Abstract
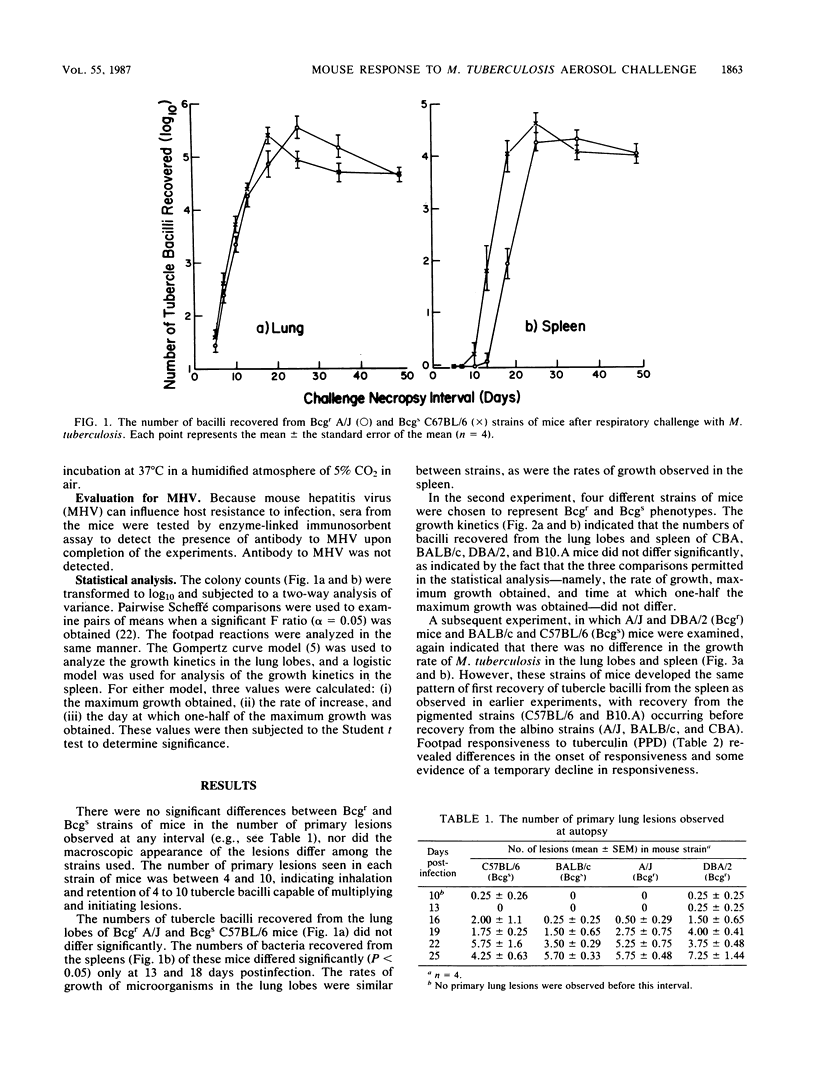
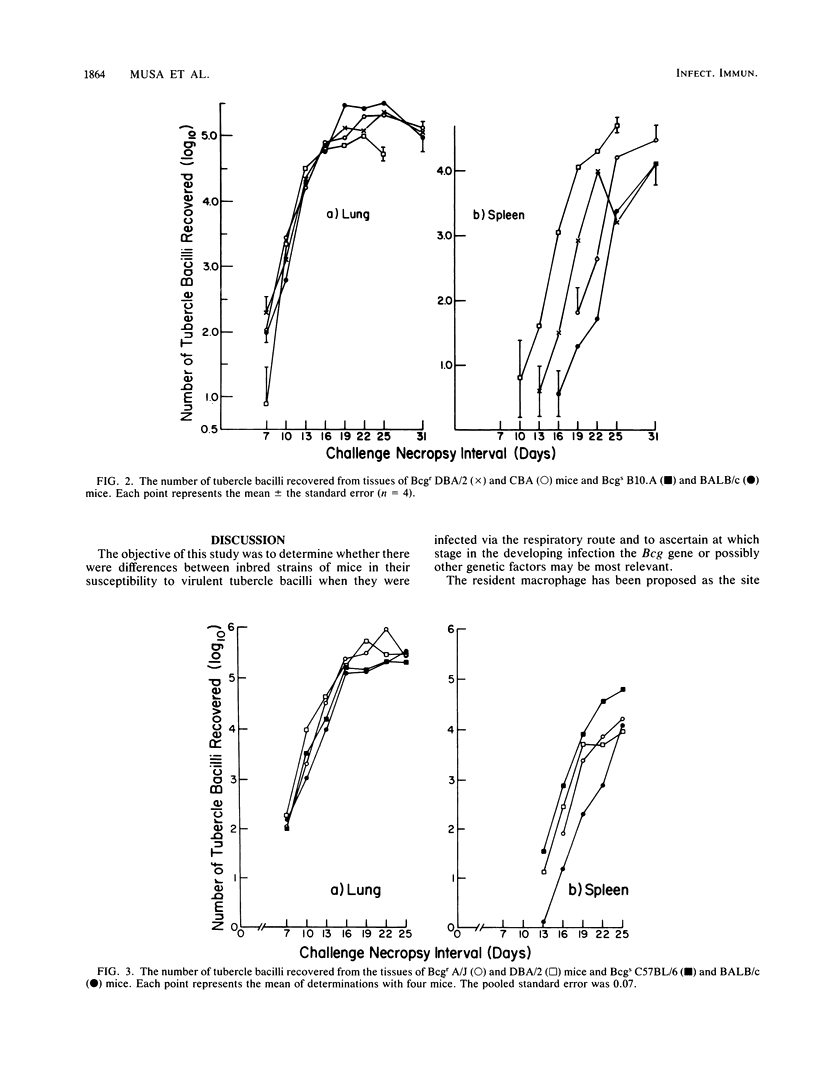
An autosomal dominant gene (Bcg), which maps to mouse chromosome 1, has been shown to confer on mice resistance to attenuated Mycobacterium bovis BCG Montreal, Salmonella typhimurium, and Leishmania donovani. Most animal models used for the study of the Bcg gene have involved intravenous injection of a large number of microorganisms (greater than 10(4) CFU). The present study examines the effect of the Bcg gene on the resistance of inbred mice to challenge via the respiratory route with 5 to 10 CFU of virulent Mycobacterium tuberculosis. The number of tubercle bacilli recovered from the lung lobes indicates that the growth kinetics of the microorganism did not differ between BCG-resistant and BCG-susceptible strains of mice. The number of tubercle bacilli recovered from the spleen was also similar among strains. Although there were reproducible differences in the time of first recovery of bacilli from the spleen, these differences appeared to be unrelated to the expression of the Bcg gene. When mice were challenged with purified protein derivative, all strains responded similarly as observed by measurements of footpad swelling.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradley D. J. Regulation of Leishmania populations within the host. II. genetic control of acute susceptibility of mice to Leishmania donovani infection. Clin Exp Immunol. 1977 Oct;30(1):130–140. [PMC free article] [PubMed] [Google Scholar]
- Bradley D. J., Taylor B. A., Blackwell J., Evans E. P., Freeman J. Regulation of Leishmania populations within the host. III. Mapping of the locus controlling susceptibility to visceral leishmaniasis in the mouse. Clin Exp Immunol. 1979 Jul;37(1):7–14. [PMC free article] [PubMed] [Google Scholar]
- Cheers C., McKenzie I. F. Resistance and susceptibility of mice to bacterial infection: genetics of listeriosis. Infect Immun. 1978 Mar;19(3):755–762. doi: 10.1128/iai.19.3.755-762.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degré M. Phagocytic and bactericidal activities of peritoneal and alveolar macrophages from mice. J Med Microbiol. 1969 Aug;2(3):353–357. doi: 10.1099/00222615-2-3-353. [DOI] [PubMed] [Google Scholar]
- Forget A., Skamene E., Gros P., Miailhe A. C., Turcotte R. Differences in response among inbred mouse strains to infection with small doses of Mycobacterium bovis BCG. Infect Immun. 1981 Apr;32(1):42–47. doi: 10.1128/iai.32.1.42-47.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gros P., Skamene E., Forget A. Cellular mechanisms of genetically controlled host resistance to Mycobacterium bovis (BCG). J Immunol. 1983 Oct;131(4):1966–1972. [PubMed] [Google Scholar]
- Gros P., Skamene E., Forget A. Genetic control of natural resistance to Mycobacterium bovis (BCG) in mice. J Immunol. 1981 Dec;127(6):2417–2421. [PubMed] [Google Scholar]
- Grover A. A., Kim H. K., Wiegeshaus E. H., Smith D. W. Host-parasite relationships in experimental airborne tuberculosis. II. Reproducible infection by means of an inoculum preserved at -70 C. J Bacteriol. 1967 Oct;94(4):832–835. doi: 10.1128/jb.94.4.832-835.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groves M. G., Osterman J. V. Host defenses in experimental scrub typhus: genetics of natural resistance to infection. Infect Immun. 1978 Feb;19(2):583–588. doi: 10.1128/iai.19.2.583-588.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groves M. G., Rosenstreich D. L., Taylor B. A., Osterman J. V. Host defenses in experimental scrub typhus: mapping the gene that controls natural resistance in mice. J Immunol. 1980 Sep;125(3):1395–1399. [PubMed] [Google Scholar]
- Johnson S. C., Zwilling B. S. Continuous expression of I-A antigen by peritoneal macrophages from mice resistant to Mycobacterium bovis (strain BCG). J Leukoc Biol. 1985 Nov;38(5):635–647. doi: 10.1002/jlb.38.5.635. [DOI] [PubMed] [Google Scholar]
- LYNCH C. J., PIERCE-CHASE C. H., DUBOS R. A GENETIC STUDY OF SUSCEPTIBILITY TO EXPERIMENTAL TUBERCULOSIS IN MICE INFECTED WITH MAMMALIAN TUBERCLE BACILLI. J Exp Med. 1965 Jun 1;121:1051–1070. doi: 10.1084/jem.121.6.1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrer R. I., Szklarek D., Selsted M. E., Fleischmann J. Increased content of microbicidal cationic peptides in rabbit alveolar macrophages elicited by complete Freund adjuvant. Infect Immun. 1981 Sep;33(3):775–778. doi: 10.1128/iai.33.3.775-778.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lissner C. R., Swanson R. N., O'Brien A. D. Genetic control of the innate resistance of mice to Salmonella typhimurium: expression of the Ity gene in peritoneal and splenic macrophages isolated in vitro. J Immunol. 1983 Dec;131(6):3006–3013. [PubMed] [Google Scholar]
- Lissner C. R., Weinstein D. L., O'Brien A. D. Mouse chromosome 1 Ity locus regulates microbicidal activity of isolated peritoneal macrophages against a diverse group of intracellular and extracellular bacteria. J Immunol. 1985 Jul;135(1):544–547. [PubMed] [Google Scholar]
- Nakamura R. M., Tokunaga T. Strain difference of delayed-type hypersensitivity to BCG and its genetic control in mice. Infect Immun. 1978 Dec;22(3):657–664. doi: 10.1128/iai.22.3.657-664.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orme I. M., Collins F. M. Demonstration of acquired resistance in Bcgr inbred mouse strains infected with a low dose of BCG montreal. Clin Exp Immunol. 1984 Apr;56(1):81–88. [PMC free article] [PubMed] [Google Scholar]
- Pelletier M., Forget A., Bourassa D., Gros P., Skamene E. Immunopathology of BCG infection in genetically resistant and susceptible mouse strains. J Immunol. 1982 Nov;129(5):2179–2185. [PubMed] [Google Scholar]
- Plant J., Glynn A. A. Locating salmonella resistance gene on mouse chromosome 1. Clin Exp Immunol. 1979 Jul;37(1):1–6. [PMC free article] [PubMed] [Google Scholar]
- Skamene E. Genetic regulation of host resistance to bacterial infection. Rev Infect Dis. 1983 Sep-Oct;5 (Suppl 4):S823–S832. doi: 10.1093/clinids/5.supplement_4.s823. [DOI] [PubMed] [Google Scholar]
- Skamene E., Gros P., Forget A., Kongshavn P. A., St Charles C., Taylor B. A. Genetic regulation of resistance to intracellular pathogens. Nature. 1982 Jun 10;297(5866):506–509. doi: 10.1038/297506a0. [DOI] [PubMed] [Google Scholar]
- Taylor B. A., O'Brien A. D. Position on mouse chromosome 1 of a gene that controls resistance to Salmonella typhimurium. Infect Immun. 1982 Jun;36(3):1257–1260. doi: 10.1128/iai.36.3.1257-1260.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiegeshaus E. H., McMurray D. N., Grover A. A., Harding G. E., Smith D. W. Host-parasite relationships in experimental airborne tuberculosis. 3. Relevance of microbial enumeration to acquired resistance in guinea pigs. Am Rev Respir Dis. 1970 Sep;102(3):422–429. doi: 10.1164/arrd.1970.102.3.422. [DOI] [PubMed] [Google Scholar]



