Abstract
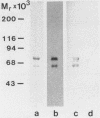
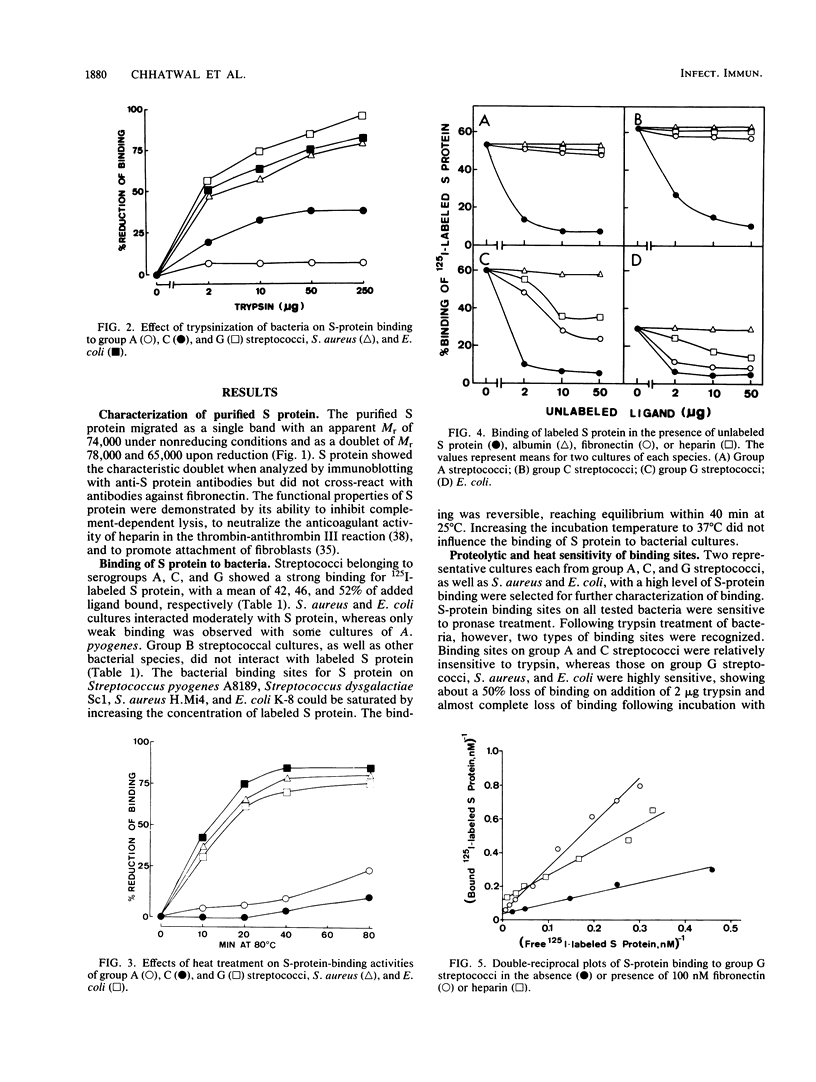
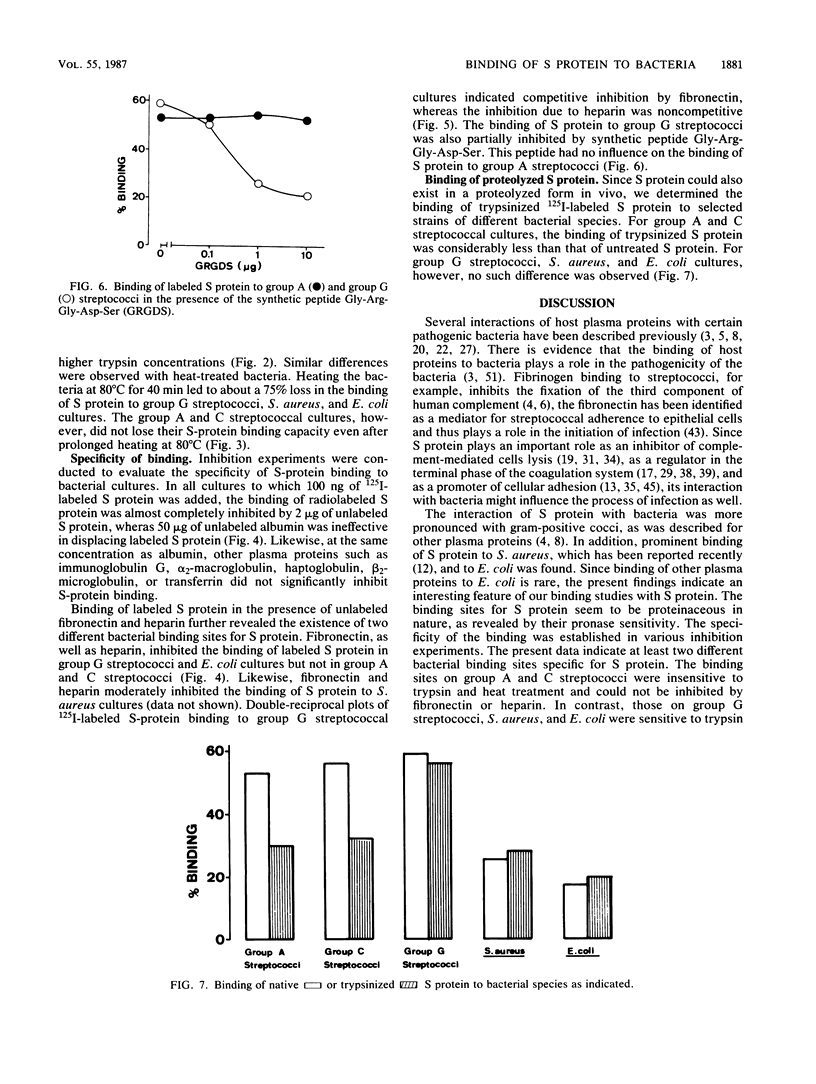
Specific binding of the 125I-labeled human S protein (vitronectin) which has been shown to be identical with serum-spreading factor, was observed with group A, C, and G streptococci as well as with Staphylococcus aureus and Escherichia coli. The specific binding of S protein to group A, C, and G streptococci was high, whereas the binding to S. aureus and E. coli cultures was moderate. In contrast, group B streptococci and a number of other bacterial species tested did not interact with S protein. The binding of S protein to bacteria was saturable and could be inhibited only by unlabeled S protein but not by albumin. Trypsinization and heat treatment of bacteria destroyed the S-protein binding capacity for group G streptococci, S. aureus, and E. coli but not for group A and C streptococci. Likewise, unlabeled human fibronectin and heparin inhibited the binding of labeled S protein to group G streptococci, S. aureus, and E. coli, but did not influence the binding to group A and C streptococci. Double-reciprocal plots of S-protein binding to group G streptococci indicated that fibronectin inhibited the binding in a competitive manner, while heparin acts in a noncompetitive manner. Moreover, the binding of S protein to G streptococci could be partially by the synthetic peptide Gly-Arg-Gly-Asp-Ser, which contains the cell attachment site of S protein. Trypsin-treated S protein had similar binding activity as untreated S protein for group G streptococci, S. aureus, and E. coli, but showed reduced binding to group A and C streptococci. The present data are indicative of two different types of bacterial binding sites in S protein. The binding to group G streptococci, S. aureus, and E. coli is mediated in part through a domain in the S protein containing the sequence Arg-Gly-Asp, whereas a different site is responsible for the binding to group A and C streptococci.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham S. N., Beachey E. H., Simpson W. A. Adherence of streptococcus pyogenes, Escherichia coli, and Pseudomonas aeruginosa to fibronectin-coated and uncoated epithelial cells. Infect Immun. 1983 Sep;41(3):1261–1268. doi: 10.1128/iai.41.3.1261-1268.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes D., Wolfe R., Serrero G., McClure D., Sato G. Effects of a serum spreading factor on growth and morphology of cells in serum-free medium. J Supramol Struct. 1980;14(1):47–63. doi: 10.1002/jss.400140106. [DOI] [PubMed] [Google Scholar]
- Chhatwal G. S., Blobel H. Isolation and properties of a novel IgG-binding protein from streptococci of serological group U. Med Microbiol Immunol. 1987;176(1):1–12. doi: 10.1007/BF00189403. [DOI] [PubMed] [Google Scholar]
- Chhatwal G. S., Dutra I. S., Blobel H. Fibrinogen binding inhibits the fixation of the third component of human complement on surface of groups A, B, C, and G streptococci. Microbiol Immunol. 1985;29(10):973–980. doi: 10.1111/j.1348-0421.1985.tb02961.x. [DOI] [PubMed] [Google Scholar]
- Chhatwal G. S., Dutra I. S., Blobel H. Specific binding sites for monomeric and aggregated beta 2-microglobulin on surface of groups A, B, C, and G streptococci. Microbiol Immunol. 1986;30(2):155–164. doi: 10.1111/j.1348-0421.1986.tb00930.x. [DOI] [PubMed] [Google Scholar]
- Chhatwal G. S., Lämmler C., Blobel H. Interactions of plasma proteins with group A, B, C and G streptococci. Zentralbl Bakteriol Mikrobiol Hyg A. 1985 Apr;259(2):219–227. doi: 10.1016/s0176-6724(85)80053-5. [DOI] [PubMed] [Google Scholar]
- Chhatwal G. S., Müller H. P., Blobel H. Characterization of binding of human alpha 2-macroglobulin to group G streptococci. Infect Immun. 1983 Sep;41(3):959–964. doi: 10.1128/iai.41.3.959-964.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlbäck B., Podack E. R. Characterization of human S protein, an inhibitor of the membrane attack complex of complement. Demonstration of a free reactive thiol group. Biochemistry. 1985 Apr 23;24(9):2368–2374. doi: 10.1021/bi00330a036. [DOI] [PubMed] [Google Scholar]
- Fröman G., Switalski L. M., Faris A., Wadström T., Hök M. Binding of Escherichia coli to fibronectin. A mechanism of tissue adherence. J Biol Chem. 1984 Dec 10;259(23):14899–14905. [PubMed] [Google Scholar]
- Fuquay J. I., Loo D. T., Barnes D. W. Binding of Staphylococcus aureus by human serum spreading factor in an in vitro assay. Infect Immun. 1986 Jun;52(3):714–717. doi: 10.1128/iai.52.3.714-717.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUNTER W. M., GREENWOOD F. C. Preparation of iodine-131 labelled human growth hormone of high specific activity. Nature. 1962 May 5;194:495–496. doi: 10.1038/194495a0. [DOI] [PubMed] [Google Scholar]
- Hayman E. G., Engvall E., A'Hearn E., Barnes D., Pierschbacher M., Ruoslahti E. Cell attachment on replicas of SDS polyacrylamide gels reveals two adhesive plasma proteins. J Cell Biol. 1982 Oct;95(1):20–23. doi: 10.1083/jcb.95.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes R. Preparation from human serum of an alpha-one protein which induces the immediate growth of unadapted cells in vitro. J Cell Biol. 1967 Feb;32(2):297–308. doi: 10.1083/jcb.32.2.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes R. O., Yamada K. M. Fibronectins: multifunctional modular glycoproteins. J Cell Biol. 1982 Nov;95(2 Pt 1):369–377. doi: 10.1083/jcb.95.2.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenne D., Hugo F., Bhakdi S. Interaction of complement S-protein with thrombin-antithrombin complexes: a role for the S-protein in haemostasis. Thromb Res. 1985 May 15;38(4):401–412. doi: 10.1016/0049-3848(85)90138-0. [DOI] [PubMed] [Google Scholar]
- Jenne D., Stanley K. K. Molecular cloning of S-protein, a link between complement, coagulation and cell-substrate adhesion. EMBO J. 1985 Dec 1;4(12):3153–3157. doi: 10.1002/j.1460-2075.1985.tb04058.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolb W. P., Muller-Eberhard H. J. The membrane attack mechanism of complement. Isolation and subunit composition of the C5b-9 complex. J Exp Med. 1975 Apr 1;141(4):724–735. [PMC free article] [PubMed] [Google Scholar]
- Kronvall G. A surface component in group A, C, and G streptococci with non-immune reactivity for immunoglobulin G. J Immunol. 1973 Nov;111(5):1401–1406. [PubMed] [Google Scholar]
- Kronvall G., Myhre E. B., Björck L., Berggård I. Binding of aggregated human beta2-microglobulin to surface protein structure in group A, C, and G streptococci. Infect Immun. 1978 Oct;22(1):136–142. doi: 10.1128/iai.22.1.136-142.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronvall G., Schönbeck C., Myhre E. Fibrinogen binding structures in beta-hemolytic streptococci group A, C, and G. Comparisons with receptors for IgG and aggregated beta 2-microglobulin. Acta Pathol Microbiol Scand B. 1979 Oct;87(5):303–310. [PubMed] [Google Scholar]
- Kuusela P., Vartio T., Vuento M., Myhre E. B. Binding sites for streptococci and staphylococci in fibronectin. Infect Immun. 1984 Aug;45(2):433–436. doi: 10.1128/iai.45.2.433-436.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Miekka S. I., Ingham K. C., Menache D. Rapid methods for isolation of human plasma fibronectin. Thromb Res. 1982 Jul 1;27(1):1–14. doi: 10.1016/0049-3848(82)90272-9. [DOI] [PubMed] [Google Scholar]
- Mosher D. F., Proctor R. A. Binding and factor XIIIa-mediated cross-linking of a 27-kilodalton fragment of fibronectin to Staphylococcus aureus. Science. 1980 Aug 22;209(4459):927–929. doi: 10.1126/science.7403857. [DOI] [PubMed] [Google Scholar]
- Myhre E. B., Kronvall G. Demonstration of specific binding sites for human serum albumin in group C and G streptococci. Infect Immun. 1980 Jan;27(1):6–14. doi: 10.1128/iai.27.1.6-14.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myhre E. B., Kuusela P. Binding of human fibronectin to group A, C, and G streptococci. Infect Immun. 1983 Apr;40(1):29–34. doi: 10.1128/iai.40.1.29-34.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podack E. R., Dahlbäck B., Griffin J. H. Interaction of S-protein of complement with thrombin and antithrombin III during coagulation. Protection of thrombin by S-protein from antithrombin III inactivation. J Biol Chem. 1986 Jun 5;261(16):7387–7392. [PubMed] [Google Scholar]
- Podack E. R., Kolb W. P., Müller-Eberhard H. J. The C5b-6 complex: formation, isolation, and inhibition of its activity by lipoprotein and the S-protein of human serum. J Immunol. 1978 Jun;120(6):1841–1848. [PubMed] [Google Scholar]
- Podack E. R., Kolb W. P., Müller-Eberhard H. J. The SC5b-7 complex: formation, isolation, properties, and subunit composition. J Immunol. 1977 Dec;119(6):2024–2029. [PubMed] [Google Scholar]
- Podack E. R., Müller-Eberhard H. J. Binding of desoxycholate, phosphatidylcholine vesicles, lipoprotein and of the S-protein to complexes of terminal complement components. J Immunol. 1978 Sep;121(3):1025–1030. [PubMed] [Google Scholar]
- Podack E. R., Preissner K. T., Müller-Eberhard H. J. Inhibition of C9 polymerization within the SC5b-9 complex of complement by S-protein. Acta Pathol Microbiol Immunol Scand Suppl. 1984;284:89–96. [PubMed] [Google Scholar]
- Preissner K. T., Heimburger N., Anders E., Müller-Berghaus G. Physicochemical, immunochemical and functional comparison of human S-protein and vitronectin. Evidence for the identity of both plasma proteins. Biochem Biophys Res Commun. 1986 Jan 29;134(2):951–956. doi: 10.1016/s0006-291x(86)80512-5. [DOI] [PubMed] [Google Scholar]
- Preissner K. T., Müller-Berghaus G. S protein modulates the heparin-catalyzed inhibition of thrombin by antithrombin III. Evidence for a direct interaction of S protein with heparin. Eur J Biochem. 1986 May 2;156(3):645–650. doi: 10.1111/j.1432-1033.1986.tb09626.x. [DOI] [PubMed] [Google Scholar]
- Preissner K. T., Wassmuth R., Müller-Berghaus G. Physicochemical characterization of human S-protein and its function in the blood coagulation system. Biochem J. 1985 Oct 15;231(2):349–355. doi: 10.1042/bj2310349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preissner K. T., Zwicker L., Müller-Berghaus G. Formation, characterization and detection of a ternary complex between S protein, thrombin and antithrombin III in serum. Biochem J. 1987 Apr 1;243(1):105–111. doi: 10.1042/bj2430105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruoslahti E., Engvall E., Hayman E. G. Fibronectin: current concepts of its structure and functions. Coll Relat Res. 1981;1(1):95–128. doi: 10.1016/s0174-173x(80)80011-2. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E., Pierschbacher M. D. Arg-Gly-Asp: a versatile cell recognition signal. Cell. 1986 Feb 28;44(4):517–518. doi: 10.1016/0092-8674(86)90259-x. [DOI] [PubMed] [Google Scholar]
- Simpson W. A., Beachey E. H. Adherence of group A streptococci to fibronectin on oral epithelial cells. Infect Immun. 1983 Jan;39(1):275–279. doi: 10.1128/iai.39.1.275-279.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson W. A., Hasty D. L., Mason J. M., Beachey E. H. Fibronectin-mediated binding of group A streptococci to human polymorphonuclear leukocytes. Infect Immun. 1982 Aug;37(2):805–810. doi: 10.1128/iai.37.2.805-810.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki S., Oldberg A., Hayman E. G., Pierschbacher M. D., Ruoslahti E. Complete amino acid sequence of human vitronectin deduced from cDNA. Similarity of cell attachment sites in vitronectin and fibronectin. EMBO J. 1985 Oct;4(10):2519–2524. doi: 10.1002/j.1460-2075.1985.tb03965.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki S., Pierschbacher M. D., Hayman E. G., Nguyen K., Ohgren Y., Ruoslahti E. Domain structure of vitronectin. Alignment of active sites. J Biol Chem. 1984 Dec 25;259(24):15307–15314. [PubMed] [Google Scholar]
- Switalski L. M., Ljungh A., Rydén C., Rubin K., Hök M., Wadström T. Binding of fibronectin to the surface of group A, C, and G streptococci isolated from human infections. Eur J Clin Microbiol. 1982 Dec;1(6):381–387. doi: 10.1007/BF02019939. [DOI] [PubMed] [Google Scholar]
- Tomasini B. R., Mosher D. F. On the identity of vitronectin and S-protein: immunological crossreactivity and functional studies. Blood. 1986 Sep;68(3):737–742. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschopp J., Müller-Eberhard H. J., Podack E. R. Formation of transmembrane tubules by spontaneous polymerization of the hydrophilic complement protein C9. Nature. 1982 Aug 5;298(5874):534–538. doi: 10.1038/298534a0. [DOI] [PubMed] [Google Scholar]
- Vercellotti G. M., Lussenhop D., Peterson P. K., Furcht L. T., McCarthy J. B., Jacob H. S., Moldow C. F. Bacterial adherence to fibronectin and endothelial cells: a possible mechanism for bacterial tissue tropism. J Lab Clin Med. 1984 Jan;103(1):34–43. [PubMed] [Google Scholar]
- Whitnack E., Beachey E. H. Antiopsonic activity of fibrinogen bound to M protein on the surface of group A streptococci. J Clin Invest. 1982 Apr;69(4):1042–1045. doi: 10.1172/JCI110508. [DOI] [PMC free article] [PubMed] [Google Scholar]