Abstract
Terminal differentiation of epithelial cells into more specialized cell types is a critical step of organogenesis. Throughout the process of terminal differentiation, epithelial progenitors acquire or up-regulate expression of renal function genes and cease to proliferate, while expression of embryonic genes is repressed. This exquisite coordination of gene expression is accomplished by signaling networks and transcription factors which couple the external environment with the new functional demands of the cell. While there has been much progress in understanding the early steps involved in renal epithelial cell differentiation, a major gap remains in our knowledge of the factors that control the steps of terminal differentiation. A number of signaling molecules and transcription factors have been recently implicated in determining segmental nephron identity and functional differentiation. While some of these factors (the p53 gene family, HNF1β) promote the terminal epithelial differentiation fate, others (Notch, Brn-1, IRX, KLF4 and Foxi1) tend to regulate differentiation of specific nephron segments and individual cell types. This article summarizes current knowledge related to these transcription factors and discusses how diverse cellular signals are integrated to generate a transcriptional output during the process of terminal differentiation. Since these transcriptional processes are accompanied by profound changes in nuclear chromatin structure involving the genes responsible for creating and maintaining the differentiated cell phenotype, future studies should focus on identifying the nature of these epigenetic events and factors, how they are regulated temporally and spatially, and the chromatin environment they eventually reside in.
Introduction
Terminal differentiation is a key biological process characterized by cell cycle arrest and acquisition of specialized functions by each cell type. The genetic programs that couple cellular growth to functional differentiation are a subject of intense investigation (78). There is increasing appreciation that although formation of adequate numbers of nephrons (i.e., renal mass) is critical for achieving optimal renal function capacity, the signals that determine how, when, and which renal epithelial cells should acquire a given functional phenotype are equally essential. Aberrant terminal differentiation is a hallmark of dysplasia, cyst formation and cancer and therapeutic interventions to promote differentiation and cell cycle arrest (e.g., EGFR antagonists and histone deacetylase inhibitors) are effective measures in some models of polycystic kidney disease and cancer (17, 26, 72, 73). Therefore, identification of factors that couple cell cycle arrest with functional differentiation is of great clinical importance.
Termination of nephrogenesis and differentiation are dynamic and coordinated processes
The metanephric kidney is derived from the intermediate mesoderm at embryonic (E) day 10.5 in mice and nephrogenesis continues postnatally (PN) in this species (24, 58, 68). While initial studies indicated that murine nephrogenesis ends between PN 7-10, recent molecular analysis has revealed that formation of nascent nephrons ceases by PN3 (20) and is accompanied by loss of ureteric bud (UB) ampullae and tip cell Wnt11 expression. Interestingly, the PN3 UB retains its ability to express the inductive molecule Wnt9b (at least in vitro) and to induce nascent nephrons (20).
The mechanisms and events leading to termination of nephrogenesis are not entirely clear. Hartman et al. (2007) proposed that the mechanism could involve depletion of the progenitor pool within the MM or UB cell lineages or a genetic switch in response to a physiologic sensor. It was found that there was no appreciable change in apoptosis that might explain the loss of metanephric mesenchyme and there was no evidence to support that capping mesenchyme had differentiated into another mesenchymal lineage. Accordingly, the loss of nephrogenic mesenchyme occurred because of conversion to nephrons without replenishment from the progenitor pool and not by cell death. The idea that onset of glomerular filtration provides an off-on switch to induce the terminal differentiation program in the tubular compartment is intuitively appealing. However, this hypothesis is challenged by activation and sustained expression of terminal differentiation genes in the non-filtering ex vivo cultured embryonic kidney (63). Nevertheless, tubular fluid flow is likely to provide important physiological clues by acting as a mechanosensory stimulus of the primary monocilium and downstream calcium-dependent signaling pathways (e.g., Stat-p21waf1) (5, 41, 75).
Transcriptional networks in nephron patterning
Genetic and molecular studies in mice have shown that Wnt9b, which is strongly expressed in the UB branches, is essential for the induction of nephrogenesis (9). Wnt9b activates the canonical β-catenin pathway in surrounding MM and induces expression of early markers of nephron formation such as Pax8, FGF8, the LIM homeobox protein LIM1 (also known as LHX1), and Wnt4 (9). Wnt4 induces mesenchyme-epithelium transition and formation of the renal vesicle (11, 33, 34, 57) (Fig. 1). Mice with a conditional deletion of FGF8 or LIM1 fail to express proximal and distal-patterning markers such as the Notch ligand, Delta-like 1 (DLL1) and BRN1 (11, 34, 67).
Figure 1.
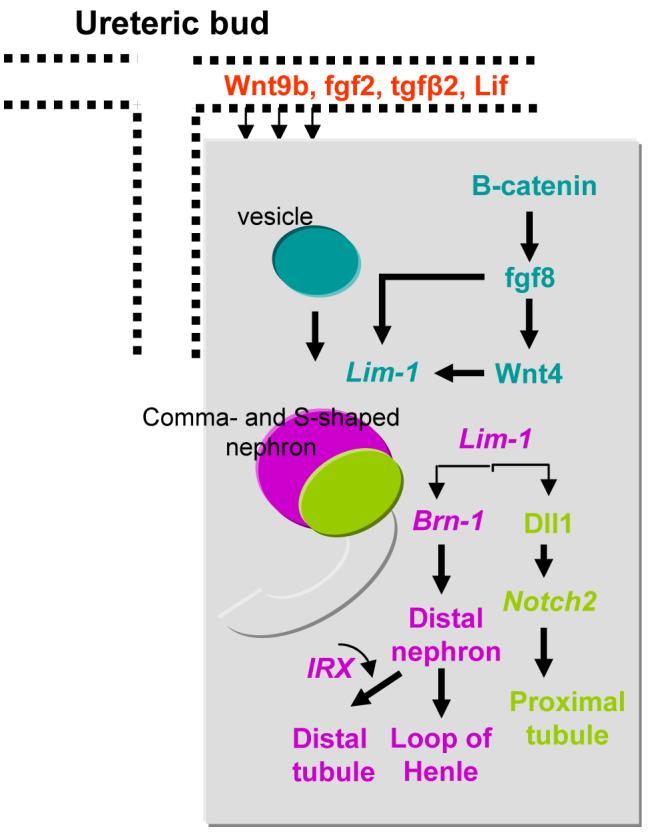
Transcriptional pathways which regulate the formation of the renal vesicle and its transition to the segmented early nephron. Wnt9b is synthesized and released from the ureteric bud branches. Wnt9b in cooperation with inducing factors (e.g., LIF, FGF2, TGFβ2) converge on β-catenin signaling to stimulate mesenchyme-epithelium transition and subsequently the conversion of the renal vesicle (blue) to comma- and S-shaped bodies (pink/green/grey). The transcription factor Lim-1 promotes further patterning by stimulating expression Brn1 (pink) and Dll1 (green). Brn1 in cooperation with Iroquois transcription factors IRX specify distal nephron fate, whereas Dll1-Notch2 signaling specifies the proximal tubule fate. Data presented in this figure summarizes results published in the following articles: Dev Cell 9: 283-292, 2005; Development 132: 2809-2823, 2005; Dev Biol 297: 103-117, 2006; Development 134: 801-811, 2007; Development 130: 4751-4759, 2003; Genes Dev 21: 2358-2370, 2007.
The mature nephron is subdivided into segments that are specialized for specific tasks. This specialization, which is acquired at terminal differentiation, is reflected in protein expression profiles of various segments that complement function. In several developmental systems, overlapping and differential expression of transcription factors generate combinations of interacting proteins that regulate cell-specific gene expression as well as developmental fate (4, 15, 30, 56, 60). With respect to the developing kidney, such interactions might also explain how segment identity and functional status are acquired. While there has been much progress in understanding the early steps involved in renal epithelial cell differentiation, a major gap remains in our knowledge of the factors that drive segmental nephron identity. Advances in studying model systems such as Xenopus and Zebrafish pronephros as well as in mice have been instrumental in understanding proximal-distal specification of the nephron (35, 66, 77).
LIM1 is required to induce the initial stages of patterning in the renal vesicle, by controlling the expression of the POU-domain transcription factor BRN1 and the Notch ligand DLL1 at the pole of the vesicle that lies in close proximity to the ureter (11, 34). DLL1 contributes via NOTCH2 in the specification of the proximal tubule fate (12, 35). Under the control of transcription factors BRN1 and Iroquois-class homeodomain proteins IRX 1–3, distal segments further extend and differentiate towards distal tubule and the Henle’s loop (46, 49, 76) (Fig. 1). Brn1–/– mutant mice show disrupted development of the Henle’s loop and formation of distal convoluted tubules (46). In X. laevis, a subset of Irx transcription factors (Irx1, Irx2 and Irx3) are specifically expressed in a medial zone of the developing pronephric anlagen, which will give rise to the intermediate tubule. Loss- and gain-of-function experiments in X. laevis revealed that Irx3 (but not Irx1 or Irx2) is required and sufficient to direct early distal tubule fate (49). In the mouse, expression of the same subset of Irx genes marks the future early distal tubule compartment in the S-shaped bodies of the developing metanephros. However, the function of Irx proteins in mouse kidney patterning remains to be defined and may be redundant, since Irx-2 null mutant are phenotypically normal (37).
The p53 family regulates both cell cycle progression and terminal differentiation markers
The p53 gene encodes a sequence-specific DNA-binding protein/tumor suppressor that maintains genomic integrity via its ability to induce cell cycle arrest or apoptosis, depending on the type and magnitude of the stress and the cell type (47, 71). Previous studies have demonstrated a developmental role for p53 in several organisms including Xenopus (2, 23, 64, 65, 69), and mouse (13, 16, 18, 19, 21, 32, 38, 39, 44, 59). We recently reported that terminal nephron differentiation is accompanied by p53 phosphorylation and acetylation, protein stabilization, and enhanced DNA binding activity (54). Moreover, we identified several terminal differentiation genes, including the bradykinin B2 receptor (Bdkrb2), aquaporin-2 (AQP-2), Na,K-ATPase a1, and angiotensin II type 1 receptor (Agtr1), as a novel group of p53-target genes (42, 52, 54) (Fig. 2). In contrast, the proliferating cell nuclear antigen (PCNA), which encodes a protein expressed in dividing cells and is necessary for cell cycle progression and DNA replication, is directly repressed by p53, and is therefore excluded from the differentiation zone of the developing renal cortex (55). In keeping with these findings, we found that p53-deficient newborn mice exhibit persistent renal cell proliferation, impaired cell cycle control, and disorganized spatial expression of nephron differentiation markers (17, 24). Interestingly expression of terminal differentiation genes is attenuated but not abrogated in p53-null kidneys (52), raising the possibility of compensatory regulation by other developmentally regulated transcription factors with overlapping functions.
Figure 2. Role of p53 family in terminal nephron differentiation.
A. Terminal nephron differentiation is accompanied by p53 stabilization and enhanced DNA binding activity (J Clin Invest 109: 1021-1030, 2002). In the nephrogenic zone, p53 levels are kept low since p53 is repressed transcriptionally by Pax2 (EMBO J 14: 5638-45, 1995). The promoter of proliferating cell nuclear antigen (PCNA), which encodes a protein expressed in dividing cells and is necessary for cell cycle progression and DNA replication, is directly repressed by p53, and is therefore excluded from the differentiation zone (Am J Physiol Renal Physiol 283: F727-733, 2002). The genes encoding the CDK inhibitor, p21 (Cip1/Waf1) and KLF4, are direct targets of p53 (Cell 75: 817-825, 1993; J. Biol. Chem. 278: 2101-2105, 2003). In the differentiating zone, p53 binds to the promoters of terminal differentiation genes (also called renal function genes), including the bradykinin B2 receptor, aquaporin-2, and Na,K-ATPase a1. All of these genes have composite cis-regulatory elements for p53, KLF4 and CREB. B. Angiotensin II, acting via AT1 receptor-mediated signaling, stimulates the phosphorylation of CREB on Ser-133. The latter modification allows CREB to interact with p53 and the co-activator CBP. CBP acts as a bridging molecule with the basal transcriptional machinery as well as a histone acetyltransferase. The end result is enhanced BdkrB2 transcription (J Am Soc Nephrol 18: 1140-1149, 2007). C. p53 and its homologue p73 are capable of binding to the promoters of renal function genes and activating their expression. This function requires additional transcription factors including KLF4 or Foxi1. Fig. 2A is adapted with permission from Am J Physiol Renal Physiol 283: F727-733, 2002.
p53 cooperates with at least two other transcription factors, CREB (cyclic AMP response element-binding protein) and KLF4 (Kruppel-like factor 4), and several renal function genes (e.g. BdkrB2, AQP2, ENaC) contain p53-CRE-KLF binding sites in their promoter regions. In one case, the promoter for the BdkrB2 gene (which encodes for the bradykinin B2 receptor) has contiguous binding sites for p53, CREB and KLF4 extending from position -44 to -82 bp, relative to the transcription start site. Disruption of function and/or binding of any of these factors impair the activity of the BdkrB2 promoter suggesting that the three factors act as an enhanceosome-like fashion (53). Chromatin immunoprecipitation confirmed that the p53/KLF4/CREB complex assembles in vivo on the mouse BdkrB2 gene in a developmentally regulated manner. Moreover, the complex is bridged together by a large co-factor, CBP (CREB-binding protein), which also has a histone acetyltransferase activity. Recruitment of CBP by the enhanceosome complex results in hyperacetylation of promoter-associated histones and thus further enhances gene transcription (53). Interestingly, angiotensin II, acting via AT1 receptor, stimulates and kinase cascade leading to phosphorylation of CREB on serine 133. p-CREB has a higher affinity to DNA as well as to p53 and is capable of recruiting CBP as well. As a result, angiotensin II is a strong stimulus of BdkrB2 gene transcription (61) (Fig. 2B).
Recent studies have identified two homologues of p53, p63 and p73 (10, 14, 29, 45, 62). Both genes encode transcription factors with significant sequence homology to p53. Highest homology lies in the DNA-binding domain allowing p63 and p73 to bind and activate transcription of p53 target genes and to induce apoptosis and/or growth arrest. However, unlike the p53 gene, p73 and p63 are not induced by most DNA-damaging agents and are rarely mutated in tumors. On the other hand, there is strong genetic evidence supporting an important role for p63 and p73 in embryonic development. p63-null mice exhibit severe defects in limb, craniofacial, and epithelial development (36, 40, 43, 45), whereas p73-null mice have central nervous system defects and pheromonal abnormalities (45, 48, 50, 74). Unlike p53, transcription of the p73 gene yields multiple full-length (transactivation (TA) domain) and amino terminus-truncated (ΔN) isoforms. ΔNp73 acts in a dominant negative fashion to inhibit the actions of TAp73 and p53 on their target genes, promoting cell survival and proliferation and suppressing apoptosis (25). The balance between TAp73 and its negative regulator, ΔNp73, may therefore represent an important determinant of developmental cell fate.
Studies from our laboratory have shown that TAp73 and ΔNp73 exhibit reciprocal spatiotemporal expression and functions during nephrogenesis (51). TAp73 was predominantly expressed in the differentiation domain of the renal cortex in an overlapping manner with the vasopressin-sensitive water channel aquaporin-2 (AQP-2). Chromatin immunoprecipitation assays demonstrated that the endogenous AQP-2 promoter was occupied by TAp73 in a developmentally regulated manner. Furthermore TAp73 stimulated AQP-2 promoter-driven reporter expression. The transcriptional effects of TAp73 on AQP-2 were independent of p53. In marked contrast to TAp73, ΔNp73 isoforms were induced early in development and were preferentially expressed in proliferating nephron precursors. Moreover ΔNp73 was a potent repressor of AQP-2 gene transcription. We speculate that the spatiotemporal switch from ΔNp73 to TAp73 may play an important role in the terminal differentiation program of the developing nephron. These results led to the emergence of a new paradigm for terminal nephron differentiation in which the p53/p73 transcriptional network induces renal function gene expression while simultaneously inhibiting cell cycle progression. This contrasts to the role of the retinoblastoma gene product Rb, which is essential for enteroendocrine cells to undergo cell cycle arrest as they terminally differentiate but is not required for the expression of gastrointestinal hormones (70).
In the collecting duct, the forkhead transcription factor, Foxi1, mediates differentiation of intercalated cells from a precursor epithelial cell and is upstream of intercalated cell-specific genes (1, 7) (Fig. 2C). Foxi1-/- mice exhibit impaired expression of intercalated cell genes such as Pendrin, H-ATPase and AE1 but unaltered expression of principal cell markers. Preliminary results from our laboratory indicate that Foxi1 is a target for p53-mediated transcriptional activation. Therefore, it appears that p53/p73 are capable of activating both principal and intercalated cell genes, arguing that a general function of the p53 gene family is to promote terminal differentiation rather than to specify a specific lineage or fate.
Hepatocyte nuclear factor 1β (HNF1β) controls a gene regulatory network necessary for epithelial differentiation
HNF1β is a transcription factor that regulates tissue-specific gene expression in the kidney, liver, pancreas, and other organs (8). During embryonic development, HNF-1β is expressed in the branching ureteric bud as well as in comma- and S-shaped bodies (28) and can regulate genes in various nephron segments. Mutations of HNF-1β in humans produce maturity onset diabetes of the young, type 5 (MODY5), a disorder that is frequently associated with congenital cystic abnormalities of the kidney (6).
HNF1β is a direct regulator of at least two collecting duct genes: ksp-Cadherin (3), a kidney specific cadherin, and PKHD1, the gene mutated in autosomal recessive PKD (22, 27). Kidney-specific inactivation of HNF-1β uncovered a transcriptional network involving HNF-1β-Pkhd1-Umod-Pkd2 (28). In vivo chromatin immunoprecipitation experiments showed that HNF-1β binds to several DNA elements in the 5′-flanking regions of the Umod, Pkhd1, and Pkd2 genes. Similar to the mechanism by which p53 and p73 activate renal function genes, HNF-1β activates Pkhd1 transcription by recruiting coactivators that promote histone acetylation and chromatin remodeling at the promoter (28).
In summary, the formation of terminally differentiated cell types requires withdrawal from the cell cycle and repression of many genes involved in cell cycle control as well as activation of cell type-specific genes. A major question is how a cell can coordinate cell cycle control with functional differentiation? As discussed above, transcription factors such as p53 performs dual functions by inducing cell cycle arrest genes (e.g., p21Cip1), repressing proliferation genes (e.g., PCNA and CDC2), and activating renal function genes (e.g., GPCRs and water channels). Cooperation with other segment-specific transcription factors and co-factors eventually ensures expression of segmental identity. As an example, the differential presence of Foxi1/p53 promotes intercalated cell identity, whereas KLF4/p53 favors principal cell gene expression. As recently discussed (31), these transcriptional processes are accompanied by profound changes in nuclear chromatin structure involving the transcription factors/co-factors needed to regulate the genes responsible for creating and maintaining the differentiated cell phenotype . Future studies should focus on identifying the nature of these epigenetic events and factors, how they are regulated temporally and spatially, and the chromatin environment they eventually reside in.
Acknowledgments
The authors apologize for not including many excellent articles on the subject due to lack of space. This work was supported by NIH grants DK-62550 and DK-56264 to S.E.D. Z.S. is supported by the Carl W. Gottschalk Research Scholar Grant from the American Society of Nephrology and COBRE 1P20 RR017659 to Z.S. The authors thank the members of the Tulane Renal Development Group and Dr. Oliver Wessely’s laboratory for insightful discussions.
References
- 1.Al-Awqati Q, Schwartz GJ. A fork in the road of cell differentiation in the kidney tubule. J Clin Invest. 2004;113:1528–1530. doi: 10.1172/JCI22029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amariglio F, Tchang F, Prioleau MN, Soussi T, Cibert C, Mechali M. A functional analysis of p53 during early development of Xenopus laevis. Oncogene. 1997;15:2191–2199. doi: 10.1038/sj.onc.1201395. [DOI] [PubMed] [Google Scholar]
- 3.Bai Y, Pontoglio M, Hiesberger T, Sinclair AM, Igarashi P. Regulation of kidney-specific Ksp-cadherin gene promoter by hepatocyte nuclear factor-1beta. Am J Physiol Renal Physiol. 2002;283:F839–851. doi: 10.1152/ajprenal.00128.2002. [DOI] [PubMed] [Google Scholar]
- 4.Barak H, Rosenfelder L, Schultheiss TM, Reshef R. Cell fate specification along the anterior-posterior axis of the intermediate mesoderm. Dev Dyn. 2005;232:901–914. doi: 10.1002/dvdy.20263. [DOI] [PubMed] [Google Scholar]
- 5.Bhunia AK, Piontek K, Boletta A, Liu L, Qian F, Xu PN, Germino FJ, Germino GG. PKD1 induces p21(waf1) and regulation of the cell cycle via direct activation of the JAK-STAT signaling pathway in a process requiring PKD2. Cell. 2002;109:157–168. doi: 10.1016/s0092-8674(02)00716-x. [DOI] [PubMed] [Google Scholar]
- 6.Bingham C, Ellard S, Allen L, Bulman M, Shepherd M, Frayling T, Berry PJ, Clark PM, Lindner T, Bell GI, Ryffel GU, Nicholls AJ, Hattersley AT. Abnormal nephron development associated with a frameshift mutation in the transcription factor hepatocyte nuclear factor-1 beta. Kidney Int. 2000;57:898–907. doi: 10.1046/j.1523-1755.2000.057003898.x. [DOI] [PubMed] [Google Scholar]
- 7.Blomqvist SR, Vidarsson H, Fitzgerald S, Johansson BR, Ollerstam A, Brown R, Persson AE, Bergstrom GG, Enerback S. Distal renal tubular acidosis in mice that lack the forkhead transcription factor Foxi1. J Clin Invest. 2004;113:1560–1570. doi: 10.1172/JCI20665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boj SF, Parrizas M, Maestro MA, Ferrer J. A transcription factor regulatory circuit in differentiated pancreatic cells. Proc Natl Acad Sci USA. 2001;98:14481–14486. doi: 10.1073/pnas.241349398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carroll TJ, Park JS, Hayashi S, Majumdar A, McMahon AP. Wnt9b plays a central role in the regulation of mesenchymal to epithelial transitions underlying organogenesis of the mammalian urogenital system. Dev Cell. 2005;9:283–292. doi: 10.1016/j.devcel.2005.05.016. [DOI] [PubMed] [Google Scholar]
- 10.Chen X. The p53 family: same response, different signals? Mol Med Today. 1999;5:387–392. doi: 10.1016/s1357-4310(99)01545-2. [DOI] [PubMed] [Google Scholar]
- 11.Chen YT, Kobayashi A, Kwan KM, Johnson RL, Behringer RR. Gene expression profiles in developing nephrons using Lim1 metanephric mesenchyme-specific conditional mutant mice. BMC Nephrol. 2006;7:1. doi: 10.1186/1471-2369-7-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cheng HT, Kim M, Valerius MT, Surendran K, Schuster-Gossler K, Gossler A, McMahon AP, Kopan R. Notch2, but not Notch1, is required for proximal fate acquisition in the mammalian nephron. Development. 2007;134:801–811. doi: 10.1242/dev.02773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Christophorou MA, Martin-Zanca D, Soucek L, Lawlor ER, Brown-Swigart L, Verschuren EW, Evan GI. Temporal dissection of p53 function in vitro and in vivo. Nat Genet. 2005;37:718–726. doi: 10.1038/ng1572. [DOI] [PubMed] [Google Scholar]
- 14.Courtois S, de Fromentel CC, Hainaut P. p53 protein variants: structural and functional similarities with p63 and p73 isoforms. Oncogene. 2004;23:631–638. doi: 10.1038/sj.onc.1206929. [DOI] [PubMed] [Google Scholar]
- 15.Cripps RM, Olson EN. Control of cardiac development by an evolutionarily conserved transcriptional network. Dev Biol. 2002;246:14–28. doi: 10.1006/dbio.2002.0666. [DOI] [PubMed] [Google Scholar]
- 16.de Rozieres S, Maya R, Oren M, Lozano G. The loss of mdm2 induces p53-mediated apoptosis. Oncogene. 2000;19:1691–1697. doi: 10.1038/sj.onc.1203468. [DOI] [PubMed] [Google Scholar]
- 17.Deshpande A, Sicinski P, Hinds PW. Cyclins and cdks in development and cancer: a perspective. Oncogene. 2005;24:2909–2915. doi: 10.1038/sj.onc.1208618. [DOI] [PubMed] [Google Scholar]
- 18.Dey DC, Bronson RP, Dahl J, Carroll JP, Benjamin TL. Accelerated development of polyoma tumors and embryonic lethality: different effects of p53 loss on related mouse backgrounds. Cell Growth Differ. 2000;11:231–237. [PubMed] [Google Scholar]
- 19.Halevy O. p53 gene is up-regulated during skeletal muscle cell differentiation. Biochem Biophys Res Commun. 1993;192:714–719. doi: 10.1006/bbrc.1993.1473. [DOI] [PubMed] [Google Scholar]
- 20.Hartman HA, Lai HL, Patterson LT. Cessation of renal morphogenesis in mice. Dev Biol. 2007;310:379–387. doi: 10.1016/j.ydbio.2007.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hettmann T, Barton K, Leiden JM. Microphthalmia due to p53-mediated apoptosis of anterior lens epithelial cells in mice lacking the CREB-2 transcription factor. Dev Biol. 2000;222:110–123. doi: 10.1006/dbio.2000.9699. [DOI] [PubMed] [Google Scholar]
- 22.Hiesberger T, Shao X, Gourley E, Reimann A, Pontoglio M, Igarashi P. Role of the hepatocyte nuclear factor-1beta (HNF-1beta) C-terminal domain in Pkhd1 (ARPKD) gene transcription and renal cystogenesis. J Biol Chem. 2005;280:10578–10586. doi: 10.1074/jbc.M414121200. [DOI] [PubMed] [Google Scholar]
- 23.Hoever M, Clement JH, Wedlich D, Montenarh M, Knochel W. Overexpression of wild-type p53 interferes with normal development in Xenopus laevis embryos. Oncogene. 1994;9:109–120. [PubMed] [Google Scholar]
- 24.Horster M, Huber S, Tschop J, Dittrich G, Braun G. Epithelial nephrogenesis. Pflugers Arch. 1997;434:647–660. doi: 10.1007/s004240050448. [DOI] [PubMed] [Google Scholar]
- 25.Huttinger-Kirchhof N, Cam H, Griesmann H, Hofmann L, Beitzinger M, Stiewe T. The p53 family inhibitor DeltaNp73 interferes with multiple developmental programs. Cell Death Differ. 2006;13:174–177. doi: 10.1038/sj.cdd.4401809. [DOI] [PubMed] [Google Scholar]
- 26.Ibraghimov-Beskrovnaya O. Targeting dysregulated cell cycle and apoptosis for polycystic kidney disease therapy. Cell Cycle. 2007;6:776–779. doi: 10.4161/cc.6.7.4047. [DOI] [PubMed] [Google Scholar]
- 27.Igarashi P. Following the expression of a kidney-specific gene from early development to adulthood. Nephron Exp Nephrol. 2003;94:e1–6. doi: 10.1159/000070812. [DOI] [PubMed] [Google Scholar]
- 28.Igarashi P, Shao X, McNally BT, Hiesberger T. Roles of HNF-1beta in kidney development and congenital cystic diseases. Kidney Int. 2005;68:1944–1947. doi: 10.1111/j.1523-1755.2005.00625.x. [DOI] [PubMed] [Google Scholar]
- 29.Ikawa S, Nakagawara A, Ikawa Y. p53 family genes: structural comparison, expression and mutation. Cell Death Differ. 1999;6:1154–1161. doi: 10.1038/sj.cdd.4400631. [DOI] [PubMed] [Google Scholar]
- 30.Jasper H, Benes V, Atzberger A, Sauer S, Ansorge W, Bohmann D. A genomic switch at the transition from cell proliferation to terminal differentiation in the Drosophila eye. Dev Cell. 2002;3:511–521. doi: 10.1016/s1534-5807(02)00297-6. [DOI] [PubMed] [Google Scholar]
- 31.Jones KA. Transcription strategies in terminally differentiated cells: shaken to the core. Genes Dev. 2007;21:2113–2117. doi: 10.1101/gad.1598007. [DOI] [PubMed] [Google Scholar]
- 32.Jones SN, Roe AE, Donehower LA, Bradley A. Rescue of embryonic lethality in Mdm2-deficient mice by absence of p53. Nature. 1995;378:206–208. doi: 10.1038/378206a0. [DOI] [PubMed] [Google Scholar]
- 33.Kispert A, Vainio S, McMahon AP. Wnt-4 is a mesenchymal signal for epithelial transformation of metanephric mesenchyme in the developing kidney. Development. 1998;125:4225–4234. doi: 10.1242/dev.125.21.4225. [DOI] [PubMed] [Google Scholar]
- 34.Kobayashi A, Kwan KM, Carroll TJ, McMahon AP, Mendelsohn CL, Behringer RR. Distinct and sequential tissue-specific activities of the LIM-class homeobox gene Lim1 for tubular morphogenesis during kidney development. Development. 2005;132:2809–2823. doi: 10.1242/dev.01858. [DOI] [PubMed] [Google Scholar]
- 35.Kopan R, Cheng HT, Surendran K. Molecular insights into segmentation along the proximal-distal axis of the nephron. J Am Soc Nephrol. 2007;18:2014–2020. doi: 10.1681/ASN.2007040453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Koster MI, Roop DR. p63 and epithelial appendage development. Differentiation. 2004;72:364–370. doi: 10.1111/j.1432-0436.2004.07208002.x. [DOI] [PubMed] [Google Scholar]
- 37.Lebel M, Agarwal P, Cheng CW, Kabir MG, Chan TY, Thanabalasingham V, Zhang X, Cohen DR, Husain M, Cheng SH, Bruneau BG, Hui CC. The Iroquois homeobox gene Irx2 is not essential for normal development of the heart and midbrain-hindbrain boundary in mice. Mol Cell Biol. 2003;23:8216–8225. doi: 10.1128/MCB.23.22.8216-8225.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lengner CJ, Steinman HA, Gagnon J, Smith TW, Henderson JE, Kream BE, Stein GS, Lian JB, Jones SN. Osteoblast differentiation and skeletal development are regulated by Mdm2-p53 signaling. J Cell Biol. 2006;172:909–921. doi: 10.1083/jcb.200508130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Leveillard T, Gorry P, Niederreither K, Wasylyk B. MDM2 expression during mouse embryogenesis and the requirement of p53. Mech Dev. 1998;74:189–193. doi: 10.1016/s0925-4773(98)00074-4. [DOI] [PubMed] [Google Scholar]
- 40.Levrero M, De Laurenzi V, Costanzo A, Gong J, Wang JY, Melino G. The p53/p63/p73 family of transcription factors: overlapping and distinct functions. J Cell Sci. 2000;113(Pt 10):1661–1670. doi: 10.1242/jcs.113.10.1661. [DOI] [PubMed] [Google Scholar]
- 41.Liu W, Murcia NS, Duan Y, Weinbaum S, Yoder BK, Schwiebert E, Satlin LM. Mechanoregulation of intracellular Ca2+ concentration is attenuated in collecting duct of monocilium-impaired orpk mice. Am J Physiol Renal Physiol. 2005;289:F978–988. doi: 10.1152/ajprenal.00260.2004. [DOI] [PubMed] [Google Scholar]
- 42.Marks J, Saifudeen Z, Dipp S, El-Dahr SS. Two functionally divergent p53-responsive elements in the rat bradykinin B2 receptor promoter. J Biol Chem. 2003;278:34158–34166. doi: 10.1074/jbc.M304543200. [DOI] [PubMed] [Google Scholar]
- 43.Mills AA, Zheng B, Wang XJ, Vogel H, Roop DR, Bradley A. p63 is a p53 homologue required for limb and epidermal morphogenesis. Nature. 1999;398:708–713. doi: 10.1038/19531. [DOI] [PubMed] [Google Scholar]
- 44.Mo L, Cheng J, Lee EY, Sun TT, Wu XR. Gene deletion in urothelium by specific expression of Cre recombinase. Am J Physiol Renal Physiol. 2005;289:F562–568. doi: 10.1152/ajprenal.00368.2004. [DOI] [PubMed] [Google Scholar]
- 45.Moll UM, Slade N. p63 and p73: roles in development and tumor formation. Mol Cancer Res. 2004;2:371–386. [PubMed] [Google Scholar]
- 46.Nakai S, Sugitani Y, Sato H, Ito S, Miura Y, Ogawa M, Nishi M, Jishage K, Minowa O, Noda T. Crucial roles of Brn1 in distal tubule formation and function in mouse kidney. Development. 2003;130:4751–4759. doi: 10.1242/dev.00666. [DOI] [PubMed] [Google Scholar]
- 47.Poyurovsky MV, Prives C. Unleashing the power of p53: lessons from mice and men. Genes Dev. 2006;20:125–131. doi: 10.1101/gad.1397506. [DOI] [PubMed] [Google Scholar]
- 48.Pozniak CD, Radinovic S, Yang A, McKeon F, Kaplan DR, Miller FD. An anti-apoptotic role for the p53 family member, p73, during developmental neuron death. Science. 2000;289:304–306. doi: 10.1126/science.289.5477.304. [DOI] [PubMed] [Google Scholar]
- 49.Reggiani L, Raciti D, Airik R, Kispert A, Brandli AW. The prepattern transcription factor Irx3 directs nephron segment identity. Genes Dev. 2007;21:2358–2370. doi: 10.1101/gad.450707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rentzsch F, Kramer C, Hammerschmidt M. Specific and conserved roles of TAp73 during zebrafish development. Gene. 2003;323:19–30. doi: 10.1016/j.gene.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 51.Saifudeen Z, Diavolitsis V, Stefkova J, Dipp S, Fan H, El-Dahr SS. Spatiotemporal switch from DeltaNp73 to TAp73 isoforms during nephrogenesis: impact on differentiation gene expression. J Biol Chem. 2005;280:23094–23102. doi: 10.1074/jbc.M414575200. [DOI] [PubMed] [Google Scholar]
- 52.Saifudeen Z, Dipp S, El-Dahr SS. A role for p53 in terminal epithelial cell differentiation. J Clin Invest. 2002;109:1021–1030. doi: 10.1172/JCI13972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Saifudeen Z, Dipp S, Fan H, El-Dahr SS. Combinatorial control of the bradykinin B2 receptor promoter by p53, CREB, KLF-4, and CBP: implications for terminal nephron differentiation. Am J Physiol Renal Physiol. 2005;288:F899–909. doi: 10.1152/ajprenal.00370.2004. [DOI] [PubMed] [Google Scholar]
- 54.Saifudeen Z, Du H, Dipp S, El-Dahr SS. The bradykinin type 2 receptor is a target for p53-mediated transcriptional activation. J Biol Chem. 2000;275:15557–15562. doi: 10.1074/jbc.M909810199. [DOI] [PubMed] [Google Scholar]
- 55.Saifudeen Z, Marks J, Du H, El-Dahr SS. Spatial repression of PCNA by p53 during kidney development. Am J Physiol Renal Physiol. 2002;283:F727–733. doi: 10.1152/ajprenal.00114.2002. [DOI] [PubMed] [Google Scholar]
- 56.Sajithlal G, Zou D, Silvius D, Xu PX. Eya 1 acts as a critical regulator for specifying the metanephric mesenchyme. Dev Biol. 2005;284:323–336. doi: 10.1016/j.ydbio.2005.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Saulnier DM, Ghanbari H, Brandli AW. Essential function of Wnt-4 for tubulogenesis in the Xenopus pronephric kidney. Dev Biol. 2002;248:13–28. doi: 10.1006/dbio.2002.0712. [DOI] [PubMed] [Google Scholar]
- 58.Schedl A. Renal abnormalities and their developmental origin. Nat Rev Genet. 2007;8:791–802. doi: 10.1038/nrg2205. [DOI] [PubMed] [Google Scholar]
- 59.Schmitt CA, Fridman JS, Yang M, Baranov E, Hoffman RM, Lowe SW. Dissecting p53 tumor suppressor functions in vivo. Cancer Cell. 2002;1:289–298. doi: 10.1016/s1535-6108(02)00047-8. [DOI] [PubMed] [Google Scholar]
- 60.Sears RC, Nevins JR. Signaling networks that link cell proliferation and cell fate. J Biol Chem. 2002;277:11617–11620. doi: 10.1074/jbc.R100063200. [DOI] [PubMed] [Google Scholar]
- 61.Shen B, Harrison-Bernard LM, Fuller AJ, Vanderpool V, Saifudeen Z, El-Dahr SS. The Bradykinin B2 receptor gene is a target of angiotensin II type 1 receptor signaling. J Am Soc Nephrol. 2007;18:1140–1149. doi: 10.1681/ASN.2006101127. [DOI] [PubMed] [Google Scholar]
- 62.Strano S, Rossi M, Fontemaggi G, Munarriz E, Soddu S, Sacchi A, Blandino G. From p63 to p53 across p73. FEBS Lett. 2001;490:163–170. doi: 10.1016/s0014-5793(01)02119-6. [DOI] [PubMed] [Google Scholar]
- 63.Sweet DH, Eraly SA, Vaughn DA, Bush KT, Nigam SK. Organic anion and cation transporter expression and function during embryonic kidney development and in organ culture models. Kidney Int. 2006;69:837–845. doi: 10.1038/sj.ki.5000170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Takebayashi-Suzuki K, Funami J, Tokumori D, Saito A, Watabe T, Miyazono K, Kanda A, Suzuki A. Interplay between the tumor suppressor p53 and TGF beta signaling shapes embryonic body axes in Xenopus. Development. 2003;130:3929–3939. doi: 10.1242/dev.00615. [DOI] [PubMed] [Google Scholar]
- 65.Tchang F, Gusse M, Soussi T, Mechali M. Stabilization and expression of high levels of p53 during early development in Xenopus laevis. Dev Biol. 1993;159:163–172. doi: 10.1006/dbio.1993.1230. [DOI] [PubMed] [Google Scholar]
- 66.Tran U, Pickney LM, Ozpolat BD, Wessely O. Xenopus Bicaudal-C is required for the differentiation of the amphibian pronephros. Dev Biol. 2007;307:152–164. doi: 10.1016/j.ydbio.2007.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Urban AE, Zhou X, Ungos JM, Raible DW, Altmann CR, Vize PD. FGF is essential for both condensation and mesenchymal-epithelial transition stages of pronephric kidney tubule development. Dev Biol. 2006;297:103–117. doi: 10.1016/j.ydbio.2006.04.469. [DOI] [PubMed] [Google Scholar]
- 68.Vainio S, Lin Y. Coordinating early kidney development: lessons from gene targeting. Nat Rev Genet. 2002;3:533–543. doi: 10.1038/nrg842. [DOI] [PubMed] [Google Scholar]
- 69.Wallingford JB, Seufert DW, Virta VC, Vize PD. p53 activity is essential for normal development in Xenopus. Curr Biol. 1997;7:747–757. doi: 10.1016/s0960-9822(06)00333-2. [DOI] [PubMed] [Google Scholar]
- 70.Wang Y, Ray SK, Hinds PW, Leiter AB. The retinoblastoma protein, RB, is required for gastrointestinal endocrine cells to exit the cell cycle, but not for hormone expression. Dev Biol. 2007;311:478–486. doi: 10.1016/j.ydbio.2007.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Woods DB, Vousden KH. Regulation of p53 function. Exp Cell Res. 2001;264:56–66. doi: 10.1006/excr.2000.5141. [DOI] [PubMed] [Google Scholar]
- 72.Woolf AS, Price KL, Scambler PJ, Winyard PJ. Evolving concepts in human renal dysplasia. J Am Soc Nephrol. 2004;15:998–1007. doi: 10.1097/01.asn.0000113778.06598.6f. [DOI] [PubMed] [Google Scholar]
- 73.Xu WS, Parmigiani RB, Marks PA. Histone deacetylase inhibitors: molecular mechanisms of action. Oncogene. 2007;26:5541–5552. doi: 10.1038/sj.onc.1210620. [DOI] [PubMed] [Google Scholar]
- 74.Yang A, Walker N, Bronson R, Kaghad M, Oosterwegel M, Bonnin J, Vagner C, Bonnet H, Dikkes P, Sharpe A, McKeon F, Caput D. p73-deficient mice have neurological, pheromonal and inflammatory defects but lack spontaneous tumours. Nature. 2000;404:99–103. doi: 10.1038/35003607. [DOI] [PubMed] [Google Scholar]
- 75.Yoder BK. Role of primary cilia in the pathogenesis of polycystic kidney disease. J Am Soc Nephrol. 2007;18:1381–1388. doi: 10.1681/ASN.2006111215. [DOI] [PubMed] [Google Scholar]
- 76.Yu J, McMahon AP, Valerius MT. Recent genetic studies of mouse kidney development. Curr Opin Genet Dev. 2004;14:550–557. doi: 10.1016/j.gde.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 77.Zhou X, Vize PD. Proximo-distal specialization of epithelial transport processes within the Xenopus pronephric kidney tubules. Dev Biol. 2004;271:322–338. doi: 10.1016/j.ydbio.2004.03.036. [DOI] [PubMed] [Google Scholar]
- 78.Zhu L, Skoultchi AI. Coordinating cell proliferation and differentiation. Curr Opin Genet Dev. 2001;11:91–97. doi: 10.1016/s0959-437x(00)00162-3. [DOI] [PubMed] [Google Scholar]



