Abstract
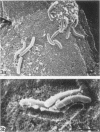
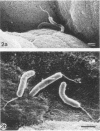
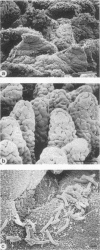
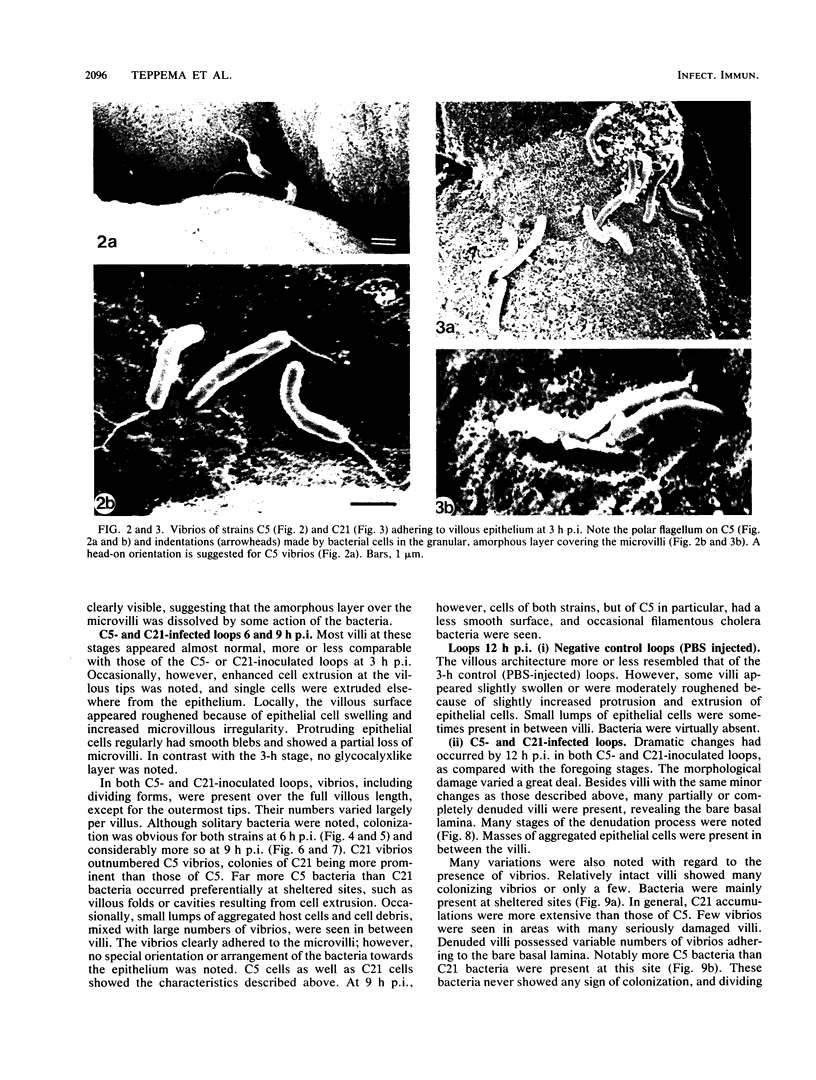
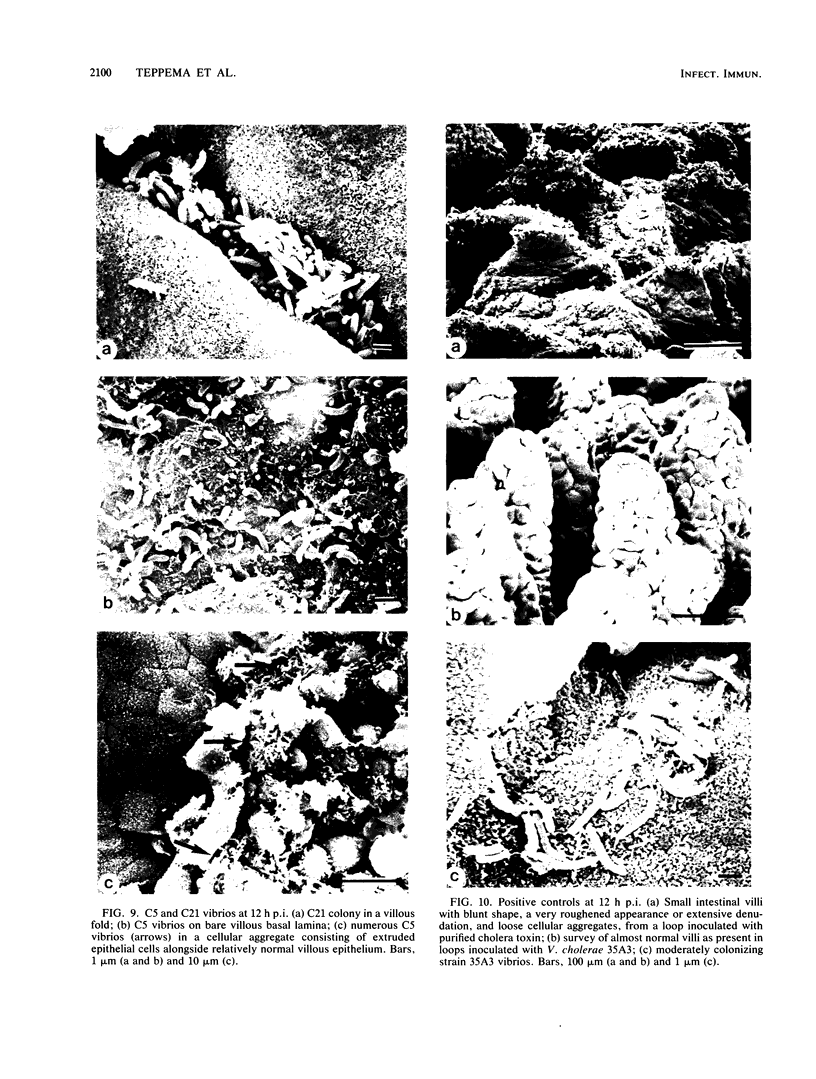
A scanning electron microscopic study was carried out to compare the in vivo pathogenicity of two strains of Vibrio cholerae in an adult rabbit ligated-gut test model. V. cholerae C5 (serotype Ogawa, biotype El Tor), a motile strain possessing hemagglutinating activity in vitro, and C21 (serotype Ogawa, classical biotype), a nonmotile strain possessing no hemagglutinating activity, were tested. Tissue samples from small intestinal loops were examined 3, 6, 9, and 12 h postinoculation. Contradictory to most published data, neither hemagglutinating activity nor motility appeared to be essential prerequisites for the pathogenesis of cholera in the experimental animal model used: nonmotile hemagglutinin-negative strain C21 adhered to and colonized the small intestine at least to the same extent as did motile hemagglutinin-positive strain C5. Maximum colonization was seen at 9 h postinoculation for both strains. C5 and C21 vibrios caused comparable damage to the villi of the small intestine. The villous epithelium showed only mild changes during the first 9 h postinoculation. However, after 12 h the epithelium was seriously damaged concomitant with a decrease in the number of vibrios. Many villi showed partial or total denudation, owing to repelled epithelium, leaving a bare basal lamina with only some to moderate numbers of vibrios attached. Since similar changes were induced by pure cholera enterotoxin, these changes were likely the result of excessive fluid accumulation. From this study it is concluded that, at least in the animal model used, factors other than hemagglutinating activity and motility may also play a role in the association of V. cholerae with the small intestinal surface.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asakura H., Morita A., Morishita T., Tsuchiya M., Fukumi H., Oashi M., Uylangco C., Castro A. Pathologic findings from intestinal biopsy specimens in human cholera. Am J Dig Dis. 1973 Apr;18(4):271–278. doi: 10.1007/BF01070987. [DOI] [PubMed] [Google Scholar]
- Attridge S. R., Rowley D. The role of the flagellum in the adherence of Vibrio cholerae. J Infect Dis. 1983 May;147(5):864–872. doi: 10.1093/infdis/147.5.864. [DOI] [PubMed] [Google Scholar]
- BARUA D., CHATTERJEE S. N. ELECTRON MICROSCOPY OF EL TOR VIBRIOS. Indian J Med Res. 1964 Aug;52:828–830. [PubMed] [Google Scholar]
- Beachey E. H. Bacterial adherence: adhesin-receptor interactions mediating the attachment of bacteria to mucosal surface. J Infect Dis. 1981 Mar;143(3):325–345. doi: 10.1093/infdis/143.3.325. [DOI] [PubMed] [Google Scholar]
- Bhattacharjee J. W., Srivastava B. S. Mannose-sensitive haemagglutinins in adherence of Vibrio cholerae eltor to intestine. J Gen Microbiol. 1978 Aug;107(2):407–410. doi: 10.1099/00221287-107-2-407. [DOI] [PubMed] [Google Scholar]
- Chatterjee S. N., Das J. Electron microscopic observations on the excretion of cell-wall material by Vibrio cholerae. J Gen Microbiol. 1967 Oct;49(1):1–11. doi: 10.1099/00221287-49-1-1. [DOI] [PubMed] [Google Scholar]
- Cray W. C., Jr, Tokunaga E., Pierce N. F. Successful colonization and immunization of adult rabbits by oral inoculation with Vibrio cholerae O1. Infect Immun. 1983 Aug;41(2):735–741. doi: 10.1128/iai.41.2.735-741.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DE S. N., CHATTERJE D. N. An experimental study of the mechanism of action of Vibriod cholerae on the intestinal mucous membrane. J Pathol Bacteriol. 1953 Oct;66(2):559–562. doi: 10.1002/path.1700660228. [DOI] [PubMed] [Google Scholar]
- Evans D. G., Evans D. J., Jr, Gorbach S. L. Identification of enterotoxigenic Escherichia coli and serum antitoxin activity by the vascular permeability factor assay. Infect Immun. 1973 Nov;8(5):731–735. doi: 10.1128/iai.8.5.731-735.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein R. A., Boesman-Finkelstein M., Holt P. Vibrio cholerae hemagglutinin/lectin/protease hydrolyzes fibronectin and ovomucin: F.M. Burnet revisited. Proc Natl Acad Sci U S A. 1983 Feb;80(4):1092–1095. doi: 10.1073/pnas.80.4.1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein R. A., Hanne L. F. Purification and characterization of the soluble hemagglutinin (cholera lectin)( produced by Vibrio cholerae. Infect Immun. 1982 Jun;36(3):1199–1208. doi: 10.1128/iai.36.3.1199-1208.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foo Eng Sim A., Riganti M., Pongponratn E., Chaicumpa W. Time of maximum colonisation by Vibrio cholerae El Tor in 5-6 day old mice as an experimental cholera model. Southeast Asian J Trop Med Public Health. 1981 Dec;12(4):609–610. [PubMed] [Google Scholar]
- Foster J. W., Kinney D. M. ADP-ribosylating microbial toxins. Crit Rev Microbiol. 1985;11(4):273–298. doi: 10.3109/10408418409105905. [DOI] [PubMed] [Google Scholar]
- Freter R., Allweiss B., O'Brien P. C., Halstead S. A., Macsai M. S. Role of chemotaxis in the association of motile bacteria with intestinal mucosa: in vitro studies. Infect Immun. 1981 Oct;34(1):241–249. doi: 10.1128/iai.34.1.241-249.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freter R., Jones G. W. Adhesive properties of Vibrio cholerae: nature of the interaction with intact mucosal surfaces. Infect Immun. 1976 Jul;14(1):246–256. doi: 10.1128/iai.14.1.246-256.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freter R., O'Brien P. C., Macsai M. S. Role of chemotaxis in the association of motile bacteria with intestinal mucosa: in vivo studies. Infect Immun. 1981 Oct;34(1):234–240. doi: 10.1128/iai.34.1.234-240.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaastra W., de Graaf F. K. Host-specific fimbrial adhesins of noninvasive enterotoxigenic Escherichia coli strains. Microbiol Rev. 1982 Jun;46(2):129–161. doi: 10.1128/mr.46.2.129-161.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guentzel M. N., Berry L. J. Motility as a virulence factor for Vibrio cholerae. Infect Immun. 1975 May;11(5):890–897. doi: 10.1128/iai.11.5.890-897.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guinée P. A., Jansen W. H., Peters P. W. Vibrio cholerae infection and acquired immunity in an adult rabbit model. Zentralbl Bakteriol Mikrobiol Hyg A. 1985 Feb;259(1):118–131. doi: 10.1016/s0176-6724(85)80013-4. [DOI] [PubMed] [Google Scholar]
- Gyobu Y., Kodama H., Uetake H., Katsuda S. Studies on the enteropathogenic mechanism of non-O 1 Vibrio cholerae isolated from the environment and fish in Toyama Prefecture. Microbiol Immunol. 1984;28(7):735–745. doi: 10.1111/j.1348-0421.1984.tb00729.x. [DOI] [PubMed] [Google Scholar]
- Hanne L. F., Finkelstein R. A. Characterization and distribution of the hemagglutinins produced by Vibrio cholerae. Infect Immun. 1982 Apr;36(1):209–214. doi: 10.1128/iai.36.1.209-214.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson H. A., Lange S., Lönnroth I. Internalization in vivo of cholera toxin in the small intestinal epithelium of the rat. Acta Pathol Microbiol Immunol Scand A. 1984 Jan;92(1):15–21. doi: 10.1111/j.1699-0463.1984.tb04372.x. [DOI] [PubMed] [Google Scholar]
- Holmgren J., Svennerholm A. M. Cholera and the immune response. Prog Allergy. 1983;33:106–119. [PubMed] [Google Scholar]
- Jones G. W., Abrams G. D., Freter R. Adhesive properties of Vibrio cholerae: adhesion to isolated rabbit brush border membranes and hemagglutinating activity. Infect Immun. 1976 Jul;14(1):232–239. doi: 10.1128/iai.14.1.232-239.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones G. W., Freter R. Adhesive properties of Vibrio cholerae: nature of the interaction with isolated rabbit brush border membranes and human erythrocytes. Infect Immun. 1976 Jul;14(1):240–245. doi: 10.1128/iai.14.1.240-245.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabir S., Ali S. Characterization of surface properties of Vibrio cholerae. Infect Immun. 1983 Mar;39(3):1048–1058. doi: 10.1128/iai.39.3.1048-1058.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine M. M., Kaper J. B., Black R. E., Clements M. L. New knowledge on pathogenesis of bacterial enteric infections as applied to vaccine development. Microbiol Rev. 1983 Dec;47(4):510–550. doi: 10.1128/mr.47.4.510-550.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden J. M., Nematollahi W. P., Hill W. E., McCardell B. A., Twedt R. M. Virulence of three clinical isolates of Vibrio cholerae non-O-1 serogroup in experimental enteric infections in rabbits. Infect Immun. 1981 Aug;33(2):616–619. doi: 10.1128/iai.33.2.616-619.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson E. T., Clements J. D., Finkelstein R. A. Vibrio cholerae adherence and colonization in experimental cholera: electron microscopic studies. Infect Immun. 1976 Aug;14(2):527–547. doi: 10.1128/iai.14.2.527-547.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottow J. C. Ecology, physiology, and genetics of fimbriae and pili. Annu Rev Microbiol. 1975;29:79–108. doi: 10.1146/annurev.mi.29.100175.000455. [DOI] [PubMed] [Google Scholar]
- Pierce N. F., Kaper J. B., Mekalanos J. J., Cray W. C., Jr Role of cholera toxin in enteric colonization by Vibrio cholerae O1 in rabbits. Infect Immun. 1985 Dec;50(3):813–816. doi: 10.1128/iai.50.3.813-816.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potten C. S., Allen T. D. Ultrastructure of cell loss in intestinal mucosa. J Ultrastruct Res. 1977 Aug;60(2):272–277. doi: 10.1016/s0022-5320(77)80071-3. [DOI] [PubMed] [Google Scholar]
- Rozee K. R., Cooper D., Lam K., Costerton J. W. Microbial flora of the mouse ileum mucous layer and epithelial surface. Appl Environ Microbiol. 1982 Jun;43(6):1451–1463. doi: 10.1128/aem.43.6.1451-1463.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrank G. D., Verwey W. F. Distribution of cholera organisms in experimental Vibrio cholerae infections: proposed mechanisms of pathogenesis and antibacterial immunity. Infect Immun. 1976 Jan;13(1):195–203. doi: 10.1128/iai.13.1.195-203.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spira W. M., Sack R. B., Froehlich J. L. Simple adult rabbit model for Vibrio cholerae and enterotoxigenic Escherichia coli diarrhea. Infect Immun. 1981 May;32(2):739–747. doi: 10.1128/iai.32.2.739-747.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava R., Sinha V. B., Srivastava B. S. Events in the pathogenesis of experimental cholera: role of bacterial adherence and multiplication. J Med Microbiol. 1980 Feb;13(1):1–9. doi: 10.1099/00222615-13-1-1. [DOI] [PubMed] [Google Scholar]
- Tweedy J. M., Park R. W., Hodgkiss W. Evidence for the presence of fimbriae (pili) on vibrio species. J Gen Microbiol. 1968 Apr;51(2):235–244. doi: 10.1099/00221287-51-2-235. [DOI] [PubMed] [Google Scholar]
- Yancey R. J., Willis D. L., Berry L. J. Role of motility in experimental cholera in adult rabbits. Infect Immun. 1978 Nov;22(2):387–392. doi: 10.1128/iai.22.2.387-392.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]