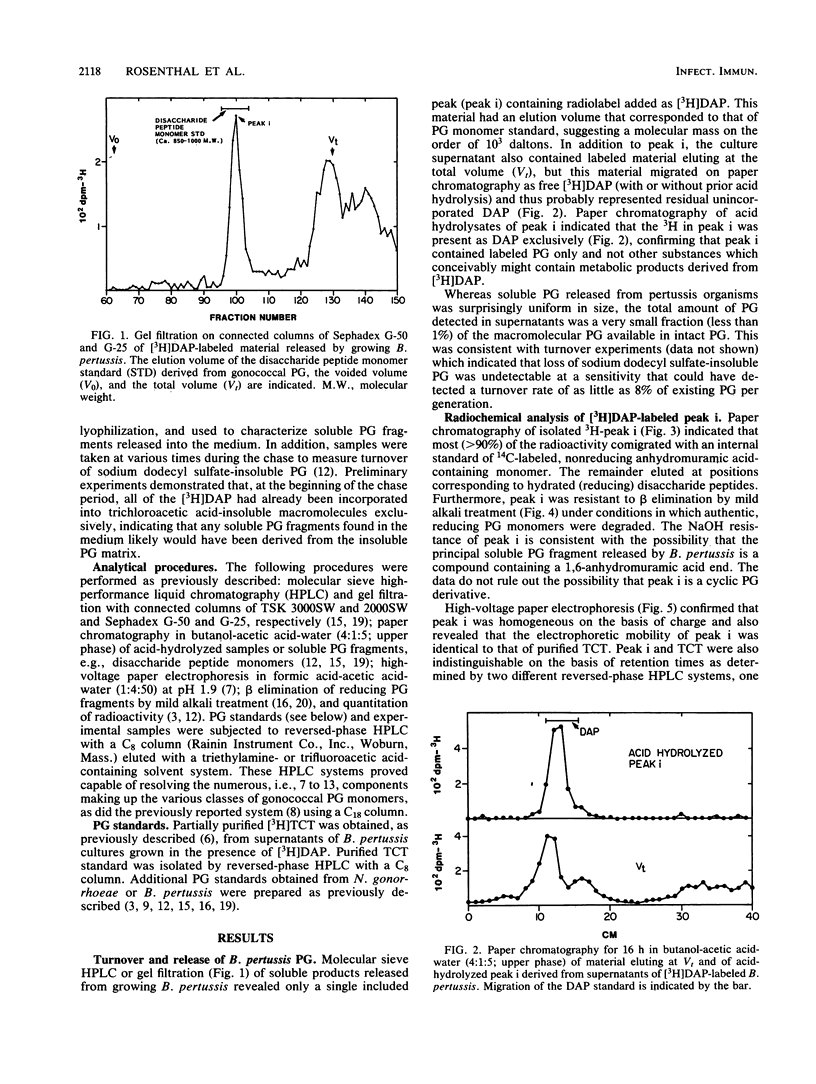
Abstract
Bordetella pertussis is known to release a factor which promotes the loss of ciliated respiratory epithelium and copurifies with a soluble peptidoglycan (PG) fragment termed tracheal cytotoxin (TCT). The objective of this study was to determine whether pertussis organisms turn over and release PG derivatives in addition to TCT. B. pertussis Tohama (phase III) was grown in liquid Stainer-Scholte medium containing [3H]diaminopimelic acid (DAP) to label PG specifically, washed to remove free label, and suspended in fresh medium without [3H]DAP. Molecular sieve chromatography of supernatants obtained from such cultures revealed a single included peak of 3H, the elution volume of which corresponded roughly to a disaccharide peptide monomer standard (ca. 10(3) daltons). This material (i) contained [3H]DAP in acid-hydrolyzable linkage, (ii) comigrated with 1,6-anhydro-N-acetylmuramic acid-containing disaccharide peptides on paper chromatography, (iii) was resistant to degradation by mild alkali, and (iv) was indistinguishable from authentic TCT by high-voltage paper electrophoresis and two reversed-phase high-performance liquid chromatography systems. Together, the data suggest that B. pertussis releases a markedly homogeneous set of PG fragments, consisting principally of TCT, and that TCT is possibly a nonreducing, anhydromuramic acid-containing fragment or a cyclic PG derivative.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dougherty T. J. Analysis of Neisseria gonorrhoeae peptidoglycan by reverse-phase, high-pressure liquid chromatography. J Bacteriol. 1985 Jul;163(1):69–74. doi: 10.1128/jb.163.1.69-74.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming T. J., Wallsmith D. E., Rosenthal R. S. Arthropathic properties of gonococcal peptidoglycan fragments: implications for the pathogenesis of disseminated gonococcal disease. Infect Immun. 1986 May;52(2):600–608. doi: 10.1128/iai.52.2.600-608.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folkening W. J., Nogami W., Martin S. A., Rosenthal R. S. Structure of Bordetella pertussis peptidoglycan. J Bacteriol. 1987 Sep;169(9):4223–4227. doi: 10.1128/jb.169.9.4223-4227.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman W. E., Herwaldt L. A. Bordetella pertussis tracheal cytotoxin. Dev Biol Stand. 1985;61:103–111. [PubMed] [Google Scholar]
- Goldman W. E., Klapper D. G., Baseman J. B. Detection, isolation, and analysis of a released Bordetella pertussis product toxic to cultured tracheal cells. Infect Immun. 1982 May;36(2):782–794. doi: 10.1128/iai.36.2.782-794.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin S. A., Rosenthal R. S., Biemann K. Fast atom bombardment mass spectrometry and tandem mass spectrometry of biologically active peptidoglycan monomers from Neisseria gonorrhoeae. J Biol Chem. 1987 Jun 5;262(16):7514–7522. [PubMed] [Google Scholar]
- Melly M. A., McGee Z. A., Rosenthal R. S. Ability of monomeric peptidoglycan fragments from Neisseria gonorrhoeae to damage human fallopian-tube mucosa. J Infect Dis. 1984 Mar;149(3):378–386. doi: 10.1093/infdis/149.3.378. [DOI] [PubMed] [Google Scholar]
- Petersen B. H., Rosenthal R. S. Complement consumption gonococcal peptidoglycan. Infect Immun. 1982 Feb;35(2):442–448. doi: 10.1128/iai.35.2.442-448.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisabarro A. G., de Pedro M. A., Vázquez D. Structural modifications in the peptidoglycan of Escherichia coli associated with changes in the state of growth of the culture. J Bacteriol. 1985 Jan;161(1):238–242. doi: 10.1128/jb.161.1.238-242.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal R. S., Folkening W. J., Miller D. R., Swim S. C. Resistance of O-acetylated gonococcal peptidoglycan to human peptidoglycan-degrading enzymes. Infect Immun. 1983 Jun;40(3):903–911. doi: 10.1128/iai.40.3.903-911.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal R. S., Gfell M. A., Folkening W. J. Influence of protein synthesis inhibitors on regulation of extent of O-acetylation of gonococcal peptidoglycan. Infect Immun. 1985 Jul;49(1):7–13. doi: 10.1128/iai.49.1.7-13.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal R. S. Release of soluble peptidoglycan from growing gonococci: hexaminidase and amidase activities. Infect Immun. 1979 Jun;24(3):869–878. doi: 10.1128/iai.24.3.869-878.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal R. S., Wright R. M., Sinha R. K. Extent of peptide cross-linking in the peptidoglycan of Neisseria gonorrhoeae. Infect Immun. 1980 Jun;28(3):867–875. doi: 10.1128/iai.28.3.867-875.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R. K., Rosenthal R. S. Effect of penicillin G on release of peptidoglycan fragments by Neisseria gonorrhoeae: characterization of extracellular products. Antimicrob Agents Chemother. 1981 Jul;20(1):98–103. doi: 10.1128/aac.20.1.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R. K., Rosenthal R. S. Release of soluble peptidoglycan from growing conococci: demonstration of anhydro-muramyl-containing fragments. Infect Immun. 1980 Sep;29(3):914–925. doi: 10.1128/iai.29.3.914-925.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stainer D. W., Scholte M. J. A simple chemically defined medium for the production of phase I Bordetella pertussis. J Gen Microbiol. 1970 Oct;63(2):211–220. doi: 10.1099/00221287-63-2-211. [DOI] [PubMed] [Google Scholar]
- Swim S. C., Gfell M. A., Wilde C. E., 3rd, Rosenthal R. S. Strain distribution in extents of lysozyme resistance and O-acetylation of gonococcal peptidoglycan determined by high-performance liquid chromatography. Infect Immun. 1983 Nov;42(2):446–452. doi: 10.1128/iai.42.2.446-452.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]



