. [PubMed] [Google Scholar]
- Zenser T. V., Metzger J. F. Comparison of the action of Escherichia coli enterotoxin on the thymocyte adenylate cyclase-cyclic adenosine monophosphate system to that of cholera toxin and prostaglandin E1. Infect Immun. 1974 Sep;10(3):503–509. doi: 10.1128/iai.10.3.503-509.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Heyningen S. Cholera toxin. Biol Rev Camb Philos Soc. 1977 Nov;52(4):509–509. doi: 10.1111/j.1469-185x.1977.tb00858.x. [DOI] [PubMed] [Google Scholar]




