Abstract
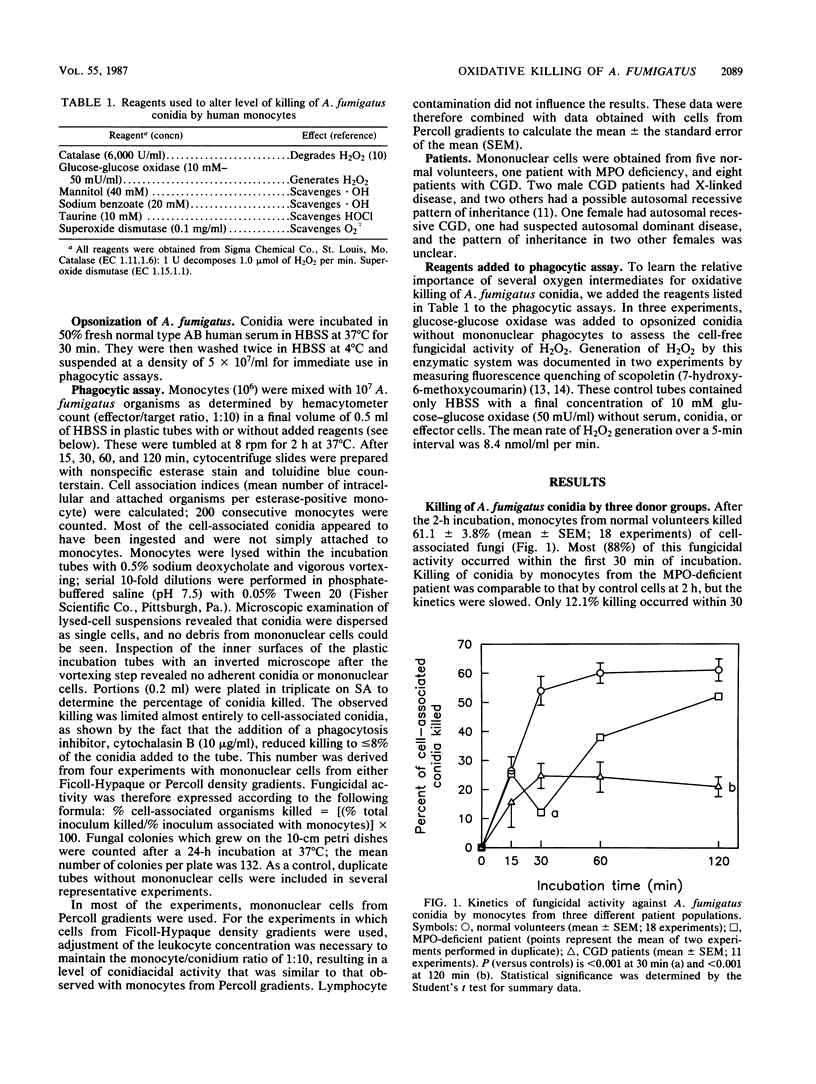
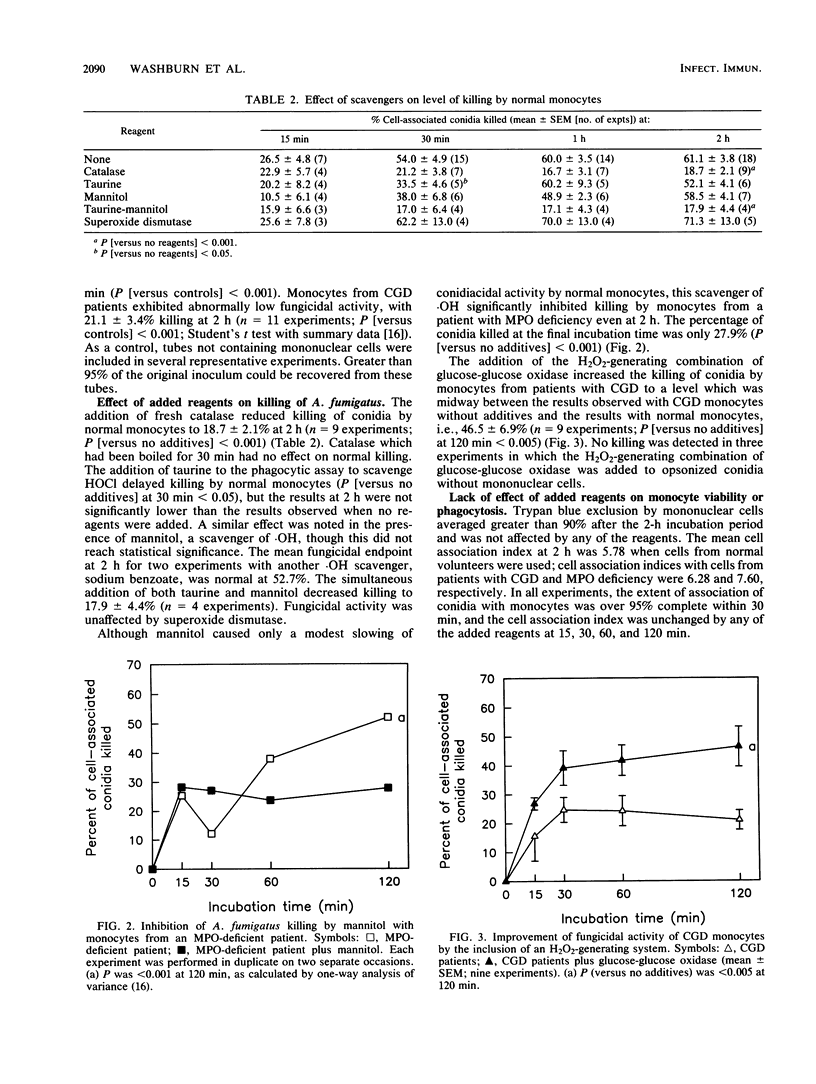
The relative importance of several oxygen intermediates in fungicidal action against opsonized Aspergillus fumigatus conidia was investigated with monocytes from normal volunteers and patients with either chronic granulomatous disease or myeloperoxidase (MPO) deficiency. Results from experiments in which catalase, taurine, mannitol, or glucose-glucose oxidase were added to these phagocytes indicated that the MPO-hydrogen peroxide-halide system and an MPO-independent oxidative system exerted comparable conidiacidal activity. These findings offer a plausible explanation for the susceptibility of patients with chronic granulomatous disease to invasive Aspergillus infections; their phagocytes fail to generate hydrogen peroxide, a substrate necessary for both systems. Patients with MPO deficiency are not known to be predisposed to invasive aspergillosis, suggesting that an MPO-independent oxidative system may provide an alternative mechanism for the oxidative killing of Aspergillus spp.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cohen M. S., Isturiz R. E., Malech H. L., Root R. K., Wilfert C. M., Gutman L., Buckley R. H. Fungal infection in chronic granulomatous disease. The importance of the phagocyte in defense against fungi. Am J Med. 1981 Jul;71(1):59–66. doi: 10.1016/0002-9343(81)90259-x. [DOI] [PubMed] [Google Scholar]
- Diamond R. D., Huber E., Haudenschild C. C. Mechanisms of destruction of Aspergillus fumigatus hyphae mediated by human monocytes. J Infect Dis. 1983 Mar;147(3):474–483. doi: 10.1093/infdis/147.3.474. [DOI] [PubMed] [Google Scholar]
- Diamond R. D., Krzesicki R., Epstein B., Jao W. Damage to hyphal forms of fungi by human leukocytes in vitro. A possible host defense mechanism in aspergillosis and mucormycosis. Am J Pathol. 1978 May;91(2):313–328. [PMC free article] [PubMed] [Google Scholar]
- Gmelig-Meyling F., Waldmann T. A. Separation of human blood monocytes and lymphocytes on a continuous Percoll gradient. J Immunol Methods. 1980;33(1):1–9. doi: 10.1016/0022-1759(80)90077-0. [DOI] [PubMed] [Google Scholar]
- Lehrer R. I., Cline M. J. Interaction of Candida albicans with human leukocytes and serum. J Bacteriol. 1969 Jun;98(3):996–1004. doi: 10.1128/jb.98.3.996-1004.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrer R. I., Jan R. G. Interaction of Aspergillus fumigatus Spores with Human Leukocytes and Serum. Infect Immun. 1970 Apr;1(4):345–350. doi: 10.1128/iai.1.4.345-350.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitz S. M., Diamond R. D. Killing of Aspergillus fumigatus spores and Candida albicans yeast phase by the iron-hydrogen peroxide-iodide cytotoxic system: comparison with the myeloperoxidase-hydrogen peroxide-halide system. Infect Immun. 1984 Mar;43(3):1100–1102. doi: 10.1128/iai.43.3.1100-1102.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitz S. M., Diamond R. D. Mechanisms of resistance of Aspergillus fumigatus Conidia to killing by neutrophils in vitro. J Infect Dis. 1985 Jul;152(1):33–42. doi: 10.1093/infdis/152.1.33. [DOI] [PubMed] [Google Scholar]
- Mandell G. L. Catalase, superoxide dismutase, and virulence of Staphylococcus aureus. In vitro and in vivo studies with emphasis on staphylococcal--leukocyte interaction. J Clin Invest. 1975 Mar;55(3):561–566. doi: 10.1172/JCI107963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno Y., Buescher E. S., Roberts R., Metcalf J. A., Gallin J. I. Reevaluation of cytochrome b and flavin adenine dinucleotide in neutrophils from patients with chronic granulomatous disease and description of a family with probable autosomal recessive inheritance of cytochrome b deficiency. Blood. 1986 Apr;67(4):1132–1138. [PubMed] [Google Scholar]
- Root R. K., Metcalf J. A. H2O2 release from human granulocytes during phagocytosis. Relationship to superoxide anion formation and cellular catabolism of H2O2: studies with normal and cytochalasin B-treated cells. J Clin Invest. 1977 Dec;60(6):1266–1279. doi: 10.1172/JCI108886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Root R. K., Metcalf J., Oshino N., Chance B. H2O2 release from human granulocytes during phagocytosis. I. Documentation, quantitation, and some regulating factors. J Clin Invest. 1975 May;55(5):945–955. doi: 10.1172/JCI108024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schønheyder H., Andersen P. Fractionation of Aspergillus fumigatus antigens by hydrophobic interaction chromatography and gel filtration. Int Arch Allergy Appl Immunol. 1984;73(3):231–236. doi: 10.1159/000233473. [DOI] [PubMed] [Google Scholar]
- Wagner D. K., Collins-Lech C., Sohnle P. G. Inhibition of neutrophil killing of Candida albicans pseudohyphae by substances which quench hypochlorous acid and chloramines. Infect Immun. 1986 Mar;51(3):731–735. doi: 10.1128/iai.51.3.731-735.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young R. C., Bennett J. E., Vogel C. L., Carbone P. P., DeVita V. T. Aspergillosis. The spectrum of the disease in 98 patients. Medicine (Baltimore) 1970 Mar;49(2):147–173. doi: 10.1097/00005792-197003000-00002. [DOI] [PubMed] [Google Scholar]


