Abstract
The link between a new variant form of Creutzfeldt-Jakob disease (vCJD) and the consumption of prion contaminated cattle meat as well as recent findings showing that vCJD can be transmitted by blood transfusion have raised public health concerns. Currently, a reliable test to identify prions in blood samples is not available. The purpose of this study was to evaluate the possibility to remove scrapie prion protein (PrPSc) and infectivity from red blood cell (RBC) suspensions by a simple washing procedure using a cell separation and washing device. The extent of prion removal was assessed by Western blot, PMCA and infectivity bioassays. Our results revealed a substantial removal of infectious prions (≥ 3 logs of infectivity) by all techniques used. This data suggest that a significant amount of infectivity present in RBC preparations can be removed by a simple washing procedure. This technology may lead to increased safety of blood products and reduce the risk of further propagation of prion diseases.
Transmissible spongiform encephalopaties (TSEs), also known as prion diseases, are a group of fatal neurodegenerative disorders affecting several mammalian species [1]. The main features of these diseases include the spongiform degeneration of the brain and the accumulation of an abnormal isoform of the prion protein, termed PrPSc [2]. PrPSc appear to be the main (or sole) component of the infectious agent [2; 3]. Although prion diseases are rare in humans, the established link between the new variant form of Creutzfeldt Jakob disease (vCJD) and the consumption of cattle meat contaminated by bovine spongiform encephalopathy (BSE) have raised concern about a possible outbreak of a large epidemic in the human population. Over the past few years, BSE has become a significant health and economic problem affecting many countries, principally in Europe. In addition to BSE epidemics, the incidence of other prionopaties in economically relevant species such as sheep, goats and cervids presents an alarming scenario to the animal farming industry. Moreover, the possible spread of BSE in other species (i.e. sheep, goats, porcine) has raised a concern about the emergence of multiple new sources of prions that may possibly affect human population [4].
The identification of PrPSc and infectivity in blood opens a new source of public health concern [5-9]. This alarming information urge for the development of methodologies able to remove prions from organs and fluids designated for transplant or transfusion. Exacerbating this state of affairs is that the lack of a reliable test to identify individuals incubating the disease during the long and silent period from the infection to the appearance of clinical symptoms [10]. Recent studies had demonstrated that vCJD can be iatrogenically transmitted from human to human by blood transfusion [11; 12]. Transmission of prion disease through blood transfusion has also been described in sheep and experimental rodents [8; 9]. Since blood used for transfusion was taken from animals and humans months or years before the onset of clinical disease, and because millions of people have been exposed to BSE infected material, there is a large concern that a portion of donated blood units might be contaminated with prions. The lack of routine methodologies approved to detect infectious prions in blood creates a need for prion elimination devices, which must be suitable for high throughput and effectiveness without affecting the quality of blood components.
The dynamic and distribution of PrPSc and infectivity in different blood fractions is unknown, but recent studies suggest that at pre-symptomatic stages of the disease PrPSc may be mostly attached to white blood cells (WBCs) originating from early peripheral prion replication in lymphoid tissues [6]. Later in the disease, a substantial amount of PrPSc is present in plasma and red blood cells (RBCs) where it seems to be mostly not cell associated and coming from brain leakage [6; 13] (Marcelo Barria and CS, unpublished data). These findings suggest that there are two pools of infectious PrPSc in blood; one that is cell-associated that can be mostly eliminated by efficient leukoreduction and a second pool freely circulating in plasma and contaminating RBC preparations. Indeed, it was reported that leucofiltration removed 42% of the total TSE infectivity in endogenously infected blood [14]. Leucoreduction is efficient for the removal of WBC-associated TSE infectivity from blood; however, it is not, by itself, sufficient to remove all blood-borne TSE infectivity [14].
Every year, about 75 million units of blood are collected worldwide [15]. RBC transfusion is one of the treatments most extensively used in clinical practice. To reduce the risk for infection, RBCs can be filtered, washed, frozen, or irradiated for specific indications [15]. It has been suggested that PrPSc in RBCs can be depleted with specific adsorptive ligand resins. Indeed, a recent study reported that PrP-binding resins were able to reduce infectivity titer by 3 to more than 4 ID50 units [16]. We hypothesize that PrPSc in RBCs preparations can be removed by a simple washing of this fraction, since the infectious agent appears not to be tightly bound to RBCs. Various procedures to wash RBCs preparations are currently being used to purify the cells before use for blood transfusion [15]. In order to assess PrPSc removal from the final pRBCs fraction we used a hamster experimental model of prion diseases. We assessed the removal of prions by infectivity bioassays as well as by studying the levels of PrPSc using Western blot and protein misfolding cyclic amplification (PMCA) technology.
Material and Methods
Preparation of PrPSc samples
Symptomatic 263K infected hamsters were sacrificed by CO2 inhalation and brains were collected. 10% brain homogenates (w/v) were prepared in phosphate buffer saline (PBS) plus Complete™ cocktail of protease inhibitors (PI) (Boehringer Mannheim, Mannheim, Germany). The samples were clarified by a brief, low-speed centrifugation (1500 rpm for 30s). Brain homogenates were mixed with 1 volume of 20% sarkosyl and the mixture was homogenized and sonicated using a Bandelin Sonoplus sonicator. This sample was centrifuged at 100,000 × g for 1 hr at 4°C. Supernatant was discarded and 2 volumes of PBS plus PI were added to pellets. Ultracentrifugation step was repeated. Supernatants were discarded and pellets were resuspended in 1 volume of PBS by pipetting and sonication. The sample was stored at -80 °C until use.
Treatment of spiked blood samples with the prototype cell separation and washing device
The prototype cell separation and washing device was capable of processing two partial units of whole blood at one time. Three units of whole blood from Interstate Blood Bank (in Tennessee) were used in this study. Each unit was leukoreduced using a Pall™ Leukotrap Filter. 235 mL of each unit was transferred into a centrifugation bag. 5 mL of the remaining whole blood from each unit was transferred into a 15 mL conical tube to serve as a negative control. Each of the three centrifugation bags was spiked with 5 × 106 LD50/mL of PrPSc. 5 mL of the mixture were removed from each bag and transferred to a 15 mL conical tube to serve as a positive control. Two units of prion spiked whole blood were loaded into the centrifuge (Figure 1). Plasma was expressed by centrifugation of the whole blood for approximately 15 minutes at 3000 RPM bringing the pRBC hematocrit to ≥ 95%. Each pRBC unit was washed with 120 mL of normal saline solution. Saline was expressed by centrifugation of the RBCs for approximately 15 minutes at 3000 RPM bringing the pRBC hematocrit to ≥ 95%. 5 mL pRBCs final fraction were removed from each bag and transferred to a 15 mL conical tube to serve as a post-treatment sample (Figure 1). The entire process can be performed in approximately 30 minutes.
Figure 1. Schematic representation of the RBC washing procedure.
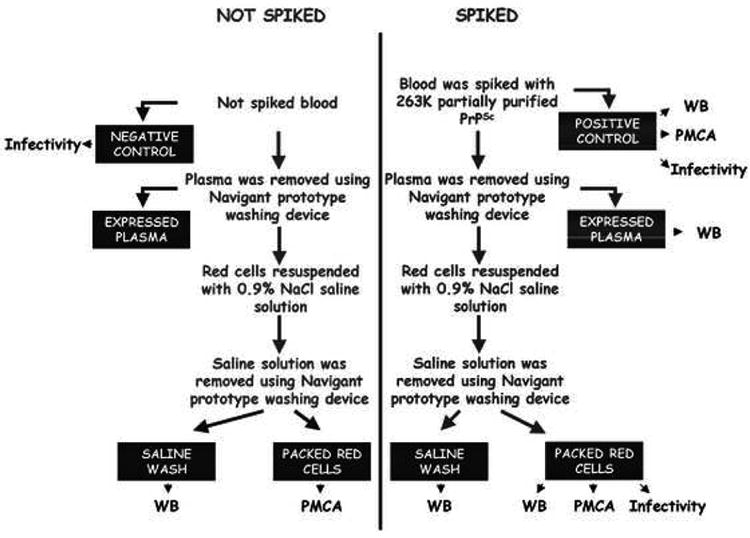
Different units of human whole blood were spiked with partially purified 263K PrPSc and samples were treated using the prototype device to separate and wash RBCs. From this purification protocol Complete Spiked Blood (Positive Control), Expressed Plasma, Saline Wash and pRBCs suspensions were obtained. PrPSc levels in each fraction were evaluated by Western blot, PMCA and/or infectivity studies. Determinations were done by triplicate in independent experiments.
Analysis of the quality of packed red blood cells
To assess RBC quality after washing, whole blood was washed as previously described without a PrPSc spike. After, the saline was expressed and discarded, and a full volume bag of AS-3 (GNI AS-3 Storage Solution, Larne, UK), approximately 107 mL, was added for storage at 4°C. Stored RBCs were monitored on days 35 and 42 of storage to assess blood gases (pO2, pCO2), CBC (Becton-Dickinson Coulter AcT Diff, Fullerton, CA), and biochemistry; pH with Accemet research AR20 meter, lactate, glucose, Na+, K+, methemoglobin, plasma free hemoglobin and hemolysis (calculated: % Hemolysis = [(hemoglobinfree mg/dL) × (1 − Hct)/(hemoglobinTotal g/dL)] × 100).
PrPSc concentration by sarkosyl precipitation
For detection of PrPSc in blood fractions, it was first necessary to concentrate diluted samples, which cannot be loaded directly into a gel or that interfere with electrophoresis and blotting. The concentration procedure consists in adding 1 volume of 20% sarkosyl, incubate the mixture for 10 min at room temperature and centrifuge it at 100,000 × g for 1h at 4°C. Supernatants are discarded and pellets are resuspended into 2 volumes of 10% sarkosyl. Centrifugation process is repeated in order to assure the complete removal of blood components. Supernatants are discarded and pellets are resuspended in PBS by sonication. Following this protocol, PrPSc is recovered in the pellet fraction with a yield higher than 90%.
PrPSc detection
Samples are first digested with 12.5 μg/ml of proteinase K (PK) at 37°C for 1h and the reaction was stopped by adding NuPAGE LDS Sample buffer. Proteins were then fractionated by electrophoresis using 4-12 % sodium dodecyl sulphate-polyacrylamide gels (SDS-PAGE) under reducing conditions, electroblotted into Hybond-ECL nitrocellulose membrane and probed with the 3F4 antibody (Covance, Emeryville, CA) (dilution 1:5,000). The immunoreactive bands were visualized by ECL Plus Western blotting detection system and quantified by densitometry using a UVP Bioimaging system EC3 apparatus (UVP, Upland, CA).
PMCA procedure
The detailed PMCA protocol, including troubleshooting, has been published elsewhere [17]. Briefly, samples were mixed with 10% normal brain homogenate prepared in conversion buffer and loaded onto 0.2-ml PCR tubes. Tubes were positioned on an adaptor placed on the plate holder of a microsonicator (Misonix Model 3000, Farmingdale, NY) and programmed to perform cycles of 30 min incubation at 37°C followed by a 20 sec pulse of sonication set at 60% potency.
In Vivo Infectivity Studies
Four to six week old female Syrian Golden hamsters were anesthesized and stereotaxically injected in the right hippocampus with 4 μL of the sample. The onset of clinical disease was measured by scoring the animals three times a week as described [3]. Brains were extracted and the right cerebral hemisphere was frozen and stored at -70°C for biochemical examination of PrPSc using Western blot analysis and the left hemisphere was fixed in 10% formaldehyde solution, sectioned and embedded in paraffin. Serial sections (6μm thick) from each block were stained with hematoxylin-eosin, using standard protocols or incubated with an antibody recognizing the glial fibrillary acidic protein (GFAP) (DakoCytomation, Carpinteria, CA). Immunoreactions were developed using the peroxidase-antiperoxidase method, following manufacturer's specifications.
Results
The experimental procedure consisted of spiking two groups of entire blood units with partially purified hamster PrPSc equivalent to a 5 × 106 LD50. One group was washed by the prototype washing device and the other was left untreated (Figure 1). The final RBC pellets were resuspended in PBS and aliquots used for Western blot, PMCA and to infect wild type hamsters. Washed, packed human RBCs produced by this procedure were able to be stored in standard additive solutions (AS-3) for at least 42 days while still meeting all in vitro blood bank standards for acceptable RBC quality (Table 1).
Table 1. Red blood cells quality after storage in AS-3.
Biochemical parameters of packed red blood cells 35 and 42 days after treatment with the washing procedure. Values represent average ± standard deviation.
| Storage | Lactate (mmol/L) | Glucose (mmol/L) | pH | pO2 (mm Hg) | pCO2 (mm Hg) | Plasma Hb (mg/dL) | % Hemolysis | |
|---|---|---|---|---|---|---|---|---|
| Day 35 (Mean ± SD) | 14.3 ± 1.9 | 18.0 ± 5.1 | 6.3 ± 0.1 | 54.4 ± 12.8 | 51.9 ± 8.5 | 187.9 ± 96.1 | 0.4 ± 0.2 | |
| Day 42 (Mean ± SD) | 16.6 ± 2.4 | 16.2 ± 3.8 | 6.2 ± 0.1 | 58.1 ± 13.2 | 53.2 ± 8.3 | 244.1 ± 157.0 | 0.6 ± 0.3 | |
| Storage | Na+ (mmol/L) | K+ (mmol/L) | WBC (106/mL) | Hb (g/dL) | % Hct | MCV (fL) | MCH (pg) | MCHC (g/dL) |
| Day 35 (Mean ± SD) | 106.2 ± 11.9 | 41.5 ± 3.4 | 0.1 ± 0.0 | 18.5 ± 09 | 57.1 ± 2.4 | 88.1 ± 7.8 | 28.7 ± 3.1 | 32.4 ± 1.0 |
| Day 42 (Mean ± SD) | 101.2 ± 4.1 | 45.9 ± 3.6 | 0.1 ± 0.0 | 18.4 ± 0.9 | 57.1 ± 2.0 | 88.1 ± 7.1 | 28.3 ± 3.0 | 32.3 ± 1.1 |
We first evaluated the amount of PrPSc present in different fractions by Western blot analysis. Blood components interfere with electrophoresis and blotting. To overcome this problem we implemented a procedure to clean and concentrate PrPSc. To make sure that this procedure does not result in any loss of PrPSc, we performed a control experiment in which the different blood fractions obtained during the washing procedure were first prepared and then spiked with the same quantity of PrPSc. Each of these fractions was subjected to the sarkosyl precipitation procedure and the pellet was analyzed by Western blot. As shown in figure 2A, all samples have similar quantity of PrPSc, which was equivalent to the amount added, indicating that the sarkosyl precipitation method recovered >95% of PrPSc and no differences were observed in the distinct blood fractions in which the protocol was tested. Using this procedure we were able to assess the presence and quantity of PrPSc in each of the steps of the washing procedure including the whole spiked blood, expressed plasma, saline wash and pRBCs. The results indicate that the majority of PrPSc was eliminated in the plasma, whereas only a faint signal was found on the saline wash and no signal was observed in the RBC fraction (figure 2B).
Figure 2. Levels of PrPSc in different fractions of spiked blood obtained by the fractionation and washing procedure.
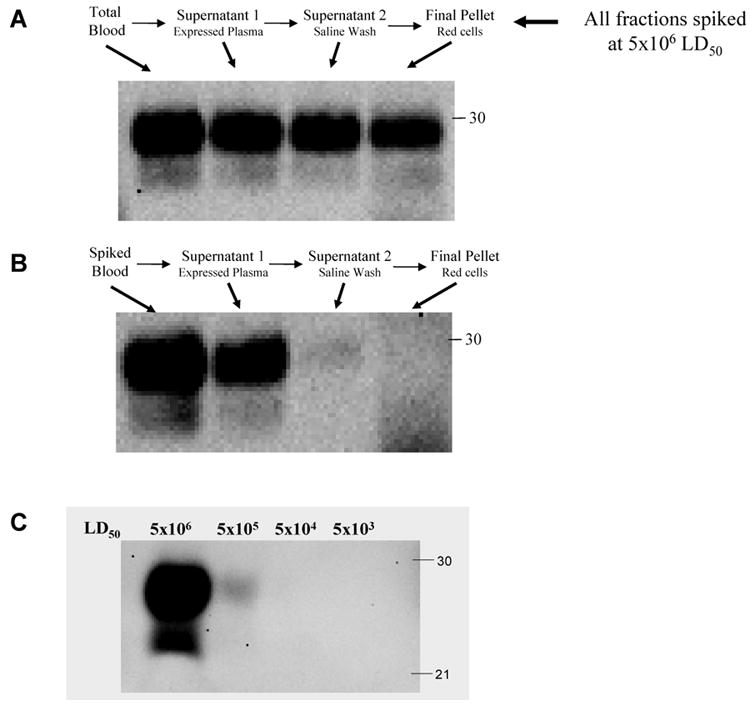
A: Control experiment to evaluate the yield of PrPSc recovery by the sarkosyl precipitation procedure. Blood samples were subjected to the protocol to separate and wash RBCs and all the fractions in the procedure were collected and spiked with PrPSc amount equivalent to 5×106 LD50. Thereafter samples were precipitated with sarkosyl and equal amounts of the pellet were analyzed by Western blot. B: Representative Western blot showing PrPSc levels in different fractions after treatment with the prototype cell separation and washing procedure. C: Detection of PrPSc by Western blot at different dilutions. The first lane represents the quantity of PrPSc added to the blood samples, which contains the equivalent to 5×106 LD50. This concentration was serially diluted by 10-folds to assess the limit of detection of Western blot assay.
Densitometric evaluation of 3 replicates, indicate that 87.8% of PrPSc was present in plasma, whereas only 5.8% was detected in the saline washing (table 2). The later amount is probably less reliable than the first, because of the weak signal observed. Assuming that both centrifugation and washing steps removed a similar amount of PrPSc (around 88%), our estimation from the Western blot studies would be that the RBC pellet contain around 1.5% of PrPSc. In other words the conclusion is that the prototype cell separation and washing device removes around 98.5% of PrPSc from the RBC preparation. This estimation is further supported by an experiment in which different dilutions of PrPSc were directly loaded into the gel to assess the limit of detection of the Western blot with the concentrations of PrPSc used in this study. As shown in figure 2C, a 100-fold dilution (or 1%) of a sample containing 5×106 LD50 of PrPSc is no longer detectable by Western blot.
Table 2. Densitometric analysis of PrPSc removal by Western blot analysis.
Percentage of remaining PrPSc in each fraction, obtained by densitometric analysis of three independent Western blots, as the one shown in figure 2. Values represent the mean ± standard deviation.
| Fraction | % PrPSc remaining |
|---|---|
| Spiked blood | 100% |
| Expressed plasma | 87.8% ± 7.6 |
| Saline wash | 5.8% ± 3.5 |
| pRBC | 0% ± 0 |
In order to experimentally estimate the quantity of PrPSc present in the RBC fraction, we subjected the samples to serial PMCA [3; 5; 18]. A first round of 144 cycles did not show signal in any of the 3 pRBCs fractions subjected to the prototype cell separation and washing device (figure 3A). However, when a second round of PMCA was performed (figure 3B), we observed signal in all of the samples, indicating that PrPSc was indeed present in RBCs, albeit in very low quantity. As we previously reported [18], one round of 144 PMCA cycles enable to detect hamster PrPSc up to a brain dilution of 1 ×10-6, which for this material corresponds to around 5×103 LD50. Therefore, we conclude that pRBCs fraction contains less than 5×103 LD50 or <0.1% of PrPSc spiked into the blood. In other words, the PMCA results indicate that the blood processing by the prototype cell separation and washing device enable to remove >99.9% (∼ 3 logs) of infectious prions.
Figure 3. Detection of PrPSc by PMCA in pRBCs samples obtained by the fractionation and washing procedure.
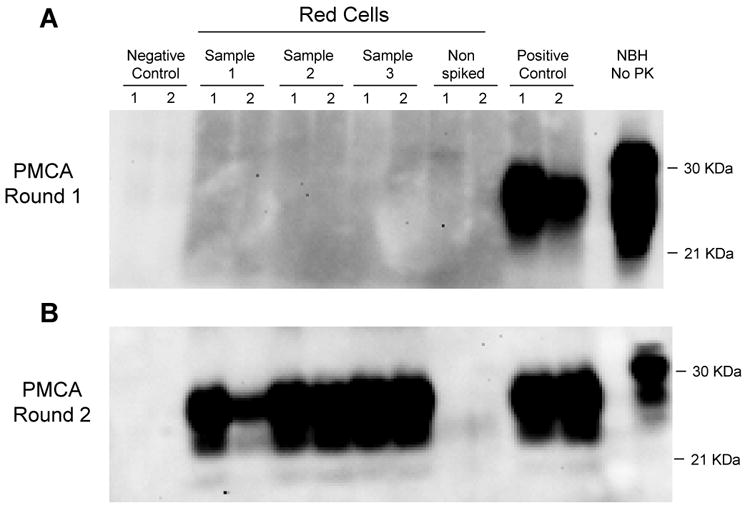
RBC suspensions obtained from three independent experiments were subjected to two rounds of serial PMCA. Each PMCA reaction was performed in duplicate (numbers 1 and 2). For the negative control, we used normal Brain Homogenate (NBH) where no PrPSc is present and pRBCs obtained from non-spiked blood was used. For the positive control, Whole Blood spiked with 5 × 106 LD50 PrPSc was used. HaNBH represents control PrPC without proteinase K (PK) digestion.
Lastly, we assessed the reduction of infectivity from washed pRBC fractions by bioassay in experimental hamsters. Three groups of hamsters were injected intracerebrally with samples from three replicates of washed pRBCs. We also inoculated hamsters with blood spiked with 5 × 106 LD50 (three independent spiking experiments) and as negative controls we injected animals with non-spiked blood from three different donors (figure 1). We monitored the time that took for the animals in each group to exhibit typical clinical signs of scrapie. Brains from these animals were extracted for histological and biochemical analysis to confirm the disease. As expected, none of the negative control hamsters (animals inoculated with non-spiked blood) showed signs of scrapie up to 350 days after inoculation (table 3). The disease onset in animals inoculated with an aliquot of whole blood spiked with 5 × 106 LD50, the positive control was in average 100 days, which is well within the expected for this quantity of prions. The attack rate (percentage of animals showing the disease) was 100%. The animals inoculated with pRBCs prepared by the washing procedure, showed a much longer incubation period (136 days in average) (table 3). Strikingly of the 15 animals inoculated with washed spiked pRBCs only 6 developed clinical disease, representing an attack rate of 40%. These results indicate that the process of pRBCs preparation significantly removed infectious prions from the blood. Histopathological studies of the brain of all clinically sick animals showed the typical damage observed in scrapie animals, including spongiform degeneration (figure 4A) and astroglyosis (figure 4B). It is important to mention that from the 9 animals inoculated with washed pRBCs that did not developed clinical signs of the disease, some (2 of the 9) showed spongiform degeneration and astroglyosis when they were sacrificed at 350 days post-inoculation (figure 4A and B, lower panels). This result suggest that these correspond to sub-clinical animals. The other 7 non-clinical animals did not show pathology at the time when they were sacrificed. The presence of PrPSc in the brain of infected animals was assessed by the detection of the PK resistant fragment of the prion protein (PrP27-30) by Western blot. As shown in figure 4C, PrP27-30 was detected in all hamsters inoculated with 5 × 106 LD50 spiked blood. Misfolded protein was also detected in clinically affected washed pRBC inoculated hamsters. As before 2 of the 9 subclinical animals have PrP27-30 in their brains (Fig. 4C).
Table 3. Incubation time in animals infected with diverse blood preparations.
Each group represents the data of three replicates that were combined to obtain the average ± standard error. The experiment was terminated at 350 days after inoculation. Attack rate represent the percentage of animals developing characteristic prion clinical signs.
| Group | Attack rate (%) | Incubation time - days (Mean ± st. error) |
|---|---|---|
| Non-spiked blood | 0/15 (0%) | - |
| Spiked whole blood | 13/13 (100%) | 99.7 ± 1.84 |
| Spiked RBC washed | 6/15 (40%) | 135.8 ± 6.67 |
Figure 4. Histological and biochemical characteristics of the brain of animals.
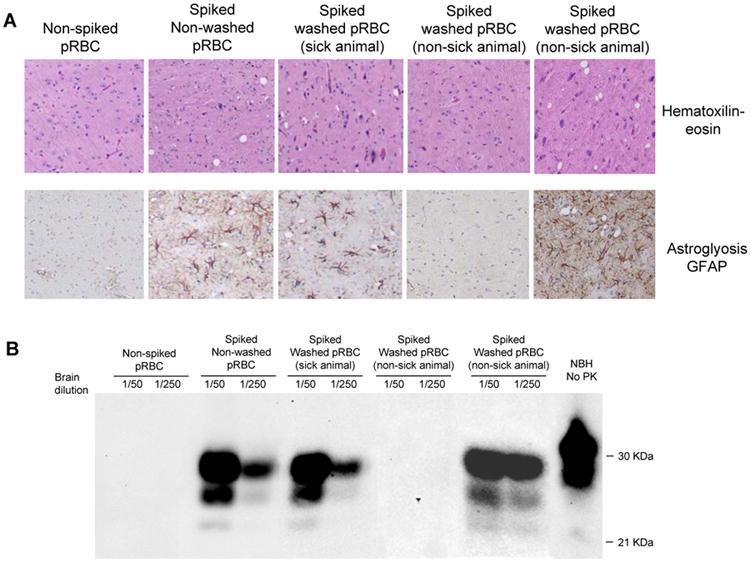
Spongiform degeneration was assessed after staining with hematoxilin-eosin (A), astrogliosis was evaluated by immunohistochemistry with anti-GFAP antibodies (B) and Western blot showing the PrP27-30 (C). The figure includes representative pictures of animals inoculated with: non-spiked pRBCs (negative control), spiked blood not washed (positive control), and spiked washed pRBCs (3 different animals from this group are shown to illustrate sick animals, non-clinically sick animals that developed brain pathology and non-sick animals that did not exhibited brain damage).
To estimate the extent of prion removal we compared the number of days in which hamsters developed clinical signs of scrapie after inoculation with known quantities of infectious material (supplementary figure 1). From this comparison it is possible to appreciate that administration of 5 × 106 LD50 should produce disease around 95 days, validating the results obtained in our experiment with 5 × 106 LD50 spiked blood. Extrapolation from this curve allows estimating that an incubation period of 135 days is produced when the equivalent to 3 × 103 LD50 is inoculated into hamsters. This result indicates that pRBCs prepared according to the cell separation and washing procedure, contains approximately 0.06% of infectivity spiked into the blood. Therefore, the in vivo results indicate that the cell separation and washing procedure removed 99.94% of infectious prions, which represents more than 3 orders of magnitude (logs of infectivity). This estimation only considers the incubation period and not the reduced attack rate, thus it is most likely that the rate of reduction of infectivity is even larger.
Discussion
Infectivity studies have shown that blood carries prions both in the symptomatic and pre-symptomatic stages of the disease in several animal species both in experimentally-infected and naturally-produced disease [5-9]. Recently, four cases of vCJD have been associated with blood transfusion from asymptomatic donors who subsequently died from vCJD [11; 19]. Because the incubation period of human prion diseases may be several decades, it is currently unknown how many people may be in an asymptomatic phase of vCJD infection. In addition, it is possible that some infected patients may never develop clinical symptoms but will remain asymptomatic carriers who can potentially transmit the disease to other individuals. All these considerations emphasize the importance of developing technologies capable to detect prions in blood and to remove the infectious protein from the blood supply.
In the current study we analyzed whether a simple cell separation and washing procedure to prepare RBCs was sufficient to reduce PrPSc and infectivity from pRBCs used for transfusion. Similar washing procedures are currently being used to wash RBCs from other microbial agents and thus it would be very useful if simultaneously they can remove infectious prions. The results obtained on the biochemical estimation of PrPSc removal by Western blot and PMCA indicated that the washing procedure removed >98.5% and >99.9% of the infectious protein spiked into the blood. The in vivo results were even more encouraging, estimating that the procedure eliminated 99.94% of infectious material. Therefore, the overall conclusion of this study corroborated by the three different and independent techniques employed is that simple washing of blood fractions removed around or more than 3 logs of infectious material. This removal is highly significant and may well be enough to protect completely the blood supply, since the estimated quantities of prions in blood are close to the minimum required to transmit the disease. However, it remains to be shown that this procedure will perform similar removal with endogenously infected blood. A better understanding of this phenomenon will help to develop effective blood purification procedures that can efficiently reduce the risk of further spreading the disease through blood transfusion.
Supplementary Material
Acknowledgments
This work was supported by NIH grant NS049173 and a grant from Navigant LLC to CS and by a project from the Department of Defense to Navigant LLC.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Collinge J. Prion diseases of humans and animals: their causes and molecular basis. Annu Rev Neurosci. 2001;24:519–550. doi: 10.1146/annurev.neuro.24.1.519. [DOI] [PubMed] [Google Scholar]
- 2.Prusiner SB. Prions. Proc Natl Acad Sci U S A. 1998;95:13363–13383. doi: 10.1073/pnas.95.23.13363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Castilla J, Saá P, Hetz C, Soto C. In vitro generation of infectious scrapie prions. Cell. 2005;121:195–206. doi: 10.1016/j.cell.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 4.Morales R, Abid K, Soto C. The prion strain phenomenon: molecular basis and unprecedented features. Biochim Biophys Acta. 2007;1772:681–691. doi: 10.1016/j.bbadis.2006.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Castilla J, Saa P, Soto C. Detection of prions in blood. Nat Med. 2005;11:982–985. doi: 10.1038/nm1286. [DOI] [PubMed] [Google Scholar]
- 6.Saa P, Castilla J, Soto C. Presymptomatic detection of prions in blood. Science. 2006;313:92–94. doi: 10.1126/science.1129051. [DOI] [PubMed] [Google Scholar]
- 7.Brown P. Blood infectivity, processing and screening tests in transmissible spongiform encephalopathy. Vox Sang. 2005;89:63–70. doi: 10.1111/j.1423-0410.2005.00683.x. [DOI] [PubMed] [Google Scholar]
- 8.Holada K, Vostal JG, Theisen PW, MacAuley C, Gregori L, Rohwer RG. Scrapie infectivity in hamster blood is not associated with platelets. J Virol. 2002;76:4649–4650. doi: 10.1128/JVI.76.9.4649-4650.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Houston F, Foster JD, Chong A, Hunter N, Bostock CJ. Transmission of BSE by blood transfusion in sheep. Lancet. 2000;356:999–1000. doi: 10.1016/s0140-6736(00)02719-7. [DOI] [PubMed] [Google Scholar]
- 10.Soto C. Diagnosing prion diseases: needs, challenges and hopes. Nat Rev Microbiol. 2004;2:809–819. doi: 10.1038/nrmicro1003. [DOI] [PubMed] [Google Scholar]
- 11.Llewelyn CA, Hewitt PE, Knight RS, Amar K, Cousens S, Mackenzie J, Will RG. Possible transmission of variant Creutzfeldt-Jakob disease by blood transfusion. Lancet. 2004;363:417–421. doi: 10.1016/S0140-6736(04)15486-X. [DOI] [PubMed] [Google Scholar]
- 12.Peden AH, Head MW, Ritchie DL, Bell JE, Ironside JW. Preclinical vCJD after blood transfusion in a PRNP codon 129 heterozygous patient. Lancet. 2004;264:527–529. doi: 10.1016/S0140-6736(04)16811-6. [DOI] [PubMed] [Google Scholar]
- 13.Murayama Y, Yoshioka M, Okada H, Takata M, Yokoyama T, Mohri S. Urinary excretion and blood level of prions in scrapie-infected hamsters. J Gen Virol. 2007;88:2890–2898. doi: 10.1099/vir.0.82786-0. [DOI] [PubMed] [Google Scholar]
- 14.Gregori L, McCombie N, Palmer D, Birch P, Sowemimo-Coker SO, Giulivi A, Rohwer RG. Effectiveness of leucoreduction for removal of infectivity of transmissible spongiform encephalopathies from blood. Lancet. 2004;364:529–531. doi: 10.1016/S0140-6736(04)16812-8. [DOI] [PubMed] [Google Scholar]
- 15.Klein HG, Spahn DR, Carson JL. Red blood cell transfusion in clinical practice. Lancet. 2007;370:415–426. doi: 10.1016/S0140-6736(07)61197-0. [DOI] [PubMed] [Google Scholar]
- 16.Gregori L, Lambert BC, Gurgel PV, Gheorghiu L, Edwardson P, Lathrop JT, MacAuley C, Carbonell RG, Burton SJ, Hammond D, Rohwer RG. Reduction of transmissible spongiform encephalopathy infectivity from human red blood cells with prion protein affinity ligands. Transfusion. 2006;46:1152–1161. doi: 10.1111/j.1537-2995.2006.00865.x. [DOI] [PubMed] [Google Scholar]
- 17.Castilla J, Saa P, Morales R, Abid K, Maundrell K, Soto C. Protein misfolding cyclic amplification for diagnosis and prion propagation studies. Methods Enzymol. 2006;412:3–21. doi: 10.1016/S0076-6879(06)12001-7. [DOI] [PubMed] [Google Scholar]
- 18.Saa P, Castilla J, Soto C. Ultra-efficient replication of infectious prions by automated protein misfolding cyclic amplification. J Biol Chem. 2006;281:35245–35252. doi: 10.1074/jbc.M603964200. [DOI] [PubMed] [Google Scholar]
- 19.Ironside JW. Variant Creutzfeldt-Jakob disease: risk of transmission by blood transfusion and blood therapies. Haemophilia. 2006;12 1:8–15. doi: 10.1111/j.1365-2516.2006.01195.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


