Abstract
Determining the brain adaptations that underlie complex tool-use skills is an important component in understanding the physiological bases of human material culture. It is argued here that the ways in which humans skilfully use tools and other manipulable artefacts is possible owing to adaptations that integrate sensory–motor and cognitive processes. Data from brain-injured patients and functional neuroimaging studies suggest that the left cerebral hemisphere, particularly the left parietal cortex, of modern humans is specialized for this purpose. This brain area integrates dynamically representations that are computed in a distributed network of regions, several of which are also left-lateralized. Depending on the nature of the task, these may include conceptual knowledge about objects and their functions, the actor's goals and intentions, and interpretations of task demands. The result is the formation of a praxis representation that is appropriate for the prevailing task context. Recent evidence is presented that this network is organized similarly in the right- and left-handed individuals, and participates in the representation of both familiar tool-use skills and communicative gestures. This shared brain mechanism may reflect common origins of the human specializations for complex tool use and language.
Keywords: praxis, tool use, gesture, apraxia, neuroimaging, hand dominance
1. Introduction
The Palaeolithic record indicates that our ancestors began modifying rocks for pounding at least 2.5 Myr ago (Ambrose 2001). Subsequently, rocks were attached to sticks to create compound tools that were capable of generating higher impact forces. These hammers, as well as many other manipulable artefacts, have been refined into their various modern forms through a process of cumulative cultural evolution (Basalla 1988; Tomasello 1999). Though difficult to trace in the fossil record, advancing technology was accompanied by the evolution of specific manual skills involved in the use of these implements. Given that all primates possess the ability to dexterously reach, grasp and manipulate objects with their hands, why is it that only humans have developed such an extensive and universal material culture?
One possibility is that modifications to the neural circuits of the primate brain involved in the sensory–motor control of manual prehension underlie these human specializations. Training macaques to use simple tools that extend their normal manual abilities (e.g. reaching with a stick (Iriki et al. 1996) or grasping with a set of tongs (Umilta` et al. 2008)) induces experience-dependent modifications in sensory–motor representations. Likewise, while the gross functional organization of areas involved in the manual prehension may be conserved (Rizzolatti & Craighero 2004; Grefkes & Fink 2005), there are regional differences between monkey and human brains (Preuss et al. 1996; Orban et al. 2004). Yet, can human tool-use skills really be understood strictly in terms of sensory–motor adaptations? Consider the use of the modern claw hammer. The most stable way to grasp this, or any other object, is at its centre of mass (Blake et al. 1992), and this location can be rapidly and often very accurately estimated on the basis of an object's perceived shape (Goodale et al. 1994; Johnson-Frey 2005b). If the actor's goal is pounding a nail, however, grasping the hammer by its head may not be ideal. Instead, an actor possessing knowledge about the functionality of the hammer would probably select a less stable grip, but one that is better suited for pounding. By grasping the handle at different distances from the centre of mass, the skilled carpenter can further optimize the trade-off between force and precision in accordance with the prevailing task demands. If bent by an errant blow, it might even become necessary to reverse the grip in order to remove the compromised nail with the hammer's claw. Or, the actor may decide to pass the hammer to a colleague in which case grasping the hammer by its head might actually be preferred.
As this example makes clear, the ways in which we skilfully interact with tools and other artefacts are highly context dependent, and aspects of this context are not specified by the physical properties of the task or actor's body. Though certainly necessary, sensory–motor processes alone are therefore insufficient to account for this flexibility. These skills are also influenced by conceptual knowledge about objects and their functions, the actor's intended goals and interpretations of prevailing task demands. These various sources of sensory–motor and cognitive information are somehow integrated to form internal representations that constrain and guide manual praxis, engendering a flexibility that is absent even in the tool-using behaviours of our nearest living relatives (Povinelli 2000; Johnson-Frey 2003). Determining the neural mechanisms that make this possible is elemental to understanding the origins of human material culture.
2. The left cerebral hemisphere and praxis
It has been known for more than a century that damage, especially to the left cerebral hemisphere of humans, can lead to apraxia—an impairment in the representation of acquired skills that cannot be attributed to difficulties in linguistic, sensory or lower level motor functions such as weakness or paralysis (Geschwind & Kaplan 1962; Heilman & Rothi 1997; Leiguarda & Marsden 2000). The difficulties experienced by these patients are not primarily with the sensory–motor control, as they often perform well when allowed to actually use objects. Instead, their impairments come to the fore when faced with tasks such as pantomime or imitation that require accessing stored action representations under circumstances that provide minimal contextual support. The fact that their problems occur when using either upper limb indicates that the affected internal representations are at a level of abstraction that is independent of the limb involved in producing the movements.
The ability to pantomime familiar tool or object use skills in response to a verbal command has long been considered a critical test for diagnosing apraxia because it isolates the retrieval of stored praxis representations in response to minimally informative stimuli. Several investigations have used functional neuroimaging to examine the neural mechanisms underlying the performance of this task (for reviews see Johnson-Frey 2004 and Lewis 2006). Consistent with lesion loci in apraxia (Haaland et al. 2000), retrieval and planning of these actions is consistently associated with increased activity in a distributed network of areas in the left cerebral hemisphere that includes a combination of areas within parietal, premotor, prefrontal and posterior temporal cortices (Moll et al. 2000; Choi et al. 2001; Ohgami et al. 2004; Rumiati et al. 2004; Johnson-Frey et al. 2005c; Fridman et al. 2006). This left cerebral asymmetry is observed regardless of whether the forthcoming actions involve the use of the left or right hands (Moll et al. 2000; Choi et al. 2001; Ohgami et al. 2004; Johnson-Frey et al. 2005c). By contrast, sensory–motor functions involved in actually pantomiming these skills are represented in a largely symmetrical system. Both hemispheres show increased activity in parietal and premotor areas when executing these actions, with pronounced engagement of the primary sensory–motor cortex contralateral to the involved limb (Johnson-Frey et al. 2005c).
3. The praxis representation network
Together, the patient and neuroimaging data converge on the hypothesis that functions involved in representing manual praxis skills are asymmetrically organized in the modern human brain. Retrieval and planning of these actions reliably activates a distributed network in the left cerebral hemisphere and damage in these areas frequently results in apraxia. Though several proposals have been offered on the basis of neuropsychological data (Rothi et al. 1991; Cubelli et al. 2000; Buxbaum 2001), a complete understanding of the processes taking place within this architecture is lacking.
An alternative approach is to consider what modifications to the sensory–motor control of prehension in primates might be necessary to accommodate human tool-use skills. A useful starting point is a computational model developed to explain how objects' visual affordances constrain the selection of grasping actions (Fagg & Arbib 1998). The foundation of the Fagg, Arbib, Rizzolatti and Sakata (FARS) model is anatomical and physiological data indicate that grasping actions are represented in a network consisting of interconnected areas of the macaque inferior parietal lobule and ventral premotor cortex (Rizzolatti & Matelli 2003). Parietal cortex is viewed as playing a critical role in selecting manual actions through the integration of multiple sources of information received from other regions distributed throughout the cerebral cortex. For instance, in addition to sensory information about objects' spatial attributes (e.g. form, size, orientation) and the properties of the hand and the arm, parietal cortex also receives input about the objects' identities from the inferotemporal cortex. Likewise, areas of the prefrontal cortex provide information on the prevailing task demands. These inputs bias the selection of an appropriate grasp representation in the parietal cortex, and this choice then determines which motor prototypes in the ventral premotor cortex are activated and passed to the primary motor cortex for execution.
Aspects of this framework can be extended to accommodate human praxis where the selection of actions is heavily influenced by cognition. This view is a departure from traditional conceptualizations of the left parietal cortex as a repository for stored praxis representations (for a history, see Leiguarda & Marsden 2000 and Goldenberg 2003). Instead, this region is cast in the role of dynamically assembling praxis representations to satisfy constraints that are provided by computations undertaken in a multitude of other brain regions. The exact computations, and thus the other brain areas involved, will be highly dependent on the particular demands of the task (i.e. context dependent) that, as illustrated earlier for the use of a hammer, often evolve through time. Tool-use pantomime, for instance, requires access to conceptual knowledge of manipulable objects and their functions, and the left posterior temporal cortex appears to play a role in representing this information (Damasio et al. 2001; Mahon et al. 2007; Martin 2007). Likewise, the relationship between a verbally cued object and any associated movements is arbitrary, and the dorsal premotor cortex is involved in representing these conditional stimulus–response associations (Grafton et al. 1998; Picard & Strick 2001). The middle frontal gyrus may likewise contribute information pertaining to the actor's prospective goals (Duncan & Owen 2000; Rowe et al. 2000; Buccino et al. 2004). If visual objects were provided as cues, then areas of the fusiform gyrus in the temporal cortex involved in coding object's visual properties would be engaged (Chao et al. 1999), and so forth. If this system is really so flexible, then it might be expected to also participate in constructing representations even of those meaningful praxis skills that do not involve using artefacts.
4. Tool use and communicative gestures
It is believed that advanced tool use preceded and may have played a role in the evolution of gestural communication (Bradshaw & Nettleton 1982; Gibson 1993). If so, then one might expect to find common neurological substrates involved in representing both types of praxis skills. Yet, the prevailing wisdom in neuropsychology is that acquired tool-use skills and communicative gestures involve two distinct representational systems (Rothi et al. 1991; Cubelli et al. 2000; Buxbaum 2001). This view is based on observations that apraxic patients who falter at tool-use pantomime are often less impaired (Roy et al. 1991; Foundas et al. 1999), or completely unaffected (Rapcsak et al. 1993; Dumont et al. 1999), when performing communicative gestures (e.g. waving hello). To date, only a single neuroimaging study has attempted to identify areas involved in representing these skills (Fridman et al. 2006). Given the data from brain-lesioned patients, it is surprising that no differences were detected in the parietal cortex. Instead, only the left ventral premotor cortex responded more when planning tool-use actions versus communicative gestures. The interpretation of these findings is challenging due to the fact that only the right hand was tested. The difference in the left ventral premotor cortex could be specific to the use of the contralateral right hand, for example.
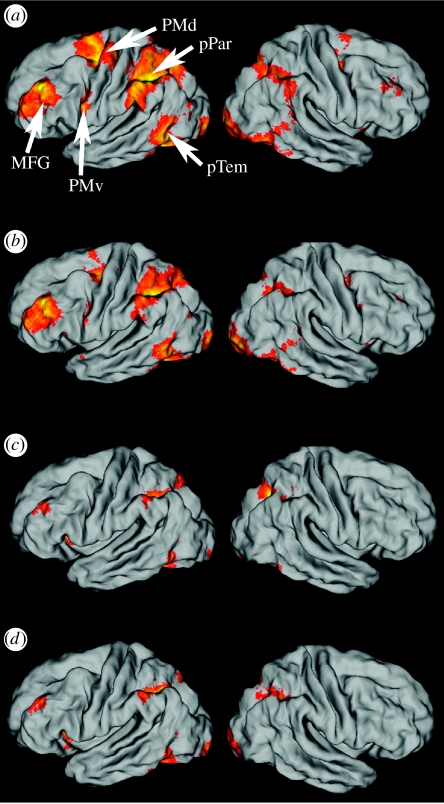
Recently, we revisited this issue (Kroliczak & Frey 2007). The brain activity of 12 healthy right-handed adults was monitored by functional magnetic resonance imaging (fMRI) while they retrieved stored praxis representations in response to randomly ordered verbs denoting familiar tool-use actions (e.g. ‘cutting’) or communicative gestures (e.g. ‘beckoning’). In conditions designed to control for linguistic stimulus processing, participants were presented with verbs denoting familiar mental actions (e.g. ‘believing’). On each trial, they were instructed to read the stimulus word and, in the case of verbs denoting physical actions, prepare to undertake the associated movements using either their right (experiment 1) or left (experiment 2) hands. After a variable length delay interval, they were cued to reproduce the planned actions gently, taking care not to destabilize their heads. As in the previous studies introduced above, we found that retrieving and planning transitive actions for subsequent production with either hand increased activity in the left parietal, dorsal premotor, middle frontal and posterior temporal cortices (figure 1). Importantly, these very same areas showed increased activity when retrieving and planning intransitive gestures for subsequent production with either limb. In fact, no brain areas showed significantly greater activity when planning either behaviour relative to the other. That apraxic patients are sometimes less affected when performing communicative gestures may indicate that tool-use pantomime is simply a task that places more demands on this system, perhaps because it requires participants to represent both the absent objects (a key piece of contextual information) and the associated actions.
Figure 1.
Brain areas in healthy right-handed adults which show increased activity relative to resting baseline when planning familiar tool-use pantomimes or communicative gestures. To facilitate visualization, activation maps are displayed on a partially unfolded template brain. (a) Planning tool-use pantomimes for production with the right hand is associated with greater activity in the left posterior parietal (pPar), dorsal (PMd) and ventral (PMv) premotor cortices, middle frontal gyrus (MFG) and frontal and posterior temporal (pTem) cortices. Effects are smaller in the homologous regions of the right cerebral hemisphere. (b) A similar network is engaged when planning communicative gestures for production with the right hand. Though less pronounced, these same areas also show increased activity when planning (c) tool-use pantomimes or (d) communicative gestures for production with the left hand.
These findings are also important in that they provide neurological evidence consistent with hypothesized links between the origins of tool use and language (Greenfield 1991), including the suggestion that tool use may have played a causal role in the evolution of gestural communication (Bradshaw & Nettleton 1982; Gibson 1993). The acquisition of these behaviours, particularly through imitation (Buccino et al. 2004) and/or observational (Frey & Gerry 2006) learning, may involve Broca's area. Yet, contrary to what might be expected on the basis of previous models (Greenfield 1991), we find no evidence to suggest that Broca's area is a critical substrate for representing these skills once established, at least not in the adult modern human brain (figure 1). Instead, the neural overlap between tool use and communicative gestures is actually much more extensive and distributed. As for why this might be, an interesting possibility to consider hinges on the fact that both classes of behaviour can be viewed as goal-directed, manipulative acts. Rather than affecting inanimate objects through direct application of force, communicative gestures often target individuals in whom we hope to evoke a certain behavioural response. Gallagher & Frith (2004) found that observing such instrumental gestures activated many of these same left-lateralized areas, while observing gestures that expressed the actor's internal emotional state engaged a distinct network in regions of temporal and paracingulate cortices implicated in mentalizing tasks. Though our lack of cues for expressive gestures in the present experiments precludes us from addressing this issue, whether or not such a dissociation is observed when retrieving representations for subsequent production is an interesting question for future work.
All of the studies discussed thus far have exclusively involved right-handed (i.e. left-hemisphere motor dominant) individuals. Whether or not a similar pattern of left cerebral asymmetry is present in left-handers is a question whose answer could shed additional light on the origins and nature of the praxis system.
5. Hand dominance and praxis
Other primates do show hand preferences under certain circumstances (Hopkins & Pearson 2000; Hopkins & Russell 2004). Yet, none are known to display the population-level bias evident in humans where approximately 90% of individuals consistently favour their right hand for fine motor tasks (Coren & Porac 1977; Annett 2006). Evidence from the fossil and archaeological records suggests that this right-hand (i.e. left-hemisphere) bias for manual control existed very early in our lineage (Steele 2006). Our understanding of the relationship between hand dominance and acquired praxis skills at the neurological level is, however, incomplete.
One possibility is that our right-hand dominance is a reflection of the left-lateralized system for representing manual praxis (Geschwind & Galaburda 1985; Heilman 1997). It could also be true that hand dominance and praxis rely on separable mechanisms, but that there is an advantage to having them co-located in the same cerebral hemisphere. This arrangement would, for example, eliminate the need for interhemispheric transfer by allowing praxis representations to be accessed directly by areas in the left hemisphere which are involved in controlling distal movements of the contralateral right hand. In either case, these hypotheses predict a strong linkage between hand dominance and praxis skills. Accordingly, left-handers should represent acquired manual skills in their motor dominant right hemispheres. Evidence is surprisingly scarce. While it is true that some left-handers do show signs of apraxia following right hemisphere lesions (Poeck & Kerschensteiner 1971; Valenstein & Heilman 1979; Dobato et al. 2001), the same can be said for some right-handed patients (Marchetti & Della Sala 1997; Raymer et al. 1999). On the neuroimaging side, left-handed participants do show greater recruitment of right parietal, frontal and temporal cortices than right-handers when listening to the sounds made by using handheld tools versus animals, and this might reflect automatic activation of right-lateralized praxis representations (Lewis et al. 2006).
Alternatively, it could be that the mechanisms responsible for hand dominance and praxis are relatively independent (Lausberg et al. 1999). If so, then most left-handers should also represent praxis skills in their left hemispheres. This view receives support from a large study of adults undergoing unilateral inactivation of the cerebral hemispheres presurgically (i.e. Wada testing). Results indicate that the ability to pantomime actions is more closely associated with the laterality of language functions than hand dominance (Meador et al. 1999). Over 90% of right-handers are also left-hemisphere dominant for language (Knecht et al. 2000a). In left-handers, there is greater variability with approximately 70% showing left-hemisphere dominance and the rest displaying either a bilateral or right-lateralized organization (Kimura 1983; Knecht et al. 2000b). Additional support for the separation of mechanisms involved in praxis and hand dominance can be found in our earlier investigations of tool-use pantomime in left- and right-handed patients who had undergone complete surgical transections of the corpus callosum to treat medically intractable epilepsy (i.e. ‘split brain’ surgery; Johnson-Frey 2005a). Both individuals in the study are left-hemisphere dominant for language. Despite their handedness differences, both were also found to be most accurate when stimuli were presented to their isolated left hemispheres and pantomimes were produced with their right hands.
We recently completed an fMRI project using the same procedure described above to look at the representation of familiar tool-use actions and communicative gestures in a sample of strongly left-handed individuals. The preliminary results are largely similar to those of the right-handed individuals discussed earlier. Retrieving and planning both types of actions was associated with increased activity within the same left-lateralized regions. Along with our earlier split brain results, these findings suggest that, in modern humans, praxis representations and hand dominance may rely on relatively independent neurological mechanisms.
6. Summary
Though difficult to investigate through the fossil record, the manual skills involved in using complex tools and other manipulable artefacts are a key component of human material culture. Determining the brain mechanisms that underlie these human specializations has the potential to yield critical insights into their origins, and the methods of cognitive neuroscience make such inquiry possible in the modern human brain. I have argued that sensory–motor adaptations in systems that control prehension are necessary, but not sufficient to explain the complex and flexible ways in which humans routinely use tools and other manipulable artefacts. These praxis behaviours are influenced as well by cognitive processes that provide contextual information beyond what is available to the senses. Conceptual knowledge about objects and their functions, the actors' goals and interpretations of task demands all influence the selection of these praxis behaviours. Evidence from apraxic patients and functional neuroimaging studies converge on the hypothesis that the human left cerebral hemisphere is asymmetrically involved in the processes that make this possible. Rather than serving as a repository for skill memories, the left parietal cortex is said to assemble praxis representations dynamically in order to fit the multiple constraints provided by a variety of computations distributed throughout the left hemisphere and possibly beyond. The result is the formation of an internal praxis representation that can be used to guide contextually appropriate actions. There are reasons to believe that critical aspects of this system are organized similarly in both right- and left-handed individuals, and therefore these functions may be relatively independent of those responsible for hand dominance. Furthermore, the very same brain areas involved in representing familiar tool-use skills also show increased activity when retrieving and planning communicative gestures. This is consistent with the hypothesis that human specializations for tool use and language have common origins.
Acknowledgments
This research was conducted with approval from the Institutional Review Board at the University of Oregon.
S.H.F. has also published as ‘Scott H. Johnson and Scott H. Johnson-Frey’. This work was supported by a grant (NS053962) from the NIH/NINDS. The author thanks Gregory Kroliczak for his assistance with figure preparation.
Footnotes
One contribution of 14 to a Theme Issue ‘The sapient mind: archaeology meets neuroscience’.
References
- Ambrose S.H. Paleolithic technology and human evolution. Science. 2001;291:1748–1753. doi: 10.1126/science.1059487. doi:10.1126/science.1059487 [DOI] [PubMed] [Google Scholar]
- Annett M. The distribution of handedness in chimpanzees: estimating right shift in Hopkins' sample. Laterality. 2006;11:101–109. doi: 10.1080/13576500500376500. [DOI] [PubMed] [Google Scholar]
- Basalla G. Cambridge University Press; New York, NY: 1988. The evolution of technology. [Google Scholar]
- Blake A, Brady J.M, Blake A. Computational modeling of hand eye coordination. Phil. Trans. R. Soc. B. 1992;337:351–360. doi:10.1098/rstb.1992.0113 [Google Scholar]
- Bradshaw J.L, Nettleton N.C. Language lateralization to the dominant hemisphere: tool use, gesture and language in hominid evolution. Curr. Psychol. Rev. 1982;2:171–192. doi:10.1007/BF02684498 [Google Scholar]
- Buccino G, Vogt S, Ritzl A, Fink G.R, Zilles K, Freund H.J, Rizzolatti G. Neural circuits underlying imitation learning of hand actions: an event-related fMRI study. Neuron. 2004;42:323–334. doi: 10.1016/s0896-6273(04)00181-3. doi:10.1016/S0896-6273(04)00181-3 [DOI] [PubMed] [Google Scholar]
- Buxbaum L.J. Ideomotor apraxia: a call to action. Neurocase. 2001;7:445–458. doi: 10.1093/neucas/7.6.445. doi:10.1093/neucas/7.6.445 [DOI] [PubMed] [Google Scholar]
- Chao L.L, Haxby J.V, Martin A. Attribute-based neural substrates in temporal cortex for perceiving and knowing about objects. Nat. Neurosci. 1999;2:913–919. doi: 10.1038/13217. doi:10.1038/13217 [DOI] [PubMed] [Google Scholar]
- Choi S.H, Na D.L, Kang E, Lee K.M, Lee S.W, Na D.G. Functional magnetic resonance imaging during pantomiming tool-use gestures. Exp. Brain Res. 2001;139:311–317. doi: 10.1007/s002210100777. doi:10.1007/s002210100777 [DOI] [PubMed] [Google Scholar]
- Coren S, Porac C. Fifty centuries of right-handedness: the historical record. Science. 1977;198:631–632. doi: 10.1126/science.335510. doi:10.1126/science.335510 [DOI] [PubMed] [Google Scholar]
- Cubelli R, Marchetti C, Boscolo G, Della Sala S. Cognition in action: testing a model of limb apraxia. Brain Cogn. 2000;44:144–165. doi: 10.1006/brcg.2000.1226. doi:10.1006/brcg.2000.1226 [DOI] [PubMed] [Google Scholar]
- Damasio H, Grabowski T.J, Tranel D, Ponto L.L, Hichwa R.D, Damasio A.R. Neural correlates of naming actions and of naming spatial relations. Neuroimage. 2001;13:1053–1064. doi: 10.1006/nimg.2001.0775. doi:10.1006/nimg.2001.0775 [DOI] [PubMed] [Google Scholar]
- Dobato J.L, Baron M, Barriga F.J, Pareja J.A, Vela L, Sanchez Del Rio M. Apraxia cruzada secundaria a infarto parietal derecho. Rev. Neurol. 2001;33:725–728. [PubMed] [Google Scholar]
- Dumont C, Ska B, Schiavetto A. Selective impairment of transitive gestures: an unusual case of apraxia. Neurocase. 1999;5:447–458. [Google Scholar]
- Duncan J, Owen A.M. Common regions of the human frontal lobe recruited by diverse cognitive demands. Trends Neurosci. 2000;23:475–483. doi: 10.1016/s0166-2236(00)01633-7. doi:10.1016/S0166-2236(00)01633-7 [DOI] [PubMed] [Google Scholar]
- Fagg A.H, Arbib M.A. Modeling parietal–premotor interactions in primate control of grasping. Neural Netw. 1998;11:1277–1303. doi: 10.1016/s0893-6080(98)00047-1. doi:10.1016/S0893-6080(98)00047-1 [DOI] [PubMed] [Google Scholar]
- Foundas A.L, Macauley B.L, Raymer A.M, Maher L.M, Rothi L.J, Heilman K.M. Ideomotor apraxia in Alzheimer disease and left hemisphere stroke: limb transitive and intransitive movements. Neuropsychiatry Neuropsychol. Behav. Neurol. 1999;12:161–166. [PubMed] [Google Scholar]
- Frey S.H, Gerry V.E. Modulation of neural activity during observational learning of actions and their sequential orders. J. Neurosci. 2006;26:13 194–13 201. doi: 10.1523/JNEUROSCI.3914-06.2006. doi:10.1523/JNEUROSCI.3914-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridman E.A, Immisch I, Hanakawa T, Bohlhalter S, Waldvogel D, Kansaku K, Wheaton L, Wu T, Hallett M. The role of the dorsal stream for gesture production. Neuroimage. 2006;29:417–428. doi: 10.1016/j.neuroimage.2005.07.026. doi:10.1016/j.neuroimage.2005.07.026 [DOI] [PubMed] [Google Scholar]
- Gallagher H.L, Frith C.D. Dissociable neural pathways for the perception and recognition of expressive and instrumental gestures. Neuropsychologia. 2004;42:1725–1736. doi: 10.1016/j.neuropsychologia.2004.05.006. doi:10.1016/j.neuropsychologia.2004.05.006 [DOI] [PubMed] [Google Scholar]
- Geschwind N, Galaburda A.M. Cerebral lateralization. Biological mechanisms, associations, and pathology: I. A hypothesis and a program for research. Arch. Neurol. 1985;42:428–459. doi: 10.1001/archneur.1985.04060050026008. [DOI] [PubMed] [Google Scholar]
- Geschwind N, Kaplan E.A. Human cerebral disconnection syndromes. Neurology. 1962;12:675–685. doi: 10.1212/wnl.12.10.675. [DOI] [PubMed] [Google Scholar]
- Gibson, K. R. 1993 The evolution of lateral asymmetries, language, tool-use, and intellect (eds J. Bradshaw & L. Rogers). Am. J. Phys. Anthropol 92, 123–124.
- Goldenberg G. Apraxia and beyond: life and work of Hugo Liepmann. Cortex. 2003;39:509–524. doi: 10.1016/s0010-9452(08)70261-2. [DOI] [PubMed] [Google Scholar]
- Goodale M.A, Meenan J.P, Bulthoff H.H, Nicolle D.A, Murphy K.J, Racicot C.I. Separate neural pathways for the visual analysis of object shape in perception and prehension. Curr. Biol. 1994;4:604–610. doi: 10.1016/s0960-9822(00)00132-9. doi:10.1016/S0960-9822(00)00132-9 [DOI] [PubMed] [Google Scholar]
- Grafton S.T, Fagg A.H, Arbib M.A. Dorsal premotor cortex and conditional movement selection: a PET functional mapping study. J. Neurophysiol. 1998;79:1092–1097. doi: 10.1152/jn.1998.79.2.1092. [DOI] [PubMed] [Google Scholar]
- Greenfield P.M. Language, tools, and brain—the ontogeny and phylogeny of hierarchically organized sequential behaviour. Behav. Brain Sci. 1991;14:531–550. [Google Scholar]
- Grefkes C, Fink G.R. The functional organization of the intraparietal sulcus in humans and monkeys. J. Anat. 2005;207:3–17. doi: 10.1111/j.1469-7580.2005.00426.x. doi:10.1111/j.1469-7580.2005.00426.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haaland K.Y, Harrington D.L, Knight R.T. Neural representations of skilled movement. Brain. 2000;123:2306–2313. doi: 10.1093/brain/123.11.2306. doi:10.1093/brain/123.11.2306 [DOI] [PubMed] [Google Scholar]
- Heilman, K. M. 1997 Handedness. In Apraxia: the neuropsychology of action (eds L. J. G. Rothi & K. M. Heilman), pp. 19–28. Hove, UK: Psychology Press.
- Heilman, K. M. & Rothi, L. J. G. 1997 Limb apraxia: a look back. In Apraxia: the neuropsychology of action (eds L. J. G. Rothi & K. M. Heilman), pp. 7–18. Hove, UK: Psychology Press.
- Hopkins W.D, Pearson K. Chimpanzee (Pan troglodytes) handedness: variability across multiple measures of hand use. J. Comp. Psychol. 2000;114:126–135. doi: 10.1037/0735-7036.114.2.126. doi:10.1037/0735-7036.114.2.126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins W.D, Russell J.L. Further evidence of a right hand advantage in motor skill by chimpanzees (Pan troglodytes) Neuropsychologia. 2004;42:990–996. doi: 10.1016/j.neuropsychologia.2003.11.017. doi:10.1016/j.neuropsychologia.2003.11.017 [DOI] [PubMed] [Google Scholar]
- Iriki A, Tanaka M, Iwamura Y. Coding of modified body schema during tool use by macaque postcentral neurones. Neuroreport. 1996;7:2325–2330. doi: 10.1097/00001756-199610020-00010. [DOI] [PubMed] [Google Scholar]
- Johnson-Frey S.H. What's so special about human tool use? Neuron. 2003;39:201–204. doi: 10.1016/s0896-6273(03)00424-0. doi:10.1016/S0896-6273(03)00424-0 [DOI] [PubMed] [Google Scholar]
- Johnson-Frey S.H. The neural bases of complex tool use in humans. Trends Cogn. Sci. 2004;8:71–78. doi: 10.1016/j.tics.2003.12.002. doi:10.1016/j.tics.2003.12.002 [DOI] [PubMed] [Google Scholar]
- Johnson-Frey S.H, Funnell M.G, Gerry V.E, Gazzaniga M.S. A dissociation between tool use skills and hand dominance: insights from left and right-handed callosotomy patients. J. Cogn. Neurosci. 2005a;17:262–272. doi: 10.1162/0898929053124974. doi:10.1162/0898929053124974 [DOI] [PubMed] [Google Scholar]
- Johnson-Frey S.H, Vinton D, Norlund R.N, Grafton S.G. Cortical topography of the human anterior intraparietal area. Cogn. Brain Res. 2005b;23:397–405. doi: 10.1016/j.cogbrainres.2004.11.010. doi:10.1016/j.cogbrainres.2004.11.010 [DOI] [PubMed] [Google Scholar]
- Johnson-Frey S.H, Newman-Norlund R, Grafton S.T. A distributed left hemisphere network active during planning of everyday tool use skills. Cereb. Cortex. 2005c;15:681–695. doi: 10.1093/cercor/bhh169. doi:10.1093/cercor/bhh169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura D. Speech representation in an unbiased sample of left-handers. Hum. Neurobiol. 1983;2:147–154. [PubMed] [Google Scholar]
- Knecht S, Deppe M, Drager B, Bobe L, Lohmann H, Ringelstein E, Henningsen H. Language lateralization in healthy right-handers. Brain. 2000a;123:74–81. doi: 10.1093/brain/123.1.74. doi:10.1093/brain/123.1.74 [DOI] [PubMed] [Google Scholar]
- Knecht S, Drager B, Deppe M, Bobe L, Lohmann H, Floel A, Ringelstein E.B, Henningsen H. Handedness and hemispheric language dominance in healthy humans. Brain. 2000b;123:2512–2518. doi: 10.1093/brain/123.12.2512. doi:10.1093/brain/123.12.2512 [DOI] [PubMed] [Google Scholar]
- Kroliczak, G. & Frey, S. H. 2007 Role of left parietal cortex in representation of familiar transitive and intransitive manual actions. In Thirteenth Annual Meeting of the Organization for Human Brain Mapping, Chicago
- Lausberg H, Gottert R, Munssinger U, Boegner F, Marx P. Callosal disconnection syndrome in a left-handed patient due to infarction of the total length of the corpus callosum. Neuropsychologia. 1999;37:253–265. doi: 10.1016/s0028-3932(98)00079-7. doi:10.1016/S0028-3932(98)00079-7 [DOI] [PubMed] [Google Scholar]
- Leiguarda R.C, Marsden C.D. Limb apraxias: higher-order disorders of sensorimotor integration. Brain. 2000;123:860–879. doi: 10.1093/brain/123.5.860. doi:10.1093/brain/123.5.860 [DOI] [PubMed] [Google Scholar]
- Lewis J.W. Cortical networks related to human use of tools. Neuroscientist. 2006;12:211–231. doi: 10.1177/1073858406288327. doi:10.1177/1073858406288327 [DOI] [PubMed] [Google Scholar]
- Lewis J.W, Phinney R.E, Brefczynski-Lewis J.A, DeYoe E.A. Lefties get it “right” when hearing tool sounds. J. Cogn. Neurosci. 2006;18:1314–1330. doi: 10.1162/jocn.2006.18.8.1314. doi:10.1162/jocn.2006.18.8.1314 [DOI] [PubMed] [Google Scholar]
- Mahon B.Z, Milleville S.C, Negri G.A, Rumiati R.I, Caramazza A, Martin A. Action-related properties shape object representations in the ventral stream. Neuron. 2007;55:507–520. doi: 10.1016/j.neuron.2007.07.011. doi:10.1016/j.neuron.2007.07.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchetti C, Della Sala S. On crossed apraxia. Description of a right-handed apraxic patient with right supplementary motor area damage. Cortex. 1997;33:341–354. doi: 10.1016/s0010-9452(08)70010-8. [DOI] [PubMed] [Google Scholar]
- Martin A. The representation of object concepts in the brain. Annu. Rev. Psychol. 2007;58:25–45. doi: 10.1146/annurev.psych.57.102904.190143. doi:10.1146/annurev.psych.57.102904.190143 [DOI] [PubMed] [Google Scholar]
- Meador K.J, Loring D.W, Lee K, Hughes M, Lee G, Nichols M, Heilman K.M. Cerebral lateralization: relationship of language and ideomotor praxis. Neurology. 1999;53:2028–2031. doi: 10.1212/wnl.53.9.2028. [DOI] [PubMed] [Google Scholar]
- Moll J, de Oliveira-Souza R, Passman L.J, Cunha F.C, Souza-Lima F, Andreiuolo P.A. Functional MRI correlates of real and imagined tool-use pantomimes. Neurology. 2000;54:1331–1336. doi: 10.1212/wnl.54.6.1331. [DOI] [PubMed] [Google Scholar]
- Ohgami Y, Matsuo K, Uchida N, Nakai T. An fMRI study of tool-use gestures: body part as object and pantomime. Neuroreport. 2004;15:1903–1906. doi: 10.1097/00001756-200408260-00014. doi:10.1097/00001756-200408260-00014 [DOI] [PubMed] [Google Scholar]
- Orban G.A, Van Essen D, Vanduffel W. Comparative mapping of higher visual areas in monkeys and humans. Trends Cogn. Sci. 2004;8:315–324. doi: 10.1016/j.tics.2004.05.009. doi:10.1016/j.tics.2004.05.009 [DOI] [PubMed] [Google Scholar]
- Picard N, Strick P.L. Imaging the premotor areas. Curr. Opin. Neurobiol. 2001;11:663–672. doi: 10.1016/s0959-4388(01)00266-5. doi:10.1016/S0959-4388(01)00266-5 [DOI] [PubMed] [Google Scholar]
- Poeck K, Kerschensteiner M. Ideomotor apraxia following right-sided cerebral lesion in a left-handed subject. Neuropsychologia. 1971;9:359–361. doi: 10.1016/0028-3932(71)90032-7. doi:10.1016/0028-3932(71)90032-7 [DOI] [PubMed] [Google Scholar]
- Povinelli D.J. Oxford University Press; New York, NY: 2000. Folk physics for apes: the chimpanzee's theory of how the world works. [Google Scholar]
- Preuss T.M, Stepniewska I, Kaas J.H. Movement representation in the dorsal and ventral premotor areas of owl monkeys: a microstimulation study. J. Comp. Neurol. 1996;371:649–676. doi: 10.1002/(SICI)1096-9861(19960805)371:4<649::AID-CNE12>3.0.CO;2-E. doi:10.1002/(SICI)1096-9861 (19960805)371:4<649::AID-CNE12>3.0.CO;2-E [DOI] [PubMed] [Google Scholar]
- Rapcsak S.Z, Ochipa C, Beeson P.M, Rubens A.B. Praxis and the right hemisphere. Brain Cogn. 1993;23:181–202. doi: 10.1006/brcg.1993.1054. doi:10.1006/brcg.1993.1054 [DOI] [PubMed] [Google Scholar]
- Raymer A.M, Merians A.S, Adair J.C, Schwartz R.L, Williamson D.J, Rothi L.J, Poizner H, Heilman K.M. Crossed apraxia: implications for handedness. Cortex. 1999;35:183–199. doi: 10.1016/s0010-9452(08)70793-7. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Craighero L. The mirror-neuron system. Annu. Rev. Neurosci. 2004;27:169–192. doi: 10.1146/annurev.neuro.27.070203.144230. doi:10.1146/annurev.neuro.27.070203.144230 [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Matelli M. Two different streams form the dorsal visual system: anatomy and functions. Exp. Brain Res. 2003;153:146–157. doi: 10.1007/s00221-003-1588-0. doi:10.1007/s00221-003-1588-0 [DOI] [PubMed] [Google Scholar]
- Rothi L.J, Ochipa C, Heilman K.M. A cognitive neuropsychological model of limb praxis. Cogn. Neuropsychol. 1991;8:443–458. doi:10.1080/02643299108253382 [Google Scholar]
- Rowe J.B, Toni I, Josephs O, Frackowiak R.S, Passingham R.E. The prefrontal cortex: response selection or maintenance within working memory? Science. 2000;288:1656–1660. doi: 10.1126/science.288.5471.1656. doi:10.1126/science.288.5471.1656 [DOI] [PubMed] [Google Scholar]
- Roy E.A, Square-Storer P, Hogg S, Adams S. Analysis of task demands in apraxia. Int. J. Neurosci. 1991;56:177–186. doi: 10.3109/00207459108985414. doi:10.3109/00207459108985414 [DOI] [PubMed] [Google Scholar]
- Rumiati R.I, Weiss P.H, Shallice T, Ottoboni G, Noth J, Zilles K, Fink G.R. Neural basis of pantomiming the use of visually presented objects. Neuroimage. 2004;21:1224–1231. doi: 10.1016/j.neuroimage.2003.11.017. doi:10.1016/j.neuroimage.2003.11.017 [DOI] [PubMed] [Google Scholar]
- Steele J.U.N. Humans, tools and handedness. In: Roux V, Bril B, editors. Stone knapping: the necessray conditions for a uniquely hominin behaviour. MacDonald Institute; Cambridge, UK: 2006. pp. 217–239. [Google Scholar]
- Tomasello M. The human adaptation for culture. Annu. Rev. Anthropol. 1999;28:509–529. doi:10.1146/annurev.anthro.28.1.509 [Google Scholar]
- Umilta` M.A, Escola L, Intskirveli I, Grammont F, Rochat M, Caruana F, Jezzini A, Gallese V, Rizzolatti G. When pliers become fingers in the monkey motor system. Proc. Natl Acad. Sci. USA. 2008;105:2209–2213. doi: 10.1073/pnas.0705985105. doi:10.1073/pnas.0705985105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenstein E, Heilman K.M. Apraxic agraphia with neglect-induced paragraphia. Arch. Neurol. 1979;36:506–508. doi: 10.1001/archneur.1979.00500440076016. [DOI] [PubMed] [Google Scholar]