SUMMARY
Mouse models of cystic fibrosis (CF) indicate that sulfotransferase (SULT) 1E1 is significantly induced in livers of many mice lacking cystic fibrosis transmembrane receptor (CFTR) activity. Increased SULT1E1 activity results in the alteration of estrogen-regulated protein expression in the livers of these mice. In this study, human MMNK-1 cholangiocytes with repressed CFTR function were used to induce SULT1E1 expression in human HepG2 hepatocytes to investigate whether SULT1E1 can be increased in human CF liver. CFTR expression was inhibited in MMNK-1 cholangiocytes using CFTR-siRNA, then the MMNK-1 and HepG2 cells were co-cultured in a membrane-separated Transwell system. Expression of SULT1E1 and selected estrogen-regulated proteins were then assayed in the HepG2 cells. Results demonstrate that inhibition of CFTR expression in MMNK-1 cells results in the induction SULT1E1 message and activity in HepG2 cells in the Transwell system. The expression of estrogen-regulated proteins including insulin-like growth factor (IGF)-1, glutathione S-transferase (GST) P1 and carbonic anhydrase (CA) II expression are repressed in the HepG2 cells cultured with the CFTR-siRNA-MMNK-1 cells apparently in response to the increased sulfation of β-estradiol. Thus, we have shown that co-culture of HepG2 hepatocytes with MMNK-1 cholangiocytes with siRNA repressed CFTR expression results in the selective induction of SULT1E1 in the HepG2 cells. Loss of CFTR function in cholangiocytes may have a paracrine regulatory effect on hepatocytes via the induction of SULT1E1 and the increased sulfation of β-estradiol. Experiments are presently underway in our laboratory to elucidate the identity of these paracrine regulatory factors.
Keywords: Sulfotransferase, SULT1E1, cystic fibrosis, cystic fibrosis transmembrane conductance regulator, hepatocyte, cholangiocyte
INTRODUCTION
Cystic fibrosis (CF) is a chronic, progressive, genetic disease that affects approximately 1 of every 2500 Caucasian newborns [1] and is caused by mutations in the cystic fibrosis transmembrane receptor (CFTR) gene. Although lung disease is the primary cause of morbidity and mortality, non-pulmonary disorders including pancreatic, liver and intestinal diseases are also becoming more prevalent as the lifespan of CF patients increases. With improved pulmonary therapy CF patient survival often extends into adulthood and liver disease (LD) has become the second leading cause of death in CF [2, 3]. The initiating event in CFLD development is unknown; however, CFLD appears as a progressive fibrogenic process that leads to cirrhosis in some patients [4]. Approximately 15–20% of CF patients are diagnosed with CFLD in childhood and the progression of the disease occurs within this group [1, 3]. Genetic and environmental factors may modify this process and be factors in explaining the heterogeneity in the liver response to abnormal CFTR function [5]. To date, a cohesive mechanism explaining the pathogenesis of CFLD is lacking.
In mouse models of CF generated by expression of improperly processed CFTR (Δ508) or lacking CFTR expression (knockout, KO), there is a selective induction of sulfotransferase 1E1 (SULT1E1) in liver hepatocytes [6]. SULT1E1 catalyzes the conjugation of estrogens with a charged sulfonate group that represents an important metabolic reaction in the inactivation of estrogens at physiological concentrations [7–9]. Sulfated estrogens cannot bind to and activate the estrogen receptors and thus lose their estrogenic activities. SULT1E1 is a cytosolic enzyme in mammalian hepatocytes whose expression is readily detectable in mouse hepatocytes and human hepatoma HepG2 cells and liver specimens [7, 10, 11].
Whereas SULT1E1 is expressed in hepatocytes in the liver, CFTR is selectively expressed in cholangiocytes in the apical membrane [12]. The mechanism by which the absence of CFTR in the apical plasma membrane of cholangiocytes leads to the clinical manifestations of CFLD is unknown, and functional studies with primary CF cholangiocytes are limited [4]. The secretion of bile by the liver depends upon functional interactions between hepatocytes and cholangiocytes, and a deficit in bile flow is one problem encountered in CF liver [13]. The induction of SULT1E1 in hepatocytes of CFTR(−/−) mice indicates an apparent separate interaction between the loss of CFTR function in cholangiocytes and hepatocytes. Cholangiocytes in CFTR(−/−) mice are capable of increasing the expression of SULT1E1 in hepatocytes by an unknown mechanism. The induction of SULT1E1 activity in the livers of CFTR(−/−) mice also provides a mechanism for the disruption of the hormonal regulation of liver function via a significant increase in E2 inactivation and alteration of the expression of E2-regulated genes [7].
The present study utilizes two human cell lines to investigate the interaction between hepatocytes and cholangiocytes in human CF. It is not known whether the loss of CFTR function in human cholangiocytes is associated with the induction of SULT1E1 in human liver. This analysis is hindered by the very limited availability of human CF liver specimens. Therefore, a co-culture system utilizing human cholangiocytes and hepatocytes was developed to investigate the paracrine interactions between these cells in the regulation of SULT1E1 expression. MMNK-1 cells are an immortalized human cholangiocyte cell line that expresses CFTR [7, 14]. HepG2 hepatocytes express SULT1E1 and the stable over-expression of SULT1E1 in these cells disrupts E2-regulated processes including growth hormone stimulated STAT5b phosphorylation and IGF-1 expression (unpublished observation). CFTR expression in MMNK-1 cells was inhibited using siRNA and the effect of the loss of CFTR function on expression of SULT1E1 in HepG2 cells investigated in a membrane separated co-culture Transwell system.
MATERIALS AND METHODS
HepG2 cells were purchased from the American Type Culture Collection (Rockville, MD). MMNK-1 cells were from Dr. Melissa Runge-Morris (Wayne State University, Detroit, MI). [3H]-E2 (56 Ci/mmol) was purchased from DuPont–NEN (Boston, MA). 3′-Phosphoadenosine 5′-phosphosulphate (PAPS) was from Dr. Sanford Singer (University of Dayton, Dayton, OH). RNA STAT-60 was purchased from Tel-Test (Friendswood, TX). Supersensitive Link-Label IHC Detection System was from BioGenex (San Ramon, CA). Mouse monoclonal anti-human CFTR antibody (MM13-4) was from GeneTex (San Antonio, TX). The siRNA expression vector pRNA-U6.1/Zeo was obtained from GenScript Corp. (Piscataway, NJ). TaqMan® Universal PCR Master Mix and TaqMan® Gene Expression Assays for human SULT1E1 (Hs00192690), SULT1A1 (Hs00742033), IGF-1 (00153126) and eukaryotic 18S ribosomal RNA (4319413E) were obtained from Applied Biosystems (Foster City, CA). Cell culture Transwell Inserts were from Corning Incorporated (Acton, MA). Biotin-SP-conjugated affiniPure Goat anti-Mouse IgG(H+L) and peroxidase-conjugated streptavidin were purchased from Jackson ImmunoResearch (West Grove, PA). Flash-frozen human liver tissues were surgical remnant specimens obtained form the Tissue Procurement Service of the Comprehensive Cancer Center at the University of Alabama at Birmingham after pathological diagnosis with UAB Institutional Review Board approval. Liver tissues without pathological involvement were utilized for this study.
Cell culture
Human HepG2 hepatocellular carcinoma cells were maintained in Eagle’s Minimum Essential Medium (MEM) containing 7% fetal bovine serum, supplemented with non-essential amino acids and sodium pyruvate. Human MMNK-1 cholangiocytes were maintained in Dulbecco’s Modified Eagle Medium (DMEM) containing 7% fetal bovine serum. Both cell lines were maintained in an atmosphere of 5%CO2/95% air and passed at approximately 90% confluency.
Expression of SULT1E1 and CFTR in HepG2 and MMNK-1 cells
CFTR and SULT1E1 levels were evaluated in HepG2 and MMNK-1 cells by immunoblot analysis. Cell lysates were prepared from confluent 60 mm plates of HepG2 and MMNK-1 cells with mammalian protein extraction reagent (M-PER, Pierce) as per the manufacturer’s instructions. Protein concentrations were estimated using the Bradford assay with γ-globulin as the standard. Cell and tissue lysates (120 μg protein) were resolved by 10% SDS-PAGE and electrotransferred to nitrocellulose membranes. Membranes were blocked with 5% bovine serum albumin then incubated with rabbit polyclonal anti-SULT1E1 antibody (1/500) [8] or mouse monoclonal anti-CFTR antibody (1/200) for 2 h. Goat anti-rabbit IgG horseradish peroxidase (HRP) conjugate (1:60,000) or goat anti-mouse IgG HRP conjugate (1:30,000), as appropriate, was used as the secondary antibody followed by visualization of bound proteins with the SuperSignal West Pico System (Pierce).
Inhibition of CFTR expression in MMNK-1 cells by CFTR siRNA
In order to inhibit CFTR expression in MMNK-1 cells using the stable expression of siRNA, two complementary oligonucleotides were synthesized targeting the CFTR sequence at nucleotides 225–243 (5′-CCTGGAATTGTCAGACATA-3′), then annealed and ligated into the pRNA-U6.1/Zeo expression vector. A control siRNA vector was also generated. MMNK-1 cells were transformed with either the pRNA-U6.1/Zeo-CFTR-siRNA or pRNA-U6.1/Zeo-control-siRNA constructs using Lipofectamine 2000 (Invitrogen). Transfected colonies were isolated following zeocin selection (200 μg/ml) and expanded to generate stably transformed MMNK-1 cells for experimental use.
To evaluate CFTR protein expression in MMNK-1 cells, cell lysates were prepared from CFTR-siRNA MMNK-1 cells and control-siRNA U6.1 MMNK-1 cells with the M-PER reagent. Cell lysates (120 μg protein) were resolved by SDS-PAGE and immunoblotted with the mouse monoclonal anti-CFTR antibody. CFTR expression was also analyzed in CFTR-siRNA MMNK-1 cells and control-siRNA MMNK-1 cells by immunohistochemical analysis. MMNK-1 cells were plated on coverslips and incubated for 24 h. Attached cells were fixed in 70% ethanol overnight, permeabilized and quenched with 3% H2O2. Cells were then blocked with 3% goat serum and incubated with mouse monoclonal anti-CFTR IgG (1:50) for 2 h. Samples were stained using the BioGenex Liquid DAB Kit following the addition of biotin-SP-conjugated affiniPure Goat anti-Mouse IgG(H+L) and peroxidase-conjugated streptavidin, then counterstained, dehydrated and mounted. Photographs were taken using a Nikon 20×40 imaging system.
HepG2 and MMNK-1 cell co-culture
Co-culture experiments were performed with HepG2 cells and either CFTR-siRNA or control-siRNA transfected MMNK-1 cells to evaluate the effect of decreased CFTR levels on SULT1E1 expression in HepG2 cells. Prior to co-culture, HepG2 cells were plated into 6-well plates and allowed to attach overnight. MMNK-1 cells were placed in 24 mm Transwell inserts and cultured separately. The Transwell inserts possess a polycarbonate membrane with 0.4 μm pores permitting separate co-culture of the two cell lines with free exchange of media between the two cell types. The Transwell inserts with CFTR- or control-siRNA transfected MMNK-1 cells were placed into HepG2 culture wells and cultured for different time intervals. After co-incubation, the Transwell inserts with the MMNK-1 cells were removed and HepG2 cells were analyzed for SULT1E1 expression.
SULT1E1 expression analysis using quantitative RT-PCR
After HepG2 cells were co-cultured with stably CFTR-siRNA transfected or control-MMNK-1 cells for different times, the cells were separated and total RNA was extracted with STAT-60. Quantitative RT-PCR was performed in an ABI 7500 thermal cycler using TaqMan® Gene Expression Assays for human SULT1E1 and SULT1A1. SULT1A1 is the major xenobiotic phenol SULT in human liver and was analyzed for comparative purposes. 18S rRNA was the endogenous control for total RNA normalization. The PCR cycling protocol included 1 cycle each of 50°C ×2 min and 95°C × 10 min and then 40 cycles of 95°C × 15 sec and 6 0°C × 1 min. Control HepG2 cells with no co-culture were used as a calibrator sample. The amount of SULT1E1 and SULT1A1 mRNA expression in these HepG2 cells was used for comparison of expression in the co-cultured samples. Relative expression was determined with the comparative method using the ABI software.
Estradiol sulfation in co-cultured HepG2 cells
To evaluate E2 sulfation activity in HepG2 cells, cells were cultured alone or co-cultured with CFTR-siRNA or control-siRNA MMNK-1 for 8 h. MMNK-1 cell-containing inserts were removed and the HepG2 cells incubated in fresh medium for 24 or 48 h. Lysate was then prepared from the HepG2 cells with M-PER and assayed for E2 sulfation with our standard chloroform extraction method [8]. Briefly, lysate was incubated in Tris buffer, pH 7.4, with 20 nM [3H]-E2 and 3′-phosphoadenosine 5′-phosphosulfate (the sulfonate donor) for 20 min, then the reaction was extracted with chloroform to remove unsulfated E2. Radioactivity was determined in the aqueous phase to quantify [3H]-E2 sulfate.
Alteration of estrogen-regulated gene expression in co-cultured HepG2 cells
HepG2 cells were co-cultured with MMNK-1 cells by placing Transwell Inserts containing either control- or CFTR-siRNA MMNK-1 cells into HepG2 culture plates for 8 h. After removal of the MMNK-1 cell inserts, HepG2 cells were incubated for 48 h at which time total RNA was extracted using STAT-60. Carbonic anhydrase isoforms II and III (CA II, CA III), glutathione-S-transferase-Pi (GST-Pi) and β-actin messages were amplified via RT-PCR. The CA II primers were; forward 5′-GGACAAGGTTCAGAGCATACTGTGG-3′ and reverse 5′-ACATTCCAGAAGAGGAGGGTG-3′. The CA III primers were; forward 5′-AATTGCCAAGGGGGAAAACC-3′ and reverse CATCAAGGAAAATCTGGAACTCGC-3′. The GST-Pi primers were; forward 5′-AAGCCTTTTGAGACCCTGCTGTCC-3′ and reverse 5′-GTTTCCCGTTGCCATTGATG-3′. Specific PCR product formation was analyzed in different cycles. Product formation was generally in the linear range of synthesis in cycles 24–25. PCR products in the linear synthesis range were resolved in 1% agarose gels and visualized with ethidium bromide staining. Bands were quantified by scanning densitometry.
Induction of IGF-1 expression in HepG2 cells after co-culture
The induction of IGF-1 message in HepG2 cells co-cultured with CFTR- or control-MMNK-1 cells was investigated after co-culture for 8 h. The MMNK-1 cells were removed and HepG2 cells were incubated for 24 or 48 h in fresh medium. Total RNA was extracted and IGF-1 message was analyzed by qRT-PCR using Applied Biosystems primers for human IGF-1. 18S rRNA was used as the endogenous control for total RNA normalization.
Effect of MMNK-1 conditioned medium on HepG2 cell SULT1E1 expression
To confirm that a secreted paracrine factor is involved in the induction of SULT1E1 in HepG2 cells after co-culture with Transwell-separated MMNK-1 cells, medium from control siRNA or CFTR siRNA cells was prepared and used as growth medium for HepG2 cells. Four 100 mm plates each of control-siRNA and CFTR-siRNA MMNK-1 cells were prepared and allowed to achieve approximately 60% confluency. At this point, 20 ml of fresh medium was added for 48 h then removed and applied to 60 mm plates of HepG2 cells at approximately 60% confluency (5 ml/plate). Fifteen plates of HepG2 cells were prepared with each the control- and CFTR-siRNA medium for triplicate preparations of cell lysate with M-PER at times 0, 12, 24, 36 and 48 h. Lysate protein was determined by the Bradford method, then the E2 sulfation assay was performed for each lysate with equivalent amounts of protein.
RESULTS
Differential expression of SULT1E1 and CFTR in HepG2 hepatocytes and MMNK-1 cholangiocytes
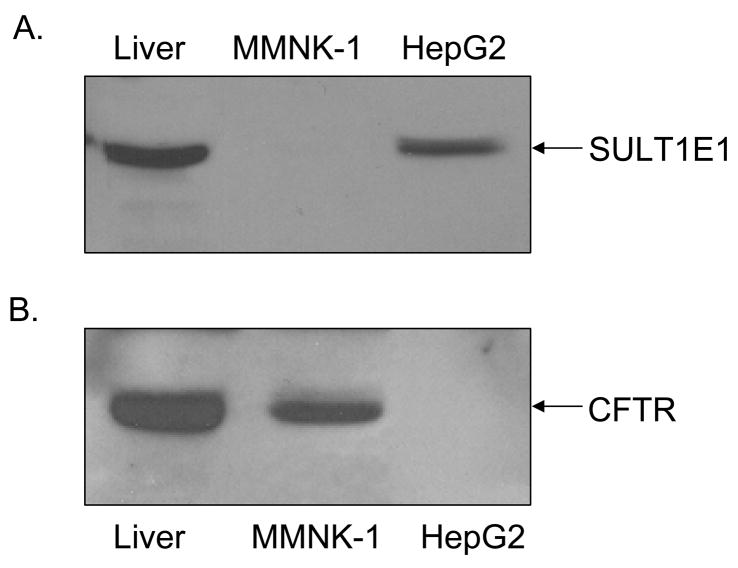
The expression of CFTR and SULT1E1 in MMNK-1 cells and HepG2 cells was analyzed. Figure 1 shows the immunoblot analysis of CFTR and SULT1E1 expression in HepG2 and MMNK-1 cells. SULT1E1 is readily detectable in HepG2 cell lysate as well as in human liver lysate whereas no SULT1E1 expression is detectable in MMNK-1 cell lysate (Figure 1A). In contrast, Figure 1B demonstrates that CFTR protein is detectable in liver lysate and in MMNK-1 cell lysate but not in HepG2 cell lysate, consistent with previous reports that CFTR is expressed in cholangiocytes but not hepatocytes [15].
Figure 1.
Differential expression of SULT1E1 and CFTR in HepG2 and MMNK-1 cells. Lysates from HepG2 and MMNK-1 cells (120 μg protein) as well as from human liver (100 μg protein) were prepared and resolved by SDS-PAGE then electrotransferred to nitrocellulose membranes. The membrane-bound proteins were incubated with rabbit polyclonal anti-SULT1E1 antibodies and mouse monoclonal anti-CFTR antibodies (GeneTex). SULT1E1 immunoreactivity is shown in panel A and CFTR immunoreactivity is shown in panel B.
Inhibition of CFTR expression in CFTR siRNA transfected MMNK-1 cells
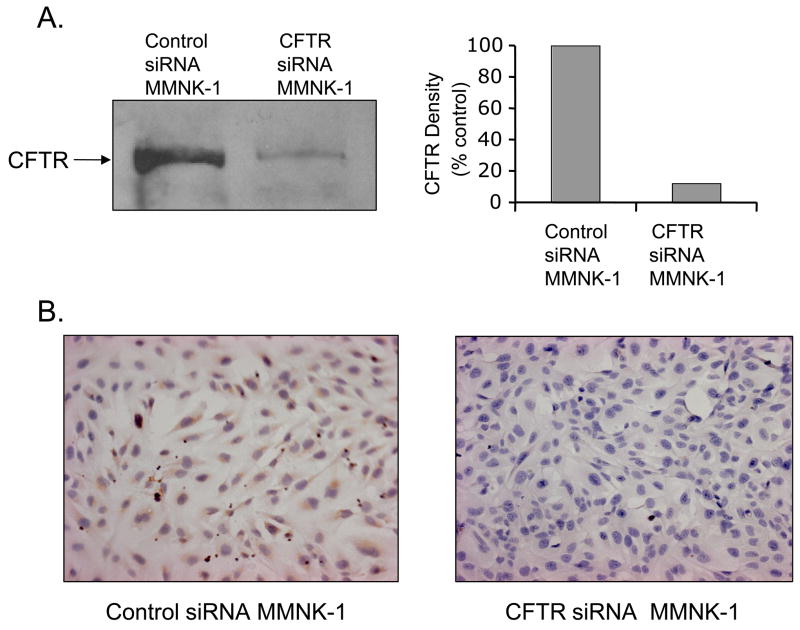
CFLD derives from a genetic dysfunction of CFTR expression or structure resulting in the loss of activity [4]. Because human cholangiocytes express significant levels of CFTR [16], MMNK-1 cholangiocytes that natively express CFTR were used to establish a cholangiocyte model to evaluate the loss of CFTR function. To repress CFTR expression, MMNK-1 cells were stably transformed with the pRNA-U6.1/Zeo-CFTR-siRNA plasmid. MMNK-1 cells for controls were transformed with the pRNA-U6.1/Zeo-control-siRNA plasmid. Cell lysates were prepared from both control and CFTR-siRNA MMNK-1 cells. Levels of CFTR were evaluated by immunoblot analysis in the cell lysates using a mouse monoclonal anti-CFTR antibody. Figure 2A shows that CFTR protein levels in CFTR-siRNA transfected MMNK-1 cells were significantly repressed in comparison with control-siRNA transfected MMNK-1 cells. Relative quantification demonstrated that CFTR expression in MMNK-1 cells was inhibited >80% as compared to control cells.
Figure 2.
Immunoblot analysis of CFTR in siRNA inhibited MMNK-1 cells. CFTR expression in MMNK-1 cells was repressed using the stable expression of specific siRNA as described in Methods. Lysates (120 μg protein) from control- and CFTR-siRNA transfected MMNK-1 cells were resolved by SDS-PAGE. CFTR levels were evaluated by immunoblot analysis using mouse anti-CFTR monoclonal antibodies. Panel A, left, shows the immunoreactivity of the control-siRNA MMNK-1 lysate (left lane) and CFTR-siRNA MMNK-1 lysate (right lane). Panel A, right, presents the results of the densitometric analysis of immunoblot results on the left. (B) Immunohistochemical analysis of CFTR levels in control-siRNA and CFTR-siRNA MMNK-1 cells. MMNK-1 cells were plated on coverslips in 6-well plates and analyzed immunohistochemically for CFTR expression as described in Methods. Photographs were taken using a Nikon 20×40 imaging system. Panel B, left, shows the CFTR staining in control-siRN MMNK-1 cells. Panel B, right, shows the staining in CFTR-siRNA MMNK-1 cells.
Immunohistochemical analysis of intact control and CFTR-siRNA MMNK-1 cells plated on glass cover slips carried out using a mouse monoclonal anti-CFTR antibody verified the loss of CFTR expression observed by immunoblot analysis. Control-siRNA transformed MMNK-1 cells showed intense CFTR staining in the plasma membrane (Figure 2B, left). In contrast, few CFTR-siRNA MMNK-1 cells were stained with the anti-CFTR IgG (Figure 2B, right). Also, CFTR staining was detected in only a few of the CFTR-siRNA MMNK-1 cells indicating that the majority of the cells had CFTR levels below the limit of detection.
SULT1E1 expression analysis using real-time quantitative RT-PCR
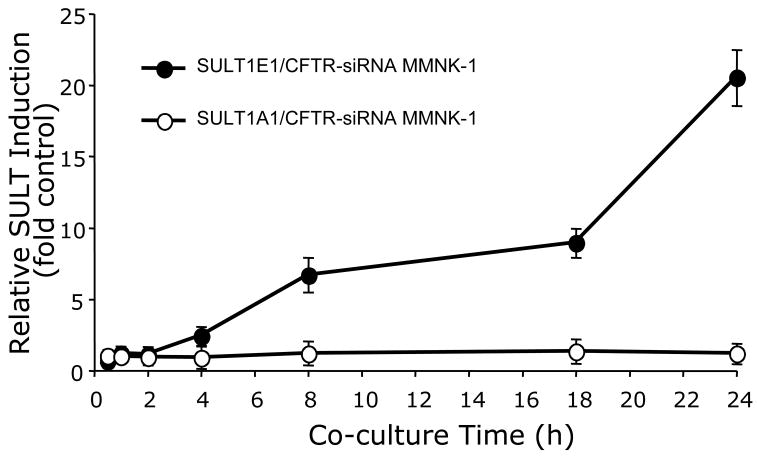
To determine whether the loss of CFTR expression in cholangiocytes alters the expression of SULT1E1 in HepG2 hepatocytes in a paracrine mechanism, a membrane-separated Transwell co-culture system was used. Control or CFTR-siRNA MMNK-1 cultured in Transwell inserts were co-cultured with HepG2 cells for different time intervals prior to the isolation of HepG2 cell RNA for the analysis of SULT1E1 and SULT1A1 message qRT-PCR. As shown in Figure 3, SULT1E1 message was significantly up-regulated in a time-dependent manner in HepG2 cells co-cultured with CFTR-repressed MMNK-1 cells for 4 h or longer. In contrast, SULT1A1 message, a major SULT isoform in human liver [16] was not altered under these same conditions indicating that this induction is specific to SULT1E1. SULT2A1 and cytochrome P450 3A4 message expression were also not induced by co-culture with the CFTR-siRNA MMNK-1 cells (data not shown). No significant increase in the expression of either SULT1E1 or SULT1A1 was detected in HepG2 cells co-cultured with control-siRNA MMNK-1 cells or with normal, untransformed MMNK-1 cells.
Figure 3.
Time course of SULT1E1 and SULT1A1 expression in HepG2 cells during co-culture with control- or CFTR-siRNA MMNK-1 cells. HepG2 cells were co-cultured with either control-siRNA or CFTR-siRNA MMNK-1 cells for 30 min, 1 h, 2 h, 4 h, 8 h, 18 h or 24 h in triplicate wells. Total RNA was then extracted and utilized for qRTPCR analysis of SULT1E1 or SULT1A1 as described in Methods with ribosomal 18S RNA as the endogenous control for total RNA normalization. mRNA expression levels were calculated using the Ct method, where the calibrators were samples from normal HepG2 cells with no MMNK-1 co-culture. Relative expression of SULT1E1 and SULT1A1 was expressed as fold-change between normal HepG2 cells and co-culture with control- or CFTR siRNA MMNK-1 cells, using the mean value from 3 replicate wells. The results are expressed as fold change ± s.d. and the standard deviations for the replicate wells were no larger than 6%.
SULT1E1 activity in co-cultured HepG2 cells
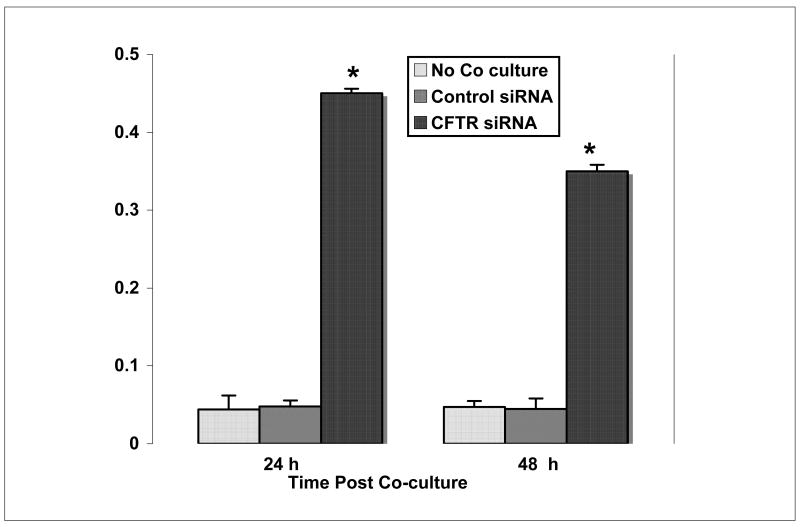
To evaluate the effect of HepG2 cell co-culture with CFTR-siRNA MMNK-1 cells on SULT1E1 activity, HepG2 cells were cultured alone or co-cultured for 8 h with CFTR-siRNA or control-siRNA cells then maintained in fresh medium for 24 h or 48 h after removal of Transwell inserts. Figure 4 shows that lysates prepared from HepG2 cells cultured alone or with control-siRNA MMNK-1 cells for 24 h or 48 h demonstrate similar rates of E2 sulfation. In contrast, HepG2 cells co-cultured with CFTR-siRNA MMNK-1 cells show significantly elevated E2 sulfation activity at both 24 and 48 h after removal of Transwell inserts compared to control-siRNA HepG2 cells. Furthermore, greater E2 sulfation activity was observed in these HepG2 cells 24 h after separation from the CFTR-siRNA MMNK-1 cells at 48 h after separation. The decrease in SULT1E1 activity in these HepG2 cells 48 h after removal from co-culture suggests that co-culture of the HepG2 and MMNK-1 cells is required for continued induction of SULT1E1 expression.
Figure 4.
Selective induction of SULT1E1 activity in HepG2 cells co-cultured with CFTR-siRNA MMNK-1 cells. HepG2 cells were cultured alone, or co-cultured with control-siRNA or CFTR-siRNA-MMNK-1 cells for 8 h then transferred to fresh medium and incubated for 24 or 48 h alone. Cellular lysate was then prepared with M-PER and assayed for E2 sulfation activity as described in Methods. E2 sulfation activity is expressed as pmol/min/mg protein. Each point represents the mean of SULT1E1 activity in lystae prepared from three separate wells and each lysate was assayed in triplicate. SULT1E1 activity is expressed as pmol E2 sulfated per min per mg protein ± s.d. The asterisk denote a significant difference at p<0.01 from the SULT1E1 sulfation rate in HepG2 cells with no co-culture using the Student’s T-test.
Alteration of estrogen-regulated gene expression in co-cultured HepG2 cells
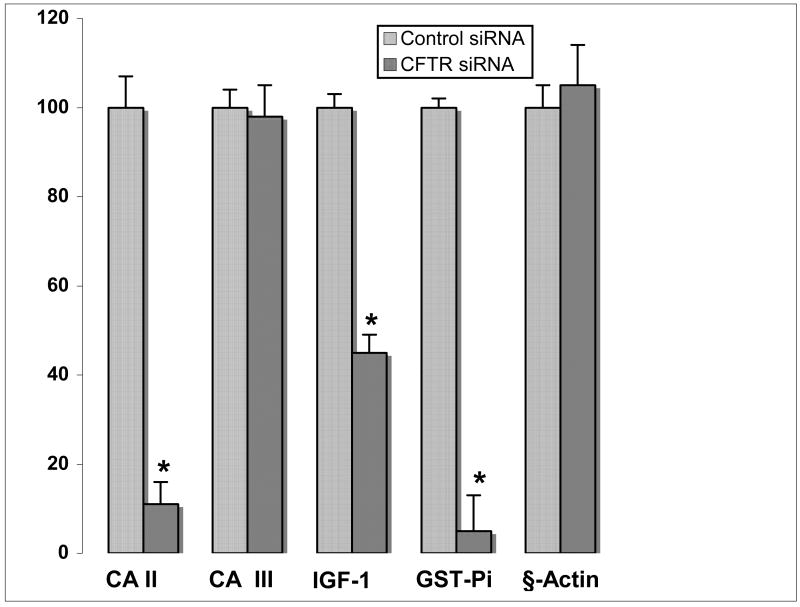
CFTR (−/−) mice demonstrate altered expression of several E2-regulated proteins in liver that correlate with the levels of SULT1E1 activity [7]. To investigate whether co-culture of HepG2 cells with CFTR-siRNA MMNK-1 cells resulted in changes in expression of E2-regulated proteins, control- or CFTR-siRNA MMNK-1 cells were co-cultured with HepG2 cells for 8 h. MMNK-1 cells were then removed and HepG2 cells were incubated for an additional 48 h prior to the isolation of total RNA. CA II, CA III, GST-Pi, IGF-1 and β-actin messages were amplified via RT-PCR with the appropriate primers. PCR products from the linear range of the amplification curves were resolved by agarose gel electrophoresis and visualized by staining with ethidium bromide. Figure 5 shows that CA II, GST-Pi and IGF-1 messages in HepG2 cells were decreased in the cells co-cultured with CFTR-siRNA MMNK-1 cells in comparison to HepG2 cells co-cultured with control-siRNA MMNK-1 cells. There was no apparent difference in CA III and β-actin message levels between these same cells. Both CA and GST-Pi also demonstrated decreased expression in the livers of the CFTR(−/−) mice [7]. Additionally, hepatic IGF-1 message levels were decreased in the CFTR(−/−) mice and were inversely correlated with SULT1E1 activity.
Figure 5.
Effect of co-culture with CFTR-siRNA-MMNK-1 cells on estrogen-regulated gene expression in HepG2 cells. HepG2 cells were co-cultured with control- or CFTR-siRNA-MMNK-1 cells for 8 h. MMNK-1 cells were removed and HepG2 cells were incubated in fresh medium for 48 h. Total RNA was prepared and utilized for RT-PCR amplification of CA II, CA III, GST-Pi and β-actin messages. PCR reactions from the linear phase of the amplification cycle (24–25 cycles) were resolved in 1% agarose gels, visualized with ethidium bromide staining and quantified by scanning densitometry. IGF-1 message was analyzed by qRT-PCR as described in Methods. IGF-1 mRNA expression levels were calculated using the Ct method, where the calibrators were the samples from normal HepG2 cells without co-culture with MNNK-1. Relative IGF-1 message levels are expressed as percent change between co-culture with CFTR-siRNA or control-siRNA MMNK-1 cells and control HepG2 cells that were not co-cultured. Each point represents the mean ± S.D. of four reactions and the asterisk denotes significance at p<0.01 as compared to message levels in control cells using the Student’s T-test.
Effect of MMNK-1 conditioned medium on HepG2 cell SULT1E1 expression
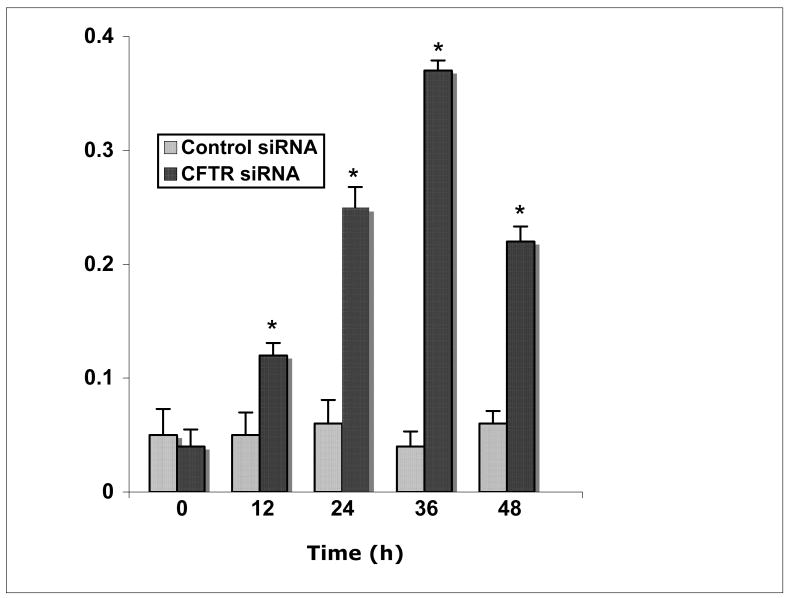
To support our hypothesis that CFTR-siRNA MMNK-1 cells secrete a paracrine factor that affects SULT1E1 in HepG2 cells, SULT1E1 activity was evaluated in lysate prepared from HepG2 cells grown in conditioned medium from either control-siRNA or CFTR-siRNA MMNK-1 cells. As shown in Figure 6, SULT1E1 activity is significantly elevated in SULT1E1 cells maintained in CFTR-siRNA MMNK-1 cells at 12, 24, 36 and 48 h, with the most significant elevation at 36 h of culture. By 48 h of culture, SULT1E1 activity begins to decline, suggesting that the paracrine factor secreted by the CFTR-siRNA MMNK-1 cells is either depleted or labile, and may require continuous production to maintain elevated SULT1E1 levels.
Figure 6.
SULT1E1 activity in HepG2 cells after co-culture in MMNK-1 cell conditioned medium. Lysate was prepared from HepG2 cells maintained in conditioned medium from control- or CFTR-siRNA MMNK-1 cells for 0, 12, 24, 36 or 48 h, then assayed for 20 nM E2 sulfation activity. Results are expressed as pmol E2 sulfated/min/mg protein ± s.d., and represent the means of triplicate SULT1E1 assays in each lysate from triplicate wells. The asterisk represents a significant difference from HepG2 cells maintained in control-siRNA MMNK-1 conditioned medium, at p<0.01 with the Student’s T-test.
DISCUSSION
SULT1E1 in both mice and humans is responsible for the sulfation and inactivation of E2 at physiological concentrations [16, 17]. Human SULT1E1 has a Km for E2 of 4 nM and is capable of inhibiting the activation of estrogen receptor-α by physiological concentrations of E2 in human endometrial cells [18]. Therefore, significant changes in SULT1E1 activity would be expected to decrease the levels of free active E2 in hepatocytes and as a consequence alter estrogen-regulated processes. Our laboratory has previously reported that most CFTR-ΔF508 and CFTR (−/−) mice exhibit increased levels of liver SULT1E1 activity [6, 7]. SULT1E1 activity in livers of CFTR(−/−) mice is generally 20–30-fold greater than the levels in CFTR wild type or (+/−) littermates [6]. The increases in SULT1E1 expression in CFTR (−/−) mouse liver correlate with changes in E2-regulated proteins such as GST-Pi, CYP2B9 and CA II [7]. These proteins have significant roles in altering the effects of oxidative stress, the metabolism of toxic xenobiotics and the generation of reactive oxygen species [19].
CF liver pathology is associated with the functional loss of CFTR in cholangiocytes, the intrahepatic biliary epithelial cells [4]. CFTR is not expressed in the more abundant hepatocytes [12] nor was CFTR expression detected in HepG2 cells (Fig. 1). In contrast, SULT1E1 is expressed in hepatocytes and is not detectable in MMNK-1 cholangiocytes (Fig.1) [7]. Loss of CFTR function in cholangiocytes has several physiological consequences in the liver. There are changes in the composition and viscosity of bile limiting its role in lipid absorption [15]. There are alterations in the composition and interhepatic circulation of bile acids [20]. In addition, overt LD is a relatively frequent and early complication of CF occurring in approximately 20% of patients [20]. The initiating event is unknown although it appears to involve steatosis and a progressive fibrogenic process leading to cirrhosis and portal hypertension [4, 21]. Alterations in liver function associated with the loss of CFTR activity may be responsible for part of the variability in the clinical manifestations of CFLD.
In liver, CF is characterized by the loss of CFTR function in cholangiocytes. In the CFTR (−/−) mouse models the loss of CFTR in cholangiocytes is associated with an induction of SULT1E1 activity in hepatocytes. Whether an induction of SULT1E1 occurs in human CF liver is not known. The investigation of SULT1E1 expression in CFLD is significantly limited by the severe lack of availability of human CF liver tissue. Therefore, to establish that the repression of CFTR function in human cholangiocytes was capable of eliciting the induction of SULT1E1 in adjacent hepatocyes, a membrane separated co-culture system was used. The hypothesis was that the loss of CFTR activity in cholangiocytes results in release of a factor that acts via a paracrine mechanism to induce SULT1E1 expression in hepatocytes. CFTR expression in MMNK-1 cholangiocytes was stably repressed using siRNA then the MMNK-1 cells were co-cultured with HepG2 cells in a Transwell system wherein the cells were separated by a 0.4 μM semi-permeable membrane. CFTR-repressed MMNK-1 cells selectively induced the expression of SULT1E1 in the co-cultured HepG2 cells. The induction of SULT1E1 indicates that increased SULT1E1 expression in the livers of CF patients is possible. These characteristics are indicative of transcriptional regulation of SULT1E1. Also, as observed in the CFTR(−/−) mice and HepG2 cells stably expressing SULT1E1 [7], the expression of IGF-1, CA II and GST-Pi were altered in the HepG2 cells co-cultured with CFTR-siRNA MMNK-1 cells. In CFTR(−/−) mice CA III expression in the liver was down-regulated whereas the expression of CA II which is localized exclusively in cholangiocytes [22] was not analyzed. In human liver, both the CA II and CA III isoforms are expressed in hepatocytes [23] and only the expression of CA II was decreased in the presence of increased SULT1E1 activity (Figure 5).
The co-culture model provides evidence for the paracrine regulation of hepatocytes by CF cholangiocytes. The underlying mechanism for the induction of SULT1E1 expression in the HepG2 cells by CFTR-deficient MMNK-1 cells is unknown although several mechanisms are possible. Dysfuntional CFTR potentiates cholangiocyte involvement in inflammatory reactions [4] that could activate inflammatory factors in co-cultured hepatocytes and thus promote transcription of SULT1E1. Disruption of CFTR in cholangiocytes may result in abnormal cAMP-stimulated Cl−, K+ and HCO3− secretion [24]. Second message systems in co-cultured hepatocytes may be affected by an imbalance in ion and water across the cell membrane with a consequent stimulation of transcription. The disruptions in bile acid synthesis and metabolism could result in the production and efflux of sterol compounds capable of activating orphan nuclear receptors in hepatocytes. Activation of the Liver X receptor (LXR) has been reported to regulate SULT1E1 expression in mice [25].
Consistent with the findings in CFTR-KO mice, IGF-1 expression is decreased in the HepG2 cells with SULT1E1 activity induced by co-culture with CFTR-siRNA MMNK-1 cells (Figure 5). Our previous results suggest that increased SULT1E1 activity in mouse liver and HepG2 hepatocytes results in a reduction of IGF-1 message expression. In HepG2 cells, E2 increases STAT5b activation following stimulation by growth hormone. Increased SULT1E1 activity in HepG2 cells is associated with a decrease in both STAT5b activation and IGF-1 message levels. IGF-1 has been reported to have protective effects on cholangiocyte survival during primary biliary cirrhosis [26] and to interact with E2 to stimulate cholangiocyte proliferation in a growth hormone stimulated process [27, 28]. An increase in SULT1E1 activity in hepatocytes may then be associated with reduced IGF-1 synthesis resulting in greater intrahepatic cholangiocyte damage and biliary dysfunction. Moreover, estrogens may target the biliary tree, where they modulate proliferative and secretory activities in cholangiocytes [24]. Estrogens have an important function in growth and cytokine signaling by modulating the proliferative response of cholangiocytes to damage via estrogen receptor activation [28]. Thus, in addition to its effects in the hepatocytes, increased hepatic SULT1E1 activity may result in alteration of cholangiocyte activity by decreasing liver estrogen levels.
Results found with the MMNK-1/HepG2 co-culture model indicate that the loss of CFTR function in cholangiocytes has the capability of eliciting paracrine effects on hepatocytes. Paracrine regulation of SULT1E1 activity generates a mechanism for altering many E2-regulated processes in liver. In addition, the induction of SULT1E1 activity and disruption in E2-regulated protein expression may have subtle or obvious effects on hepatocyte metabolism and function. The sensitivity of SULT1E1 to paracrine induction may be a factor in the variable appearance of LD or liver problems in CF patients. The role for increased E2 sulfation in the development of LD requires further examination. The paracrine mechanism for the induction of SULT1E1 has not been established and it is not known whether SULT1E1 expression is induced in other human tissues including breast, endometrium, testis or gastrointestinal tract. However, the results indicate that the loss of CFTR activity in a tissue may have important specific paracrine regulatory effects in different and distant tissues.
Acknowledgments
This research was supported in part by NIH grant GM38953 to CNF and a grant from Cystic Fibrosis Research, Inc. to CNF.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Colombo C, Battezzati PM. Liver involvement in cystic fibrosis: primary organ damage or innocent bystander? J Hepatol. 2004;41:1041–1044. doi: 10.1016/j.jhep.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 2.Sokol RJ, Durie PR. Recommendations for management of liver and biliary tract disease in cystic fibrosis. Cystic Fibrosis Foundation Hepatobiliary Disease Consensus Group. J Pediatr Gastroenterol Nutr. 1999;1(28 Suppl):S1–13. doi: 10.1097/00005176-199900001-00001. [DOI] [PubMed] [Google Scholar]
- 3.FitzSimmons SC. The changing epidemiology of cystic fibrosis. J Pediatr. 1993;122:1–9. doi: 10.1016/s0022-3476(05)83478-x. [DOI] [PubMed] [Google Scholar]
- 4.FeranchakAP AP, Sokol RJ. Cholangiocyte biology and cystic fibrosis liver disease. Seminars Liver Dis. 2001;21:471–488. doi: 10.1055/s-2001-19030. [DOI] [PubMed] [Google Scholar]
- 5.Feranchak AP. Hepatobiliary complications of cystic fibrosis. Curr Gastroenterol Rep. 2004;6:231–239. doi: 10.1007/s11894-004-0013-6. [DOI] [PubMed] [Google Scholar]
- 6.Falany JL, Greer H, Kovacs T, Sorscher EJ, Falany CN. Elevation of hepatic sulphotransferase activities in mice with resistance to cystic fibrosis. Biochem J. 2002;364:115–120. doi: 10.1042/bj3640115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li L, Falany CN. Elevated hepatic SULT1E1 activity in mouse models of cystic fibrosis alters the regulation of estrogen responsive proteins. J Cystic Fibrosis. 2007;6:23–30. doi: 10.1016/j.jcf.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 8.Falany CN, Krasnykh V, Falany JL. Bacterial expression and characterization of a cDNA for human liver estrogen sulfotransferase. J Steroid Biochem Mol Biol. 1995;52:529–539. doi: 10.1016/0960-0760(95)00015-r. [DOI] [PubMed] [Google Scholar]
- 9.Zhang H, Varmalova O, Vargas FM, Falany CN, Leyh TS. The catalytic mechanism of the human estrogen sulfotransferase. J Biol Chem. 1998;273:10888–10892. doi: 10.1074/jbc.273.18.10888. [DOI] [PubMed] [Google Scholar]
- 10.Falany CN. Human Cytosolic Sulfotransferases: Properties, Physiological Functions, and Toxicology. In: Lash LH, editor. Methods in Pharmacology and Toxicology. Drug Metabolism and Transport: Molecular Methods and Mechanism. Humana Press; Totowa, NJ: 2005. pp. 341–378. [Google Scholar]
- 11.Duanmu Z, Weckle A, Koukouriyaki SB, Hines RN, Falany CN, Kocarek TA, Runge-Morris M. Developmental expression of aryl, estrogen and hydroxysteroid sulfotransferases in pre- and postnatal human liver. J Pharm Exp Ther. 2006;316:1310–1317. doi: 10.1124/jpet.105.093633. [DOI] [PubMed] [Google Scholar]
- 12.Cohn JA, Strong TV, Picciotto MR, Nairn AC, Collins FS, Fitz JG. Localization of the cystic fibrosis transmembrane conductance regulator in human bile duct epithelial cells. Gastroenterology. 1993;105:1857–1864. doi: 10.1016/0016-5085(93)91085-v. [DOI] [PubMed] [Google Scholar]
- 13.Diwakar V, Pearson L, Beath S. Liver disease in children with cystic fibrosis. Paediatr Respir Rev. 2001;2:340–349. doi: 10.1053/prrv.2001.0170. [DOI] [PubMed] [Google Scholar]
- 14.Maruyama M, Kobayashi N, Westerman KA, Sakaguchi M, Allain JE, Totsugawa T, Okitsu T, Fukazawa T, Weber A, Stolz DB, Leboulch P. Establishment of a highly differentiated immortalized human cholangiocyte cell line with SV40T and hTERT. Transplantation. 2004;77:446–451. doi: 10.1097/01.TP.0000110292.73873.25. [DOI] [PubMed] [Google Scholar]
- 15.Colombo C, Battezzati PM, Strazzabosco M, Podda M. Liver and biliary problems in cystic fibrosis. Seminars Liver Dis. 1998;18:227–235. doi: 10.1055/s-2007-1007159. [DOI] [PubMed] [Google Scholar]
- 16.Falany CN. Enzymology of human cytosolic sulfotransferases. FASEB J. 1997;11:206–216. doi: 10.1096/fasebj.11.4.9068609. [DOI] [PubMed] [Google Scholar]
- 17.Kakuta Y, Pedersen LG, Carter CW, Negishi M, Pedersen LC. Crystal structure of estrogen sulphotransferase. Nat Struct Biol. 1997;4:904–908. doi: 10.1038/nsb1197-904. [DOI] [PubMed] [Google Scholar]
- 18.Falany JL, Azziz RR, Falany CN. Identification and characterization of cytosolic sulfotransferases in normal human endometrium. Chem Biol Interact. 1998;109:329–339. doi: 10.1016/s0009-2797(97)00143-9. [DOI] [PubMed] [Google Scholar]
- 19.Rushmore TH, Kong AN. Pharmacogenomics, regulation and signaling pathways of phase I and II drug metabolizing enzymes. Curr Drug Metab. 2002;3:481–490. doi: 10.2174/1389200023337171. [DOI] [PubMed] [Google Scholar]
- 20.Colombo C, Russo MC, Zazzeron L, Romano G. Liver disease in cystic fibrosis. J Pediatr Gastroenterol Nutr. 2006;1(43 Suppl):S49–55. doi: 10.1097/01.mpg.0000226390.02355.52. [DOI] [PubMed] [Google Scholar]
- 21.Lamireau T, MonnereauS S, Martin JE, Marcotte JE, Winnock M, Alvarez F. Epidemiology of liver disease in cystic fibrosis: a longitudinal study. J Hepatol. 2004;41:920–925. doi: 10.1016/j.jhep.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 22.Ueno Y, Ishii M, Takahashi S, Igarashi T, Toyota T, LaRusso NF. Different susceptability of mice to immune-mediated cholangitis induced by immunization with carbonic anhydrase II. Lab Invest. 1998;78:629–637. [PubMed] [Google Scholar]
- 23.Kuo WH, Chiang WL, Yang SF, Yeh KT, Yeh CM, Hsieh YS, Chu SC. The differential expression of cytosolic carbonic anhydrase in human hepatocellular carcinoma. Life Sci. 2003;73:2211–2223. doi: 10.1016/s0024-3205(03)00597-6. [DOI] [PubMed] [Google Scholar]
- 24.Zsembery A, Jessner W, Sitter G, Spirli C, Strazzabosco M, Graf J. Correction of CFTR malfunction and stimulation of Ca-activated Cl- channels restore HCO3- secretion in cystic fibrosis bile ductular cells. Hepatology. 2002;35:95–104. doi: 10.1053/jhep.2002.30423. [DOI] [PubMed] [Google Scholar]
- 25.Gong H, Guo P, Zhai Y, Zhou J, Uppal H, Jarzynka MJ, Song WC, Cheng SY, Xie W. Estrogen deprivation and inhibition of breast cancer growth in vivo through activation of the orphan nuclear liver X receptor. Mol Endocrinol. 2007;21:1781–1790. doi: 10.1210/me.2007-0187. [DOI] [PubMed] [Google Scholar]
- 26.Onori P, AlvaroD D, Floreani AR, Mancino MG, Franchitto A, Guido M, Carpino G, De Santis A, Angelico M, Attili AF, Gaudio E. Activation of the IGF1 system characterizes cholangiocyte survival during progression of primary biliary cirrhosis. J Histochem Cytochem. 2007;55:327–334. doi: 10.1369/jhc.6R7125.2006. [DOI] [PubMed] [Google Scholar]
- 27.Alvaro D, Metalli VD, Alpini G, Onori P, Franchitto A, Barbaro B, Glaser SS, Francis H, Cantafora A, Blotta I, Attili AF, Gaudio E. The intrahepatic biliary epithelium is a target of the growth hormone/insulin-like growth factor 1 axis. J Hepatol. 2005;43:875–883. doi: 10.1016/j.jhep.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 28.Alvaro D, Mancino MG, Onori P, Franchitto A, Alpini G, Francis H, Glaser S, Gaudio E. Estrogens and the pathophysiology of the biliary tree. World J Gastroenterol. 2006;12:3537–3545. doi: 10.3748/wjg.v12.i22.3537. [DOI] [PMC free article] [PubMed] [Google Scholar]