Abstract
How does complex social behavior evolve? What are the developmental building blocks of division of labor and specialization, the hallmarks of insect societies? Studies have revealed the developmental origins in the evolution of division of labor and specialization in foraging worker honeybees, the hallmarks of complex insect societies. Selective breeding for a single social trait, the amount of surplus pollen stored in the nest (pollen hoarding) revealed a phenotypic architecture of correlated traits at multiple levels of biological organization in facultatively sterile female worker honeybees. Verification of this phenotypic architecture in “wild-type” bees provided strong support for a “pollen foraging syndrome” that involves increased senso-motor responses, motor activity, associative learning, reproductive status, and rates of behavioral development, as well as foraging behavior. This set of traits guided further research into reproductive regulatory systems that were co-opted by natural selection during the evolution of social behavior. Division of labor, characterized by changes in the tasks performed by bees, as they age, is controlled by hormones linked to ovary development. Foraging specialization on nectar and pollen results also from different reproductive states of bees where nectar foragers engage in prereproductive behavior, foraging for nectar for self-maintenance, while pollen foragers perform foraging tasks associated with reproduction and maternal care, collecting protein.
I. Introduction
Advanced societies of insects display marked patterns of behavior where reproduction is restricted to elite individuals (queens) who are often anatomically differentiated from nonreproductive individuals (the workers) (Wheeler, 1928). Workers are often further differentiated into anatomically and/or behaviorally differentiated individuals that specialize on the performance of specific behavioral tasks for at least some part of their adult lives. In the honeybee, this differentiation is behavioral without any obvious anatomical differences and expressed by changes in behavior associated with age and change of location in the nest, an age-related polyethism (Seeley, 1982). Typically, bees perform tasks in the center of the brood nest soon after emergence including cleaning brood cells and feeding larvae. After about 1 week they make a transition to performing tasks outside the brood nest area such as comb construction and food processing. When they are in about their third week of life, they make a final transition to foraging outside the nest after which they are seldom observed performing tasks within the nest other than those directly related to foraging, such as unloading pollen and nectar, and performing recruitment dances.
When a worker honeybee makes the transition to foraging, she usually collects pollen (a source of protein) and nectar (a carbohydrate source), though a minority of workers collect water and propolis, a resinous substance collected from plants and used in nest construction. Most food foragers collect both pollen and nectar on a single foraging trip, however, many collect only a single substance (Hunt et al., 1995; Page et al., 2000). The total load collected by a forager is constrained. A maximum nectar load is about 60 mg, while a maximum load of pollen is about 30 mg. So, each 1 mg of pollen “costs” about 2 mg of nectar. Nectar is carried inside the crop, the first chamber of the alimentary canal (Snodgrass, 1956), while pollen is carried on the hind legs and may impose aerodynamic drag, perhaps explaining the differences in maximum load sizes.
Returning nectar foragers pass their nectar loads to younger bees in the nest through trophallaxis. The younger bees then distribute the nectar to other bees, or deposit it in open cells in the comb where it is eventually processed by other bees into honey. Returning pollen foragers deposit their loads directly into empty cells or cells containing pollen close to the area of the nest where young larvae are raised (Dreller and Tarpy, 2000). Stored pollen is consumed by young bees (Crailsheim et al., 1992). The pollen proteins are converted into glandular secretions that are fed directly to larvae (Crailsheim, 1990). Stored pollen inhibits pollen foraging in colonies (Dreller et al., 1999) while pheromones produced by larvae stimulate pollen foraging (Pankiw et al., 1998). Colonies regulate the amount of stored pollen (Fewell and Winston, 1992), probably through a combination of the inhibiting effects of pollen and stimulating effects of brood. At “equilibrium” pollen intake into the colony should equal pollen consumption, and meet the protein demands of developing larvae.
The brood nest is organized spatially with the brood (eggs, larvae, and pupae) located centrally (Winston, 1987). Pollen is stored close to the brood, and honey is stored at the periphery of the nest (Fig. 1). The amount of pollen stored in the comb represents a complex colony-level trait that is a consequence of the interactions of thousands of individual colony members. Younger workers consume pollen and feed the protein to larvae, older workers respond to the foraging stimuli, forage, and recruit other foragers to their resources. The stored pollen phenotype can be selected by artificial selection and is assumed to be under natural selection (Page and Fondrk, 1995).
Figure 1.
A diagram of a comb drawn from near the center of a honeybee nest showing the spatial orientation of honey, pollen, and brood.
II. Effects of Selection on Pollen Hoarding
A. Colony Level Selection
Hellmich et al. (1985) conducted two-way selection for the amount of pollen stored in the comb (pollen hoarding) and demonstrated a strong selective response. Subsequent studies showed that when fostered in the same colony, workers from the high-pollen hoarding strain were more likely to forage for pollen than were bees from the low strain (Calderone and Page, 1988, 1992). Bees from the high strain also foraged about 1 day earlier in life (Calderone and Page, 1988). Page and Fondrk (1995) repeated the selection from a different commercial population and also demonstrated a strong response to selection. After just three generations, colonies of the high strain contained about six times more pollen. Like Hellmich et al. (1985), they selected for a single trait, pollen hoarding, however, they also looked at other individual behavioral and physiological traits that might have changed as a consequence of selection on the colony-level phenotype. This enabled them to look for mechanisms at different levels of biological organization that causally underlie the differences in the colony-level phenotype (Page and Erber, 2002).
B. Foraging Behavior of High-and Low-Strain Bees
High-strain bees initiate foraging earlier in life than low-strain bees. Pankiw and Page (2001) demonstrated an average difference of about 10 days in a study of 12 host colonies. High-strain bees are more likely to specialize on pollen, while low-strain bees are more likely to specialize on nectar (Fewell and Page, 2000; Page and Fondrk, 1995; Pankiw and Page, 2001). High-and low-strain bees were raised together in “wild-type” colonies (commercial colonies not derived from the pollen hoarding strains). Workers of each strain were marked with paint on the thorax to identify their strain origins and then were placed into the same wild type test colonies, a type of “common garden,” experiment. Colony entrances were examined daily. Marked, returning foragers were captured, and their nectar and pollen loads analyzed. High-strain bees were more likely to collect pollen and collected larger pollen loads and smaller nectar loads than low-strain bees. High-strain bees were also more likely to collect water, and when they collected nectar, accepted nectar with lower sugar content than did bees of the low strain. Low-strain bees were much more likely to return empty from foraging trips (Page et al., 1998).
Differences in pollen load sizes were expected and represented by differences between the strains in their responses to pollen foraging stimuli. Fewell and Winston (1992) showed that colonies respond to changes in quantities of stored pollen by changing the allocation of foraging effort between collecting nectar and pollen. When colonies were presented with additional stored pollen beyond what they had already stored, they responded by reducing the number of pollen foragers and the sizes of the pollen loads. The opposite effect on foraging behavior was observed when stored pollen was removed. Colonies regulate the amount of stored pollen around a homeorhetic set point. Studies by Dreller et al. (1999) and Dreller and Tarpy (2000) demonstrated that foragers directly assess the amount of pollen stored in the combs and adjust their foraging behavior accordingly. The mechanism appears to involve the assessment of empty cells near the areas of the nest where larvae and pupae are located. Therefore, the regulatory mechanism involves individual assessment of stored pollen and individual “decisions” with respect to what to collect on a foraging trip (Fewell and Page, 2000). High-strain colonies reach a regulated set point with much larger quantities of stored pollen than do low-strain colonies. Therefore, high-strain bees have a threshold for stored pollen (or empty cells near the brood) that is different from low-strain bees. When cofostered in a wild-type colony, where high-and low-strain bees are much fewer than the resident bees, high-strain bees perceive the amount of stored pollen below their optimal set point, while the low-strain bees perceive it above theirs. As a result, high-strain bees are much more likely to forage for pollen, and low-strain bees are much more likely to forage for nectar.
Young larvae and hexane rinses of young larvae stimulate pollen-specific foraging behavior (Pankiw et al., 1998). Increasing the numbers of larvae in a nest, or augmenting the larvae with larval rinses, results in the recruitment of new pollen foragers and larger pollen loads but does not affect nectar foraging (Dreller et al., 1999; Pankiw et al., 1998). When foragers are not allowed direct contact with larvae, they do not change their foraging behavior with changes in larval quantities (Dreller et al., 1999). Selection for high-and low-pollen hoarding could have resulted in differences in quantities of brood, differences in brood pheromone levels in colonies, or differences in the perception/response systems coupled to pollen foraging stimuli. High-and low-strain bees do not differ in quantities of brood except under space-limited conditions where brood areas are reduced by excess pollen hoarding (Page and Fondrk, 1995).
High-and low-strain bees respond differently to changes in the pollen and brood stimuli in colonies. Pankiw and Page (2001) cofostered high-and low-strain bees in colonies with high-and low-pollen hoarding stimuli. High-stimulus colonies were experimentally manipulated to contain more larvae and less stored pollen than the low-stimulus colonies. Foragers in the high-stimulus colonies were more likely to collect pollen, collected larger loads of pollen, and smaller loads of nectar. High-strain bees demonstrated a larger difference in foraging behavior between treatments, demonstrating a genotype x–environment interaction where high-strain bees are more sensitive to the foraging stimulus environment. In summary, selection for the colony-level trait—the amount of pollen stored in the comb—resulted in changes in behavior at the individual level. Workers from colonies selected for storing more pollen initiated foraging earlier in life, foraged more successfully, were more likely to collect pollen, collected larger pollen loads and smaller nectar loads, and were more likely to collect water and nectar with lower concentrations of sugar. High-and low-strain bees respond to changes in foraging stimuli. Based on what we know about the regulation of stored pollen, a pollen foraging inhibiting stimulus, and the effects of brood on the release of pollen foraging behavior, it seems likely that high-and low-strain bees differ in their responsiveness to these important stimuli.
C. Sensory Responses
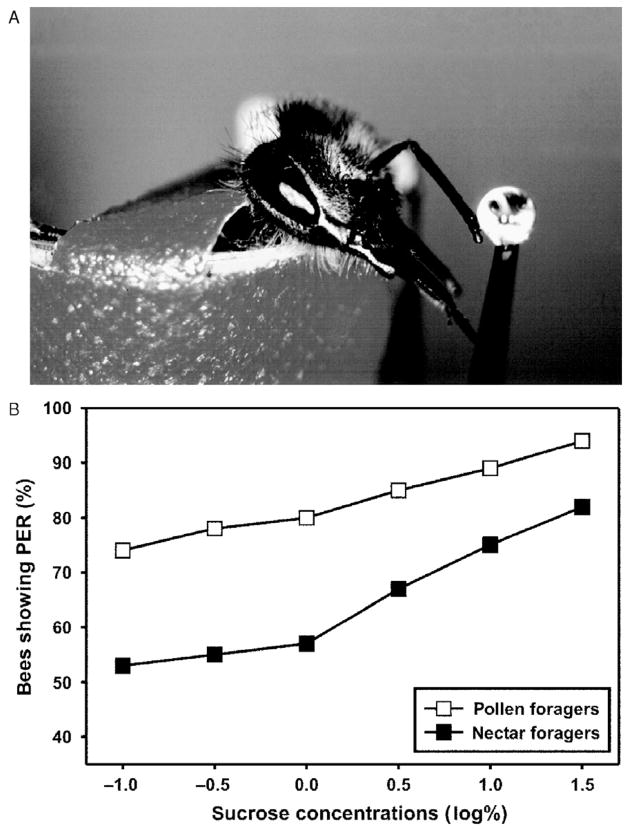
Changes in foraging behavior related to collecting pollen were expected of selection for pollen hoarding. However, high-strain bees are also more likely to forage for water than low-strain bees (Page et al., 1998). When high-strain bees forage for nectar, they accept nectar with lower concentrations of sugar than low-strain bees. There was no obvious physiological or behavioral mechanism to explain these relationships until Page et al. (1998) looked at the responses of pollen and nectar foragers to sucrose solutions under controlled laboratory conditions. Bees can respond to antennal stimulation with sucrose by extending the proboscis (Kunze, 1933; Marshall, 1935). Page et al. (1998) used an increasing concentration series of sucrose solutions to determine the sucrose responsiveness of wild type pollen and nectar foragers. Bees were placed into small tubes to restrict their movement. Then they were sequentially tested at each antenna with a droplet of sucrose solution (Fig. 2A). Sucrose concentrations increased with a logarithmic sequence of 0.1%, 0.3%, 1%, 3%, 10%, and 30%. Their response was recorded as “yes” (proboscis extension response, PER) or “no” (no PER) for each of the trials, which provided a measure of responsiveness to sucrose. The average proboscis responses of several bees to different sucrose concentrations are represented by the concentration–response curve (Fig. 2B). This curve can be used to estimate bees’ sucrose response threshold or their sensitivity for sucrose (Page et al., 1998). Bees that are more responsive have lower thresholds and are more sensitive. The results were surprising: pollen foragers were more likely than nectar foragers to respond to water and lower concentrations of sucrose (Fig. 2B). Apparently, pollen foragers have lower thresholds for water and sucrose and are, therefore, more sensitive for these stimuli.
Figure 2.
Measuring of sucrose responsiveness in honeybee foragers. (A) Fixed honeybee showing the proboscis extension response (PER). When the antenna of a bee is touched with a droplet of sucrose solution of sufficient concentration, the bee extends her proboscis in expectation of food. This response can be used to measure responsiveness to different sucrose concentrations. (B) Sucrose-concentration response curve of pollen and nectar foragers. The x-axis presents the log(%) of the sucrose concentrations tested. The y-axis displays the percentage of bees showing the PER. Pollen foragers are more responsive to all sucrose concentrations tested than nectar foragers (Scheiner et al., 2003a).
Responsiveness to sucrose depends on a number of external and internal parameters. Feeding bees, under laboratory conditions with sucrose, generally reduces responsiveness, but the differences between pollen and nectar foragers remain (Page et al., 1998). In free flying bees, responsiveness to sucrose is modulated by feeding and foraging experience (Pankiw et al., 2001). Even the sucrose responsiveness of hive bees changes with changing concentrations of nectar brought back by returning foragers (Pankiw et al., 2004). Sucrose responsiveness varies during the foraging season in pollen and nectar foragers. Nevertheless, pollen foragers consistently show higher sensitivity than nectar foragers (Scheiner et al., 2003a). The effects of genotype on sucrose responsiveness were shown by testing young bees of the high-and low-strain before they initiated foraging (Pankiw and Page, 1999; Pankiw et al., 2002; Scheiner et al., 2001a). In all age groups high-strain bees were more responsive to sucrose solutions and water than low-strains bees. This finding suggests that selection for pollen hoarding behavior had resulted in selection for the gustatory response system, which correlates with foraging behavior. These experiments demonstrate that gustatory sensitivity and foraging behavior are closely related.
If water and sucrose responses are related to nectar and pollen foraging, we should be able to test wild type bees before they start to forage and predict their foraging behavior 2–3 weeks later. Pankiw and Page (2000) tested wild type bees for their responses to water and sucrose within their first week of adult life, before they initiated foraging. Bees were marked for individual identification and placed back into their colony. Colony entrances were observed, returning foragers were collected, and their foraging loads were analyzed. Bees displaying the highest responsiveness to water and sucrose solutions when they were up to 7 days old were most likely to collect water on a foraging trip. The next most responsive group was very likely to collect pollen. Bees with lower responsiveness would later collect nectar or both nectar and pollen. The group with the lowest responsiveness would later in life return empty to the hive (Fig. 3).
Figure 3.
Sucrose responses of 1-week-old bees predict their foraging behavior later in life. The x-axis shows the foraging material of the bees tested for their sucrose responses at the age of 1 week, brought back by them when they reached foraging age. The y-axis shows the lowest sucrose concentrations (log10) at which 1-week-old bees responded with proboscis extension. Bees with the highest sucrose responsiveness (i.e., the lowest threshold) at young age will later forage for water or pollen. Individuals with low-sucrose responsiveness (i.e., a high threshold) perhaps collect nectar, nectar and pollen, or return empty. From Fig. 1 Pankiw and Page (2000) with the kind permission of Springer Science and Business Media.
The function of gustatory responsiveness for the division of foraging labor is not clear a priori. Why should a pollen forager be very sensitive to sucrose when she is mainly collecting pollen? Why is a water collector simply sensitive to water and insensitive to sucrose stimuli? A number of studies have clarified these questions. Bees who are sensitive to sucrose are also sensitive to stimuli of other modalities, and they show higher stimulus-related motor activity.
Bees that are highly responsive to sucrose are also highly responsive to pollen stimuli (Scheiner et al., 2004a). In these experiments, the gustatory responsiveness of bees was measured first. Then the same bees were stimulated with different pollen concentrations, which were produced by mixing pollen with cellulose grains of the same size. Bees that were highly responsive to sucrose, also responded with proboscis extension to the pollen stimulus, provided the concentration of pollen was higher than 6.3%, while bees with low responsiveness to sucrose did not respond to the same pollen concentration. Over 40% of the sensitive animals showed the proboscis response when stimulated with pure pollen, while less than 10% of the sucrose-insensitive bees responded to pure pollen. In another experiment, bees were tested in an olfactometer after measuring their sucrose responsiveness (Scheiner et al., 2004a). Again, bees that were sensitive to sucrose were also more sensitive to olfactory stimuli than were animals that were relatively insensitive to sucrose. Phototactic behavior of bees was tested in a round arena that allowed stimulation of a single bee with small monochromatic light sources (520 nm) of relative intensities between 3% and 100%. Stimulated bees walked toward the light. Walking behavior was recorded by an infrared camera that was mounted above the arena. Bees with high responsiveness to sucrose were also more sensitive to light stimuli in the arena. All these experiments demonstrate that sucrose responsiveness correlates with sensitivities for other stimulus modalities. Pollen foraging bees are not only sensitive to sucrose but also to pollen, odors, and light stimuli.
The behavioral responses to stimuli that can be measured in honeybees are the result of complex neuronal processes that integrate sensory information and produce motor output. Motor patterns of the proboscis, the antennae, or the legs are controlled by specific motor systems consisting of different types of neurons and often different types of muscles. Therefore, it is important to ask whether the motor system is tuned differently in bees that differ in their sensory responses. Several experiments have shown that sensory input can influence motor output in honeybees. Bees whose eyes are covered by paint scan an object within the range of the antennae with rapid antennal movements. The mechanical stimuli produced during antennal contact with an object initiate motor activity that even shows motor learning (Erber et al., 1997). Antennal scanning activity is significantly higher in bees that are responsive to sucrose compared to animals that are not responsive (Scheiner et al., 2005). This experiment demonstrates that there is a correlation between gustatory responsiveness and stimulus-evoked motor activity.
Responsiveness to sucrose correlates with locomotor activity under ambient light conditions when bees first emerge as adults. Humphries et al. (2005) tested locomotion in newly emerged wild type bees by measuring their walking activity in an enclosed arena under ambient light. They then determined their response to sucrose using the proboscis extension response protocols. Bees that were more responsive to sucrose were also more active in the light. A number of independent experiments with wild type nectar foragers have shown that the velocity of walking in the dark is not correlated with gustatory responsiveness but with foraging role (independent unpublished experiments by Hoormann, Erber, and Franz). Pollen foragers walk faster than nectar foragers. High-strain workers were more active than low-strain workers, consistent with the results from wild-type bees (Humphries et al., 2005). Rueppell et al., 2005 tested high-and low-strain males (drones) for locomotor activity under light and dark conditions. High-strain drones were more active under both conditions, which is consistent with the results from workers. Thus, these experiments suggest that wild type pollen and nectar foragers differ in locomotor activity and that the same relations are found in high-and low-strain bees.
In summary, the gustatory responses of bees to sucrose solutions are related to foraging behavior and to sensory responses to odor, pollen, and light. Pollen forages are more sensitive to sensory stimuli than nectar foragers. As a consequence of sensory sensitivities, stimulus evoked motor patterns are different in sensitive and insensitive animals. Locomotor activity differs between pollen and nectar foragers and also between high-and low-strain bees. Sucrose responsiveness can be used as a robust indicator for general differences of processing sensory information in the central nervous system.
D. Learning and Memory in Wild-Type Bees and Selected Strains
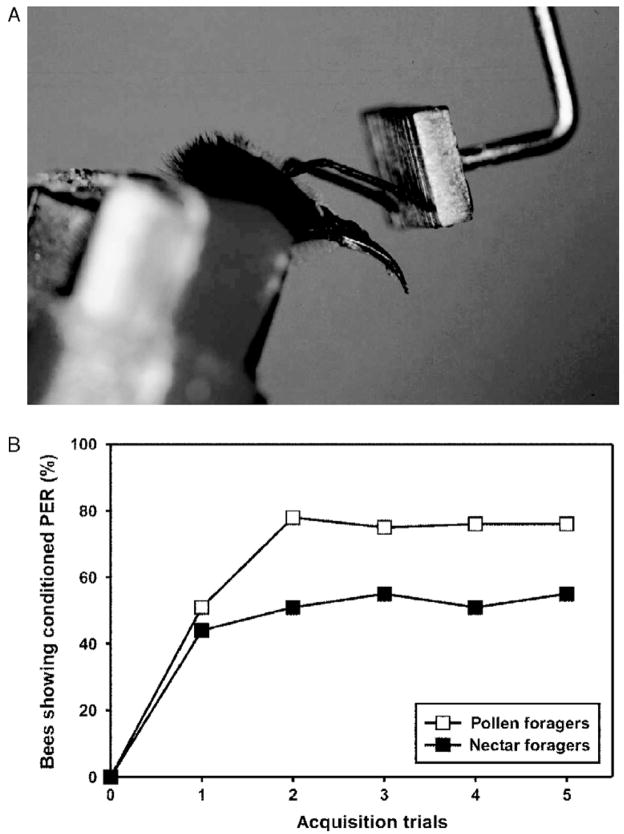
Division of foraging labor correlates with associative learning performance. In different laboratory learning paradigms, pollen foragers were shown to perform better than nectar foragers (Scheiner et al., 1999, 2001b, 2003a). In the tactile learning assay which was employed by Scheiner et al. (1999) to compare the learning performance of pollen and nectar foragers, bees were trained to associate the characteristics of a small metal plate with a sucrose reward. Returning pollen and nectar foragers were constrained in small tubes and their eyes were occluded with black paint to block visual inputs. The tactile object was brought into the scanning range of a bee. After the bees began scanning the target plate (the conditioned stimulus, CS) a droplet of sucrose solution was touched to one antenna (the unconditioned stimulus, US), eliciting the proboscis extension response (PER). A droplet of sucrose solution was then presented briefly to the tip of the proboscis as a reward (Erber et al., 1998). After few trials, most bees learned to respond to the plate without the US (Fig. 4). Pollen foragers learned faster than nectar foragers and reached a higher plateau in their acquisition function. These findings were later also demonstrated for olfactory learning, in which the bees have to associate an odor with a sucrose reward (Scheiner et al., 2003a).
Figure 4.
Tactile learning. (A) Honeybee showing conditioned proboscis extension response during tactile antennal conditioning. In this learning paradigm, the bee is rewarded for scanning a tactile object. The sucrose reward is briefly presented to the antenna. When the bee extends her proboscis after antennal stimulation with sucrose, she can imbibe a droplet of sucrose solution. After few conditioning trials, the bee shows conditioned proboscis extension while scanning the tactile target plate. (B) Acquisition curves of pollen and nectar foragers in tactile antennal learning. The x-axis shows the acquisition trials. The y-axis shows the percentage of bees displaying the conditioned proboscis extension response (PER). Both groups have reached the plateau of their acquisition function after three acquisition trials. However, the level of acquisition is higher in pollen foragers than in nectar foragers.
Learning differences were not only described for pollen and nectar foragers. High-strain bees perform better in tactile and olfactory learning tests than low-strain bees (Scheiner et al., 2001a,b). Because this is true for bees that have not yet initiated foraging, it demonstrates that it is not a function of foraging experience but has genetic determinants.
In general, bees that are more responsive to sucrose learn faster and reach a higher asymptote of learning than bees that are less responsive (Scheiner et al., 1999, 2001a,Scheiner et al., b,c, 2003a, 2004a, 2005). The probability of showing the conditioned response in retrieval tests 24 hours after conditioning is higher in bees that are more responsive to sucrose (Scheiner et al., 2004a, 2005).
If bees with a similar responsiveness to sucrose are tested for tactile or olfactory learning, they do not differ in their learning performance, regardless of their genotype or foraging role (Scheiner et al., 1999, 2001a,Scheiner et al., b, 2003a). These findings led to the hypothesis that learning performance is directly related to the evaluation of the sucrose stimulus used during conditioning. If this hypothesis is correct, it should be possible to induce an equal learning performance in bees with very different responsiveness to sucrose by giving them equal subjective rewards, based on their individual sucrose responsiveness. For individuals with high-sucrose responsiveness, a low-sucrose concentration should have the same subjective reward value as a high-sucrose concentration would have for a bee with low-responsiveness. This hypothesis was tested by Scheiner et al. (2005). A mathematical model for the individual reward value of sucrose was developed for bees that differ in gustatory sensitivity. Individuals were placed in classes according to their sucrose responsiveness. Based on their sucrose responsiveness, equal subjective reward concentrations were estimated. The performance and memory of all bees during conditioning and in the retrieval tests was very similar.
The correlation between learning performance and individual evaluation of the reward explains why pollen foragers learn better than nectar foragers and why high-strain bees perform better than low-strain bees. Pollen foragers are more responsiveness to sucrose than are nectar foragers; and high-strains bees are more responsive than low-strain bees. Bees with higher responsiveness place a higher reward value on sucrose and, therefore, reach a higher performance level (Page et al., 1998; Pankiw and Page, 2000; Scheiner et al., 1999, 2001b, 2003a, 2005). A similar relationship has been shown for nonassociative learning. Individuals with high-sucrose responsiveness need more trials for habituation of the proboscis extension response and display stronger sensitization by a sucrose stimulus than bees with low-sucrose responsiveness. Because individual sucrose responsiveness increases with age, older bees need more trials for habituation than younger bees (Scheiner, 2004).
E. Transmitter Systems and Neurochemical Signaling Cascades
1. Nervous System Signaling and Sensory Sensitivity
The set of variable correlated traits observed between pollen and nectar foragers, and between high-and low-strain bees, are centered on differences in sensory and motor response (see in an earlier section). As a consequence, differences in signaling cascades affecting sensory and motor response systems are prime candidates for understanding the neurobiochemical and genetic origin of variation in foraging behavior. Central components of nervous system signaling include biogenic amines, protein kinases, and second messengers that interact to affect sensory input, signal processing, and motor response.
a. Biogenic Amines
Biogenic amines modulate sensory and motor responses, traits that vary between pollen and nectar foragers, and bees from the high-and low-pollen hoarding strains. In honeybees, the four biogenic amines—dopamine, serotonin, octopamine, and tyramine have important functions in nervous system signaling (Blenau and Baumann, 2001, 2003). Their capability to modulate sensory sensitivity makes them candidates for the regulation of foraging behavior. Octopamine, which has been studied most extensively, generally increases sensitivity and related behavioral responses. Responsiveness to gustatory stimuli that are applied to the antenna, for example, is strongly increased after octopamine application (Braun and Bicker, 1992; Menzel et al., 1988, 1990; Scheiner et al., 2002). This amine also increases olfactory sensitivity (Menzel et al., 1991, 1994). Octopamine can also act on the visual system. It enhances, for example, the direction-specific visual antennal reflex (Erber and Kloppenburg, 1995; Erber et al., 1993a). Tyramine has a similar effect as octopamine on gustatory sensitivity (Scheiner et al., 2002). Otherwise, the behavioral role of this amine is less clear because until recently it was mainly considered as the biochemical precursor of octopamine rather than being a neurotransmitter itself. But interest in this amine has been growing since the first tyramine receptor of the bee was cloned (Blenau et al., 2000). Serotonin and dopamine often act antagonistically to octopamine in sensory systems. Dopamine, for example, reduces gustatory responsiveness (Scheiner et al., 2002). Serotonin, which has no effect on gustatory responses, decreases the direction-specific visual antennal reflex (Erber and Kloppenburg, 1995; Erber et al., 1993a, b).
Because sensory sensitivity correlates with different aspects of foraging behavior and because amines can modulate sensory sensitivity, we assume that biogenic amines are involved in division of foraging labor by modulating response-thresholds to foraging-related stimuli (see in a later section).
b. Protein Kinases and Second Messengers
Sensory responses involve complex signaling cascades of which the biogenic amines are only one part. Other important signaling molecules are second messengers, such as cAMP or cGMP, and protein kinases, which activate target proteins by phosphorylation of their threonine or serine residues. Stimulation of the antenna with sucrose, for example, increases the activity of cAMP-dependent protein kinase (PKA) (Hildebrandt and Müller, 1995). Octopamine injections can mimic antennal sucrose stimulation and lead to an increase in PKA activity. This suggests a close interaction of octopamine and PKA during sensing of gustatory stimuli presented to the antenna.
Responsiveness to sucrose correlates with activity of PKA in the antennal lobes (Scheiner et al., 2003b). Bees with high responsiveness to sucrose stimuli applied to the antenna have a higher baseline PKA activity than bees with low-sucrose responsiveness. Activation of PKA by application of 8-Br-cAMP increases responsiveness to sucrose (Scheiner et al., 2003b). High-and low-strain bees differ in their sucrose responsiveness and differ in brain titers for PKA, making cAMP activation of PKA a likely cause of this difference. Humphries et al. (2003) showed that bees selected for high-pollen hoarding have significantly higher titers of PKA than low-pollen hoarding bees at the time they emerge as adults and at 5 days of age. Whether high-and low-strain bees also differ in their PKA activity in the antennal lobes remains to be tested. Together, these findings imply a strong role of PKA in sensory responsiveness to gustatory stimuli applied to the antenna.
cGMP-dependent protein kinase (PKG) also appears to be involved in the perception of sucrose stimuli in insects. The two Drosophila variants—sitters and rovers, which differ in their PKG activity (Osborne et al., 1997) also differ in their responsiveness to sucrose (Scheiner et al., 2004b); and we have first indications that feeding of the PKG activator 8-Br-cGMP increases responsiveness to sucrose in honeybees (R. Scheiner and J. Erber, unpublished). Rueppell et al. (2004a,b), mapped a quantitative trait locus that affects responsiveness to sucrose close to Amfor, the honeybee gene for PKG, suggesting that variation in PKG between the high-and low-pollen hoarding strains may be affecting observed differences in sucrose responses. In addition, Ben-Shahar et al. (2003) showed that cGMP increases responsiveness to light. J. Tsuruda and R. E. Page (unpublished data) demonstrated that high-strain bees and wild-type bees with higher sucrose responsiveness are more responsive to light stimuli than low-strain bees and wild-type bees that are less responsive to sucrose. Differences in cGMP signaling provide a plausible explanation for these correlations. It is likely that significant cross talk occurs between the cAMP and cGMP pathways. PKA is activated by cGMP as well as by cAMP (Jiang et al., 2002), and some proteins can act as substrates for both PKA and PKG (Wang and Robinson, 1997).
These examples show the important role of biogenic amines, protein kinases, and second messengers in the modulation of sensory response thresholds. Individual differences in behavioral response thresholds are assumed to be at the basis of division of labor in insect colonies (Beshers and Fewell, 2001; Beshers et al., 1999; Page and Erber, 2002; Robinson, 1992; Theraulaz et al., 1998). Therefore, we can hypothesize that changes in the division of labor profile of a colony are, to some extent, induced by a complex interaction of biogenic amines, second messengers, and protein kinases. A number of studies show how these neuromodulators change division of labor, although the exact mechanisms behind these changes are still poorly understood.
2. Nervous System Signaling and Learning
Pollen and nectar foragers, and bees of the high-and low-pollen hoarding strains, differ in associative learning performance (see in an earlier section). Sensory system inputs are linked to motor-response systems through learning processes that alter behavior. Associative learning is an important part of honeybee behavior. Foragers, for example, have to remember the location of their hive and different food sources. Once they arrive at a flower, they have to remember how to find and handle the nectar and pollen that is presented. All bees of a colony must remember the odors of their hive and of their nest-mates. These are just a few examples. There are many more situations when bees perform associative learning tasks. Honeybees learn conditioned stimuli of different modalities very fast and establish long-lasting memories under free-flying conditions and in the laboratory (Bitterman et al., 1983; Giurfa, 2003; Menzel and Müller, 1996).
Biogenic amines, especially octopamine (OA) are important modulators of associative learning. Application of this amine improves olfactory acquisition, memory formation, and retrieval traits that distinguish pollen and nectar foragers; and bees from the high-and low-pollen hoarding strains. Octopamine injections into the calyx or the alpha-lobe of the mushroom bodies, which are assumed to be the centers of olfactory learning, enhance memory formation (Menzel et al., 1990), whereas injections of the octopamine-receptor antagonist mianserine into the antennal lobes or downregulation of the expression of the octopamine receptor AmOA1 strongly decrease acquisition and retrieval (Farooqui et al., 2003). Octopamine has also an important function at the cellular level of associative learning. It was shown in associative learning under laboratory conditions that the ventral unpaired median neuron 1 of the maxillary neuromere (VUMmx1 neuron; Hammer, 1993) depolarizes in response to the presentation of sucrose rewards to antennae and proboscis. Current injection into the VUMmx1 neuron can substitute for the sucrose reward during olfactory conditioning (Hammer, 1993; Hammer and Menzel, 1998). VUMmx1 belongs to a group of octopamine-immunoreactive neurons (Kreissl et al., 1994), and it is assumed that VUM neurons release octopamine, which could mediate the reward in some forms of associative conditioning (Hammer, 1997; Hammer and Menzel, 1998).
In contrast to octopamine, dopamine inhibits retrieval of information without affecting acquisition (Bicker and Menzel, 1989; Macmillan and Mercer, 1987; Menzel et al., 1988, 1990, 1994, 1999; Mercer and Menzel, 1982; Michelsen, 1988). Serotonin can reduce both acquisition and retrieval when injected prior to conditioning (Bicker and Menzel, 1989; Mercer and Menzel, 1982; Menzel et al., 1990, 1994). The effect of tyramine on associative learning has not been studied.
These examples imply that biogenic amines are involved in different pathways of associative learning in honeybees. Among the different signaling cascades, the PKA and the PKC signaling pathways are involved in memory formation (Grünbaum and Müller, 1998; Müller, 2000). High-and low-strain bees differ both in their brain content of these protein kinases (Humphries et al., 2003) and in their associative learning performance (Scheiner et al., 2001a,b), suggesting that these pathways are involved in foraging division of labor.
It can be assumed that biogenic amines affect associative learning performance by changing the sensory sensitivity for the unconditioned and conditioned stimuli because sensory sensitivity correlates with learning performance (see earlier). However, direct experimental proof is still needed for this hypothesis.
3. Nervous System Signaling and Division of Labor
The titers of dopamine, octopamine, and 5-HT (serotonin) increase as bees’ age, with the highest titers being found in foragers (Harris and Woodring, 1992; Schulz and Robinson, 1999; Schulz et al., 2004; Taylor et al., 1992; Wagener-Hulme et al., 1999). Because bees of different ages normally perform different tasks, it is conceivable that biogenic amine titers are part of the regulatory network for age-dependent division of labor. Whether the differences in biogenic-amine titers between bees of different ages are related to age differences or whether they are related to the different tasks the bees perform is often difficult to test. Single-cohort colonies can be very helpful for distinguishing between these alternatives. Thus Schulz and Robinson (1999) showed that differences in the titers of dopamine, octopamine, and 5-HT in mushroom bodies of foragers and nurse bees were related to age, whereas in the antennal lobes the differences were related to different tasks. Another way of studying the role of biogenic amines in division of labor is to manipulate amine titers and to determine the behavioral effects. Thus it was shown that octopamine induced bees to forage precociously, whereas tyramine had the opposite effect (Schulz and Robinson, 2001).
There are also some examples of how biogenic amines affect division of labor among same-aged bees. Božic and Woodring (1998) showed that bees who perform waggle dances after they returned from a foraging bout have higher titers of dopamine, octopamine, and 5-HT throughout the season than bees who followed the dancers. Another example comes from Taylor et al. (1992). They showed that pollen foragers had higher titers of dopamine in the optic lobes than in nectar foragers. Whereas pollen foragers also had 5-HT in the optic lobes, no serotonin was found in the optic lobes of nectar foragers.
OA has also been shown to affect response thresholds to brood pheromone. When hive bees were fed with OA for several days, their responsiveness to brood pheromone increased, and the bees subsequently increased their foraging activity (Barron et al., 2002). OA treatment did not increase responsiveness to queen mandibular pheromone, which would have resulted in a higher attendance in the queen’s retinue (Barron and Robinson, 2005). This implies that OA can modulate specific olfactory thresholds in different individuals and could thus be a major modulator of division of labor (Schulz and Robinson, 2001; Schulzet al., 2002a).
Because the selected high-and low-pollen hoarding strains of Page and Fondrk (1995) differ systematically in their responsiveness to sucrose and sensitivities for other stimulus modalities, it can be assumed that these strains differ in the titer of biogenic amines. However, Schulz et al. (2004) demonstrated that these strains do not differ in their brain titers of octopa-mine, dopamine, or 5-HT. Apparently, selection for increased pollen hoarding, which led to a suite of traits modulated by these amines, did not result in detectable differences in titers of amines. It is conceivable that different degrees of receptor activation or differences in the signaling cascades downstream the biogenic amines might be responsible for the observed differences in sucrose responsiveness. As discussed in an earlier section, high-strain bees do have higher brain titers of PKA and PKC than that of low-strain bees of equivalent age.
Experimental evidence of the role of second messengers in division of labor in honeybees is rare. Ben-Shahar et al. (2002) showed that cGMP-dependent protein kinase (PKG) is involved in the initiation of honeybee foraging behavior. This kinase is encoded by Amfor, the so-called “foraging gene”. Expression of Amfor is higher in foragers than in bees that have not initiated foraging, and application of the PKG activator 8-Br-cGMP induced precocious foraging (Ben-Shahar et al., 2002). Rueppell et al. (2004a) demonstrated that differences in Amfor, or a gene or genes nearby, explain differences in age of foraging onset between bees of the high-and low-pollen hoarding strains.
F. Hormonal Signaling Cascades
The systemic hormones—ecdysone and juvenile hormone (JH)—are key modulators of insect behavior (Cayre et al., 2000; Hartfelder, 2000). Ecdysone is produced by the prothoracic gland during larval and pupal development, and by the ovary during the adult stage. Putative effects of ecdysone in adult honeybees are currently elusive (Hartfelder et al., 2002; Robinson et al., 1991). JH is a growth hormone produced by the corpora allata of insects (Hagenguth and Rembold, 1978). JH has been hypothesized to play an important role in honeybee division of labor by pacing age-related changes in behavior, especially the transition to foraging (Robinson, 1992; Robinson and Vargo, 1997). Many studies have demonstrated elevated blood titers of JH in foragers relative to bees that perform tasks in the nest (Fahrbach et al., 2003; Huang and Robinson, 1992, 1995, 1996; Huang et al., 1994; Jassim et al., 2000; Robinson, 1987; Robinson et al., 1991; Sullivan et al., 2000, 2003; Withers et al., 1995). Treatment with the JH analog methoprene also results in bees initiating foraging behavior earlier in life (Bloch et al., 2002). Other evidence suggests that JH affects aspects of adult maturation. Young bees normally do not show associative learning the first 5–6 days after emergence (Ray and Ferneyhough, 1999). When treated topically with JH within 1hour after emergence, however, they show associative olfactory learning when they are 3 days old (Maleszka and Helliwell, 2001). Application of methoprene increases sucrose responsiveness in young bees (Pankiw and Page, 2003), and also elevates responses to alarm pheromones (Robinson, 1987). These roles of JH appear to be closely linked to OA. It is has been suggested that OA and JH regulate each other, and thus modulate the onset of foraging behavior and changes in responsiveness (Kaatz et al., 1994; Schulz et al., 2002a,b). Foragers have high titers of both JH and OA, particularly in the antennal lobes (Schulz and Robinson, 1999; Spivak et al., 2003). When 1-day-old bees are treated with methoprene, their levels of OA in the antennal lobes increase and they forage precociously (Schulzet al., 2002b). When thecorpora allatacomplex is surgically removed, workers are unable to produce JH. Such bees have been observed to initiate foraging later in life than sham treated controls (Sullivan et al., 2000). When the allatectomized workers are treated with methoprene or OA, they forage at an earlier age. These experiments suggest that OA acts downstream of JH. However, OA has also been shown to increase JH release from the corpora allata in vitro in a dose-dependent manner (Kaatz et al., 1994), suggesting that OA is upstream of JH in the regulatory cascade. Interactions between JH and OA are, therefore, not well understood.
Overall, JH correlates with age-based changes in honeybee behavior and sensory sensitivity, but is it pacing behavioral development? As mentioned in an earlier section, Sullivan et al. (2000) removed the corpora allata from newly emerged bees. The allatectomized workers initiated foraging, though slightly delayed in time relative to sham treated control bees. This result, which was obtained from observations of workers that returned from presumably successfully foraging flights of more than 15-min duration, was later called into question by data that included information on activities at the entrance of the nest. In this case, the allatectomized bees were observed initiating flight at the same time as controls (Sullivan et al., 2003). Worker honeybees from the high-and low-pollen hoarding strains initiate foraging at different ages and also differ in JH titer at adult emergence, however, their JH titer is not different 12 days later (Schulz et al., 2004). Thus, it is clear that JH is not necessary for behavioral development, but that treatments with JH and JH analog nonetheless have behavioral effects.
Insights that resolve this paradox emerged with the finding that vitellogenin gene activity suppresses the JH titer of worker bees (Guidugli et al., 2005). Vitellogenin is a major yolk precursor in many insects (Babin et al., 1999; Mann et al., 1999) and is also the most abundant hemolymph protein in worker bees that perform tasks in the nest prior to foraging (Engels and Fahrenhorst, 1974; Fluri et al., 1981, 1982). Juvenile hormone is known to suppress the synthesis of honeybee vitellogenin at onset of foraging (Pinto et al., 2000), but the effect of vitellogenin gene expression on JH further suggests that these two compounds are linked in a positive feedback loop via a mutual ability to suppress each other. This regulatory relationship is uncommon in insects and was hypothesized by Amdam and Omholt (2003). They argued that the evolution of an unconventional role of honeybee vitellogenin in brood-food synthesis (Amdam et al., 2003) selected for a mechanism that retains bees in the brood nest with high-vitellogenin levels. Foraging behavior, consequently, is triggered when the vitellogenin titer drops below a certain level. The feedback action of JH on vitellogenin is a reinforcing mechanism that causes the workers to become behaviorally and physiologically locked into the forager stage. In accordance with this hypothesis, M. Nelson, K. Ihle, G. Amdam, and R. Page (unpublished data) showed that reduction of vitellogenin gene activity by RNA interference (RNAi) causes bees to forage earlier in life. Amdam et al. (2006) demonstrated that vitellogenin RNAi increases the sucrose responsiveness of worker bees, and suggested that honeybee vitellogenin is a modulator of behavior and sensory sensitivity that acts via a signaling pathway that includes JH as a downstream feedback element.
Honeybee vitellogenin is produced by the abdominal fat body, but available evidence demonstrates that this protein triggers responses in other cell types (Guidugli et al., 2005), implying that vitellogenin itself can be classified as a hormone. The documented effects of JH and JH analog treatments can be understood as direct results of suppressed vitellogenin action, and predicts that high-pollen–hoarding strain bees, which forage earlier in life than workers from the low-pollen–hoarding strain (see earlier), should demonstrate a precocious drop in vitellogenin hormone titer. Data support this prediction (Amdam et al., unpublished data).
III. Genetic and Phenotypic Architecture of Pollen Hoarding
A. Genetic Architecture
Genetic mapping studies have revealed four major quantitative trait loci (QTL) that explain significant amounts of the phenotypic variance for pollen hoarding and foraging behavior between the high-and low-pollen hoarding strains (Hunt et al., 1995; Page et al., 2000; Rueppell et al., 2004a,b). Three QTL (pln1, pln2, and pln3) were identified by directly mapping the pollen hoarding trait at the colony level. They were subsequently confirmed by marker association studies of individual foraging behavior. A fourth QTL—pln4—was revealed by marker association studies.
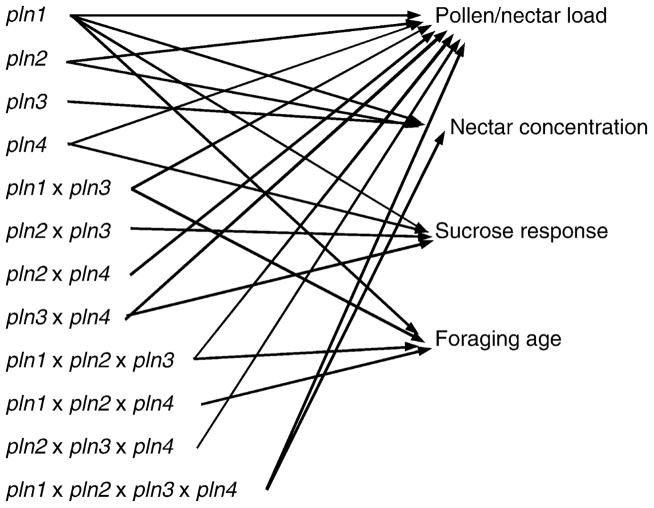
Ben-Shahar et al. (2002) demonstrated the effects of cGMP on the onset of foraging. They also demonstrated elevated titers of PKG (Amfor), a downstream target of cGMP in the signaling cascade in wild-type foragers relative to bees performing tasks in the nest. Genetic variants of PKG of Drosophila, the so-called foraging gene ( for), affect the feeding behavior of Drosophila larvae and are manifested as variation in their movement (DeBelle et al., 1989). The for gene also affects the responsiveness of Drosophila to sucrose solutions (Scheineret al., 2004b), therefore, it was a likely candidate gene for our studies. We found a polymorphism between our high-and low-strain bees in a non-coding region of Amfor and designed a marker (Rueppell et al., 2004a). Subsequent studies of bees derived from crosses of the high-and low-pollen hoarding strains demonstrated significant differences in behavior segregating with marker alleles from the two strains; (Rueppell et al., 2004a,b). This does not “prove” that Amfor itself is responsible for these effects, but it does demonstrate that Amfor or something close to it is having an effect. It is interesting that another important signaling gene—Amtyr1— mapped to pln2 (Humphries et al., unpublished), making these two signaling genes prime candidates for further research (Fig. 5).
Figure 5.
Genetic architecture of traits associated with foraging behavioral differences between the high-and low-pollen hoarding strains (Page and Fondrk, 1995).
The genetic architecture of pollen hoarding and foraging behavior is complex (Fig. 4). All QTL demonstrate pleiotropy, providing an explanation for the association of this set of traits. They are also richly epistatic, which would be expected if they are involved in complex hormonal and neuronal signaling networks. All individual QTL and most of their interactions affect pollen and nectar load sizes. All individual QTL also affect concentration of nectar collected. pln1 is central. It has a demonstrated direct effect on all behavioral traits. The combination of these QTL studies and the completed honeybee genome sequence and annotation should provide informed candidate genes for future studies of the underlying genetic basis for variation in pollen hoarding and foraging behavior.
B. Phenotypic Architecture
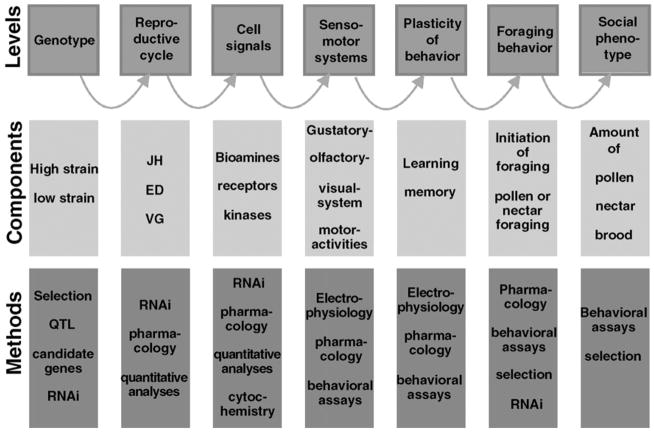
Results discussed in an earlier section, reveal a suite of covarying and interacting phenotypic traits that span behavior to neurobiochemistry that define the architecture of the pollen hoarding trait (Fig. 6). The foraging behavioral traits themselves covary. Pollen and nectar load sizes are negatively correlated as a result of constraints on maximum load sizes (Page et al., 2000). In addition, nectar load size correlates positively with nectar concentration (Page et al., 2000). Bees collect larger loads of nectar and smaller loads of pollen if the nectar has a higher concentration of sugar. Pankiw and Page (2000) showed that newly emerged wild-type bees that are more responsive to sucrose solutions forage earlier in life, collect nectar with lower concentrations of sugar, and collect larger pollen loads than those that are less responsive. This robust result was true for wild-type bees of European origin and Africanized bees (Pankiw, 2003).
Figure 6.
The phenotypic architecture of pollen hoarding behavior in honeybees. Levels of biological organization are shown on the top row spanning genotype to complex social behavior. Phenotypic traits were studied at each level and are shown in the middle row. The bottom row shows the methods that were used to study phenotypic traits at each level.
Key foci in this architecture are revealed by observed genotypic differences between the high-and low-strain bees, the rich set of correlations of gustatory sensitivity and response revealed by PER sucrose sensitivity assays, and the effects of biogenic amines on the suite of traits revealed by behavioral pharmacology. Collectively the results suggest the involvement of neuromodulatory networks involving cAMP signaling. These neuromodulatory networks affect correlated sensory response systems that include sensitivity to sugar, a central foraging stimulus for honeybees involved in foraging decision making, recruitment behavior, and associative learning. Findings that identify connections between pollen hoarding, vitellogenin hormone dynamics (Amdam et al., 2004), and ovary development (Amdam et al., 2006) suggest that pollen foraging behavior with the covarying suite of traits associated with it are modulated by a superior hierarchy of regulatory hormones involved in reproduction and reproductive behavior. It is likely that the neuromodulatory networks are themselves modulated by the reproductive hormones.
C. Reproductive Ground Plan
Amdam et al. (2004) proposed that the suite of traits associated with foraging behavior and the underlying complex genetic architecture could be explained if foraging specialization was derived from a reproductive regulatory network (West-Eberhard, 1987, 1996). In solitary insects, different stages of the female reproductive cycle (previtellogenesis, vitellogenesis, oviposition, and brood care) are linked and involve coupled physiological and behavioral changes (Finch and Rose, 1995; Lin and Lee, 1998; Miyatake, 2002). Juvenile hormone and ecdysone are key hormones controlling vitellogenesis in many insect species (Brownes, 1994; Hiremath and Jones, 1992; Ismail et al., 1998; Sankhon et al., 1999; Socha et al., 1991). In addition, they regulate behavioral transitions associated with changes in reproductive state such as the shift from foraging for nectar in previtello-genic females to protein foraging in vitellogenic individuals, as it occurs in the mosquito—Culex nigripalpus (Hancock and Foster, 2000). JH and ecdysone also modulate changes in sensory perception, locomotor activity, and reproductive physiology (Lin and Lee, 1998; Zera and Bottsford, 2001)—traits that have been shown to be different in workers from the high-and low-pollen–hoarding strains and in wild-type pollen and nectar foragers (see earlier).
In solitary insects, hormonal effects on reproductive traits typically act in mature adults (Fig. 7), following a prereproductive phase where the animals may enter diapause or aestivate and disperse (Hartfelder, 2000). In honeybees, however, these hormonal signals shifted in time (Amdam et al., 2004), occurring in the late pupal stages where they activate the production of vitellogenin (Barchuk et al., 2002). Differential amplitude of JH titers were observed in newly emerged high-and low-pollen–hoarding strain bees where high-strain workers had higher titers of JH (Schulz et al., 2004). This elevated titer correlates with a higher level of vitellogenin mRNA and a higher vitellogenin hormone titer in the blood (Amdam et al., 2004). Compared to the low-strain bees, workers of the high-pollen–hoarding strain have larger ovaries (they have more ovary filaments) that can show an active previtellogenic ovarian phenotype already at adult emergence (Amdam et al., 2006). It was proposed that if such documented markers of JH and ecdysone action are present early in honeybee adult life, then pleiotropic effects on behavior may have shifted from later life-stages as well (Amdam et al., 2004), as demonstrated by the differences in sensory responses and locomotor activity of high-and low-strain bees and the correlation of locomotor and sensory responses in wild-type workers.
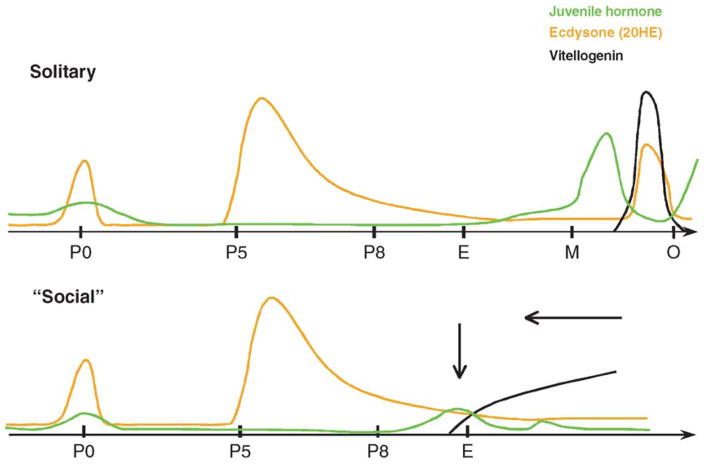
Figure 7.
A time course of blood hormone titers from early to late pupal stages (P0–P8) through emergence (E) and into mature prereproductive adults (M) to adults with activated ovaries (O) in solitary and social insects (Barchuk et al., 2002; Pinto et al., 2000). Amdam et al. (2006) hypothesized that the spikes of hormone titers seen between M and O in solitary insects have shifted with time in social insects and is homologous with the increases in titer observed just prior to adult emergence.
Reproductive signaling, early in life, can also explain the observed differences between newly emerged high-and low-strain bees in PKA and PKC titers (Humphries et al., 2003). These kinases play key roles in sensory sensitivity and learning. In addition, observed differences in Amtyr1 mRNA levels (Humphries, unpublished data) can be understood as a pleiotropic effect of a reproductive regulatory network because the tyramine pathway appears to be involved in reproductive tuning of queenless worker honeybees (Sasaki and Nagao, 2002). The finding that ovary size correlates with sensory responsiveness in 5-day-old bees (Tsuruda, unpublished data), and the known association between such sensory responses and foraging behavior 2–3 weeks later suggests that gonotrophic events in young bees have persistent effects on adult behavior. These insights are summarized in the “reproductive ground plan” hypothesis of social evolution (Amdam et al., 2004). The hypothesis proposes that the genetic and hormonal networks that govern reproductive development, physiology, and behavior in solitary species represent one fundamental regulatory module with capacity to serve as basis for evolution of social phenotypes.
IV. The Evolution of Division of Labor and Specialization
Division of labor between nest tasks and foraging activity, and foraging specialization on pollen and nectar, likely evolved from the gonotrophic cycle of solitary ancestors of the honeybee. The first step was a shift in the timing of reproductive hormonal signaling events from the mature adult stage into the late pupal stages (Amdam et al., 2004). This shift turned on the production of vitellogenin and further caused behavioral traits interlinked with reproductive maturity to be expressed in young bees. These vitellogenic females bypassed the phases of dispersal, diapause, and aestivation that characterized the ancestral prereproductive period. Instead, they expressed a coordinated set of maternal reproductive behaviors, including larval care, nest defense, and foraging (West-Eberhard, 1987, 1996). The second step was the evolution of a feedback interaction between vitellogenin and JH (Guidugli et al., 2005), apparently resulting in a regulatory mechanism that enabled vitellogenin to become a pace maker for division of labor. Higher blood titers of vitellogenin keep bees in the nest, performing maternal nonforaging tasks. Blood titers of vitellogenin decrease as a consequence of vitellogenin consumption in brood rearing (Amdam et al., 2003), and workers with low-vitellogenin levels—a state presumably incompatible with ability to nourish larvae—are triggered to leave the nest to perform foraging tasks. During this transition, a rapid increase in JH strongly suppresses remaining expression of vitellogenin, thereby producing a robust and definite differentiation of the forager phenotype (Amdam and Omholt, 2003; Guidugli et al., 2005). After the transition, bees with active ovaries preferentially forage for protein (pollen), as did their reproductively activated solitary ancestors that were provisioning their brood. Those with inactive ovaries forage primarily for nectar, as do nonreproductive solitary insect females (Dunn and Richards, 2003).
The early initiation of vitellogenesis is the necessary first step in social evolution via the subsocial route to sociality—staying and helping your mother raise siblings at the natal nest (Michener, 1974). Our model thus demystifies this first, essential step in social evolution and shows a simple, plausible mechanism by which it could have occurred. It also demonstrates that behavioral specialization can be an immediate emergent property, conferring a selective advantage to subsocial group living. Foraging specialization is, under our model, an immediate consequence of the ancestral interlinkage between reproductive tuning and foraging-preference for nectar or pollen. Temporal polyethism, furthermore, is an immediate consequence of variation in vitellogenin dynamics caused by developmental and nutritional factors. One factor that converges at the intersection between development and nutrition is worker ovary size: ovary size is determined during honeybee larval development and is influenced by nutrition (Hartfelder and Engels, 1998; Kaftanoglu et al., unpublished). In adult worker bees, ovary size is a documented component of the reproductive network that regulates social behavior (Amdam et al., 2006). Therefore, stochastic feeding events resulting in variation in ovary size could lead to differences in rates of onset of foraging behavior and foraging specialization. Finally, differences in stimulus–response sensitivities resulting from differences in reproductive states could result in a self-organized division of labor as described by Page and Mitchell, 1991, 1998; Fewell, 2003; Fewell and Page, 1999. Patterns of complex division of labor, thereby, emerged without explicit selection for task specialization.
By co-option and compartmentalization of the relationships between gonotrophic state and behavior, which was controlled originally by ancestral developmental programs, social insects evolved a division of labor and task specialization among functionally sterile individuals. Once in place, the social structure of colonies could be adapted by a fine-tuning of the pleiotropic hormonal and neuronal signaling networks that affected the behavior. However, these richly epistatic and pleiotropic networks would impose initial constraints on evolution by decreasing or masking the additive genetic variance available for natural selection, and by correlated responses to selection for traits not under direct selection. Over two decades, a concerted effort has succeeded in making these correlations transparent for the set of traits observed for the pollen-hoarding syndrome of the honeybee—in sum providing the first direct evidence for an evolutionary origin of complex social behavior.
Acknowledgments
Funding for the research presented here was provided by the National Science Foundation, National Institutes of Health, United States Department of Agriculture, Alexander von Humboldt Foundation, Deutsches Forschungsgemeinschaft, and the Norwegian Research Council.
References
- Amdam GV, Omholt SW. The hive bee to forager transition in honey bee colonies: The double repressor hypothesis. J Theor Biol. 2003;223:451–464. doi: 10.1016/s0022-5193(03)00121-8. [DOI] [PubMed] [Google Scholar]
- Amdam GV, Norberg K, Hagen A, Omholt SW. Social exploitation of vitellogenin. Proc Natl Acad Sci USA. 2003;100:1799–1802. doi: 10.1073/pnas.0333979100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amdam GV, Norberg K, Fondrk MK, Page RE. Reproductive ground plan may mediate colony-level selection effects on individual foraging behavior in honey bees. Proc Natl Acad Sci USA. 2004;101:11350–11355. doi: 10.1073/pnas.0403073101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amdam GV, Csondes A, Fondrk MK, Page RE. Complex social behavior derived from maternal reproductive traits. Nature. 2006;439:76–78. doi: 10.1038/nature04340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babin PJ, Bogerd J, Kooiman FP, Van Marrewijk WJA, Van der Horst DJ. Apolipophorin II/I, apolipoprotein B, vitellogenin, and microsomal triglyceride transfer protein genes are derived from a common ancestor. J Mol Evol. 1999;49:150–160. doi: 10.1007/pl00006528. [DOI] [PubMed] [Google Scholar]
- Barchuk AR, Bitondi MMG, Simões ZLP. Effects of juvenile hormone and ecdysone on the timing of vitellogenin appearance in hemolymph of queen and worker pupae of Apis mellifera. J Insect Sci. 2002:2.1. doi: 10.1673/031.002.0101. Available online at www.insectscience.org/2.1. [DOI] [PMC free article] [PubMed]
- Barron AB, Robinson GE. Selective modulation of task performance by octopamine in honey bee (Apis mellifera) division of labour. J Comp Physiol [A] 2005;191:659–668. doi: 10.1007/s00359-005-0619-7. [DOI] [PubMed] [Google Scholar]
- Barron AB, Schulz DJ, Robinson GE. Octopamine modulates responsiveness to foraging-related stimuli in honey bees (Apis mellifera) J Comp Physiol [A] 2002;188:603–610. doi: 10.1007/s00359-002-0335-5. [DOI] [PubMed] [Google Scholar]
- Ben-Shahar Y, Robichon A, Sokolowski MB, Robinson GE. Influence of gene action across different time scales on behavior. Science. 2002;296:741–744. doi: 10.1126/science.1069911. [DOI] [PubMed] [Google Scholar]
- Ben-Shahar Y, Leung HT, Pak WL, Sokolowski MB, Robinson GE. cGMP-dependent changes in phototaxis: A possible role for the foraging gene in honey bee division of labor. J Exp Biol. 2003;206:2507–2515. doi: 10.1242/jeb.00442. [DOI] [PubMed] [Google Scholar]
- Beshers SN, Fewell JH. Models of division of labor in social insects. Annu Rev Entomol. 2001;46:413–440. doi: 10.1146/annurev.ento.46.1.413. [DOI] [PubMed] [Google Scholar]
- Beshers SN, Robinson GE, Mittenthal JE. Response thresholds and division of labor in insect colonies. In: Detrain C, Deneubourg JL, Pasteels JM, editors. Information Processing in Social Insects. Birkhäuser Verlag; Basel, Switzerland: 1999. pp. 115–139. [Google Scholar]
- Bicker G, Menzel R. Chemical codes for the control of behavior in arthropods. Nature. 1989;337:33–39. doi: 10.1038/337033a0. [DOI] [PubMed] [Google Scholar]
- Bitterman ME, Menzel R, Fietz A, Schäfer S. Classical conditioning of proboscis extension in honey bees (Apis mellifera) J Comp Psychol. 1983;97:107–119. [PubMed] [Google Scholar]
- Blenau W, Baumann A. Molecular and pharmacological properties of insect biogenic amine receptors: Lessons from Drosophila melanogaster and Apis mellifera. Arch Insect Biochem Physiol. 2001;48(1):13–38. doi: 10.1002/arch.1055. [DOI] [PubMed] [Google Scholar]
- Blenau W, Baumann A. Aminergic signal transduction in invertebrates: Focus on tyramine and octopamine receptors. Recent Res Dev Neurochem. 2003;6:225–240. [Google Scholar]
- Blenau W, Balfanz S, Baumann A. Amtyr1: Characterization of a gene from honey bee (Apis mellifera) brain encoding a functional tyramine receptor. J Neurochem. 2000;74:900–908. doi: 10.1046/j.1471-4159.2000.0740900.x. [DOI] [PubMed] [Google Scholar]
- Bloch G, Wheeler D, Robinson GE. In: Hormones, Brain and Behavior. Pfaff D, Arnold A, Etgen A, Fahrbach S, Moss R, Rubin R, editors. Academic Press; San Diego: 2002. pp. 195–235. [Google Scholar]
- Božic J, Woodring J. Variations of brain biogenic amines in mature honey bees and induction of recruitment behavior. Comp Biochem Physiol A. 1998;120:737–744. [Google Scholar]
- Braun G, Bicker G. Habituation of an appetitive reflex in the honey bee. J Neurophysiol. 1992;67:588–598. doi: 10.1152/jn.1992.67.3.588. [DOI] [PubMed] [Google Scholar]
- Brownes M. The regulation of the yolk protein genes, a family of sex differentiation genes in Drosophila melanogaster. BioEssays. 1994;16:745–752. doi: 10.1002/bies.950161009. [DOI] [PubMed] [Google Scholar]
- Calderone NW, Page RE. Genotypic variability in age polyethism and task specialization in the honey bee, Apis mellifera (Hymenoptera: Apidae) Behav Ecol Sociobiol. 1988;22:17–25. [Google Scholar]
- Calderone NW, Page RE. Effects of interactions among genotypically diverse nestmates on task specialization by foraging honey bees (Apis mellifera) Behav Ecol Sociobiol. 1992;30:219–226. [Google Scholar]
- Cayre M, Strambi C, Strambi A, Charpin P, Ternaux J. Dual effect of ecdysone on adult cricket mushroom bodies. Eur J Neurosci. 2000;12:633–642. doi: 10.1046/j.1460-9568.2000.00947.x. [DOI] [PubMed] [Google Scholar]
- Crailsheim K. The protein balance of the honey bee worker. Apidologie. 1990;21:417–429. [Google Scholar]
- Crailsheim K, Schneider LHW, Hrassnigg N, Bühlmann G, Brosch U, Gmeinbauer R, Schöffmann B. Pollen consumption and utilization in worker honey bees (Apis mellifera carnica): Dependence on individual age and function. J Insect Physiol. 1992;38:409–419. [Google Scholar]
- Debelle JS, Hilliker AJ, Sokolowski MB. Genetic localization of foraging (for): A major gene for larval behavior in Drosophila melanogaster. Genetics. 1989;123:157–163. doi: 10.1093/genetics/123.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreller C, Tarpy DR. Perception of the pollen need by foragers in a honey bee colony. Anim Behav. 2000;59:91–96. doi: 10.1006/anbe.1999.1303. [DOI] [PubMed] [Google Scholar]
- Dreller C, Page RE, Fondrk MK. Regulation of pollen foraging in honey bee colonies: Effects of young brood, stored pollen, and empty space. Behav Ecol Sociobiol. 1999;45:227–233. [Google Scholar]
- Dunn T, Richards MH. When to bee social: Interactions among environmental constraints, incentives, guarding, and relatedness in a facultatively social carpenter bee. Behav Ecol. 2003;14:417–424. [Google Scholar]
- Engels W, Fahrenhorst H. Alters-und kastenspezifische Veräbderungen der Haemolymph-Protein-Spektren bei Apis mellificia. Roux’s Arch Dev Biol. 1974;174:285–296. doi: 10.1007/BF00573233. [DOI] [PubMed] [Google Scholar]
- Erber J, Kloppenburg P. The modulatory effects of serotonin and octopamine in the visual system of the honey bee (Apis mellifera L.): I. Behavioral analysis of the motion-sensitive antennal reflex. J Comp Physiol [A] 1995;176:111–118. [Google Scholar]
- Erber J, Pribbenow B, Grandy K, Kierzek S. Tactile motor learning in the antennal system of the honey bee (Apis mellifera) J Comp Physiol [A] 1997;181:355–365. [Google Scholar]
- Erber J, Kierzek S, Sander E, Grandy K. Tactile learning in the honey bee. J Comp Physiol A. 1998;183:737–744. [Google Scholar]
- Erber J, Kloppenburg P, Scheidler A. Neuromodulation by serotonin and octopamine in the honey bee: Behaviour, neuroanatomy and electrophysiology. Experientia. 1993a;49:1073–1083. [Google Scholar]
- Erber J, Pribbenow B, Bauer A, Kloppenburg P. Antennal reflexes in the honey bee: Tools for studying the nervous system. Apidologie. 1993b;24:283–296. [Google Scholar]
- Fahrbach SE, Farris SM, Sullivan JP, Robinson GE. Limits on volume changes in the mushroom bodies of the honey bee brain. J Neurobiol. 2003b;57:141–151. doi: 10.1002/neu.10256. [DOI] [PubMed] [Google Scholar]
- Farooqui T, Robinson K, Vaessin H, Smith BH. Modulation of early olfactory processing by an octopaminergic reinforcement pathway in the honey bee. J Neurosci. 2003;23:5370–5380. doi: 10.1523/JNEUROSCI.23-12-05370.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fewell JH. Social insect networks. Science. 2003;301:1867–1870. doi: 10.1126/science.1088945. [DOI] [PubMed] [Google Scholar]
- Fewell JH, Page RE. The emergence of division of labour in forced associations of normally solitary ant queens. Evol Ecol Res. 1999;1:537–548. [Google Scholar]
- Fewell JH, Page RE. Colony-level selection effects on individual and colony foraging task performance in honey bees, Apis mellifera L. Behav Ecol Sociobiol. 2000;48:173–181. [Google Scholar]
- Fewell JH, Winston ML. Colony state and regulation of pollen foraging in the honey bee, Apis mellifera L. Behav Ecol Sociobiol. 1992;30:387–393. [Google Scholar]
- Finch CE, Rose MR. Hormones and the physiological architecture of life history evolution. Q Rev Biol. 1995;70:1–52. doi: 10.1086/418864. [DOI] [PubMed] [Google Scholar]
- Fluri P, Sabatini AG, Vecchi MA, Wille H. Blood juvenile hormone, protein and vitellogenin titers in laying and non-laying queen honey bees. J Apicult Res. 1981;20:221–225. [Google Scholar]
- Fluri P, Lüscher M, Wille H, Gerig L. Changes in weight of the pharyngeal gland and haemolymph titers of juvenile hormone, protein and vitellogenin in worker honey bees. J Insect Physiol. 1982;28:61–68. [Google Scholar]
- Guidugli KR, Nascimento AM, Amdam GV, Barchuk AR, Angel R, Omholt SW, Simões ZLP, Hartfelder K. Vitellogenin regulates hormonal dynamics in the worker caste of a eusocial insect. FEBS Lett. 2005;579:4961–4965. doi: 10.1016/j.febslet.2005.07.085. [DOI] [PubMed] [Google Scholar]
- Giurfa M. The amazing mini-brain: Lessons from a honey bee. Bee World. 2003;84:5–18. [Google Scholar]
- Grünbaum L, Müller U. Induction of a specific olfactory memory leads to a long-lasting activation of protein kinase C in the antennal lobe of the honey bee. J Neurosci. 1998;18:4384–4392. doi: 10.1523/JNEUROSCI.18-11-04384.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagenguth H, Rembold H. Identification of juvenile hormone-3 as the only JH homolog in all developmental stages of the honey bee. Z Naturforsch [C] 1978;33C:847–850. [Google Scholar]
- Hammer M. An identified neuron mediates the unconditioned stimulus in associative olfactory learning in honey bees. Nature. 1993;366:59–63. doi: 10.1038/366059a0. [DOI] [PubMed] [Google Scholar]
- Hammer M. The neural basis of associative reward learning in honey bees. Trends Neurosci. 1997;20:245–252. doi: 10.1016/s0166-2236(96)01019-3. [DOI] [PubMed] [Google Scholar]
- Hammer M, Menzel R. Multiple sites of associative odor learning as revealed by local brain microinjections of octopamine in honey bees. Learn Mem. 1998;5:146–156. [PMC free article] [PubMed] [Google Scholar]
- Hancock RG, Foster WA. Exogenous Juvenile hormone and methoprene, but not male accessory gland substances or ovariectomy, affect the blood/nectar choice of female Culex nigiripalpus mosquitoes. Med Vet Entomol. 2000;14:373–382. doi: 10.1046/j.1365-2915.2000.00253.x. [DOI] [PubMed] [Google Scholar]
- Harris JW, Woodring J. Effects of stress, age, season, and source colony on levels of octopamine, dopamine and serotonin in the honey bee (Apis mellifera) brain. J Insect Physiol. 1992;38:29–35. [Google Scholar]
- Hartfelder K. Insect juvenile hormone: From “status quo” to high society. Braz J Med Biol Res. 2000;33:157–177. doi: 10.1590/s0100-879x2000000200003. [DOI] [PubMed] [Google Scholar]
- Hartfelder K, Engels W. Social insect polymorphism: Hormonal regulation of plasticity in development and reproduction in the honey bee. Curr Top Dev Biol. 1998;40:45–77. doi: 10.1016/s0070-2153(08)60364-6. [DOI] [PubMed] [Google Scholar]
- Hartfelder K, Bitondi MMG, Santana WC, Simões ZLP. Ecdysteroid titer and reproduction in queens and workers of the honey bee and of a stingless bee: Loss of ecdysteroid function at increasing levels of sociality? Insect Biochem Mol Biol. 2002;32:211–216. doi: 10.1016/s0965-1748(01)00100-x. [DOI] [PubMed] [Google Scholar]
- Hellmich RL, II, Kulincevic JM, Rothenbuhler WC. Selection for high and low pollen-hoarding honey bees. J Hered. 1985;76:155–158. [Google Scholar]
- Hildebrandt H, Müller U. Octopamine mediates rapid stimulation of protein kinase A in the antennal lobe of honey bees. J Neurobiol. 1995;27:44–50. doi: 10.1002/neu.480270105. [DOI] [PubMed] [Google Scholar]
- Hiremath S, Jones D. Juvenile hormone regulation of vitellogenin in the gypsy moth, Lymantria Dispar: Suppression of vitellogenin mRNA in the fat body. J Insect Physiol. 1992;38:461–474. [Google Scholar]
- Huang ZY, Robinson GE. Honey bee colony integration: Worker-worker interactions mediate hormonally regulated plasticity in division of labor. Proc Natl Acad Sci USA. 1992;89:11726–11729. doi: 10.1073/pnas.89.24.11726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang ZY, Robinson GE. Seasonal changes in juvenile hormone titers and rates of biosynthesis in honey bees. J Comp Physiol [B] 1995;165:18–28. doi: 10.1007/BF00264682. [DOI] [PubMed] [Google Scholar]
- Huang ZY, Robinson GE. Regulation of honey bee division of labor by colony age demography. Behav Ecol Sociobiol. 1996;39:147–158. [Google Scholar]
- Huang ZY, Robinson GE, Borst DW. Physiological correlates of division of labor among similarly aged honey bees. J Physiol A. 1994;174:731–739. doi: 10.1007/BF00192722. [DOI] [PubMed] [Google Scholar]
- Humphries MA, Müller U, Fondrk MK, Page RE. PKA and PKC content in the honey bee central brain differs in genotypic strains with distinct foraging behavior. J Comp Physiol A. 2003;189:555–562. doi: 10.1007/s00359-003-0433-z. [DOI] [PubMed] [Google Scholar]
- Humphries MA, Fondrk MK, Page RE. Locomotion and the pollen hoarding behavioral syndrome of the honey bee (Apis mellifera L.) J Comp Physiol A. 2005;191:669–674. doi: 10.1007/s00359-005-0624-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt GJ, Page RE, Fondrk MK, Dullum CJ. Major quantitative trait loci affecting honey bee foraging behavior. Genetics. 1995;141:1537–1545. doi: 10.1093/genetics/141.4.1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ismail SM, Satyanarayana K, Bradfield JY, Dahm KH, Bhaskaran G. Juvenile hormone acid: Evidence for a hormonal function in induction of vitellogenin in larvae of Manduca sexta. Arch Insect Biochem Physiol. 1998;37:305–314. doi: 10.1002/(SICI)1520-6327(1998)37:4<305::AID-ARCH6>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Jassim O, Huang ZY, Robinson GE. Juvenile hormone profiles of worker honey bees, Apis mellifera, during normal and accelerated behavioral development. J Insect Physiol. 2000;46:243–249. doi: 10.1016/s0022-1910(99)00176-6. [DOI] [PubMed] [Google Scholar]
- Jiang H, Colbran JL, Francis SH, Corbin JD. Direct evidence for cross-activation of cGMP-dependent protein kinase by cAMP in pig coronary arteries. J Biol Chem. 2002;267:1015–1019. [PubMed] [Google Scholar]
- Kaatz H, Eichmüller S, Kreissl S. Stimulatory effect of octopamine on juvenile hormone biosynthesis in honey bees (Apis mellifera): Physiological and immunocytochemical evidence. J Insect Physiol. 1994;40:865–872. [Google Scholar]
- Kreissl S, Eichmüller S, Bicker G, Rapus J, Eckert M. Octopamine-like immunoreactivity in the brain and subesophageal ganglion of the honey bee. J Comp Neurol. 1994;348:583–595. doi: 10.1002/cne.903480408. [DOI] [PubMed] [Google Scholar]
- Kunze G. Einige Versuche über den Geschmackssinn der Honigbiene. Zool Jahrb Allg Zool. 1933;52:465–512. [Google Scholar]
- Lin T, Lee H. Parallel control mechanisms underlying locomotor activity and sexual receptivity in the female German cockroach, Blattella germanica (L.) J Insect Physiol. 1998;44:1039–1051. doi: 10.1016/s0022-1910(98)00069-9. [DOI] [PubMed] [Google Scholar]
- Macmillan CS, Mercer AR. An investigation of the role of dopamine in the antennal lobes of the honey bee, Apis mellifera. J Comp Physiol [A] 1987;160:359–366. [Google Scholar]
- Maleszka R, Helliwell P. Effect of juvenile hormone on short-term olfactory memory in young honey bees (Apis mellifera) Horm Behav. 2001;40:403–408. doi: 10.1006/hbeh.2001.1705. [DOI] [PubMed] [Google Scholar]
- Mann CJ, Anderson TA, Read J, Chester SA, Harrison GB, Köchl S, Ritchie PJ, Bradbury P, Hussain FS, Amey J, Vanloo B, Rosseneu M, et al. The structure of vitellogenin provides a molecular model for the assembly and secretion of atherogenic lipoproteins. J Mol Biol. 1999;285:391–408. doi: 10.1006/jmbi.1998.2298. [DOI] [PubMed] [Google Scholar]
- Marshall J. On the sensitivity of the chemoreceptors on the antenna and fore-tarsus of the honey-bee, Apis mellifica L. J Exp Biol. 1935;12:17–26. [Google Scholar]
- Menzel R, Müller U. Learning and memory in honey bees: From behavior to neural substrates. Annu Rev Neurosci. 1996;19:379–404. doi: 10.1146/annurev.ne.19.030196.002115. [DOI] [PubMed] [Google Scholar]
- Menzel R, Michelsen B, Rüffer P, Sugawa M. Neuropharmacology of learning and memory in honey bees. In: Hertting G, Spatz H-C, editors. Modulation of Synaptic Transmission and Plasticity in Nervous Systems. H 19. Springer-Verlag, NATO ASI Series; Berlin, Heidelberg: 1988. pp. 333–350. [Google Scholar]
- Menzel R, Wittstock S, Sugawa M. Chemical codes of learning and memory in honey bees. In: Squire L, Lindenlaub K, editors. The Biology of Memory. Stuttgart; Schattauer: 1990. pp. 335–360. [Google Scholar]
- Menzel R, Hammer M, Braun G, Mauelshagen J, Sugawa M. Neurobiology of learning and memory in honey bees. In: Goodman LJ, Fisher RC, editors. The Behaviour and Physiology of Bees. CAB International, Wallingford; Oxon, United Kingdom: 1991. pp. 323–353. [Google Scholar]
- Menzel R, Durst C, Erber J, Eichmüller S, Hammer M, Hildebrandt H, Mauelshagen J, Müller U, Rosenboom H, Rybak J, Schäfer S, Scheidler A. The mushroom bodies in the honey bee: From molecules to behaviour. Forts Zool. 1994;39:81–103. [Google Scholar]
- Menzel R, Heyne A, Kinzel C, Gerber B, Fiala A. Pharmacological dissociation between the reinforcing, sensitizing, and response-releasing functions of reward in honey bee classical conditioning. Behav Neurosci. 1999;113:744–754. [PubMed] [Google Scholar]
- Mercer AR, Menzel R. The effects of biogenic amines on conditioned and unconditioned responses to olfactory stimuli in the honey bee Apis mellifera. J Comp Physiol. 1982;145:363–368. [Google Scholar]
- Michelsen DB. Catecholamines affect storage and retrieval of conditioned odor stimuli in honey bees. Comp Biochem Physiol [C] 1988;91:479–482. [Google Scholar]
- Michener CD. The Social Behavior of the Bees: A Comparative Study. Harvard University Press; Cambridge, MA: 1974. [Google Scholar]
- Müller U. Prolonged activation of cAMP-dependent protein kinase during conditioning induces long-term memory in honey bees. Neuron. 2000;27:7–8. doi: 10.1016/s0896-6273(00)00017-9. [DOI] [PubMed] [Google Scholar]
- Miyatake T. Circadian rhythm and time of mating in Bactrocera cucurbitae (Diptera: Tephritidae) selected of age at reproduction. Heredity. 2002;88:302–306. doi: 10.1038/sj.hdy.6800044. [DOI] [PubMed] [Google Scholar]
- Osborne KA, Robichon A, Burgess E, Butland S, Shaw RA, Coulthard A, Pereira HS, Greenspan RJ, Sokolowski MB. Natural behavior polymorphism due to a cGMP-dependent protein kinase of Drosophila. Science. 1997;277:834–836. doi: 10.1126/science.277.5327.834. [DOI] [PubMed] [Google Scholar]
- Page RE, Erber J. Levels of behavioral organization and the evolution of division of labor. Naturwissenschaften. 2002;89:91–106. doi: 10.1007/s00114-002-0299-x. [DOI] [PubMed] [Google Scholar]
- Page RE, Fondrk MK. The effects of colony-level selection on the social organization of honey bee (Apis mellifera L.) colonies: Colony-level components of pollen hoarding. Behav Ecol Sociobiol. 1995;36:135–144. [Google Scholar]
- Page RE, Mitchell SD. Self-organization and the evolution of division of labor. Apidologie. 1998;29:171–190. [Google Scholar]
- Page RE, Mitchell SD. Self organization and adaptation in insect societies. In: Fine A, Forbes M, WL, editors. PSA. Philosophy of Science Association; East Lansing, MI: 1991. pp. 289–298. [Google Scholar]
- Page RE, Erber J, Fondrk MK. The effect of genotype on response thresholds to sucrose and foraging behavior of honey bees (Apis mellifera L.) J Comp Physiol A. 1998;182:489–500. doi: 10.1007/s003590050196. [DOI] [PubMed] [Google Scholar]
- Page RE, Fondrk MK, Hunt GJ, Guzmán-Novoa E, Humphries MA, Nguyen K, Green AS. Genetic dissection of honey bee (Apis mellifera L.) foraging behavior. J Hered. 2000;91:474–479. doi: 10.1093/jhered/91.6.474. [DOI] [PubMed] [Google Scholar]
- Pankiw T. Directional change in a suite of foraging behaviors in tropical and temperate evolved honey bees (Apis mellifera L.) Behav Ecol Sociobiol. 2003;54:458–464. [Google Scholar]
- Pankiw T, Page RE. The effect of genotype, age, sex, and caste on response thresholds to sucrose and foraging behavior of honey bees (Apis mellifera L.) J Comp Physiol [A] 1999;185:207–213. doi: 10.1007/s003590050379. [DOI] [PubMed] [Google Scholar]
- Pankiw T, Page RE. Response thresholds to sucrose predict foraging division of labor in honey bees. Behav Ecol Sociobiol. 2000;47:265–267. [Google Scholar]
- Pankiw T, Page RE. Genotype and colony environment affect honey bee (Apis mellifera L.) development and foraging behavior. Behav Ecol Sociobiol. 2001;51:87–94. [Google Scholar]
- Pankiw T, Page RE. The effect of pheromones, hormones, and handling on sucrose response thresholds of honey bees (Apis mellifera L.) J Comp Physiol A. 2003;189:675–684. doi: 10.1007/s00359-003-0442-y. [DOI] [PubMed] [Google Scholar]
- Pankiw T, Page RE, Fondrk MK. Brood pheromone stimulates pollen foraging in honey bees (Apis mellifera) Behav Ecol Sociobiol. 1998;44:193–198. [Google Scholar]
- Pankiw T, Waddington KD, Page RE., Jr Modulation of sucrose response thresholds in honey bees (Apis mellifera L.): Influence of genotype, feeding and foraging experience. J Comp Physiol A. 2001;187:293–301. doi: 10.1007/s003590100201. [DOI] [PubMed] [Google Scholar]
- Pankiw T, Tarpy DR, Page RE., Jr Genotype and rearing environment affect honey bee perception and foraging behaviour. Anim Behav. 2002;64:663–672. [Google Scholar]
- Pankiw T, Nelson M, Page RE, Fondrk MK. The communal crop: Modulation of sucrose response thresholds of preforaging honey bees with incoming nectar quality. Behav Ecol Sociobiol. 2004;55:286–292. [Google Scholar]
- Pinto LZ, Bitondi MMG, Simões ZLP. Inhibition of vitellogenin synthesis in Apis mellifera workers by a juvenile hormone analogue, pyriproxyfen. J Insect Physiol. 2000;46:153–160. doi: 10.1016/s0022-1910(99)00111-0. [DOI] [PubMed] [Google Scholar]
- Ray S, Ferneyhough B. Behavioral development and olfactory learning in the honey bee (Apis mellifera) Dev Psychobiol. 1999;34:21–27. [PubMed] [Google Scholar]
- Robinson GE. Regulation of honey bee age polyethism by juvenile hormone. Behav Ecol Sociobiol. 1987;20:329–338. [Google Scholar]
- Robinson GE. Regulation of division of labor in insect societies. Annu Rev Entomol. 1992;37:637–665. doi: 10.1146/annurev.en.37.010192.003225. [DOI] [PubMed] [Google Scholar]
- Robinson GE, Vargo EL. Juvenile hormone in adult eusocial hy-menoptera: Gonadotropin and behavioral pacemaker. Arch Insect Biochem Physiol. 1997;35:559–583. doi: 10.1002/(SICI)1520-6327(1997)35:4<559::AID-ARCH13>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Robinson GE, Strambi C, Strambi A, Feldlaufer MF. Comparison of juvenile hormone and ecdysteroid haemolymph titers in adult worker and queen honey bees (Apis mellifera) J Insect Physiol. 1991;37:929–935. [Google Scholar]
- Rueppell O, Page RE, Fondrk MK. Male behavioral maturation rate responds to selection on pollen hoarding in honey bees. Anim Behav. 2005;71:227–234. doi: 10.1016/j.anbehav.2005.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rueppell O, Pankiw T, Nielson D, Fondrk MK, Beye M, Page RE. The genetic architecture of the behavioral ontogeny of honey bee workers. Genetics. 2004a;167:1767–1779. doi: 10.1534/genetics.103.021949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rueppell O, Pankiw T, Page RE. Pleiotropy, epistasis and new QTL: The genetic architecture of honey bee foraging behavior. J Hered. 2004b;95:481–491. doi: 10.1093/jhered/esh072. [DOI] [PubMed] [Google Scholar]
- Sankhon N, Lockey T, Rosell RC, Rothschild M, Coons L. Effect of methoprene and 20-hydroxyecdysone on vitellogenin production in cultured fat bodies and backless explants from unfed female Dermacentor variabilis. J Insect Physiol. 1999;45:755–761. doi: 10.1016/s0022-1910(99)00054-2. [DOI] [PubMed] [Google Scholar]
- Sasaki K, Nagao T. Brain tyramine and reproductive states of workers in honey bees. J Insect Physiol. 2002;48:1075–1085. doi: 10.1016/s0022-1910(02)00200-7. [DOI] [PubMed] [Google Scholar]
- Scheiner R. Responsiveness to sucrose and habituation of the proboscis extension response in honey bees. J Comp Physiol A. 2004;190:727–733. doi: 10.1007/s00359-004-0531-6. [DOI] [PubMed] [Google Scholar]
- Scheiner R, Erber J, Page RE. Tactile learning and the individual evaluation of the reward in honey bees (Apis mellifera L.) J Comp Physiol A. 1999;185:1–10. doi: 10.1007/s003590050360. [DOI] [PubMed] [Google Scholar]
- Scheiner R, Page RE, Erber J. Responsiveness to sucrose affects tactile and olfactory learning in preforaging honey bees of two genetic strains. Behav Brain Res. 2001a;120:67–73. doi: 10.1016/s0166-4328(00)00359-4. [DOI] [PubMed] [Google Scholar]
- Scheiner R, Page RE, Erber J. The effects of genotype, foraging role, and sucrose responsiveness on the tactile learning performance of honey bees (Apis mellifera L.) Neurobiol Learn Mem. 2001b;76:138–150. doi: 10.1006/nlme.2000.3996. [DOI] [PubMed] [Google Scholar]
- Scheiner R, Weiß A, Malun D, Erber J. Learning in honey bees with brain lesions: How partial mushroom-body ablations affect sucrose responsiveness and tactile learning. Anim Cogn. 2001c;4:227–235. [Google Scholar]
- Scheiner R, Plückhahn S, Ö ney B, Blenau W, Erber J. Behavioural pharmacology of octopamine, tyramine and dopamine in honey bees. Behav Brain Res. 2002;136:545–553. doi: 10.1016/s0166-4328(02)00205-x. [DOI] [PubMed] [Google Scholar]
- Scheiner R, Barnert M, Erber J. Variation in water and sucrose responsiveness during the foraging season affects proboscis extension learning in honey bees. Apidologie. 2003a;34:67–72. [Google Scholar]
- Scheiner R, Müller U, Heimburger S, Erber J. Activity of protein kianse A and gustatory responsiveness in the honey bee (Apis mellifera L.) J Comp Physiol A. 2003b;189:427–434. doi: 10.1007/s00359-003-0419-x. [DOI] [PubMed] [Google Scholar]
- Scheiner R, Page RE, Erber J. Sucrose responsiveness and behavioral plasticity in honey bees (Apis mellifera) Apidologie. 2004a;35:133–142. [Google Scholar]
- Scheiner R, Sokolowski M, Erber J. Activity of cGMP-dependent protein kinase (PKG) affects sucrose responsiveness and habituation in Drosophila melanogaster. Learn Mem. 2004b;11:303–311. doi: 10.1101/lm.71604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheiner R, Kuritz-Kaiser A, Menzel R, Erber J. Sensory responsiveness and the effects of equal subjective rewardson tactile learning and memory of honey bees. Learn Mem. 2005;12:626–635. doi: 10.1101/lm.98105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz DJ, Robinson GE. Biogenic amines and division of labor in honey bee colonies: Behaviorally related changes in the antennal lobes and age-related changes in the mushroom bodies. J Comp Physiol [A] 1999:488. doi: 10.1007/s003590050348. [DOI] [PubMed] [Google Scholar]
- Schulz DJ, Robinson GE. Octopamine influences division of labor in honey bee colonies. J Comp Physiol [A] 2001 doi: 10.1007/s003590000177. [DOI] [PubMed] [Google Scholar]
- Schulz DJ, Barron AB, Robinson GE. A role for octopamine in honey bee division of labor. Brain Behav Evol. 2002a;60:350–359. doi: 10.1159/000067788. [DOI] [PubMed] [Google Scholar]
- Schulz DJ, Sullivan JP, Robinson GE. Juvenile hormone and octopamine in the regulation of division of labor in honey bee colonies. Horm Behav. 2002b doi: 10.1006/hbeh.2002.1806. [DOI] [PubMed] [Google Scholar]
- Schulz DJ, Pankiw T, Fondrk MK, Robinson GE, Page RE. Comparison of juvenile hormone hemolymph and octopamine brain titers in honey bees (Hymenoptera: Apidae) selected for high and low pollen hoarding. Ann Entomol Soc Amer. 2004;97:1313–1319. [Google Scholar]
- Seeley TD. Adaptive significance of the age polyethism schedule in honey bee colonies. Behav Ecol Sociobiol. 1982;11:287–293. [Google Scholar]
- Snodgrass RE. Anatomy of the Honey Bee. Comstock Publishing Associates; Ithaca, NY: 1956. [Google Scholar]
- Socha R, Sula J, Kodrík D, Gelbic I. Hormonal control of vitellogenin synthesis in Pyrrhocoris apterus (L.) (Heteroptera) J Insect Physiol. 1991;37:805–816. [Google Scholar]
- Spivak M, Masterman R, Ross R, Mesce KA. Hygienic behavior in the honey bee (Apis mellifera L.) and the modulatory role of octopamine. J Neurobiol. 2003;55:341–354. doi: 10.1002/neu.10219. [DOI] [PubMed] [Google Scholar]
- Sullivan JP, Jassim O, Fahrbach SE, Robinson GE. Juvenile hormone paces behavioral development in the adult worker honey bee. Horm Behav. 2000;37:1–14. doi: 10.1006/hbeh.1999.1552. [DOI] [PubMed] [Google Scholar]
- Sullivan JP, Fahrbach SE, Harrison JF, Capaldi EA, Fewell JH, Robinson GE. Juvenile hormone and division of labor in honey bee colonies: Effects of allatectomy on flight behavior and metabolism. J Exp Biol. 2003;206:2287–2296. doi: 10.1242/jeb.00432. [DOI] [PubMed] [Google Scholar]
- Taylor DJ, Robinson GE, Logan BJ, Laverty R, Mercer AR. Changes in brain amine levels associated with the morphological and behavioural development of the worker honey bee. J Comp Physiol A. 1992;170:715–721. doi: 10.1007/BF00198982. [DOI] [PubMed] [Google Scholar]
- Theraulaz G, Bonabeau E, Deneubourg JL. Response threshold reinforcement and division of labour in insect societies. Proc R Soc Lond B. 1998;265:327–332. [Google Scholar]
- Wagener-Hulme C, Kuehn JC, Schulz DJ, Robinson GE. Biogenic amines and division of labor in honey bee colonies. J Comp Physiol [A] 1999;184:471–479. doi: 10.1007/s003590050347. [DOI] [PubMed] [Google Scholar]
- Wang X, Robinson PJ. Cyclic GMP-dependent protein kinase and cellular signaling in the nervous system. J Neurochem. 1997;68:443–456. doi: 10.1046/j.1471-4159.1997.68020443.x. [DOI] [PubMed] [Google Scholar]
- West-Eberhard MJ. In: Animal Societies: Theories and Fact. Itô Y, Brown JL, Kikkawa J, editors. Japan Science Press; Tokyo: 1987. pp. 35–51. [Google Scholar]
- West-Eberhard MJ. In: Natural History and Evolution of Paper Wasp. Turillazzi S, West-Eberhard MJ, editors. Oxford University Press; New York: 1996. pp. 290–317. [Google Scholar]
- Wheeler WM. The social insects. Harcourt, Brace & Co; New York: 1928. [Google Scholar]
- Winston ML. The Biology of the Honey Bee. Harvard University Press; Cambridge, MA: 1987. [Google Scholar]
- Withers GS, Fahrbach SE, Robinson GE. Effects of experience and juvenile hormone on the organization of the mushroom bodies of honey bees. J Neurobiol. 1995;26:130–144. doi: 10.1002/neu.480260111. [DOI] [PubMed] [Google Scholar]
- Zera AJ, Bottsford J. The endocrine-genetic basis of life-history variation: The relationship between the ecdysteroid titer and morph-specific reproduction in the wing-polymorphic cricket Gryllus firmus. Evolution. 2001;55:538–549. doi: 10.1554/0014-3820(2001)055[0538:tegbol]2.0.co;2. [DOI] [PubMed] [Google Scholar]