Abstract
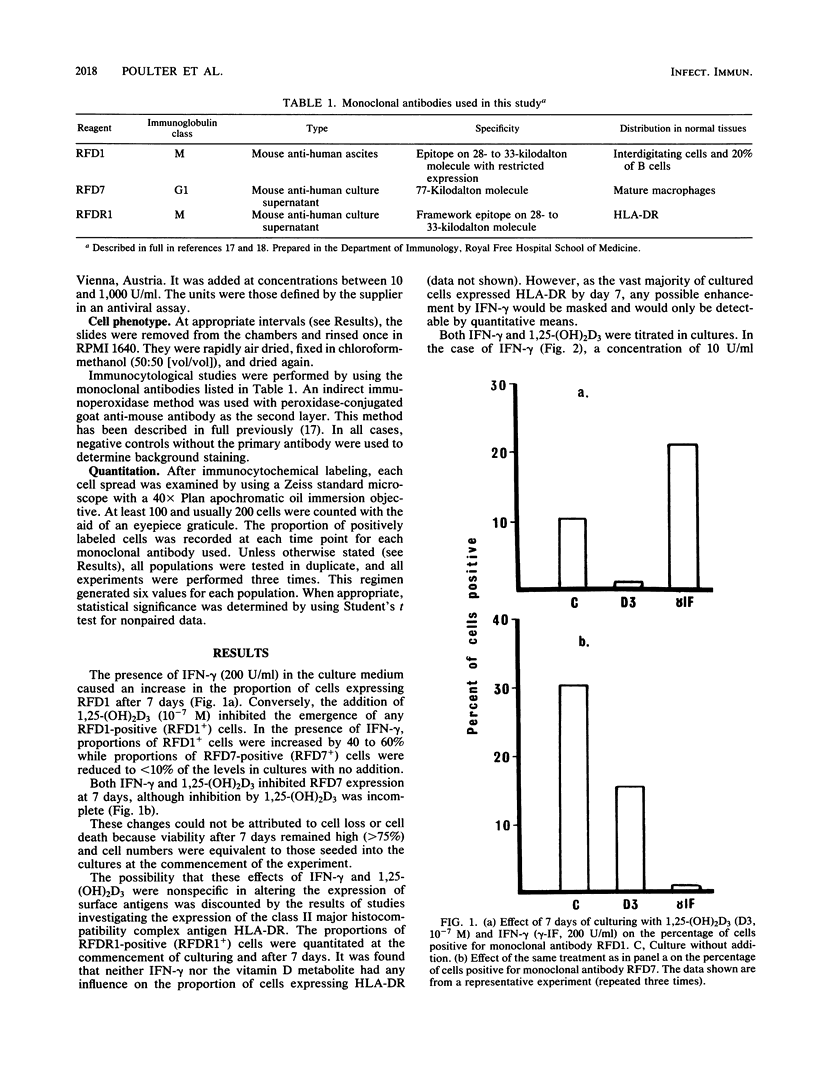
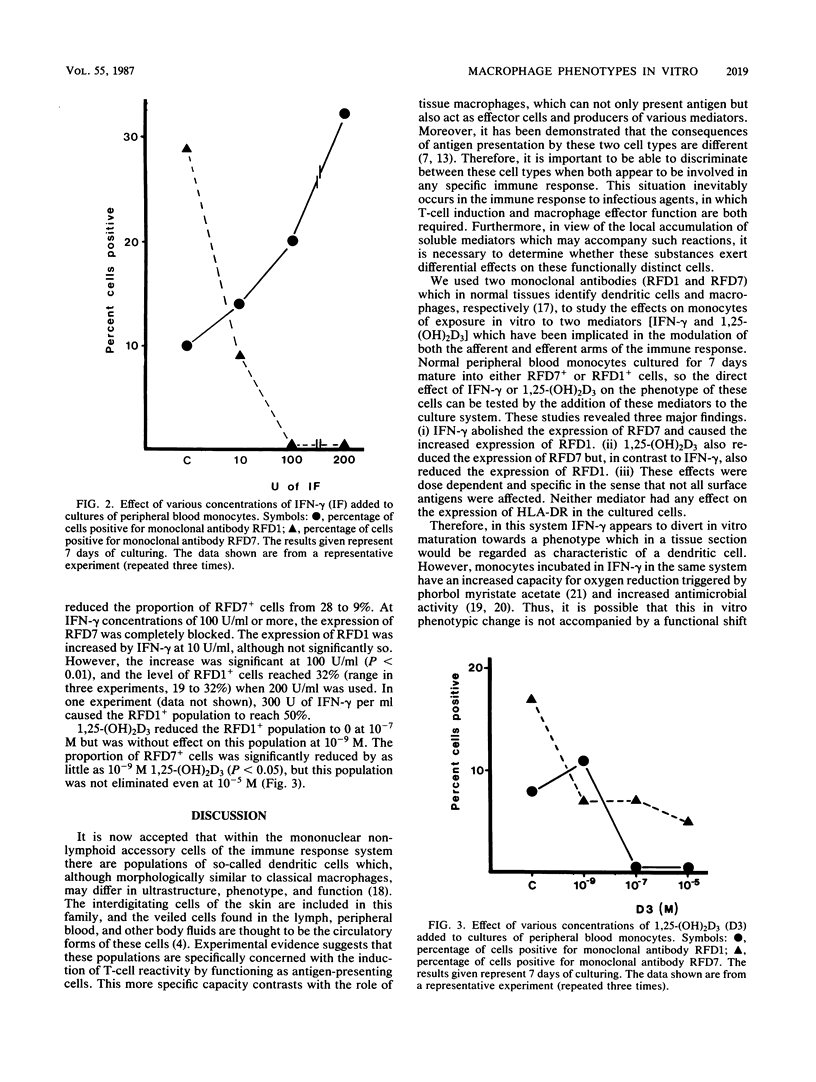
The effects of 1,25-dihydroxy vitamin D3 and gamma interferon on the phenotypic changes associated with monocyte maturation in vitro were investigated. Human monocytes separated from peripheral blood mononuclear cell populations by adherence to plastic were cultured for 7 days on glass. Immunocytological analysis was performed on monolayers fixed at various times by using monoclonal antibodies specific for mature macrophages (RFD7), interdigitating (dendritic) cells (RFD1), and class II major histocompatibility complex antigen (RFDR1). Without any addition to the culture medium, proportions of these monocytes (normally RFD1 and RFD7 negative) developed either RFD1 positivity (23%) or RFD7 positivity (49%) over 7 days of culturing. The addition of gamma interferon to these cultures markedly reduced the proportion of RFD7-positive cells (less than 10%) but increased the proportion of RFD1-positive cells (40 to 60%). In contrast, 1,25-dihydroxy vitamin D3 reduced the expression of both RFD1 and RFD7. Both of these effects were dose dependent and required at least 3 days of contact with the cells. The possibility that RFD1- and RFD7-positive cells represent functionally distinct subsets makes these effects of significance in our understanding of the role of these mediators in controlling the immunocompetence of nonlymphoid accessory cell populations and in macrophage-associated antimicrobial activity.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams J. S., Gacad M. A. Characterization of 1 alpha-hydroxylation of vitamin D3 sterols by cultured alveolar macrophages from patients with sarcoidosis. J Exp Med. 1985 Apr 1;161(4):755–765. doi: 10.1084/jem.161.4.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amento E. P., Bhalla A. K., Kurnick J. T., Kradin R. L., Clemens T. L., Holick S. A., Holick M. F., Krane S. M. 1 alpha,25-dihydroxyvitamin D3 induces maturation of the human monocyte cell line U937, and, in association with a factor from human T lymphocytes, augments production of the monokine, mononuclear cell factor. J Clin Invest. 1984 Mar;73(3):731–739. doi: 10.1172/JCI111266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreesen R., Bross K. J., Osterholz J., Emmrich F. Human macrophage maturation and heterogeneity: analysis with a newly generated set of monoclonal antibodies to differentiation antigens. Blood. 1986 May;67(5):1257–1264. [PubMed] [Google Scholar]
- Balfour B. M., Drexhage H. A., Kamperdijk E. W., Hoefsmit E. C. Antigen-presenting cells, including Langerhans cells, veiled cells and interdigitating cells. Ciba Found Symp. 1981;84:281–301. doi: 10.1002/9780470720660.ch15. [DOI] [PubMed] [Google Scholar]
- Basham T. Y., Merigan T. C. Recombinant interferon-gamma increases HLA-DR synthesis and expression. J Immunol. 1983 Apr;130(4):1492–1494. [PubMed] [Google Scholar]
- Biondi A., Rossing T. H., Bennett J., Todd R. F., 3rd Surface membrane heterogeneity among human mononuclear phagocytes. J Immunol. 1984 Mar;132(3):1237–1243. [PubMed] [Google Scholar]
- Britz J. S., Askenase P. W., Ptak W., Steinman R. M., Gershon R. K. Specialized antigen-presenting cells. Splenic dendritic cells and peritoneal-exudate cells induced by mycobacteria activate effector T cells that are resistant to suppression. J Exp Med. 1982 May 1;155(5):1344–1356. doi: 10.1084/jem.155.5.1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies P. D. A possible link between vitamin D deficiency and impaired host defence to Mycobacterium tuberculosis. Tubercle. 1985 Dec;66(4):301–306. doi: 10.1016/0041-3879(85)90068-6. [DOI] [PubMed] [Google Scholar]
- Khansari N., Chou Y. K., Fudenberg H. H. Human monocyte heterogeneity: interleukin 1 and prostaglandin E2 production by separate subsets. Eur J Immunol. 1985 Jan;15(1):48–51. doi: 10.1002/eji.1830150110. [DOI] [PubMed] [Google Scholar]
- Miyaura C., Abe E., Kuribayashi T., Tanaka H., Konno K., Nishii Y., Suda T. 1 alpha,25-Dihydroxyvitamin D3 induces differentiation of human myeloid leukemia cells. Biochem Biophys Res Commun. 1981 Oct 15;102(3):937–943. doi: 10.1016/0006-291x(81)91628-4. [DOI] [PubMed] [Google Scholar]
- Mukherjee S., Katz D. R., Rook G. A. Differing role of dendritic cells and macrophages in the induction of delayed-type hypersensitivity responses to PPD. Immunology. 1986 Oct;59(2):229–234. [PMC free article] [PubMed] [Google Scholar]
- Murray H. W., Rubin B. Y., Rothermel C. D. Killing of intracellular Leishmania donovani by lymphokine-stimulated human mononuclear phagocytes. Evidence that interferon-gamma is the activating lymphokine. J Clin Invest. 1983 Oct;72(4):1506–1510. doi: 10.1172/JCI111107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nacy C. A., Oster C. N., James S. L., Meltzer M. S. Activation of macrophages to kill rickettsiae and Leishmania: dissociation of intracellular microbicidal activities and extracellular destruction of neoplastic and helminth targets. Contemp Top Immunobiol. 1984;13:147–170. doi: 10.1007/978-1-4757-1445-6_8. [DOI] [PubMed] [Google Scholar]
- Poulter L. W., Campbell D. A., Munro C., Butcher R. G. The quantitation of HLA-DR expression on human cells using immunocytochemistry. J Immunol Methods. 1987 Apr 16;98(2):227–234. doi: 10.1016/0022-1759(87)90009-3. [DOI] [PubMed] [Google Scholar]
- Poulter L. W., Campbell D. A., Munro C., Janossy G. Discrimination of human macrophages and dendritic cells by means of monoclonal antibodies. Scand J Immunol. 1986 Sep;24(3):351–357. doi: 10.1111/j.1365-3083.1986.tb02104.x. [DOI] [PubMed] [Google Scholar]
- Poulter L. W., Janossy G. The involvement of dendritic cells in chronic inflammatory disease. Scand J Immunol. 1985 May;21(5):401–407. doi: 10.1111/j.1365-3083.1985.tb01825.x. [DOI] [PubMed] [Google Scholar]
- Rook G. A., Steele J., Ainsworth M., Champion B. R. Activation of macrophages to inhibit proliferation of Mycobacterium tuberculosis: comparison of the effects of recombinant gamma-interferon on human monocytes and murine peritoneal macrophages. Immunology. 1986 Nov;59(3):333–338. [PMC free article] [PubMed] [Google Scholar]
- Rook G. A., Steele J., Fraher L., Barker S., Karmali R., O'Riordan J., Stanford J. Vitamin D3, gamma interferon, and control of proliferation of Mycobacterium tuberculosis by human monocytes. Immunology. 1986 Jan;57(1):159–163. [PMC free article] [PubMed] [Google Scholar]
- Rook G. A., Steele J., Umar S., Dockrell H. M. A simple method for the solubilisation of reduced NBT, and its use as a colorimetric assay for activation of human macrophages by gamma-interferon. J Immunol Methods. 1985 Sep 3;82(1):161–167. doi: 10.1016/0022-1759(85)90235-2. [DOI] [PubMed] [Google Scholar]
- Schreiber A. D., Kelley M., Dziarski A., Levinson A. I. Human monocyte functional heterogeneity: monocyte fractionation by discontinuous albumin gradient centrifugation. Immunology. 1983 Jun;49(2):231–238. [PMC free article] [PubMed] [Google Scholar]
- Schreiber R. D. Identification of gamma-interferon as a murine macrophage-activating factor for tumor cytotoxicity. Contemp Top Immunobiol. 1984;13:171–198. doi: 10.1007/978-1-4757-1445-6_9. [DOI] [PubMed] [Google Scholar]



