Abstract
Group-I metabotropic glutamate receptors have been often implicated in various models of neuronal toxicity, however, the role played by the individual receptors and their putative mechanisms of action contributing to neurotoxicity or neuroprotection remain unclear. Here, using primary cultures of rat cerebellar granule cells and mouse cortical neurons, we show that conditions of trophic deprivation increased mGlu1 expression which correlated with the developing cell death. The inhibition of mGlu1 expression by specific siRNA attenuated toxicity, while adenovirus-mediated overexpression of mGlu1 resulted in increased cell death, indicating a causal relationship between the level of receptor expression and neuronal survival. In pharmacological experiments selective mGlu1 antagonists failed to protect from mGlu1-induced cell death, instead, neuronal survival was promoted by glutamate acting at mGlu1 receptors. Such properties are characteristic of a novel heterogeneous family of dependence receptors which control neuronal apoptosis. Our findings indicate that increased expression of mGlu1 in neurons creates a state of cellular dependence on the presence of its endogenous agonist glutamate. We propose a new role and a new mechanism for mGlu1 action. This receptor may play a crucial role in determining the fate of individual neurons during the development of the nervous system.
Keywords: cerebellar granule cells, metabotropic glutamate receptor, neurotoxicity, neuroprotection, trophic deprivation, dependence receptors
1. Introduction
The development of the central nervous system, the differentiation of neuronal cells, and the formation of synaptic connections depend on sustained trophic support provided by a variety of factors released from the surrounding neuronal and glial cells. Cells, deprived of such support, are eliminated by apoptosis during the course of brain development. Under experimental conditions, apoptotic cell death has been demonstrated in primary cultures of neurons subjected to trophic deprivation. A striking example are the primary cultures of cerebellar granule cells which require, for their survival, the presence of elevated K+ concentrations (Wood et al., 1997). At physiological K+ levels, granule cells die showing DNA fragmentation and other hallmarks of occurring apoptosis (D'Mello et al., 1993; Contestabile, 2002). Using competitive PCR, we have shown previously that, in rat granule cells, K+ deprivation enhanced the appearance of mGlu1 mRNA (Santi et al., 1994), suggesting that increased mGlu1 expression may contribute to the apoptotic death of these neurons.
Group-I metabotropic glutamate receptors, mGlu1 and mGlu5, known to regulate intracellular calcium homeostasis through their G protein-mediated coupling to phospholipase C (Conn and Pin, 1997), have been proposed to participate in neuronal toxicity (Nicoletti et al., 1999), The use of receptor agonists and antagonists to study these effects has provided a wide array of often contradictory data showing both toxic and protective effects of mGlu1 and mGlu5 receptors in various experimental models. Some studies point to the possibility that toxicity of mGlu1 receptors may depend on the level of receptor expression showing that overexpression of this receptor in heterologous systems leads to toxicity (Nash et al., 2001). A transient expression of mGlu1 in HEK 293 cells caused an agonist-independent cell death, while the co-expression of GRK2, which decreased mGlu1 levels, protected against mGlu1-stimulated apoptosis (Dale et al., 2000).
The aim of this work was to determine the role that mGlu1 plays in the survival of neuronal cells and to investigate the existence of a causal link between the changes in mGlu1 expression and the occurring toxicity. In this report we demonstrate a new dual mechanism of action of mGlu1 receptors involved in the control of neuronal survival. Our findings indicate that, in neurons, conditions of trophic deprivation led to increased mGlu1 expression which was directly responsible of the occurring cell death. The toxic effect of mGlu1 was not mediated by the agonist-stimulated receptor activity but depended on the level of receptor expression. Instead, the endogenous receptor agonist glutamate protected from mGlu1-induced apoptosis. These data allowed us to conclude that mGlu1 displays the properties of a dependence receptor (Mehlen and Bredesen, 2004), inducing neuronal death in the absence of glutamate and promoting neuronal survival in its presence.
2. Materials and Methods
2.1. Materials
Neurobasal culture media, B27 supplement and fetal bovine serum for neuronal cultures were purchased from Invitrogen (Grand Island, NY). EMEM, DMEM and fetal bovine serum for transfected cell cultures were purchased from Biofluids (Rockville, MD). All restriction enzymes were from New England Biolabs (Beverly, MD). Receptor agonists glutamate, (1S,3R)-1-aminocyclopentane-1,3-dicarboxylic acid (ACPD), (2R,4R)-4-Aminopyrrolidine-2,4-dicarboxylate (APDC), (±)-2-Amino-4-phosphonobutyric acid (AP4) and antagonists 7-hydroxyiminocyclopropan[b]chromen-1a-carboxylic acid ethyl ester (CPCCOEt), 6-amino-N-cyclohexyl-N,3-dimethylthiazolo[3,2-a]benzimidazole-2-carboxamide (YM298198), 2-methyl-6-(phenylethynyl)pyridine (MPEP), 2,3-dihydro-6-nitro-7-sulfamoyl-benzo(f)quinoxaline (NBQX), (5R,10S)-(-)-5-Methyl-10,11-dihydro-5H-dibenzo[a,d]cylcohepten-5,10-imine maleate (MK801), (S)-2-Amino-2-methyl-4-phosphonobutanoic acid (MAP4), (2S,3S,4S)-2-methyl-2-(carboxycyclopropyl) glycine (MCCG) were obtained from Tocris Cookson (Ellisville, MO). All other chemicals were obtained from Sigma (St. Louis, MO).
2.2. Cell cultures
Primary cultures were prepared as described previously for rat cerebellar granule cells (Wroblewski et al., 1985), mouse cortical neurons,(Bruno et al., 1998) and rat cortical astrocytes (Pshenichkin and Wise, 1995), Neuronal cultures were maintained in Neurobasal medium supplemented with B27 and 2 mM glutamine, 100 µg/ml gentamicin, and either 5 or 25 mM KCl. To prevent the growth of non-neuronal cells cytosine arabinoside (10 µM) was added next day after plating. Both cerebellar granule cells and cortical neurons were cultured in the plating medium for one week before the indicated treatment. Astrocytes were cultured in EMEM medium supplemented with 10% fetal bovine serum. PC-12 cells were permanently transfected with mGluR cDNA in pcDNA-3.1 vector (Invitrogen, Carlsbad, CA) using Effectene transfection reagent (Qiagen, Hilden, Germany). Individual cell lines were isolated and cultured in DMEM supplemented with 10% fetal bovine serum, 2 mM glutamine and 0.8 mg/ml G-418 (Mediatech, Hemdon, VA).
2.3. Treatment of cells with mGlu1 siRNA
Specific siRNA for mGlu1 (target sequence: AAAGTATTGCTGGCAGGTGCC) and nonspecific control siRNA targeting green fluorescent protein (GFP-22) sequence (CGGCAAGCTGACCCTGAAGTTCA) (Qiagen-Xeragon, Germantown, MD) were applied at 1 µM concentrations to rat cerebellar neurons in presence of 1 % Transit-TKO transfection reagent (Mirus Bio Corp, Madison, WI). Penetration into cells was confirmed using siRNA labeled with fluorescein.
2.4. Construction of Adenoviral vectors
For the construction of adenoviral vectors, rat mGlu1a cDNA was subcloned into the mammalian expression vectors pEGFP-C2 (all vectors were from Clontech, Palo Alto, CA) in frame with GFP cDNA sequence between SalI/XbaI cloning sites. Then cDNA for GFP-mGlu1 fusion protein was cloned into NheI/XbaI restriction sites of pShuttle vector containing the CMV promoter. The resulting vector was digested with I-CeuI and PI-SceI restriction enzymes and the fragment containing the sequence of GFP-mGlu1 together with CMV promoter was cloned into Adeno-X viral vector. This vector was used to transfect HEK-293 cells for production of viral particles. At final stage of large-scale viral preparation, cells were cultured in DMEM containing 2% of serum. Adenoviruses were purified from 250 ml of HEK-293 medium and the cell lysates by using BD Adeno-X virus purification kit (BD Biosciences, Palo Alto, CA). The control, GFP-expressing, viruses were obtained similarly. Adenoviral titer was determined using the BD Adeno-X Rapid Titer kit (BD Biosciences). Equal amounts (MOI) of adenoviruses delivering the GFP-mGlu1 construct or GFP alone were added to the neurons on the next day after plating.
2.5. Immunoblots
For Western blots cells were harvested in 25 mM Tris-HCl buffer, pH 7.5 containing the protease inhibitor cocktail, 1mM EDTA and 1mM Na3VO4. Membrane proteins were solubilized in the sample buffer containing 50 mM DDT, and equal amounts of sample protein were resolved by SDS-PAGE on an 8% gel (Invitrogen). Proteins were transferred to Hybond ECL nitrocellulose membrane (Amersham Pharmacia Biotech, Buckinghamshire, UK) and were probed with specific C-terminal anti-mGlu1 or anti-mGlu5 antibodies (Upstate Biotechnology, Waltham, MA). Proteins were visualized by incubation with horseradish peroxidase coupled with goat anti-rabbit secondary antibodies (Amersham) followed by exposure to a luminescent chromogen SuperSignal West Femto (Pierce Biotechnology, Rockford, IL). Loading controls were done using antibodies against β-actin (Sigma). Receptor expression was quantified by adding the densities of two receptor bands (if visible) and dividing by the density of the β-actin band.
2.6. Assessment of cell viability
To measure viability, cells cultured on 96-well plates were incubated for 1 h at 37°C with 0.2 mg/ml of 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT) and the formation of the formazan product, proportional to the number of viable cells, was measured colorimetrically at 570 nm after extraction with 70 µl DMSO (Mosmann, 1983). In some experiments cells were stained using the Live/Dead Viability/Cytotoxicity kit (Molecular Probes, Eugene, OR) and visualized under a fluorescent microscope with green fluorescence of calcein indicating live cells and red fluorescence of ethidium homodimer-1 indicating dead cells.
2.7. Measurements of PI hydrolysis
Cells, cultured in 96-well plates, were incubated overnight with 0.625 µCi/well myo-[3H]inositol (NEN, Boston, MA) to label the cell membrane phosphoinositides. After two washes with 0.1 ml of Locke’s buffer (156 mM NaCl, 5.6 mM KCl, 3.6 mM NaHCO3, 1 mM MgCl2, 1.3 mM CaCl2, 5.6 mM glucose and 20 mM Hepes, pH 7.4, all from Sigma) incubations with receptor ligands were carried out for 45 min at 37°C in Locke’s buffer containing 20 mM LiCl to block inositol phosphate degradation. The reaction was terminated by aspiration of medium and inositol phosphates were extracted with 100 µl of 0.1 M HCl for 10 min. The separation of [3H]inositol phosphates was performed by ion-exchange chromatography on AG 1-X8 resin, 200–400 mesh (Bio-Rad, Hercules, CA). The samples were diluted 10 times with water and applied onto columns equilibrated in 0.1 M formic acid. The columns were washed with 1ml of water and 1 ml of tetraborate buffer (5mM sodium tetraborate, 60 mM sodium formate). Total [3H]inositol phosphates were eluted from the columns with 0.5 ml of 0.1 M formic acid/1 M ammonium formate. The collected samples were mixed with Safety-Solve cocktail (RPI, Mount Prospect, IL) and measured by scintillation counting.
3. Results
3.1. Increase of mGlu1 expression in neurons subjected to trophic deprivation
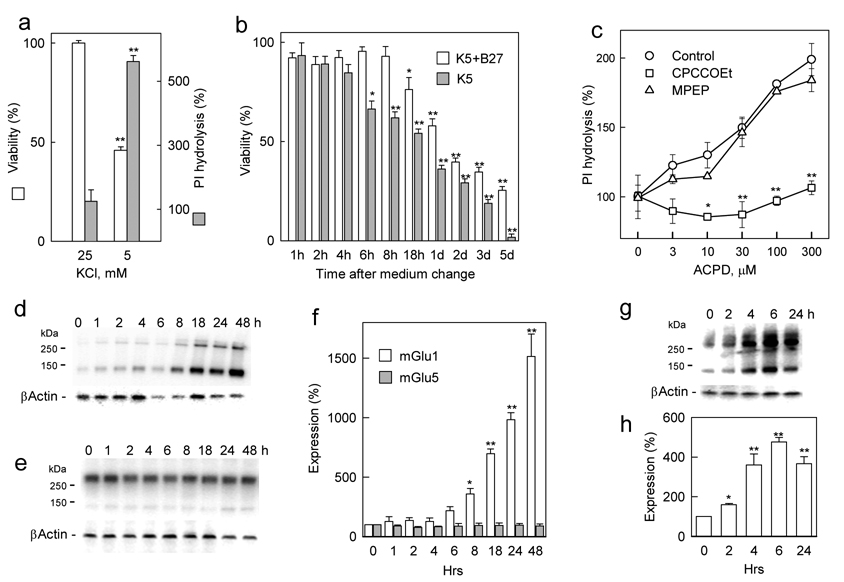
Primary cultures of cerebellar granule cells, cultured in presence of 25 mM K+ (K25 conditions), showed a high survival rate, which declined when the K+ concentration was decreased (Fig. 1a). In presence of 5 mM K+ (K5 conditions), granule cell viability decreased to about half of that observed under K25 conditions. The decrease of K+ concentrations resulted also in elevated phosphoinositide (PI) hydrolysis stimulated by the mGluR agonists ACPD, showing a large increase under K5 conditions, as compared to K25 conditions (Fig. 1a). This suggested an enhanced activity of group I mGluRs under conditions which are known to result in an apoptotic death of granule cells. To assess the correlation between the toxicity induced by trophic deprivation and the expression of group I mGluRs, granule cells were cultured in serum-free neurobasal (NB) medium, supplemented with B27 and 25 mM K+ for 7 days and then conditions of trophic deprivation were induced by transferring cells to fresh K5 medium with or without B27 (Fig. 1b). In presence of B27, K5 conditions significantly reduced cell viability after 18 h, which, after 5 days, declined to 25% of control cell viability observed under K25 conditions. In the absence of B27, significant cell death appeared already after 6 h and all cells were dead after 5 days.
Figure 1. Effect of trophic deprivation on cell viability and mGlu1 expression in primary cultures of rat cerebellar neurons.
a, Cerebellar granule cells were cultured in K25 NB medium supplemented with B27 for one week. At this time, lowering of K+ concentration for 2 days decreased viability and enhanced the expression of group I mGluRs as measured by 100 µM ACPD-stimulated PI hydrolysis (shown as % of basal PI hydrolysis). b, Time course of developing toxicity after medium change from K25+B27 to K5 in the presence and absence of B27. c, The ACPD-stimulated PI hydrolysis, enhanced 24 h after medium change from K25+B27 to K5+B27, is inhibited by the mGlu1 antagonist CPCCOEt (100 µM) but not by the mGlu5 antagonist MPEP (10 µM). Western blots showing that K5+B27 conditions induce a time-dependent increase of mGlu1 (d), but not mGlu5 (e) expression and quantification of these blots after normalization using β-actin (f). Simultaneous removal of B27 accelerates mGlu1 expression as seen on a representative Western blot (g) and quantified after normalization using β-actin (h). All values are means from at least three independent experiments with error bars representing S.E.M. * p<0.05 and **p<0.001 as compared to untreated controls using Student’s t-test.
In parallel experiments the time-course of expression of mGlu1 and mGlu5 was evaluated using Western blots with receptor-specific antibodies. In presence of B27, K5 conditions caused an increase in the expression of mGlu1 protein, seen as both monomers and dimers, which was very prominent after 18 h (Fig. 1d, f), correlating well with the time-course of toxicity observed under these conditions (Fig. 1b). In contrast, the levels of mGlu5 protein remained unchanged (Fig. 1e, f) and consisted mostly of receptor dimers. In the absence of B27, K5 conditions, which induced an accelerated toxicity (Fig. 1b), also caused a more rapid increase of mGlu1 expression, which was significantly elevated already after 4 h and showed a tendency to decline after 24 h (Fig. 1g, h). The observed differences between mGlu1 and mGlu5 expression were also confirmed in functional measurements of PI hydrolysis. The increase in mGlu1 expression was consistent with measurements of PI hydrolysis stimulated by ACPD, which was elevated after 24h in K5 conditions, and was abolished by the selective mGlu1 antagonist CPCCOEt, but not by the selective mGlu5 antagonist MPEP (Fig. 1c). Hence, both Western blots and PI measurements indicated that among group I mGluRs, mGlu1, rather than mGlu5, shows high functional expression in granule cells subjected to conditions of trophic deprivation.
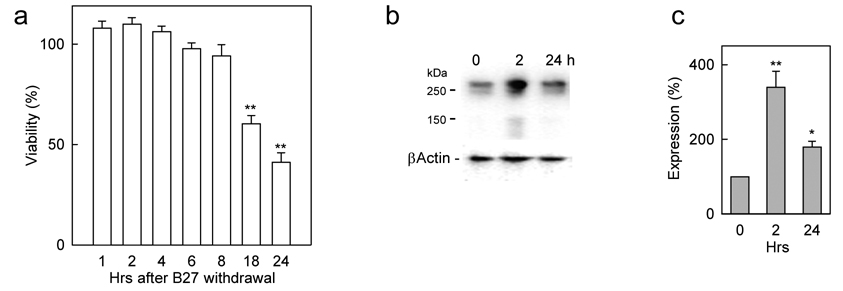
The increase in levels of mGlu1 receptors in conditions associated with trophic deprivation was not a unique feature of cerebellar granule neurons. In primary cultures of mouse cortical neurons, which do not require elevated K+ concentrations but where apoptosis can be induced by trophic deprivation (Ryu et al., 1999), the removal of B27 from the culture medium led to cell death with a time-course similar to that for cerebellar granule cells (Fig. 2a) and was accompanied by a transient increase of mGlu1 expression (Fig. 2b, c), however, after 24 h mGlu1 levels returned to control values. The above results indicate that in cerebellar and cortical neurons, conditions of trophic deprivation cause cell death which is preceded by increases in the level of mGlu1 expression.
Figure 2. Effect of trophic deprivation on cell viability and mGlu1 expression in primary cultures of mouse cortical neurons.
a, Time-dependent effect of trophic deprivation (removal of B27 supplement) on viability of mouse cortical neurons. Representative Western blot (b) and its quantification (c) from mouse cortical neurons subjected to trophic deprivation shows a transient increase in mGlu1 expression. All values are means from at least three independent experiments with error bars representing S.E.M. * p<0.05 and **p<0.001 as compared to untreated controls using Student’s t-test.
3.2. Decrease of mGlu1 expression in neurons is neuroprotective
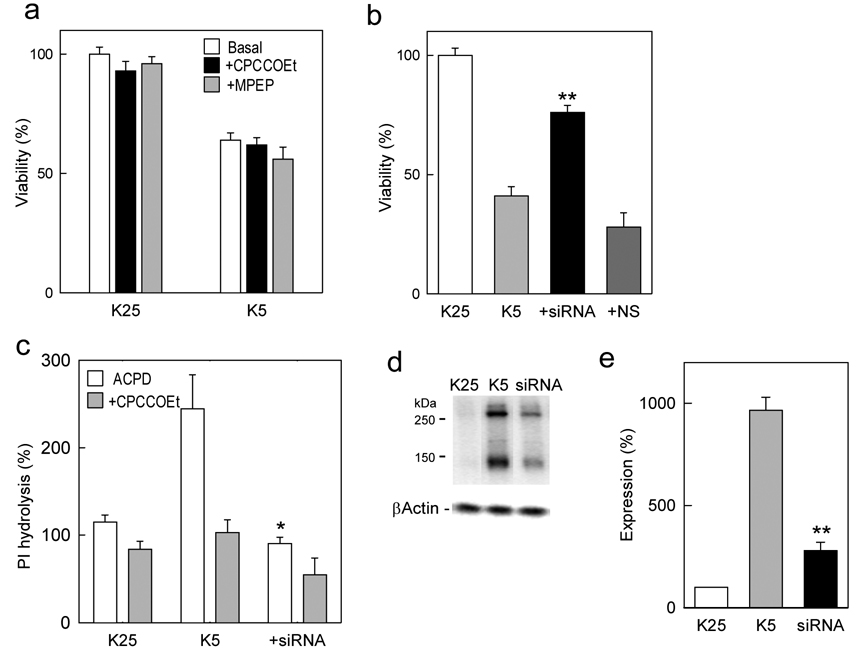
To determine whether the elevated mGlu1 expression contributes to the observed toxicity, experiments were carried out in presence of the selective receptor antagonist. However, in spite of the ability of CPCCOEt to abolish the mGlu1-mediated PI hydrolysis in granule cells (Fig. 1c), this antagonist failed to attenuate K5-induced cell death (Fig 3a), indicating that the agonist-stimulated receptor activity may not be responsible for the toxic effect. To investigate the possibility that mGlu1 toxic action may be mediated by an agonist-independent, constitutive receptor activity and, hence, may depend directly on the level of receptor expression, specific siRNA was used to downregulate the expression of native mGlu1 receptors in cerebellar granule cells. Since both cell death and increased mGlu1 expression occur after medium change from K25 to K5 conditions, the mGlu1-specific siRNA (1 µM) was applied to granule cells in presence of the Transit-TKO transfection reagent to 7 DIV granule cells at the time of medium replacement. The penetration into cells was confirmed in parallel experiments using siRNA labeled with fluorescein (not shown). When cell viability was measured 72 h after the onset of K5 conditions, the specific mGlu1 siRNA significantly increased cell viability, counteracting the toxic effect of K+ deprivation (Fig. 3b). This effect was not reproduced by the application of a nonspecific siRNA indicating that the observed protection may be due to a downregulation of mGlu1 expression. When tested in functional experiments, the specific mGlu1 siRNA virtually abolished the ACPD-induced, CPCCOEt-sensitive, activation of PI hydrolysis (Fig. 3c), demonstrating its effectiveness in eliminating mGlu1 expression. In addition, the expression of mGlu1 was evaluated by Western blots which confirmed that the large increase of mGlu1 expression under K5 conditions was significantly attenuated by mGlu1-specific siRNA (Fig. 3d, e). These results indicate that, in cerebellar neurons subjected to K+ deprivation, an increased mGlu1 expression is a significant factor contributing to neuronal death. Moreover, prevention of mGlu1 increase under conditions of trophic deprivation promoted neuronal survival.
Figure 3. Downregulation of mGlu1 expression in rat cerebellar neurons improves neuronal survival.
a, Toxicity measured 24 hrs after changing the medium from K25+B27 to K5+B27 is not prevented by either CPCCOEt (100 µM) or by MPEP (10 µM). b, Application of mGlu1-specific siRNA but not nonspecific siRNA (NS) to rat cerebellar neurons at the time of medium change from K25+B27 to K5+B27 attenuates toxicity induced by K5 conditions. c, mGlu1 siRNA abolishes ACPD (100 µM)-induced, CPCCOEt (100 µM)-sensitive, PI hydrolysis in rat cerebellar neurons measured 72 h after medium change from K25+B27 to K5+B27. Application of mGlu1-specific siRNA also attenuates enhanced mGlu1 expression as shown after 72 hrs by a representative Western blot (d), and its quantification using normalization with β-actin (e). Data are means with error bars representing S.E.M. from three to four separate experiments. *p<0.05 and **p<0.001 as compared to untreated K5 controls using Student’s t-test.
3.3. Increase of mGlu1 expression is neurotoxic
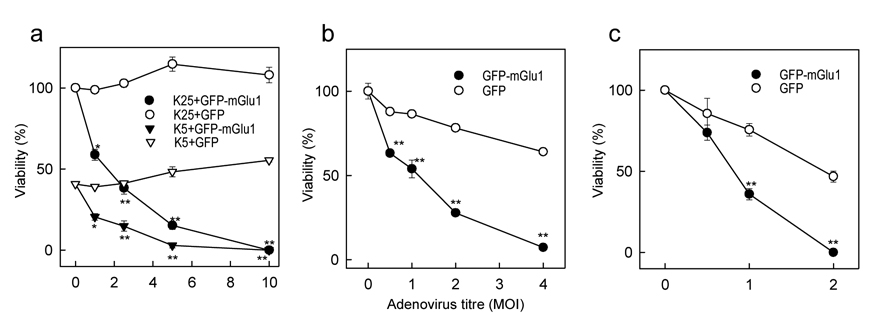
While the above results indicate that mGlu1 plays a significant role in toxicity induced in granule cells by K+ deprivation, such conditions potentially affect the expression of various genes which may be involved in the toxic mechanism. To determine whether the enhanced expression of mGlu1, alone, is a sufficient factor to induce neuronal death, experiments were performed where mGlu1 expression was increased in a specific manner without inducing other changes that may result from K+ deprivation. For this purpose we have constructed adenoviral vectors to deliver a fusion protein consisting of green fluorescent protein (GFP) linked to the N-terminus of mGlu1. This allowed us to observe the efficiency of mGlu1 expression by monitoring the fluorescence of the chimeric protein. Separate experiments in CHO cells showed that this construct yielded a fully functional receptor (data not shown). A similar vector containing only GFP cDNA was used as a control for nonspecific effects of the adenovirus. Cerebellar granule cells were infected with adenoviruses on the next day after plating in K5 or K25 medium. The efficiency of infection and the levels of expression of the delivered proteins were monitored by GFP fluorescence and were time-dependent and dose-dependent, reaching the maximum (90% of infected cells) at day 4 after infection at a virus titer of 5 MOI (multiplicity of infection). Measured at four days after infection, cell viability was reduced, depending on the titer of the virus and, hence, on the level of GFP-mGlu1 expression (Fig. 4a). The toxic effect was very strong in granule cells cultured in K25 conditions, in which the cells express very low levels of native mGlu1 and exhibit high viability. In K5 conditions, in which viability is much lower, GFP-mGlu1-expressing virus caused further toxicity. In either condition, the control adenovirus delivering GFP alone had no toxic effects (Fig. 4a). The toxic effects of mGlu1 overexpression were not unique to rat cerebellar neurons but could be replicated in primary cultures of mouse cortical neurons (Fig. 4b) and in rat cortical astrocytes (Fig. 4c). Both cell types were more sensitive than cerebellar neurons to the toxicity of the control GFP adenovirus; however, the GFP-mGlu1 adenovirus had a much stronger toxic effect. Moreover, astrocytes, which do not express measurable levels of native mGlu1, were more susceptible than neurons to the toxic effect of mGlu1 expression, displaying maximum toxicity already at a virus titer of 2 MOI. These results indicate that enhanced expression of mGlu1 is a sufficient factor to induce toxicity in neuronal and glial cells.
Figure 4. Enhanced mGlu1 expression decreases the survival of neuronal and glial cells.
a, Adenovirus-mediated overexpression of GFP-mGlu1 fusion protein, but not of GFP alone, induces toxicity in rat cerebellar neurons treated with increasing doses (MOI) of adenovirus. Similar toxic effects of GFP-mGlu1 overexpression are seen in mouse cortical neurons (b) and rat cortical astrocytes (c). The cells were infected on the next day after plating. Levels of viral infection were monitored by fluorescence imaging and cell viability was measured by the MTT assay 4 days after viral infections. Data are expressed as per cent of viable cells compared to untreated controls and are means with error bars representing S.E.M. from three separate experiments. *p<0.05 and **p<0.001 as compared to GFP-expressing controls using Student’s t-test.
3.4. Neuroprotective effects of glutamate are mediated by mGlu1
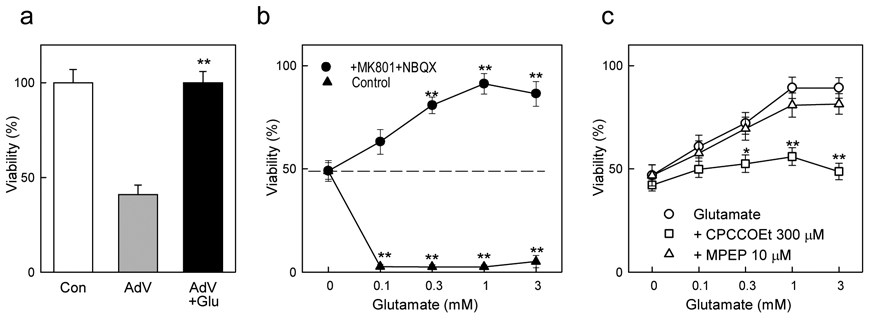
In an attempt to determine the role that mGlu1 agonists may play in regulating mGlu1 toxicity we applied high concentrations of glutamate, expecting to exacerbate the toxicity induced by adenovirus-mediated mGlu1 expression in mouse cortical neurons. To eliminate the toxic effects of glutamate mediated by ionotropic NMDA and AMPA/kainate receptors the experiments were performed in the presence of their respective antagonists, MK801 (1 µM) and NBQX (10 µM). Unexpectedly, under these conditions, 1 mM glutamate completely abolished the toxicity induced by specific mGlu1 overexpression (Fig. 5a). This effect was further investigated in mouse cortical neurons subjected to trophic deprivation by B27 removal. Under these conditions, cell viability decreased to about 50% of the untreated cultures. Addition of glutamate in the absence of antagonists of ionotropic glutamate receptors caused a complete cell death. In contrast, in presence of MK801 and NBQX, glutamate produced a dose-dependent protection which reached maximum in presence of 1 mM glutamate (Fig. 5b). The protective effect of glutamate was abolished by the mGlu1-selective antagonists CPCCOEt, but not by the mGlu5 antagonist MPEP (Fig. 5c). The inhibitory action of the selective antagonist indicates that the death of cortical neurons induced by trophic deprivation is prevented by glutamate acting at mGlu1 receptors.
Figure 5. Neuroprotective effects of glutamate in mouse cortical neurons.
a, Toxicity induced by adenoviral overexpression of mGlu1 (AdV), expressed as % of viability in untreated cells, is abolished by 1 mM glutamate (Glu), when tested in presence of inhibitors of ionotropic glutamate receptors 1 µM MK801 and 10 µM NBQX. b, Glutamate kills all cells in the absence of MK801 and NBQX, but provides a dose-dependent protection against toxicity induced by a 24h deprivation of B27 supplement (dashed line) when applied in the presence of 1µM MK801 and 10 µM NBQX. c, The protective effect of glutamate is abolished by the mGlu1 antagonist CPCCOEt but not by the mGlu5 antagonist MPEP. Data are means with error bars representing S.E.M. from at least three experiments. *p<0.05 and **p<0.001 as compared to glutamate-untreated control (a, b) or to glutamate alone (c) using Student’s t-test.
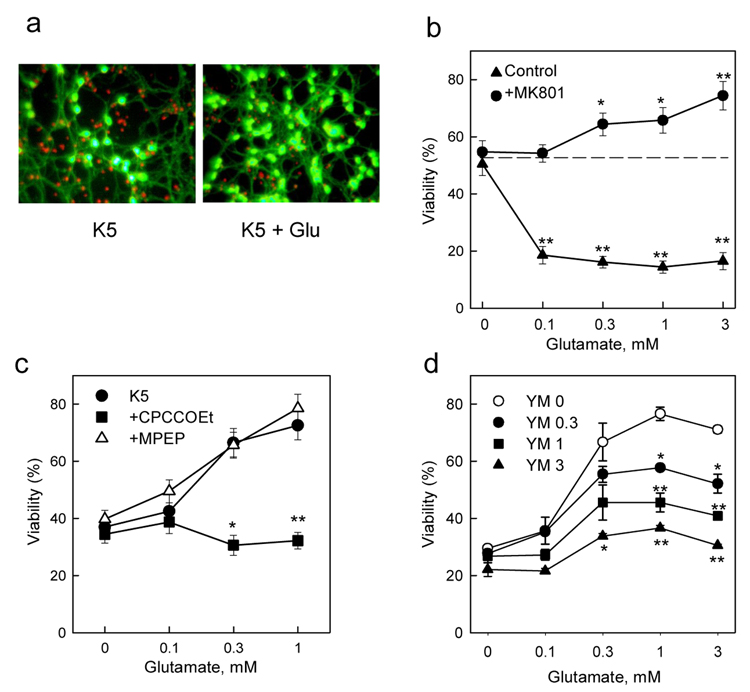
A similar protective effect of glutamate was observed in cerebellar granule cells subjected to K+ deprivation, although the conditions necessary to reveal this effect depended on the age of the cultures. In mature granule cells (18DIV), the transfer from K25 to K5 conditions decreased cell viability which could be visualized by fluorescent staining. The addition of 1 mM glutamate to K5 cultures, in presence of MK801, significantly increased the number of viable cells (green fluorescence, Fig. 6a). The protective effect of glutamate was dose-dependent and could be revealed only in the presence of the NMDA receptor antagonist (Fig. 6b), while in the absence of MK801, glutamate was neurotoxic. In young granule cells (7DIV), which were insensitive to NMDA receptor-mediated toxicity (Resink et al., 1994), the K5 conditions decreased substantially cell viability and the protective effect of glutamate was visible in the absence of MK801 (Fig. 6c). Moreover, as seen in cortical cells, the protective effect of glutamate was abolished by CPCCOEt, but not MPEP, (Fig. 6c) confirming the participation of mGlu1 in this process. To ascertain that the effect of CPCCOEt was not due to a nonspecific action we also used a different selective noncompetitive mGlu1 antagonist, YM 298198, which produced a dose-dependent inhibition of the protective effect of glutamate, yielding a pattern of dose-response curves typical for a noncompetitive antagonist (Fig. 6d). Additional pharmacological experiments (data not shown) indicated that neither the group II mGluR antagonist MCCG nor the group III antagonist MAP-4 were able to inhibit the protective effect of glutamate. Moreover, the respective group II and III agonists, APDC and AP4 failed to mimic the effect of glutamate. Together, these data indicate that the protective effect of glutamate was mediated by the activation of mGlu1 receptors and that this effect can be inhibited by the selective receptor antagonist.
Figure 6. Neuroprotective effects of glutamate in rat cerebellar neurons.
a, Fluorescent staining of cerebellar granule cells shows toxicity (red staining) under K5 conditions and an increased viability (green staining) in the presence of 1 mM glutamate. b, In mature cultures of rat granule cells (18DIV) glutamate kills all cells in the absence of MK801, but provides a dose-dependent protection against K5 toxicity (dashed line) in presence of 1 µM MK801. c, In young cerebellar neurons (7DIV) glutamate protection is visible in the absence of MK801 and is abolished by 200 µM CPCCOEt but not by 10 µM MPEP. d, The neuroprotective effect of glutamate in granule cells under K5 conditions is inhibited by the mGlu1 antagonist YM 298198 (at indicated µM concentrations). Data shown are means with error bars representing S.E.M. from at least three experiments. *p<0.05 and **p<0.001 as compared to glutamate-untreated control (b) or to glutamate alone (c, d) using Student’s t-test.
3.5. Toxic and protective effects of mGlu1 in PC12 cells
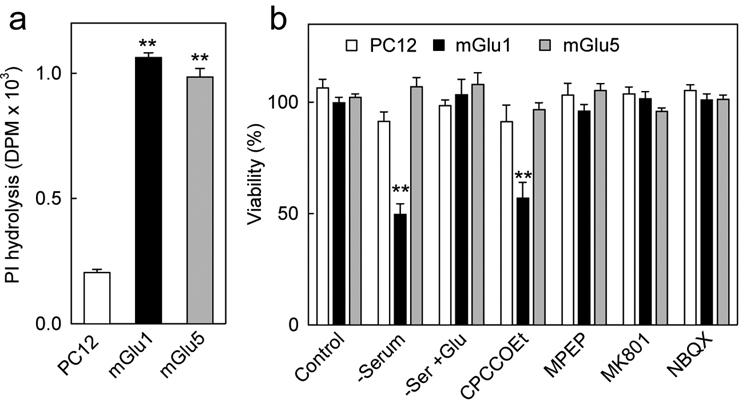
Because neuronal cells express multiple ionotropic and metabotropic glutamate receptors it could not be excluded that interactions between these receptors may contribute to the protective effects of glutamate. To address the possibility that the presence of mGlu1 alone may account for both the toxic and protective actions we used PC12 cells which are devoid of native glutamate receptors. PC12 cells were transfected with either mGlu1 or mGlu5 receptors and the levels of receptor expression were assessed by measurements of agonist-stimulated PI hydrolysis. Untransfected PC12 cells showed only a basal level of PI hydrolysis unaffected by ACPD, while cells transfected with mGlu1 or with mGlu5 showed both a 5-fold increase in PI hydrolysis in presence of 1 mM ACPD (Fig. 7a), indicating, functionally, a similar level of receptor expression. In parallel experiment, these cells were subjected to trophic deprivation by removal of serum from the culture medium for 48 h. Under those conditions, about 50 % of PC12 cells expressing mGlu1 died, while untransfected PC12 cells or cells expressing mGlu5 were unaffected (Fig. 7b). The toxicity in cells expressing mGlu1 and cultured without serum could be fully reversed by adding with 1 mM glutamate. Moreover, in the presence of serum, toxicity could be elicited in mGlu1-expressing cells by CPCCOEt, suggesting that the trophic action of serum depends on mGlu1 activated by glutamate contained in the serum. Neither the mGlu5 antagonist MPEP, nor the ionotropic glutamate receptor anatagonists, MK801 or NBQX, had any effect on the survival of transfected or untransfected PC12 cells. These experiments indicate that the introduction of mGlu1 into cells originally devoid of this receptor causes the survival of these cells to become dependent on the continuous presence of glutamate and its action at the mGlu1 receptor
Figure 7. Effects of mGlu1 expression on the viability of PC12 cells.
a , Measurements of PI hydrolysis stimulated by 100 µM ACPD in untransfected PC12 cells, and in PC 12 cells transfected with either mGlu1 or mGlu5 receptors show similar levels of functional receptor expression in mGlu1 and mGlu5-expressing cells. b, Serum deprivation (48 h) decreases viability only in mGlu1-expressing PC12 cells. This is counteracted by the addition of 1 mM glutamate, and is mimicked by 300 µM CPCCOEt, but not by 10 µM MPEP, 1 µM MK801 or 10 µM NBQX. Data shown are means with error bars representing S.E.M. from three independent experiments. **p<0.001 as compared to untransfected cells (a) or to serum containing controls (b) using Student’s t-test.
4. Discussion
In this report we have demonstrated that conditions of trophic deprivation led, in neurons, to an enhanced expression of mGlu1 receptors which correlates with the occurring cell death, while, at least in cerebellar granule cells, the expression of mGlu5 did not appear to change. These results are consistent with previous observations that under low K+ conditions granule cells undergoing apoptosis showed an enhanced immunoreactivity for mGlu1 (Copani et al., 1998). Moreover, our results indicate that the elevated mGlu1 expression is directly responsible for neuronal death. The downregulation of mGlu1 expression using receptor-specific siRNA attenuated the toxicity induced in granule cells by K+ deprivation, indicating that under these conditions mGlu1 is a major factor responsible for neuronal death. Conversely, we also show that an increase in mGlu1 expression, alone, is a sufficient factor to induce cell death, since the adenovirus-mediated overexpression of mGlu1 caused toxicity in cerebellar and cortical neurons, as well as in cortical astrocytes. Because in the above experiments both the downregulation of native mGlu1 by siRNA, which was protective, and mGlu1 overexpression with an adenoviral vector, which was toxic, were performed by specific tools unlikely to affect the expression of other genes, it may be concluded that changes in the level of mGlu1 protein alone were responsible for cell death. This was further confirmed in experiments with PC12 cells which became sensitive to short-term conditions of trophic deprivation upon the introduction of mGlu1.
While the above data left us with no doubt as to the toxic properties of mGlu1, the main problem with the interpretation of these results was the inability of the selective mGlu1 antagonist, CPCCOEt, to inhibit receptor toxicity. The lack of action of the antagonist would preclude that the action of this receptor is due to the stimulation by its agonist. One possible explanation was that the toxic effect was mediated by the receptor constitutive activity, rather than its agonist-induced activation as mGlu1 has been shown to possess an agonist-independent constitutive activity (Ango et al., 2001), which is not inhibited by CPCCOEt (Litschig et al., 1999). As a noncompetitive antagonist, CPCCOEt, inhibits the coupling between the effector and the binding domains of mGlu1 but does not affect the equilibrium between the active and inactive states of the effector domain (Parmentier et al., 2002). However, the experiments showing that it was not the antagonist but the mGlu1 agonist glutamate that protected against mGlu1 toxicity suggested that mGlu1 toxicity may not reflect a classical model of action of a G protein-coupled receptor.
The classical model of receptor action assumes that receptors are activated by their ligand but exist in an inactive state in the absence of ligand. However, a growing amount of evidence indicates the existence of a group of receptors with an alternative mechanism of action. These receptors, named dependence receptors, induce apoptosis in the absence of their ligands while ligand binding prevents the receptor-mediated toxicity, thereby creating a state of cellular dependence on the continuous presence of the agonist. One representative of this group is the low-affinity NGF receptor (p75NTR, the common neurotrophin receptor), which was found to induce toxicity in transfected cells after serum deprivation, whereas addition of NGF blocked apoptosis (Rabizadeh et al., 1993). Other family members include netrin-1 receptors (DCC and UNC5), integrins, RET, the receptor for glial-derived neurotrophic factor, the receptor for sonic hedgehog (Ptc1), and the androgen receptor (Mehlen and Bredesen, 2004). Most of the dependence receptors play crucial roles in neural development, cell migration and axon guidance in the mammalian nervous system (Porter and Dhakshinamoorthy, 2004), and have been implicated in cancer pathogenesis (Thiebault et al., 2003; Mehlen and Fearon, 2004).
If mGlu1 were to be a dependence receptor, its toxic action would be revealed in the absence of its endogenous ligand glutamate, and it would not be affected by the presence of antagonists. Instead, glutamate would be expected to prevent mGlu1-mediated toxicity and promote neuronal survival. In fact, application of glutamate to mouse cortical neurons with adenovirus-mediated overexpression of mGlu1 resulted in a complete protection from apoptosis. A similar protective effect of glutamate was revealed in cerebellar granule cells and in cortical neurons subjected to trophic deprivation. Moreover, the ability of CPCCOEt to inhibit the protective effect of glutamate confirmed that this action was mediated through the agonist-induced activation of mGlu1. This is consistent with the notion that the antagonist would not prevent the action of the receptor which depends on the absence of the agonist, instead it would block its agonist-dependent action. Hence, mGlu1 fulfills the basic criteria of a dependence receptor, inducing toxicity in the absence of glutamate and promoting cell survival in its presence. Moreover, the experiments with PC12 cells, which are similar to those performed with the p75NTR receptor (Rabizadeh et al., 1993), show that, in fact, introduction of mGlu1 creates a state of dependence on the presence of the receptor agonist.
While it is too early to propose a specific mechanism for the action of mGlu1 as a dependence receptor, some observations allow us to formulate several testable hypotheses. Although very different in structure, dependence receptors share some functional similarities including, the ability to mediate negative signaling in the absence of the agonist and positive signaling in its presence. In many cases the negative signaling is due to a caspase-mediated cleavage of the receptor C-terminal domain (CTD) which either releases a pro-apoptotic C-terminal receptor fragment (Bordeaux et al., 2000;Llambi et al., 2001), or exposes a pro-apoptotic region that presumably remains bound to the cell membrane (Mehlen et al., 1998; Forcet et al., 2001; Thibert et al., 2003). While a specific cleavage of the mGlu1 CDT has not been described, it should be pointed out that a sequence analysis of the long CTD of the mGlu1a splice variant reveals six putative caspase cleavage sites, as well as multiple sequences that may potentially interact with a variety of intracellular proteins including: seven in absentia homolog-1A (Siah-1A) and calmodulin (Ishikawa et al., 1999;Kammermeier and Ikeda, 2001), G-protein-coupled receptor kinases (Dale et al., 2000), alpha-tubulin (Ciruela et al., 1999), tamalin/cytohesin complex (Kitano et al., 2002), homer proteins (Brakeman et al., 1997;Tu et al., 1999), protein phosphatase 1C (Croci et al., 2003), protein kinase C, regulators of G-protein signaling (RGS) proteins, Src-family protein tyrosine kinase and arrestins (Valenti et al., 2002; Hermans and Challiss, 2001). Therefore, the mGlu1 CTD is well equipped to participate and interfere in various intracellular signaling processes.
It is more difficult to formulate a hypothesis proposing a mechanism for the positive signaling of mGlu1 as a dependence receptor. The existence of such a mechanism is supported by the neuroprotective action of glutamate which is blocked by selective mGlu1 antagonists. Hence, as seen with other dependence receptors, the survival-promoting action of mGlu1 should not be regarded merely as a blockade of its toxic action but, instead, as an agonists-induced switch towards positive receptor signaling. One may hypothesize that, similarly to several dependence receptors, apoptosis may be induced by mGlu1 in its monomeric state. In contrast, the binding of glutamate might stabilize the dimeric forms of the receptor (Kunishima et al., 2000;Tateyama et al., 2004) preventing receptor cleavage. This is supported by the observation that the potency of glutamate to induce the protective effect (EC50 ~ 300 µM) is about 10 times smaller that its potency to stimulate PI hydrolysis at mGlu1 receptors (EC50 ~ 30 µM). This is consistent with results from saturation studies indicating negative cooperativity of glutamate binding between the subunits of the dimeric mGlu1 (Suzuki et al., 2004) and with data reporting that the monomeric form of mGlu1 binds glutamate with a milimolar affinity (Kammermeier and Yun, 2005). Under such a hypothesis, positive signaling would be mediated by receptor dimers. Some light as to the nature of this signaling may be shed by numerous studies describing the protective effects of various agents against low K+-induced toxicity in cerebellar granule cells. Many of these agents such as BDNF (Kubo et al., 1995), GDF-15 (Subramaniam et al., 2003), and IGF-I (D'Mello et al., 1997) promote granule cell survival by mechanisms involving the phosphorylation of Akt and activation of multiple downstream cascades. A similar role for mGlu1 may be supported by the observation that DHPG, a group I mGluR agonist, increased PI3K-mediated Akt phosphorylation in mouse hippocampal slices (Hou and Klann, 2004) and by the possible coupling of group I mGluRs to PI3K mediated by the Homer/PIKE-L complex (Rong et al., 2003).
Further studies are needed to translate our in vitro observations into a comprehensive hypothesis addressing the role of mGlu1 in vivo. In its capacity as a dependence receptor, mGlu1 may mediate neuronal apoptosis in response to a reduced concentration of glutamate and may play a crucial role in controlling the fate of neuronal cells in brain development. This is consistent with the toxic effect of mGlu1 antagonist CPCCOEt reported in differentiating Purkinje cells (Catania et al., 2001) and observations that mGlu1 expression is higher in neonatal and early postnatal than in the adult brain (Casabona et al., 1997; van den Pol et al., 1994). Moreover, mGlu1 is highly expressed in Cajal-Retzius cells of rat neocortex and hippocampus which are eliminated within ten days after birth (Lopez-Bendito et al., 2002). Because mGlu1 expression has been reported in a variety of peripheral tissues (Storto et al., 2001; Zhu et al., 1999; Storto et al., 2000) and in tumor cells (Heck et al., 1997; Marin and Chen, 2004; Pollock et al., 2003) one should also consider the possibility that native mGlu1 receptors may play a role in apoptosis of non-neuronal cells, and that their expression may contribute to the neoplastic growth of cancer cells.
Acknowledgements
This work was supported by a grant from National Institutes of Health NS37436
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ango F, Prezeau L, Muller T, Tu JC, Xiao B, Worley PF, Pin JP, Bockaert J, Fagni L. Agonist-Independent Activation of Metabotropic Glutamate Receptors by the Intracellular Protein Homer. Nature. 2001;411:962–965. doi: 10.1038/35082096. [DOI] [PubMed] [Google Scholar]
- Bordeaux MC, Forcet C, Granger L, Corset V, Bidaud C, Billaud M, Bredesen DE, Edery P, Mehlen P. The RET Proto-Oncogene Induces Apoptosis: a Novel Mechanism for Hirschsprung Disease. EMBO J. 2000;19:4056–4063. doi: 10.1093/emboj/19.15.4056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brakeman PR, Lanahan AA, O'Brien R, Roche K, Barnes CA, Huganir RL, Worley PF. Homer: a Protein That Selectively Binds Metabotropic Glutamate Receptors. Nature. 1997;386:284–288. doi: 10.1038/386284a0. [DOI] [PubMed] [Google Scholar]
- Bruno V, Wroblewska B, Wroblewski JT, Fiore L, Nicoletti F. Neuroprotective Activity of N-Acetylaspartylglutamate in Cultured Cortical Cells. Neuroscience. 1998;85:751–757. doi: 10.1016/s0306-4522(97)00531-9. [DOI] [PubMed] [Google Scholar]
- Casabona G, Knopfel T, Kuhn R, Gasparini F, Baumann P, Sortino MA, Copani A, Nicoletti F. Expression and Coupling to Polyphosphoinositide Hydrolysis of Group I Metabotropic Glutamate Receptors in Early Postnatal and Adult Rat Brain. Eur J Neurosci. 1997;9:12–17. doi: 10.1111/j.1460-9568.1997.tb01348.x. [DOI] [PubMed] [Google Scholar]
- Catania MV, Bellomo M, Di Giorgi-Gerevini V, Seminara G, Giuffrida R, Romeo R, De Blasi A, Nicoletti F. Endogenous Activation of Group-I Metabotropic Glutamate Receptors Is Required for Differentiation and Survival of Cerebellar Purkinje Cells. J Neurosci. 2001;21:7664–7673. doi: 10.1523/JNEUROSCI.21-19-07664.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciruela F, Robbins MJ, Willis AC, McIlhinney RA. Interactions of the C Terminus of Metabotropic Glutamate Receptor Type 1 alpha With Rat Brain Proteins: Evidence for a Direct Interaction With Tubulin. J Neurochem. 1999;72:346–354. [PubMed] [Google Scholar]
- Conn PJ, Pin JP. Pharmacology and Functions of Metabotropic Glutamate Receptors. Annu Rev Pharmacol Toxicol. 1997;37:205–237. doi: 10.1146/annurev.pharmtox.37.1.205. [DOI] [PubMed] [Google Scholar]
- Contestabile A. Cerebellar Granule Cells As a Model to Study Mechanisms of Neuronal Apoptosis or Survival in Vivo and in Vitro. Cerebellum. 2002;1:41–55. doi: 10.1080/147342202753203087. [DOI] [PubMed] [Google Scholar]
- Copani A, Casabona G, Bruno V, Caruso A, Condorelli DF, Messina A, Di Giorgi Gerevini V, Pin JP, Kuhn R, Knopfel T, Nicoletti F. The Metabotropic Glutamate Receptor MGlu5 Controls the Onset of Developmental Apoptosis in Cultured Cerebellar Neurons. Eur J Neurosci. 1998;10:2173–2184. doi: 10.1046/j.1460-9568.1998.00230.x. [DOI] [PubMed] [Google Scholar]
- Croci C, Sticht H, Brandstatter JH, Enz R. Group I Metabotropic Glutamate Receptors Bind to Protein Phosphatase 1C. Mapping and Modeling of Interacting Sequences. J Biol Chem. 2003;278:50682–50690. doi: 10.1074/jbc.M305764200. [DOI] [PubMed] [Google Scholar]
- D'Mello SR, Borodezt K, Soltoff SP. Insulin-Like Growth Factor and Potassium Depolarization Maintain Neuronal Survival by Distinct Pathways: Possible Involvement of PI 3-Kinase in IGF-1 Signaling. J Neurosci. 1997;17:1548–1560. doi: 10.1523/JNEUROSCI.17-05-01548.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Mello SR, Galli C, Ciotti T, Calissano P. Induction of Apoptosis in Cerebellar Granule Neurons by Low Potassium: Inhibition of Death by Insulin-Like Growth Factor I and CAMP. Proc Natl Acad Sci U S A. 1993;90:10989–10993. doi: 10.1073/pnas.90.23.10989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale LB, Bhattacharya M, Anborgh PH, Murdoch B, Bhatia M, Nakanishi S, Ferguson SS. G Protein-Coupled Receptor Kinase-Mediated Desensitization of Metabotropic Glutamate Receptor 1A Protects Against Cell Death. J Biol Chem. 2000;275:38213–38220. doi: 10.1074/jbc.M006075200. [DOI] [PubMed] [Google Scholar]
- Forcet C, Ye X, Granger L, Corset V, Shin H, Bredesen DE, Mehlen P. The Dependence Receptor DCC (Deleted in Colorectal Cancer) Defines an Alternative Mechanism for Caspase Activation. Proc Natl Acad Sci U S A. 2001;98:3416–3421. doi: 10.1073/pnas.051378298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heck S, Enz R, Richter-Landsberg C, Blohm DH. Expression of Eight Metabotropic Glutamate Receptor Subtypes During Neuronal Differentiation of P19 Embryocarcinoma Cells: a Study by RT-PCR and in Situ Hybridization. Brain Res Dev Brain Res. 1997;101:85–91. doi: 10.1016/s0165-3806(97)00048-5. [DOI] [PubMed] [Google Scholar]
- Hermans E, Challiss RA. Structural, Signalling and Regulatory Properties of the Group I Metabotropic Glutamate Receptors: Prototypic Family C G-Protein-Coupled Receptors. Biochem J. 2001;359:465–484. doi: 10.1042/0264-6021:3590465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou L, Klann E. Activation of the Phosphoinositide 3-Kinase-Akt-Mammalian Target of Rapamycin Signaling Pathway Is Required for Metabotropic Glutamate Receptor-Dependent Long-Term Depression. J Neurosci. 2004;24:6352–6361. doi: 10.1523/JNEUROSCI.0995-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa K, Nash SR, Nishimune A, Neki A, Kaneko S, Nakanishi S. Competitive Interaction of Seven in Absentia Homolog-1A and Ca2+/Calmodulin With the Cytoplasmic Tail of Group 1 Metabotropic Glutamate Receptors. Genes Cells. 1999;4:381–390. doi: 10.1046/j.1365-2443.1999.00269.x. [DOI] [PubMed] [Google Scholar]
- Kammermeier PJ, Ikeda SR. A Role for Seven in Absentia Homolog (Siah1a) in Metabotropic Glutamate Receptor Signaling. BMC Neurosci. 2001;2:15. doi: 10.1186/1471-2202-2-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kammermeier PJ, Yun J. Activation of Metabotropic Glutamate Receptor 1 Dimers Requires Glutamate Binding in Both Subunits. J Pharmacol Exp Ther. 2005;312:502–508. doi: 10.1124/jpet.104.073155. [DOI] [PubMed] [Google Scholar]
- Kitano J, Kimura K, Yamazaki Y, Soda T, Shigemoto R, Nakajima Y, Nakanishi S. Tamalin, a PDZ Domain-Containing Protein, Links a Protein Complex Formation of Group 1 Metabotropic Glutamate Receptors and the Guanine Nucleotide Exchange Factor Cytohesins. J Neurosci. 2002;22:1280–1289. doi: 10.1523/JNEUROSCI.22-04-01280.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubo T, Nonomura T, Enokido Y, Hatanaka H. Brain-Derived Neurotrophic Factor (BDNF) Can Prevent Apoptosis of Rat Cerebellar Granule Neurons in Culture. Brain Res Dev Brain Res. 1995;85:249–258. doi: 10.1016/0165-3806(94)00220-t. [DOI] [PubMed] [Google Scholar]
- Kunishima N, Shimada Y, Tsuji Y, Sato T, Yamamoto M, Kumasaka T, Nakanishi S, Jingami H, Morikawa K. Structural Basis of Glutamate Recognition by a Dimeric Metabotropic Glutamate Receptor. Nature. 2000;407:971–977. doi: 10.1038/35039564. [DOI] [PubMed] [Google Scholar]
- Litschig S, Gasparini F, Rueegg D, Stoehr N, Flor PJ, Vranesic I, Prezeau L, Pin JP, Thomsen C, Kuhn R. CPCCOEt, a Noncompetitive Metabotropic Glutamate Receptor 1 Antagonist, Inhibits Receptor Signaling Without Affecting Glutamate Binding. Mol Pharmacol. 1999;55:453–461. [PubMed] [Google Scholar]
- Llambi F, Causeret F, Bloch-Gallego E, Mehlen P. Netrin-1 Acts As a Survival Factor Via Its Receptors UNC5H and DCC. EMBO J. 2001;20:2715–2722. doi: 10.1093/emboj/20.11.2715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Bendito G, Shigemoto R, Fairen A, Lujan R. Differential Distribution of Group I Metabotropic Glutamate Receptors During Rat Cortical Development. Cereb Cortex. 2002;12:625–638. doi: 10.1093/cercor/12.6.625. [DOI] [PubMed] [Google Scholar]
- Marin YE, Chen S. Involvement of Metabotropic Glutamate Receptor 1, a G Protein Coupled Receptor, in Melanoma Development. J Mol Med. 2004;82:735–749. doi: 10.1007/s00109-004-0566-8. [DOI] [PubMed] [Google Scholar]
- Mehlen P, Bredesen DE. The Dependence Receptor Hypothesis. Apoptosis. 2004;9:37–49. doi: 10.1023/B:APPT.0000012120.66221.b2. [DOI] [PubMed] [Google Scholar]
- Mehlen P, Fearon ER. Role of the Dependence Receptor DCC in Colorectal Cancer Pathogenesis. J Clin Oncol. 2004;22:3420–3428. doi: 10.1200/JCO.2004.02.019. [DOI] [PubMed] [Google Scholar]
- Mehlen P, Rabizadeh S, Snipas SJ, Assa-Munt N, Salvesen GS, Bredesen DE. The DCC Gene Product Induces Apoptosis by a Mechanism Requiring Receptor Proteolysis. Nature. 1998;395:801–804. doi: 10.1038/27441. [DOI] [PubMed] [Google Scholar]
- Mosmann T. Rapid Colorimetric Assay for Cellular Growth and Survival: Application to Proliferation and Cytotoxicity Assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Nash MS, Selkirk JV, Gaymer CE, Challiss RA, Nahorski SR. Enhanced Inducible MGlu1alpha Receptor Expression in Chinese Hamster Ovary Cells. J Neurochem. 2001;77:1664–1667. doi: 10.1046/j.1471-4159.2001.00405.x. [DOI] [PubMed] [Google Scholar]
- Nicoletti F, Bruno V, Catania MV, Battaglia G, Copani A, Barbagallo G, Cena V, Sanchez-Prieto J, Spano PF, Pizzi M. Group-I Metabotropic Glutamate Receptors: Hypotheses to Explain Their Dual Role in Neurotoxicity and Neuroprotection. Neuropharmacology. 1999;38:1477–1484. doi: 10.1016/s0028-3908(99)00102-1. [DOI] [PubMed] [Google Scholar]
- Parmentier ML, Prezeau L, Bockaert J, Pin JP. A Model for the Functioning of Family 3 GPCRs. Trends Pharmacol Sci. 2002;23:268–274. doi: 10.1016/s0165-6147(02)02016-3. [DOI] [PubMed] [Google Scholar]
- Pollock PM, Cohen-Solal K, Sood R, Namkoong J, Martino JJ, Koganti A, Zhu H, Robbins C, Makalowska I, Shin SS, Marin Y, Roberts KG, Yudt LM, Chen A, Cheng J, Incao A, Pinkett HW, Graham CL, Dunn K, Crespo-Carbone SM, Mackason KR, Ryan KB, Sinsimer D, Goydos J, Reuhl KR, Eckhaus M, Meltzer PS, Pavan WJ, Trent JM, Chen S. Melanoma Mouse Model Implicates Metabotropic Glutamate Signaling in Melanocytic Neoplasia. Nat Genet. 2003;34:108–112. doi: 10.1038/ng1148. [DOI] [PubMed] [Google Scholar]
- Porter AG, Dhakshinamoorthy S. Apoptosis Initiated by Dependence Receptors: a New Paradigm for Cell Death? Bioessays. 2004;26:656–664. doi: 10.1002/bies.20037. [DOI] [PubMed] [Google Scholar]
- Pshenichkin SP, Wise BC. Okadaic Acid Increases Nerve Growth Factor Secretion, MRNA Stability, and Gene Transcription in Primary Cultures of Cortical Astrocytes. J Biol Chem. 1995;270:5994–5999. doi: 10.1074/jbc.270.11.5994. [DOI] [PubMed] [Google Scholar]
- Rabizadeh S, Oh J, Zhong LT, Yang J, Bitler CM, Butcher LL, Bredesen DE. Induction of Apoptosis by the Low-Affinity NGF Receptor. Science. 1993;261:345–348. doi: 10.1126/science.8332899. [DOI] [PubMed] [Google Scholar]
- Resink A, Hack N, Boer GJ, Balazs R. Growth Conditions Differentially Modulate the Vulnerability of Developing Cerebellar Granule Cells to Excitatory Amino Acids. Brain Res. 1994;655:222–232. doi: 10.1016/0006-8993(94)91617-9. [DOI] [PubMed] [Google Scholar]
- Rong R, Ahn JY, Huang H, Nagata E, Kalman D, Kapp JA, Tu J, Worley PF, Snyder SH, Ye K. PI3 Kinase Enhancer-Homer Complex Couples MGluRI to PI3 Kinase, Preventing Neuronal Apoptosis. Nat Neurosci. 2003;6:1153–1161. doi: 10.1038/nn1134. [DOI] [PubMed] [Google Scholar]
- Ryu BR, Choi DW, Hartley DM, Costa E, Jou I, Gwag BJ. Attenuation of Cortical Neuronal Apoptosis by Gangliosides. J Pharmacol Exp Ther. 1999;290:811–816. [PubMed] [Google Scholar]
- Santi MR, Ikonomovic S, Wroblewski JT, Grayson DR. Temporal and Depolarization-Induced Changes in the Absolute Amounts of MRNAs Encoding Metabotropic Glutamate Receptors in Cerebellar Granule Neurons in Vitro. J Neurochem. 1994;63:1207–1217. doi: 10.1046/j.1471-4159.1994.63041207.x. [DOI] [PubMed] [Google Scholar]
- Storto M, de Grazia U, Battaglia G, Felli MP, Maroder M, Gulino A, Ragona G, Nicoletti F, Screpanti I, Frati L, Calogero A. Expression of Metabotropic Glutamate Receptors in Murine Thymocytes and Thymic Stromal Cells. J Neuroimmunol. 2000;109:112–120. doi: 10.1016/s0165-5728(00)00269-1. [DOI] [PubMed] [Google Scholar]
- Storto M, Sallese M, Salvatore L, Poulet R, Condorelli DF, Dell'Albani P, Marcello MF, Romeo R, Piomboni P, Barone N, Nicoletti F, De Blasi A. Expression of Metabotropic Glutamate Receptors in the Rat and Human Testis. J Endocrinol. 2001;170:71–78. doi: 10.1677/joe.0.1700071. [DOI] [PubMed] [Google Scholar]
- Subramaniam S, Strelau J, Unsicker K. Growth Differentiation Factor-15 Prevents Low Potassium-Induced Cell Death of Cerebellar Granule Neurons by Differential Regulation of Akt and ERK Pathways. J Biol Chem. 2003;278:8904–8912. doi: 10.1074/jbc.M210037200. [DOI] [PubMed] [Google Scholar]
- Suzuki Y, Moriyoshi E, Tsuchiya D, Jingami H. Negative Cooperativity of Glutamate Binding in the Dimeric Metabotropic Glutamate Receptor Subtype 1. J Biol Chem. 2004;279:35526–35534. doi: 10.1074/jbc.M404831200. [DOI] [PubMed] [Google Scholar]
- Tateyama M, Abe H, Nakata H, Saito O, Kubo Y. Ligand-Induced Rearrangement of the Dimeric Metabotropic Glutamate Receptor 1alpha. Nat Struct Mol Biol. 2004;11:637–642. doi: 10.1038/nsmb770. [DOI] [PubMed] [Google Scholar]
- Thibert C, Teillet MA, Lapointe F, Mazelin L, Le Douarin NM, Mehlen P. Inhibition of Neuroepithelial Patched-Induced Apoptosis by Sonic Hedgehog. Science. 2003;301:843–846. doi: 10.1126/science.1085405. [DOI] [PubMed] [Google Scholar]
- Thiebault K, Mazelin L, Pays L, Llambi F, Joly MO, Scoazec JY, Saurin JC, Romeo G, Mehlen P. The Netrin-1 Receptors UNC5H Are Putative Tumor Suppressors Controlling Cell Death Commitment. Proc Natl Acad Sci U S A. 2003;100:4173–4178. doi: 10.1073/pnas.0738063100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu JC, Xiao B, Naisbitt S, Yuan JP, Petralia RS, Brakeman P, Doan A, Aakalu VK, Lanahan AA, Sheng M, Worley PF. Coupling of MGluR/Homer and PSD-95 Complexes by the Shank Family of Postsynaptic Density Proteins. Neuron. 1999;23:583–592. doi: 10.1016/s0896-6273(00)80810-7. [DOI] [PubMed] [Google Scholar]
- Valenti O, Conn PJ, Marino MJ. Distinct Physiological Roles of the Gq-Coupled Metabotropic Glutamate Receptors Co-Expressed in the Same Neuronal Populations. J Cell Physiol. 2002;191:125–137. doi: 10.1002/jcp.10081. [DOI] [PubMed] [Google Scholar]
- van den Pol AN, Kogelman L, Ghosh P, Liljelund P, Blackstone C. Developmental Regulation of the Hypothalamic Metabotropic Glutamate Receptor MGluR1. J Neurosci. 1994;14:3816–3834. doi: 10.1523/JNEUROSCI.14-06-03816.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood AM, Tiwari P, Bristow DR. Media Composition Modulates Excitatory Amino Acid-Induced Death of Rat Cerebellar Granule Cells. Hum Exp Toxicol. 1997;16:350–355. doi: 10.1177/096032719701600702. [DOI] [PubMed] [Google Scholar]
- Wroblewski JT, Nicoletti F, Costa E. Different Coupling of Excitatory Amino Acid Receptors With Ca2+ Channels in Primary Cultures of Cerebellar Granule Cells. Neuropharmacology. 1985;24:919–921. doi: 10.1016/0028-3908(85)90046-2. [DOI] [PubMed] [Google Scholar]
- Zhu H, Ryan K, Chen S. Cloning of Novel Splice Variants of Mouse MGluR1. Brain Res Mol Brain Res. 1999;73:93–103. doi: 10.1016/s0169-328x(99)00239-9. [DOI] [PubMed] [Google Scholar]