Abstract
This study evaluated the efficacy of a contingency management (CM) intervention to promote smoking cessation in methadone-maintained patients. Twenty participants, randomized into contingent (n = 10) or noncontingent (n = 10) experimental conditions, completed the 14-day study. Abstinence was determined using breath carbon monoxide and urine cotinine levels. Contingent participants received voucher-based incentives for biochemical evidence of smoking abstinence. Noncontingent participants earned vouchers independent of smoking status. Contingent participants achieved significantly more smoking abstinence and longer durations of continuous smoking abstinence than did noncontingent participants. These results support the potential efficacy of using voucher-based CM to promote smoking cessation among methadone-maintained patients.
Keywords: contingency management, methadone maintenance, smoking cessation
Methadone maintenance (MM) represents one of the most widely used and effective treatments for opioid dependence, with more than 170,000 opioid-dependent patients currently receiving methadone in the United States (Drug and Alcohol Services Information System, 2002). Even though MM is a highly efficacious treatment for opioid dependence, ongoing abuse of other substances is common in this population, and rates of cigarette smoking are particularly high (Ball & Ross, 1991; Stitzer & Sigmon, 2006). For example, compared to 25% in the general U.S. adult population, prevalence of current smoking among MM patients is 84% to 94% (Clemmey, Brooner, Chutuape, Kidorf, & Stitzer, 1997; Nahvi, Richter, Li, Modali, & Arnsten, 2006; Richter, Gibson, Ahulwalia, & Schmelzle, 2001). As is the case in the general population, smoking in MM patients is associated with increased morbidity and mortality (Engstrom, Adamsson, Allebeck, & Rydberg, 1991; Hser, McCarthy, & Anglin, 1994). The mortality rate of opioid-dependent smokers, for example, is estimated to be four times greater than that of opioid-dependent nonsmokers (Hser et al.).
Most MM patients express serious interest in quitting smoking (Clark, Stein, McGarry, & Gogineni, 2001; Clemmey et al., 1997; Frosch, Shoptaw, Jarvik, Rawson, & Ling, 1998; Kozlowski, Skinner, Kent, & Pope, 1989; Richter et al., 2001; Sees & Clark, 1993). Furthermore, the MM treatment modality is uniquely situated to offer an ideal setting for implementing smoking-cessation interventions. Many patients achieve significant periods of stability and drug abstinence and remain engaged in treatment for long periods of time, which can support the frequent and prolonged clinical contact to enable success with smoking cessation. MM clinics also adhere to a uniform set of state and federal regulations that could support the dissemination of an effective intervention throughout clinics across the country.
Unfortunately, little is known about how to effectively help MM patients to quit smoking, and only a few published reports have focused on smoking cessation in this population. The most promising of these have used contingency management (CM), a behavioral approach widely demonstrated to reduce drug use by providing nondrug reinforcers contingent on biochemical confirmation of abstinence (Dallery & Glenn, 2005; Higgins, Alessi, & Dantona, 2002; Higgins & Silverman, 1999; Roll, 2005). In the first CM study to target smoking in MM smokers, 5 participants provided breath carbon monoxide (CO) samples twice weekly and were offered $5.00 for each sample that was 50% or less than during a prior control phase (Schmitz, Rhoades, & Grabowski, 1995). This study found no reliable effect of the intervention on smoking and raised the possibility that CM interventions for smoking cessation may not be efficacious in MM patients. In a second study, 17 MM patients provided thrice-weekly COs and earned voucher-based incentives (maximum of $73.00) for CO samples ≤ 4 parts per million (ppm; Shoptaw, Jarvik, Ling, & Rawson, 1996). Mean CO levels decreased significantly during the intervention, although only 4 participants produced more than two consecutive negative samples during the study, and all had resumed smoking by the end of the trial. Important to note, however, is that even though the treatment effects were modest, the study by Shoptaw et al. provided the first demonstration that smoking in MM patients was sensitive to CM or any other intervention.
Shoptaw et al. (2002) conducted a subsequent randomized clinical trial in which MM smokers were assigned to one of four treatments (nicotine patch, nicotine patch plus voucher-based CM, nicotine patch plus relapse prevention counseling, nicotine patch plus CM plus relapse prevention counseling). Breath COs were collected thrice weekly, and COs ≤ 8 ppm were considered abstinent. CM participants earned voucher-based incentives for abstinence using an escalating schedule of reinforcement (Higgins et al., 1991) with $447.50 in maximum earnings. Participants who received CM with and without relapse prevention counseling achieved significantly more smoking abstinence compared to those without CM, but abstinence in all groups was below 10% at 6- and 12-month follow-up.
Although these studies were able to show modest success in promoting smoking cessation in MM patients, none produced robust results or long-term abstinence. One potential explanation is that initial continuous abstinence was not achieved during the early days of the intervention. For example, the impressive levels of smoking abstinence in Shoptaw et al. (2002) were not evident until Week 3, and the majority of patients in all conditions were smoking during the first 2 weeks of the study. The literature on smoking cessation suggests that early abstinence is particularly important, with numerous reports supporting a robust association between abstinence during the initial weeks of a cessation effort and longer term outcomes (Frosch, Nahom, & Shoptaw, 2002; Gourlay, Forbes, Marriner, Pethica, & McNeil, 1994; Higgins et al., 2006; Kenford et al., 1994; Yudkin, Jones, Lancaster, & Fowler, 1996).
These data suggest that there are two factors that are likely to affect the probability of achieving initial and longer term abstinence. First, a more intensive schedule of abstinence monitoring and reinforcement is needed during the initial days of the cessation effort. Although thrice-weekly monitoring may be intense relative to many smoking-cessation interventions used with other populations, MM patients often report to the methadone clinic almost daily, which provides an opportunity to monitor them more frequently. Second, the CO cutoff of 8 ppm used in prior studies may permit intermittent or low levels of smoking to go undetected (Javors, Hatch, & Lamb, 2005). Given the importance of early, sustained abstinence to longer term outcomes, these procedural matters can be important in determining the longer term success of the intervention. Therefore, the goal of this pilot study was to examine the efficacy of an intensive 2-week CM intervention that incorporated rigorous biochemical verification methods and frequent monitoring to produce early, continuous smoking abstinence in a sample of MM smokers.
Method
Participants
Twenty MM adults were recruited from a local clinic. Participants responded to an advertisement for a smoking-cessation study, and although it was not an inclusion criterion for participating, all participants expressed an interest in quitting smoking. Eligible participants had to self-report smoking at least 10 cigarettes a day for 1 year or more and be maintained on a stable methadone dose for the past 30 days. Also, because illicit opioid and cocaine use can directly increase smoking rates (Chait & Griffiths, 1984; Higgins et al., 1994; Mello, Lukas, & Mendelson, 1985; Mello, Mendelson, Sellers, & Kuehnle, 1980; Roll, Higgins, & Tidey, 1997; Schmitz, Grabowski, & Rhoades, 1994), participants needed to be free from regular illicit opioid and cocaine use (< 30% positive urine specimens during past 30 days). Consent to confirm methadone dose and illicit drug abstinence with the clinic was obtained from each participant at the intake screening. As part of the methadone clinic's general urinalysis monitoring protocol, patients provide one to three urine samples per week, with frequency of testing based on their status in treatment. Samples were collected under observation of a same-sex laboratory technician, temperature tested for validity, and screened immediately onsite for evidence of opioids and cocaine, using an Olympus AU400 enzyme-multiplied immunoassay test analyzer. Participants had submitted a mean of 5.8 (range, 1 to 12) urine specimens during the month prior to study intake as part of their methadone treatment. Because the total number of urinalysis specimens varied across participants, a percentage was used to calculate each participant's status with illicit drug abstinence.
Assessments
At intake, participants completed a questionnaire on smoking and demographic variables and three smoking-related questionnaires. Smoking questionnaires included the Fagerstrom Test for Nicotine Dependence (Heatherton, Kozlowski, Frecker, & Fagerstrom, 1991), which is a five-item questionnaire commonly used to characterize baseline level of nicotine dependence; the Questionnaire of Smoking Urges (QSU; Tiffany & Drobes, 1991), a 32-item questionnaire assessing cigarette craving; and the Minnesota Nicotine Withdrawal Questionnaire (MNWQ; Hughes & Hatsukami, 1986), an eight-item questionnaire assessing the severity of smoking-related withdrawal (e.g., desire to smoke, anger or irritability, difficulty concentrating). Also included were two five-item Likert scales that assessed individual motivation and ability to quit smoking. Participants provided urine and breath samples for biochemical confirmation of smoking, alcohol, and illicit drug use and were compensated $35.00 for completing the intake. At each daily visit, they completed the MNWQ and a brief 10-item version of the QSU (Cox, Tiffany, & Christen, 2001). Follow-up assessments were completed on Day 14 and at 30, 60, and 90 days after quit date and included the above smoking questionnaires and collection of urine and breath samples. Participants received $35.00 per follow-up, independent of smoking status. Because the primary focus of the present study was on the efficacy of the CM intervention and biochemically verified smoking status, results from the self-report measures of nicotine withdrawal and craving will not be included here.
Study Design
All participants received a brief smoking-cessation educational intervention prior to the study (typically at the intake visit after providing informed consent) and were given a copy of the National Cancer Institute (2003) booklet, “Clearing the Air: Quit Smoking Today.” Staff reviewed its contents with the participant and answered any questions. Participants were then randomly assigned to a contingent (n = 10) or noncontingent voucher condition (n = 10), stratifying on four variables that could influence outcome: gender (male or female), age (≤ 30 years old or > 30 years old), methadone dose (≤ 100 mg or > 100 mg), and interest in pharmacotherapy (yes or no). Although pharmacotherapy was not provided during the present study, this variable was included to ensure even distribution of participants who might have an interest in pharmacotherapy for smoking cessation. It should be noted that participants were asked at each clinic visit to report any medications they had ingested in the preceding 24 hr, including all nicotine replacement products and nonnicotine pharmacotherapies (e.g., bupropion). Even though 80% (16 of 20) of participants had endorsed a general interest in pharmacotherapy at intake, no participant reported using any smoking-cessation medications throughout the 14-day intervention or at any follow-up assessment. All randomized participants were informed of their group assignment and worked with study staff to set a quit date. Beginning on their quit date, participants visited the clinic daily for 14 consecutive days.
At each study visit, participants provided breath and urine samples, reported the number of cigarettes smoked since the previous visit, and completed daily assessments of withdrawal and craving. Participants in the contingent condition earned vouchers contingent on biochemical verification of smoking abstinence (described below), and those in the noncontingent condition earned vouchers that were independent of smoking status and were yoked to a contingent partner's earnings. The yoking procedure was used to balance levels of clinic contact, monitoring, and material support across the two experimental groups and to help ensure that any differences in outcome would be attributable to the reinforcement contingency (Higgins, Wong, Badger, Ogden, & Dantona, 2000). The first three participants enrolled in the study were assigned to the contingent condition outside the randomization procedure to ensure a sufficient number of yoking partners for subsequent noncontingent participants. Failure to attend the clinic on two consecutive days resulted in dismissal from the study, although study status did not influence participants' methadone treatment, and the 4 participants who were eventually discharged continued to receive their usual methadone treatment.
Biochemical Monitoring
Breath and urine specimens were analyzed at each visit for biochemical verification of smoking status. Breath CO readings were collected using handheld meters. Breath CO is a commonly used measure in cessation studies (Society for Research on Nicotine and Tobacco [SRNT] Subcommittee on Biochemical Verification, 2002). Urine cotinine levels were measured using an onsite enzyme-multiplied immunoassay test. Urine cotinine is a metabolite of nicotine and is considered to be a sensitive biochemical measure of smoking status (Jarvis, Tunstall-Pedoe, Feyerabend, Vesey, & Saloojee, 1987).
The abstinence criterion for earning vouchers was defined as a breath CO ≤ 6 ppm on Days 1 through 5 and as a urine cotinine ≤ 80 ng/ml on Days 6 through 14. Because of the relatively short half-life of CO (4 hr), smokers can meet the 6-ppm abstinence criterion within 12 to 24 hr of stopping smoking (SRNT Subcommittee on Biochemical Verification, 2002). With the relatively long half-life of cotinine, several continuous days of abstinence are needed to meet the abstinence criterion (SRNT Subcommittee on Biochemical Verification). Therefore, CO was used early in the intervention to allow us to reinforce initial smoking abstinence, and the cotinine measure was used later to provide a more sensitive test that was likely to detect even low levels of ongoing smoking. This method of transitioning from CO to cotinine for monitoring of smoking status has been shown to be effective for promoting smoking abstinence with CM in prior research by our group (Higgins et al., 2004).
Contingent Condition
Contingent participants earned voucher-based incentives for breath samples ≤ 6 ppm during Days 1 through 5. The first negative sample earned $9.00, and values escalated by $1.50 with each subsequent negative sample. To further promote early and complete smoking abstinence, COs ≤ 4 ppm earned an additional $10.00 bonus during this 1st week. Beginning on Day 6, smoking abstinence was defined as a urine cotinine ≤ 80 ng/ml. To further encourage participants to meet this cutoff, a bonus of $50.00 was available for successfully meeting the criterion on Day 6. A positive or a missing sample resulted in no vouchers for that day and reset the value of the next negative sample to the initial $9.00. However, to encourage abstinence following a relapse, two consecutive negatives returned the schedule to the prereset value. Contingent participants could earn a maximum of $362.50 in vouchers for continuous abstinence during the 14-day study. Vouchers were redeemable for goods and services from local stores; participants received no cash, and research staff made all purchases.
Noncontingent Condition
Participants in the noncontingent condition received vouchers independent of smoking status, and voucher values were yoked to an individual in the contingent condition. Participants were told that they would receive vouchers independent of their smoking results and were not informed of their yoked status. To further emphasize that voucher delivery was not linked to smoking status, voucher earnings were provided prior to collection of biochemical samples. Noncontingent participants whose yoking partners were discharged from the study continued to earn the same voucher value as their contingent partner, such that all vouchers provided after the discharge date were $0.
Data Analysis
Participants in the contingent and noncontingent conditions were compared on demographic and drug use characteristics based on chi-square tests for dichotomous outcomes and either t tests or Wilcoxon rank sum tests for continuous variables. Chi-square tests were used to compare the two groups on the percentage of participants that were abstinent for each day during the 14-day treatment period and at each follow-up assessment. Smoking abstinence during the 14-day treatment period was based on the biochemical criteria (CO ≤ 6 ppm on Days 1 through 5 and cotinine ≤ 80 ng/ml on Days 6 through 14). Abstinence at follow-up assessments was based on a more rigorous definition, which required participants to be biochemically negative (cotinine ≤ 80 ng/ml) and report no smoking during the past 7 days. The percentage of participants in each group who completed the study was compared based on a chi-square test. T tests were used to compare groups on mean percentage of smoking-abstinent samples and the longest continuous duration of abstinence achieved during the 14-day study. Participants with missing biochemical samples for specific assessments were considered positive for smoking for that assessment. Repeated measures analyses of variance were used to compare groups on biochemical measures and self-reported smoking during the treatment period. Because the distribution of CO was heavily skewed, data were transformed using a square root transformation prior to analysis. For presentation purposes, means are presented as untransformed values. Analyses were performed using SAS statistical software. Statistical significance was based on α = .05.
Results
Baseline Demographic and Smoking Characteristics
There were no significant differences in baseline demographics, methadone dose, or smoking characteristics between the contingent and noncontingent conditions (Table 1). Of the 20 participants enrolled, the study was completed by 60% (6 of 10) and 100% (10 of 10) of those in the contingent and noncontingent conditions, respectively. Retention rate did not significantly differ between conditions (p = .09), although greater percentages of scheduled samples were missing among contingent participants than noncontingent participants (29% vs. 2%, respectively; p = .04). As noted above, any missing samples were considered to be positive for recent smoking. Reasons for leaving or for being discharged from the study included family illness (n = 1) and failure to attend for two consecutive visits (study criteria for discharge from protocol, n = 3).
Table 1.
Demographic and Smoking Variables
| Contingent |
Noncontingent |
||
| n = 10 | n = 10 | p valuea | |
| Demographics | |||
| Age (years) | 28.1 ± 6.2 | 31.4 ± 10.0 | .39 |
| Male (%) | 40 | 40 | .99b |
| Education (years) | 11.9 ± 1.7 | 12.8 ± 1.4 | .21 |
| Methadone characteristics | |||
| Methadone dose (mg) | 117.0 ± 43.2 | 95.9 ± 44.1 | .31 |
| Length of time at dose (days) | 128 (38, 234) | 47 (38, 90) | .29c |
| Smoking characteristics | |||
| Baseline CO level (ppm) | 11.8 ± 6.6 | 14.6 ± 4.0 | .26 |
| Baseline urine cotinine level (ng/ml) | 1,350.7 ± 693.1 | 1,369.9 ± 546.7 | .95 |
| Fagerstrom Test for Nicotine Dependence score | 5.8 ± 1.9 | 6.3 ± 1.2 | .49 |
| Mean age started smoking regularly (years) | 14.1 ± 2.9 | 13.7 ± 2.1 | .36 |
| Mean number of years smoking regularly | 12.2 ± 6.5 | 14.5 ± 9.5 | .54 |
| Mean number of cigarettes per day (past 7 days) | 18.0 ± 2.9 | 19.5 ± 9.6 | .64 |
| Mean nicotine yield per cigarette (mg) | 1.0 ± 0.3 | 1.0 ± 0.1 | .49 |
| Living with a smoker (%) | 40 | 80 | .07b |
| Quitting characteristics | |||
| Mean number of previous quit attempts | 4.1 ± 3.8 | 4.1 ± 3.8 | .99 |
| Longest successful quit duration (days) | 10 (0.5, 60) | 53 (7, 240) | .54c |
| Self-reported motivation to quit (5 = high, 1 = low) | 4.6 ± 0.7 | 4.4 ± 0.8 | .57 |
| Self-reported ability to quit (5 = low, 1 = high) | 3.8 ± 1.3 | 4.0 ± 1.2 | .72 |
Note. Values represent M ± SD unless otherwise noted.
p values based on two-sample t tests unless otherwise noted.
p values based on chi-square test.
Values repesent median (interquartile range). p value based on Wilcoxon rank sum test.
Smoking Abstinence
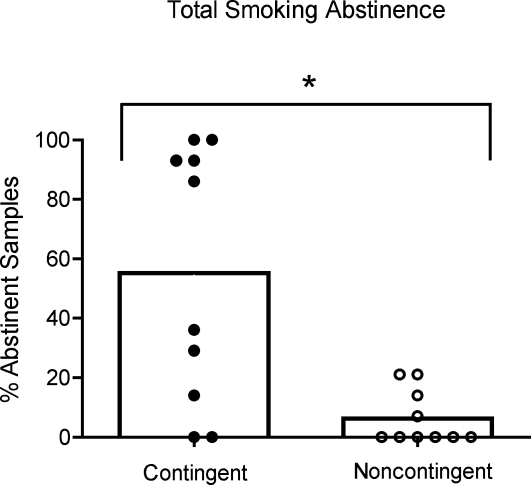
Participants in the contingent condition provided significantly more abstinent samples than those in the noncontingent condition (55% vs. 5%, respectively, p < .01; Figure 1). Inspection of individual data in Figure 1 reveals a general bimodal distribution in which approximately half of those in the contingent condition achieved nearly complete levels of abstinence and half achieved low to moderate amounts. Participants in the noncontingent condition achieved either near-zero or low levels of abstinence.
Figure 1.
Percentages of total biochemically abstinent samples, collapsed over Days 1 through 14 and shown by group, are presented for contingent and noncontingent groups (open bars). Percentages of negative samples, collapsed across Days 1 through 14 and shown for individual participants in the contingent (filled circles) and noncontingent (open circles) groups.
Participants in the contingent condition also achieved significantly longer durations of continuous smoking abstinence (6.3 vs. 0.6 days, respectively, p = .01; Figure 2). Inspection of individual data in Figure 2 shows that several participants in the contingent condition achieved nearly complete continuous abstinence, and the rest achieved a low to moderate duration of continuous abstinence. Participants in the noncontingent condition achieved near-zero or short durations of continuous smoking abstinence.
Figure 2.
Longest duration of continuous biochemical abstinence (in days) achieved during the 14-day intervention is shown for the contingent and noncontingent groups (open bars). Brackets show SEM. Number of days of longest continuous abstinence is presented for individual participants in the contingent (filled circles) and noncontingent (open circles) groups.
Biochemical Measures of Abstinence Across Study Days and at Follow-Up
Inspection of smoking status on a daily basis throughout the study showed a similar pattern (Figure 3, top). Participants in the contingent condition provided significantly more negative samples (p < .05) than those in the noncontingent condition at 12 of the 14 visits. Smoking abstinence at follow-up, defined as cotinine ≤ 80 ng/ml plus a self-report of no smoking during the past 7 days, revealed a trend towards those in the contingent condition achieving more abstinence than those in the noncontingent condition at the 30-, 60-, and 90-day follow-ups (30%, 20%, and 20% vs. 0%, 0%, and 0%, respectively); however, these differences did not reach statistical significance (p = .10).
Figure 3.
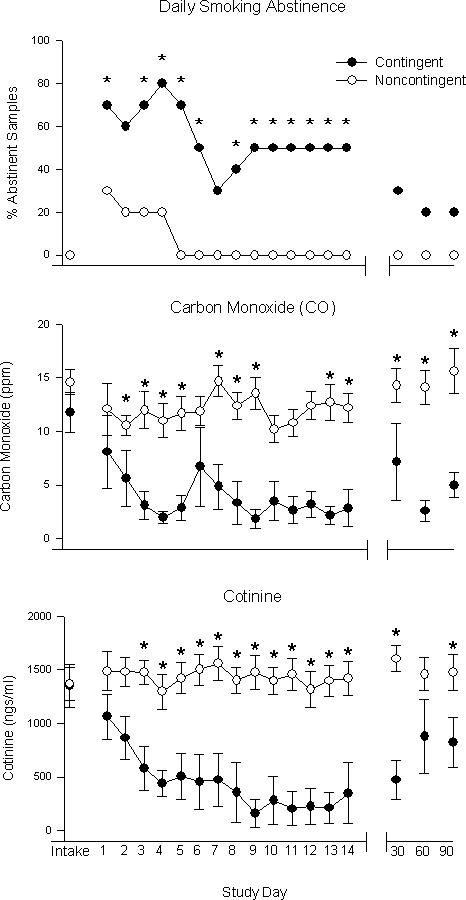
Additional measures of smoking on a daily basis are shown for the contingent (filled circles) and noncontingent (open circles) groups across the 14-day intervention and at 30-, 60-, and 90-day follow-ups. Percentages of total abstinent samples at each visit are shown for both groups (top). Mean CO levels at each visit are shown for both groups (middle). Mean cotinine values at each visit are shown for both groups (bottom). Statistical differences (p < .05) are indicated by an asterisk, and brackets indicate SEM.
A similar pattern was seen in the daily CO, cotinine, and self-report measures of smoking. Those in the contingent condition provided significantly lower breath CO levels on all but five study visits (p = .03; Figure 3, middle), and at the 30-, 60-, and 90-day follow-ups (p < .01). Those in the contingent condition also provided significantly lower cotinine levels than those in the noncontingent condition on all but two visits (p = .01; Figure 3, bottom) and at the 30- and 90-day follow-ups (p = .02). Finally, participants in the contingent condition reported smoking significantly fewer cigarettes per day during the study than those in the noncontingent condition (p < .001; data not shown). This trend was still evident at the follow-ups, although it did not reach statistical significance (p = .06).
Discussion
In the present study, we examined the efficacy of a brief but intensive voucher-based CM intervention for reducing cigarette smoking among MM patients. Despite a high prevalence of smoking in this population and numerous reports of interest among MM smokers in quitting, previous efforts at smoking cessation in this population have produced generally modest results. One likely reason for this is that procedural limitations in prior studies that have targeted smoking among MM patients may have limited their efficacy.
The present study demonstrates that a voucher-based CM intervention can promote smoking abstinence in MM patients. Participants in the contingent group provided a greater percentage of abstinent samples and achieved a longer duration of continuous abstinence than did those in the noncontingent control group. This was evident across all measures of smoking throughout the study, including breath CO levels, urine cotinine levels, and number of cigarettes smoked. These findings provide evidence that CM can be effective in promoting smoking abstinence in MM patients.
The methods used in the present study differed in several ways from those used in previous studies. First, a schedule of daily biochemical monitoring was used that was more intensive than the twice- or thrice-weekly schedule typically used in prior studies (Schmitz et al., 1995; Shoptaw et al., 1996, 2002). Second, the schedule of reinforcement in the current study included several features (e.g., escalating schedule, reset, bonuses) shown in prior studies to help promote abstinence (Roll & Higgins, 2000; Roll, Higgins, & Badger, 1996) and used a relatively high magnitude of incentives. Voucher magnitude has been shown to significantly influence amount of abstinence achieved in CM interventions (e.g., Silverman, Chutuape, Bigelow, & Stitzer, 1999). In the present 2-week study, the total earnings possible (i.e., $362.50) were substantially higher than amounts used in prior studies. For example, total possible earnings in the 2-week contingent phase of the Schmitz et al. study were $20.00, and earnings in the 4-week phase by Shoptaw et al. (1996) was $73.00. Although maximum possible earnings in the more recent 12-week study by Shoptaw et al. (2002) totaled $447.50, only approximately $29.50 was available for continuous abstinence during the important initial 2 weeks of the study. Third, the present study used a CO-to-cotinine method to carefully monitor smoking status, enabling us to reinforce initial smoking abstinence early in the study and then to move to a more sensitive test that was likely to detect even low levels of ongoing smoking later in the study. Consistent with prior research by our group (Higgins et al., 2004), this method appeared to be effective for promoting smoking abstinence in the present study. Finally, the present study employed a rigorous scientific design that included randomization to experimental groups, stratification on a subset of variables thought to potentially impact outcome, and a noncontingent control group who received vouchers independent of smoking status and were yoked to contingent partners. Overall, it is likely that these procedural features helped to improve rates of smoking abstinence compared to the outcomes seen in prior studies.
Although the intervention used in this pilot study was brief, it demonstrated the efficacy of a behavioral intervention in promoting early continuous cigarette abstinence in MM patients. Considering that a positive relation between early initial smoking abstinence and longer term outcomes has been well documented (Gourlay et al., 1994; Higgins et al., 2006; Kenford et al., 1994; Yudkin et al., 1996), the promising levels of initial smoking abstinence with this 2-week intervention bode well for longer term outcomes. Indeed, if initial continuous abstinence can be established with an intensive intervention early in the quit attempt, it may then be possible to maintain that abstinence with a monitoring schedule that becomes progressively leaner over subsequent weeks. Future efforts by our group will be aimed at developing a CM intervention that sustains smoking abstinence for the longer term.
Several limitations are relevant to this pilot study. First, this study was conducted with a limited sample size. Future studies should attempt to replicate and extend these findings with a larger number of participants. Second, relatively stringent criteria were used to ensure that participants were stable and abstinent from illicit drug use before participating in this study. It would be important to learn whether the same positive smoking outcomes would be seen in MM smokers whose illicit drug status was less stable. However, it also should be noted that this criterion did not appear to be so restrictive as to significantly limit the generality of these findings. For example, approximately 75% of this methadone clinic's general patient population met the illicit drug criterion at the time the study was conducted, and our study criteria prevented only 17% of interested patients from participating. That said, the need for this criterion should be evaluated during future efforts to replicate the current study in clinics with perhaps less stable patient populations. Third, the results of this pilot study were achieved in the absence of any nicotine replacement therapy (NRT). Use of NRT would have interfered with our use of urine cotinine as a biochemical measure of smoking, because it would have been impossible to distinguish NRT-derived cotinine from that produced by cigarettes. However, the biochemical testing methods used in this study do not preclude the use of nonnicotine pharmacotherapies for smoking cessation, such as bupropion. Bupropion has been shown to aid smoking cessation efforts to a similar degree as nicotine-based pharmacotherapies (Hughes, Stead, & Lancaster, 2004) and has been well tolerated in several prior studies with MM patients (Margolin et al., 1995; Margolin, Kosten, Petrakis, Avants, & Kosten, 1990).
It should also be noted that even though some group differences were still evident in the weeks following the study, a subset of contingent participants had relapsed to smoking. Indeed, inspection of individual data showed that some contingent participants continued to smoke during the intervention. For example, although 5 of the 10 contingent participants achieved nearly complete levels of abstinence, a subset achieved either little (3 participants) or no (2 participants) abstinence (Figure 1). Even though a core of treatment-resistant participants is typically seen with substance abuse treatments in general, it is important to explore ways to further increase the number of participants who respond favorably. Increasing incentive magnitude (e.g., Silverman et al., 1999) or using shaping procedures (e.g., Lamb, Morral, Kirby, Iguchi, & Galbicka, 2004) or adjunct pharmacotherapies may be useful approaches with particularly hard-to-treat smokers. It also would be of interest to learn whether those individuals who responded well to these relatively high voucher values might also respond to lower magnitudes. Finally, it is worth investigating the characteristics that may distinguish the individuals whose smoking is sensitive to voucher-based CM from those whose smoking is insensitive.
In summary, the present study demonstrates the efficacy of a brief voucher-based CM intervention in promoting initial smoking abstinence among MM patients. The development of effective smoking cessation programs in this treatment setting holds potential for wide dissemination. The possibility of extensive and cost-effective implementation is also enhanced by the fact that many components of routine MM treatment, such as dosing and ancillary services, can themselves function as reinforcers and be arranged contingently to promote positive behavior change (Kidorf & Stitzer, 1999). Overall, these preliminary results suggest that behavioral treatments (e.g., contingency management) offer significant promise for treating cigarette smoking among MM patients.
Acknowledgments
This research was supported in part by Grants T32 DA007242 and R01 DA019550 from the National Institute on Drug Abuse.
References
- Ball J.C, Ross A. The effectiveness of methadone maintenance treatment. New York: Springer-Verlag; 1991. [Google Scholar]
- Chait L.D, Griffiths R.R. Effects of methadone on human cigarette smoking and subjective ratings. Journal of Pharmacology and Experimental Therapeutics. 1984;229:636–640. [PubMed] [Google Scholar]
- Clark J.G, Stein M.D, McGarry K.A, Gogineni A. Interest in smoking cessation among injection drug users. The American Journal on Addictions. 2001;10((2)):159–166. doi: 10.1080/105504901750227804. [DOI] [PubMed] [Google Scholar]
- Clemmey P, Brooner R, Chutuape M.A, Kidorf M, Stitzer M. Smoking habits and attitudes in a methadone maintenance treatment population. Drug and Alcohol Dependence. 1997;44((2–3)):123–132. doi: 10.1016/s0376-8716(96)01331-2. [DOI] [PubMed] [Google Scholar]
- Cox L.S, Tiffany S.T, Christen A.G. Evaluation of the brief questionnaire of smoking urges (QSU-brief) in laboratory and clinical settings. Nicotine and Tobacco Research. 2001;3((1)):7–16. doi: 10.1080/14622200020032051. [DOI] [PubMed] [Google Scholar]
- Dallery J, Glenn I.M. Effects of an Internet-based voucher reinforcement program for smoking abstinence: A feasibility study. Journal of Applied Behavior Analysis. 2005;38:349–357. doi: 10.1901/jaba.2005.150-04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drug and Alcohol Services Information System. The DASIS report. Rockville, MD: Office of Applied Studies, Substance Abuse and Mental Health Services Administration; 2002. [Google Scholar]
- Engstrom A, Adamsson C, Allebeck P, Rydberg U. Mortality in patients with substance abuse: A follow-up in Stockholm County, 1973–1984. International Journal of Addictions. 1991;26((1)):91–106. doi: 10.3109/10826089109056241. [DOI] [PubMed] [Google Scholar]
- Frosch D.L, Nahom D, Shoptaw S. Optimizing smoking cessation outcomes among the methadone maintained. Journal of Substance Abuse Treatment. 2002;23((4)):425–430. doi: 10.1016/s0740-5472(02)00280-5. [DOI] [PubMed] [Google Scholar]
- Frosch D.L, Shoptaw S, Jarvik M.E, Rawson R.A, Ling W. Interest in smoking cessation among methadone maintained outpatients. Journal of Addictive Disorders. 1998;17((2)):9–19. doi: 10.1300/J069v17n02_02. [DOI] [PubMed] [Google Scholar]
- Gourlay S.G, Forbes A, Marriner T, Pethica D, McNeil J.J. Prospective study of factors predicting outcome of transdermal nicotine treatment in smoking cessation. British Medical Journal. 1994;309:842–846. doi: 10.1136/bmj.309.6958.842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heatherton T.F, Kozlowski L.T, Frecker R.C, Fagerstrom K.O. The Fagerstrom test for nicotine dependence: a revision of the Fagerstrom tolerance questionnaire. British Journal of Addiction. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Higgins S.T, Alessi S.M, Dantona R.L. Voucher-based incentives: A substance abuse treatment innovation. Addictive Behaviors. 2002;2:887–910. doi: 10.1016/s0306-4603(02)00297-6. [DOI] [PubMed] [Google Scholar]
- Higgins S.T, Budney A.J, Hughes J.R, Bickel W.K, Lynn M, Mortensen A. Influence of cocaine use on cigarette smoking. Journal of the American Medical Association. 1994;272((22)):1724. [PubMed] [Google Scholar]
- Higgins S.T, Delaney D.D, Budney A.J, Bickel W.K, Hughes J.R, Foerg F, et al. A behavioral approach to achieving initial cocaine abstinence. American Journal of Psychiatry. 1991;148((9)):1218–1224. doi: 10.1176/ajp.148.9.1218. [DOI] [PubMed] [Google Scholar]
- Higgins S.T, Heil S.H, Dumeer A.M, Thomas C.S, Solomon L.J, Bernstein I.M. Smoking status in the initial weeks of quitting as a predictor of smoking-cessation outcomes in pregnant women. Drug and Alcohol Dependence. 2006;85((2)):138–141. doi: 10.1016/j.drugalcdep.2006.04.005. [DOI] [PubMed] [Google Scholar]
- Higgins S.T, Heil S.H, Solomon L.J, Lussier J.P, Abel R.L, Lynch M.E, et al. A pilot study on voucher-based incentives to promote abstinence from cigarette smoking during pregnancy and postpartum. Nicotine and Tobacco Research. 2004;6((6)):1015–1020. doi: 10.1080/14622200412331324910. [DOI] [PubMed] [Google Scholar]
- Higgins S.T, Silverman K, editors. Motivating behavior change among illicit drug abusers. Washington, DC: American Psychological Association; 1999. [Google Scholar]
- Higgins S.T, Wong C.J, Badger G.J, Ogden D.E, Dantona R.L. Contingent reinforcement increases cocaine abstinence during outpatient treatment and one-year of follow-up. Journal of Consulting and Clinical Psychology. 2000;68((1)):64–72. doi: 10.1037//0022-006x.68.1.64. [DOI] [PubMed] [Google Scholar]
- Hser Y.I, McCarthy W.J, Anglin M.D. Tobacco use as a distal predictor of mortality among long-term narcotic addicts. Preventative Medicine. 1994;23((1)):61–69. doi: 10.1006/pmed.1994.1009. [DOI] [PubMed] [Google Scholar]
- Hughes J.R, Hatsukami D. Signs and symptoms of tobacco withdrawal. Archives of General Psychiatry. 1986;43((3)):289–294. doi: 10.1001/archpsyc.1986.01800030107013. [DOI] [PubMed] [Google Scholar]
- Hughes J.R, Stead L.F, Lancaster T. Antidepressants for smoking cessation. 2004. The Cochrane Database of Systematic Reviews 2004, Issue 4, Art. No. CD000031. DOI: 10.1002/14651858.CD000031.pub3. [DOI] [PubMed]
- Jarvis M.J, Tunstall-Pedoe H, Feyerabend C, Vesey C, Saloojee Y. Comparison of tests to distinguish smokers from nonsmokers. American Journal of Public Health. 1987;77((11)):1435–1438. doi: 10.2105/ajph.77.11.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javors M.A, Hatch J.P, Lamb R.J. Cut-off levels for breath carbon monoxide as a marker for cigarette smoking. Addiction. 2005;100((2)):159–167. doi: 10.1111/j.1360-0443.2004.00957.x. [DOI] [PubMed] [Google Scholar]
- Kenford S.L, Fiore M.C, Jorenby D.E, Smith S.S, Wetter D, Baker T.B. Predicting smoking cessation: Who will quit with and without the nicotine patch. Journal of the American Medical Association. 1994;271((8)):589–594. doi: 10.1001/jama.271.8.589. [DOI] [PubMed] [Google Scholar]
- Kidorf M, Stitzer M.L. Contingent access to clinic privileges reduces drug abuse in methadone maintenance patients. In: Higgins S.T, Silverman K, editors. Motivating behavior change among illicit drug abusers. Washington, DC: American Psychological Association; 1999. pp. 221–242. [Google Scholar]
- Kozlowski L.T, Skinner W, Kent C, Pope M.A. Prospects for smoking treatment in individuals seeking treatment for alcohol and other drug problems. Addictive Behavior. 1989;14((3)):273–278. doi: 10.1016/0306-4603(89)90058-0. [DOI] [PubMed] [Google Scholar]
- Lamb R.J, Morral A.R, Kirby K.C, Iguchi M.Y, Galbicka G. Shaping smoking cessation using percentile schedules. Drug and Alcohol Dependence. 2004;76:247–259. doi: 10.1016/j.drugalcdep.2004.05.008. [DOI] [PubMed] [Google Scholar]
- Margolin A, Kosten T.R, Avants S.K, Wilkins J, Ling W, Beckson M, et al. A multicenter trial of bupropion for cocaine dependence in methadone-maintained patients. Drug and Alcohol Dependence. 1995;40:125–131. doi: 10.1016/0376-8716(95)01198-6. [DOI] [PubMed] [Google Scholar]
- Margolin A, Kosten T, Petrakis I, Avants S.K, Kosten T. An open pilot study of bupropion and psychotherapy for the treatment of cocaine abuse in methadone-maintained patients. NIDA Research Monograph. 1990;105:367–368. [PubMed] [Google Scholar]
- Mello N.K, Lukas S.E, Mendelson J.H. Buprenorphine effects on cigarette smoking. Psychopharmacology. 1985;86:417–425. doi: 10.1007/BF00427902. [DOI] [PubMed] [Google Scholar]
- Mello N.K, Mendelson J.H, Sellers M.L, Kuehnle J.C. Effects of heroin self-administration on cigarette smoking. Psychopharmacology. 1980;67:45–52. doi: 10.1007/BF00427594. [DOI] [PubMed] [Google Scholar]
- Nahvi S, Richter K, Li X, Modali L, Arnsten J. Cigarette smoking and interest in quitting in methadone maintenance patients. Addictive Behaviors. 2006;31((11)):2127–2134. doi: 10.1016/j.addbeh.2006.01.006. [DOI] [PubMed] [Google Scholar]
- National Cancer Institute. Clearing the air: quit smoking today. Bethesda, MD: National Institutes of Health, U.S. Department of Health and Human Services; 2003. [Google Scholar]
- Richter K.P, Gibson C.A, Ahluwalia J.S, Schmelzle K.H. Tobacco use and quit attempts among methadone maintenance clients. American Journal of Public Health. 2001;91((2)):296–299. doi: 10.2105/ajph.91.2.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roll J.M. Assessing the feasibility of using contingency management to modify cigarette smoking by adolescents. Journal of Applied Behavior Analysis. 2005;38:463–467. doi: 10.1901/jaba.2005.114-04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roll J.M, Higgins S.T. A within-subject comparison of three different schedules of reinforcement of drug abstinence using cigarette smoking as an exemplar. Drug and Alcohol Dependence. 2000;58((1–2)):103–109. doi: 10.1016/s0376-8716(99)00073-3. [DOI] [PubMed] [Google Scholar]
- Roll J.M, Higgins S.T, Badger G.J. An experimental comparison of three different schedules of reinforcement of drug abstinence using cigarette smoking as an exemplar. Journal of Applied Behavior Analysis. 1996;29:495–505. doi: 10.1901/jaba.1996.29-495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roll J.M, Higgins S.T, Tidey J.W. Cocaine use can increase cigarette smoking: Evidence from laboratory and naturalistic settings. Experimental and Clinical Psychopharmacology. 1997;5:263–268. doi: 10.1037//1064-1297.5.3.263. [DOI] [PubMed] [Google Scholar]
- Schmitz J.M, Grabowski J, Rhoades H. The effects of high and low doses of methadone on cigarette smoking. Drug and Alcohol Dependence. 1994;34:237–242. doi: 10.1016/0376-8716(94)90162-7. [DOI] [PubMed] [Google Scholar]
- Schmitz J.M, Rhoades H, Grabowski J. Contingent reinforcement for reduced carbon monoxide levels in methadone maintenance patients. Addictive Behavior. 1995;20((2)):171–179. doi: 10.1016/0306-4603(94)00059-x. [DOI] [PubMed] [Google Scholar]
- Sees K.L, Clark H.W. When to begin smoking cessation in substance abusers. Journal of Substance Abuse Treatment. 1993;10((2)):189–195. doi: 10.1016/0740-5472(93)90044-3. [DOI] [PubMed] [Google Scholar]
- Shoptaw S, Jarvik M.E, Ling W, Rawson R.A. Contingency management for tobacco smoking in methadone-maintained opiate addicts. Addictive Behavior. 1996;21((3)):409–412. doi: 10.1016/0306-4603(95)00066-6. [DOI] [PubMed] [Google Scholar]
- Shoptaw S, Rotheram-Fuller E, Yang X, Frosch D, Nahom D, Jarvik M.E, et al. Smoking cessation in methadone maintenance. Addiction. 2002;97((10)):1317–1328. doi: 10.1046/j.1360-0443.2002.00221.x. [DOI] [PubMed] [Google Scholar]
- Silverman K, Chutuape M.A, Bigelow G.E, Stitzer M.L. Voucher-based reinforcement of cocaine abstinence in treatment-resistant methadone patients: Effects of reinforcement magnitude. Psychopharmacology. 1999;146((2)):128–138. doi: 10.1007/s002130051098. [DOI] [PubMed] [Google Scholar]
- Society for Research on Nicotine and Tobacco Subcommittee on Biochemical Verification. Biochemical verification of tobacco use and cessation. Nicotine and Tobacco Research. 2002;4((2)):149–159. doi: 10.1080/14622200210123581. [DOI] [PubMed] [Google Scholar]
- Stitzer M.L, Sigmon S.C. Other substance use disorders: Prevalence, consequences, detection and management. In: Strain E.C, Stitzer M.L, editors. The treatment of opioid dependence. Baltimore: Johns Hopkins University Press; 2006. pp. 365–397. [Google Scholar]
- Tiffany S.T, Drobes D.J. The development and initial validation of a questionnaire on smoking urges. British Journal of Addiction. 1991;86((11)):1467–1476. doi: 10.1111/j.1360-0443.1991.tb01732.x. [DOI] [PubMed] [Google Scholar]
- Yudkin P.L, Jones L, Lancaster T, Fowler G.H. Which smokers are helped to give up smoking using transdermal nicotine patches? Results from a randomized, double-blind, placebo-controlled trial. British Journal of General Practice. 1996;46:145–148. [PMC free article] [PubMed] [Google Scholar]