Abstract
This paper will broadly review the currently available twin and adoption data on antisocial behaviour (AB). It is argued that quantitative genetic research can make a significant contribution to further the understanding of how AB develops. Genetically informative study designs are particularly useful for investigating several important questions such as whether: the heritability estimates vary as a function of assessment method or gender; the relative importance of genetic and environmental influences varies for different types of AB; the environmental risk factors are truly environmental; and genetic vulnerability influences susceptibility to environmental risk. While the current data are not yet directly translatable for prevention and treatment programmes, quantitative genetic research has concrete translational potential. Quantitative genetic research can supplement neuroscience research in informing about different subtypes of AB, such as AB coupled with callous–unemotional traits. Quantitative genetic research is also important in advancing the understanding of the mechanisms by which environmental risk operates.
Keywords: antisocial behaviour, genetic, environmental risk, callous–unemotional
1. Background
Each year over 1.6 million people are killed through violence, and violence prevention is one of the most important global concerns (WHO report on Violence and Health; Krug et al. 2002). Governmental bodies everywhere in the Western world, including the UK, prioritize for prevention of antisocial behaviour (AB; Bailey 2002; Every Child Matters Green Paper, DfES 2003). The political, social or economic risk factors for AB are well studied but more recently the awareness has grown that biological risk factors, which may explain individual differences in predisposition to violence, also need to be explored. Furthermore, many environmental risk factors that are traditionally thought to be social may actually reflect genetic vulnerability (Moffitt 2005). Finally, the vulnerability factors and their ‘modus operandi’ may differ for different subtypes of AB. Thus the question is not really: ‘is it in their genes?’ or ‘what environmental factors are to blame?’ It is: how do genetic and environmental factors interact to produce specific types of AB?
This article will provide a broad and selective review of twin and adoption research into the nature and nurture of AB. Twin and adoption research has been important in not only demonstrating the relative importance of genetic and environmental factors to AB, but also in increasing our understanding of aetiological differences between subtypes of individuals with AB and how gene–environment interplay works.
2. Twin and adoption methods
The twin method is a natural experiment that relies on the different levels of genetic relatedness between monozygotic (MZ) and dizygotic (DZ) twin pairs to estimate the contribution of genetic and environmental factors to individual differences or extreme scores in a phenotype of interest. Phenotypes include any behaviour or characteristic that is measured separately for each twin, such as twins' scores on an AB checklist. The basic premise of the twin method is this: if identical twins, who share 100% of their genetic material appear more similar on an AB measure than fraternal twins, who share on average 50% of their genetic material (like any siblings), then we infer that there are genetic influences on AB. Identical twins' genetic similarity is twice that of fraternal twins. If nothing apart from genes influences behaviour, then we would expect the identical twins to be twice as similar with respect to the AB measure when compared to fraternal twins. Shared environmental influences (environmental influences that make twins similar to each other) are inferred if fraternal twins appear more similar than is expected from sharing 50% of their genes. Finally, if identical twins are not 100% similar on the measure of AB (as would be expected if only genes influenced a trait), non-shared environmental influences (environmental influences that make twins different from each other) are inferred. The non-shared environmental estimate also includes measurement error.
In adoption studies, the occurrence of behaviour/trait may be compared between adoptive and biological relatives. For example, one can compare adoptees whose biological parents/siblings are with/without AB or study adoptees reared by adoptive parents/siblings with/without AB. Genetic influences on AB are indicated by the association between adoptee and biological relative on measures of AB. Environmental influences are indicated by the association between adoptee and adoptive relative on measures of AB. Because adoptions have become less common since the 1970s most quantitative genetic studies designed in recent years use the twin method.
For both twin and adoption data, statistical model fitting techniques and regression analyses methods incorporating genetic relatedness parameters are used to investigate the aetiology of the phenotype of choice. These techniques will not be covered in this article and an interested reader is referred to Plomin et al. (2008). It is important to remember that heritability and environmental variance estimates derived from both twin and adoption data pertain to a particular population at a particular time; should the environmental circumstances change dramatically then so would the proportion of variance accounted for by genetic/environmental factors. It is equally important to note that no heritability/environmental statistic concerns a single individual but instead reflects contribution of genetic/environmental influences to individual differences or group differences on a behaviour/trait. Finally, simple heritability and environmental variance estimates also reflect gene–environment interplay. Ingenious study designs, such as ‘children of twins’ (CoT), enable researchers to conduct more fine-grain study to gene–environment interplay (see §5).
Several concerns have been raised regarding both twin and adoption methods. Just to mention a few, twin studies have been criticized on account of equal environments assumption (EEA) and representativeness of twins, while the adoption studies have been criticized on account of representativeness of adoptive families and resultant restricted environmental variance. It is beyond the scope of this paper to deal with these issues in detail and an interested reader is referred to Rutter (2005, pp. 41–44). However, a brief consideration of the concerns is warranted here. The EEA for AB assumes that the environmental variance with MZ and DZ twin pairs will be the same with respect to the environments that influence AB. In other words, the expectation is that the MZ and DZ twins share environment to the same extent. Analyses that have specifically tested the EEA by introducing environmental similarity measures (sharing friends, sharing classes, dressing alike and perceived zygosity) to a twin model have found scarce evidence for violation of EEA when assessing behavioural problems (Cronk et al. 2002). This is hardly surprising as factors such as dressing alike are unlikely to represent an environment that will influence AB. An often-heard objection to the EEA refers to the fact that MZ twins are more likely to share a chorion, which the critics of the twin method assume makes their prenatal environment more similar than that of the DZ twins. In fact, the opposite is true with monochorionic MZ twins more likely to differ in birth weight (result of the ‘transfusion syndrome’ and the following differences in availability of placental blood). To the extent that data are available, chorionicity and its consequences do not seem to threaten the logic of the twin method (Rutter 2005, p. 42). Rutter (2005, p. 43) highlights that there are genetic effects on exposure to those environments that perpetuate (via an environmental main effect) the development of behavioural problems. Some of the difference in similarity between MZ and DZ pairs may thus be due to the MZ twins (who share all their genes) being exposed to more of the similar environmental risk events. More research is required to study this phenomenon, but although it would inflate the heritability estimate somewhat, the magnitude of this EEA violation is unlikely to be sufficient to cast doubt to the whole twin strategy. Critics of the twin method have also highlighted that there may be important twin–singleton differences that would jeopardize the conclusions from the twin studies. Although twins are delayed in language development and twin pregnancies are associated with increased rate of obstetric complications, neither concern is particularly relevant for twin data on AB. Twins with obstetric complications are routinely excluded from twin analyses and language delay found in twins is very mild, representing variation in the normal range. It has been rightly pointed out that adoptive families are often carefully selected and represent only a small range of possible home environments (Moffitt 2005). Given the limitations of both twin and adoption methods, it is important to collate data across studies and methodologies.
3. Quantitative genetic studies of AB
Quantitative genetic studies can be used to estimate heritability of AB. Quantitative genetic studies have also gone beyond reporting simple heritability estimates, and investigated aetiology of different subtypes of AB and comorbidity. Recent meta-analyses of twin and adoption studies of AB suggest moderate heritability and non-shared environmental influence, as well as the modest shared environmental influence on AB in general (Rhee & Waldman 2002; Waldman & Rhee 2006).
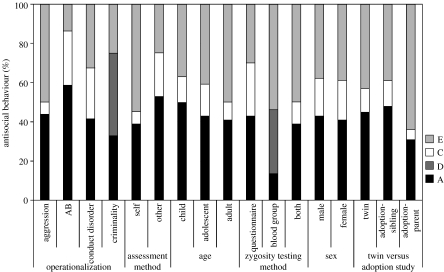
The magnitude of heritability estimate has been shown to vary as a function of operationalization (the criteria used to define AB), assessment method, age, zygosity determination method, gender and whether twin or adoption design was used (figure 1). The magnitude of heritability estimate can also vary as a function of subtype (figure 2). The magnitude of differences in heritability and environmental estimates could reflect several things. For example, different operationalization methods may produce different heritability/environmental estimates as they involve distinct age groups (e.g. child/conduct disorder versus adult/criminality), as well as use of varying assessment tools (e.g. symptom count versus arrest record). The differences in the magnitude of estimated heritability/environmental estimates can thus reflect a real finding associated with developmental period or type of AB measured, but could also reflect different levels of measurement error associated with each operationalization. Different assessment methods are also likely to have different levels of measurement error associated with them. Studies where zygosity is determined by blood generally report much lower sample sizes than studies where zygosity is determined by a questionnaire. The low sample size, in turn, limits the power of the statistical analyses and introduces sample size-dependent measurement error. Gender does not appear to make a difference to the aetiology of individual differences in AB. Thus, although there are mean differences in the average number of ABs displayed by boys and girls (with boys displaying a larger number than girls), the source of this mean difference is likely to result from factors that shift the distribution for the boys towards the risk cut-off. A recent study suggests that genetic and environmental influences on delinquency have less effect on population variation in delinquency among girls and that girls require greater causal liability for expression of delinquency than boys (Van Hulle et al. 2007). Different heritability/environmental estimates in twin versus adoption studies could relate to different N (twin studies are usually much larger), different assessment instruments (in twin studies, both twins receive the same age-appropriate instruments; in parent adoption studies, the child and the parent receive different age-appropriate instruments) and restricted variance in environmental risk factors in the adoptive families. However, what is striking is the considerable similarity in heritability estimates (mostly in the range of 40–50%) irrespective of the operationalization, assessment method, age, zygosity determination method, gender or study design used (figure 1).
Figure 1.
Genetic and environmental influences on AB appear remarkably similar in their magnitude despite different operationalization methods, assessment methods, age of assessment, zygosity assessment methods, gender of the participants or whether twin versus adoption design was used. This figure represents a selection of the statistics reported in Rhee & Waldman (2002) meta-analysis. A, additive genetic influences; D, dominant genetic influences; C, shared environmental influences; E, non-shared environmental influences.
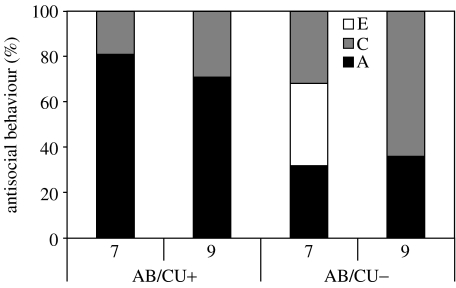
Figure 2.
The heritability of AB appears strong in those children with elevated levels of both AB and callous–unemotional traits (AB/CU+). By contrast, children with AB but lower levels of CU traits (AB/CU−) show a strong environmental influence on their AB (Viding et al. 2005, 2008). This finding holds even when the contribution of co-occurring hyperactivity is controlled for in the analyses (Viding et al. 2008). A, additive genetic influences; C, shared environmental influences; E, non-shared environmental influences.
Subtyping by callous–unemotional traits appears to index a large difference in the heritability of AB (Viding et al. 2005, 2008; figure 2). These findings from our research group are still relatively new and will require replication. We first studied teacher ratings of callous–unemotional traits and AB in approximately 7500 7-year-old twins from the Twins Early Development Study (TEDS; Viding et al. 2005). We separated children with elevated levels of AB (in the top 10% for the TEDS sample) into AB/CU+ and AB/CU− groups based on their callous–unemotional (CU) score (in the top 10% or not). AB in children with AB/CU+ was under strong genetic influence (heritability of 0.81) and no influence of shared environment. By contrast, AB in children without elevated levels of callous–unemotional traits showed moderate genetic influence (heritability of 0.30) and substantial environmental influence (shared environmental influence=0.34, non-shared environmental influence=0.26; figure 2). We have recently replicated the finding of different heritability magnitude for the AB/CU+ and AB/CU− groups using the 9-year teacher data (Viding et al. 2008). This difference in heritability magnitude holds even after hyperactivity scores of the children are controlled for, suggesting that the result is not driven by any differences in hyperactivity between the two groups. In summary, our research with pre-adolescent twins suggests that while the CU subtype is genetically vulnerable to AB, the non-CU subtype manifests a more strongly environmental aetiology to their AB (Viding et al. 2005, 2008). Other research has also suggested that the heritability of AB may vary by subtype. For example, early onset AB appears more heritable than adolescent AB (e.g. Taylor et al. 2000a,b; Arseneault et al. 2003), conduct disturbance coupled with hyperactivity shows strong genetic influence, but conduct disturbance without hyperactivity is associated with shared environmental influences (Silberg et al. 1996a,b) and finally aggressive AB appears more heritable than non-aggressive AB (Eley et al. 1999, 2003).
Recent quantitative genetic work also suggest that co-occurrence of AB and CU (Krueger et al. 2002; Taylor et al. 2003; Larsson et al. 2007; Viding et al. 2007), conduct disorder and oppositional defiant disorder (Dick et al. 2005), ADHD/hyperactivity and conduct disorder (Silberg et al. 1996a,b; Thapar et al. 2001; Nadder et al. 2002), as well as AB and substance abuse behaviours (Krueger et al. 2002) are due to common genetic factors. This research has implications for molecular genetic research. We should expect that pleiotropy (the same genes influencing many traits) and any genes showing an association with AB are also likely to influence a host of other problem behaviours.
4. Are environmental risk factors truly environmental?
Although it is well established what environment factors are associated with AB, it is still unclear whether these factors are causal (Moffitt 2005). This is partly explained by the fact that many studies cannot control for the potential impact of genetic influences on the correlation between a putative environmental risk factor and an antisocial outcome. This has led several researchers to conclude that the study of AB is ‘stuck in the risk factor stage’ (Hinshaw 2002; Moffitt 2005). One way to further examine the causal role of environmental risk factors in the development of AB is to use genetically sensitive twin and adoption designs to control for the confounding effects of parents' or children's genes on putative environmental measures (Moffitt 2005).
A powerful way of studying whether a risk factor has an environmentally mediated effect on children's AB is to use the MZ-twin differences method. Because the MZ twins share all their segregating genes and their shared environment, links between MZ-twin differences in an environmental risk factor and subsequent AB can thus be attributed to non-shared environmental processes. Careful and deliberate hypothesis testing using the MZ-twin differences design usually involves the following three steps: (i) documenting a non-shared environmental component in individual differences in the child outcome, e.g. AB, (ii) identifying specific, theoretically informative and environmental risk factors that vary between siblings and are correlated with the child outcome, e.g. negative parenting, and (iii) correlating MZ-twin differences in the environmental risk factor with MZ-twin differences in the child outcome.
Several studies have reported that MZ-twin differences in negative parenting are correlated with MZ-twin differences in AB (Asbury et al. 2003; Caspi et al. 2004; Burt et al. 2006). As a prime example, Caspi et al. (2004) used a longitudinal twin differences design to compare identical twins discordant for teacher-rated AB (i.e. identical twins who differ on AB outcome). They demonstrated that mothers' negative emotional attitudes towards their children were associated with children's AB. This association was not purely due to child effects (i.e. an effect of children's behaviour on parental treatment). In addition, the discordant identical twins design ruled out that the association between maternal expressed emotion and children's AB was genetically mediated, as maternal expressed emotion predicted differences between genetically identical individuals. Thus, these findings suggest that maternal expressed emotion is an environmentally mediated risk factor and possibly an environmental cause for AB.
There are other ways to demonstrate an environmental main effect on AB. If MZ mother's parenting predicts child's AB better than MZ aunt's parenting, then parenting has an environmental effect. On the other hand, if the MZ aunt's and the MZ mother's parenting predict the children's AB to the same extent, then parents' genes are responsible for the association between parenting and AB. If adoptive parent's bad parenting increases adoptee's AB over and above the genetic influence from the biological parents' AB, this again demonstrates a main effect of environment on AB. For a comprehensive discussion of study of environment in the context of quantitative genetic designs, the reader is referred to Moffitt (2005).
In summary, quantitative genetic studies have been useful in strongly demonstrating that environmental factors play an important role in individual differences in AB. Non-shared or child-specific environmental factors appear to be particularly important in bringing about AB. In addition to the study of environmental main effects on AB, quantitative genetic studies have also been instrumental in mapping out the effects of gene–environment interplay on AB.
5. Gene–environment interplay
There are now several ongoing twin and adoption studies that have included well-defined measures of putative environmental risk factors. These enable sophisticated extended twin models to test developmental hypothesis about different patterns of gene–environment interplay in AB (Moffitt 2005). Two broad types of gene–environment interplay operate to bring about AB: gene–environment correlation (rGE) and gene–environment interaction (G×E).
Risk environments may be a reflection of the parent's or child's genotype (Moffitt 2005), a phenomenon known as rGE. In short, rGE refers to genetic effects on individual differences in liability to exposure to environmental risk (Rutter 2005). Different types of rGE are passive, evocative and active rGE. Passive rGE is a result of parents providing their children with both genes and environments that are correlated with genetically influenced characteristics of parents. Evocative rGE appears when a child's inherited characteristics evoke a response from their environment. Active rGE refers to active genetically driven child choices of the particular environments. These phenomena are reviewed extensively by Moffitt (2005) and the reader is referred to this paper. However, a synopsis of the types of study designs that have demonstrated rGE effects on AB is presented here. To our knowledge, research is still required on active rGE, but several studies have shown passive and evocative rGE effects.
Adoption studies are a means of removing most passive rGE as adopted children are provided with environments by unrelated parents. As adoption studies show heritable effects on AB, this suggests that twin heritability estimates cannot just be a function of passive rGE (e.g. Cadoret et al. 1995). However, if we were to ask whether passive rGE exists, we would be asking whether the effect of the genes the parent shares with their child and also influence negative parenting confound the association between negative parenting and children's AB? Studies that have looked at the parenting of the adult MZ and DZ twins (Neiderhiser et al. 2004) or at the parenting of the adult MZ twins reared apart (Plomin et al. 1989) have demonstrated that parenting strategies show a modest genetic influence. The CoT design (e.g. D'Onofrio et al. 2003) has been put forward as an alternative to the adoption design to distinguish the direct effect of negative parenting from parent–child associations due to genetic effects. In other words, the CoT can be used to study whether the genetic effects go on to mediate the association between parenting and children's AB, i.e. to assess the potential impact of passive rGE for AB. The CoT design compares the offspring of the MZ and DZ twins who differ in their genetic and environmental risk factors. Appropriate statistical analyses, therefore, provide the opportunity of detecting and quantifying the environmental risk factors that are specific to the measured factor (e.g. negative parenting), genetic and environmental confounds that twins share. Using this approach, Harden et al. (2007) showed that the effect of parents' genes on marital conflict mediated the association between marital conflict and children's AB. D'Onofrio et al. (2007) used the same design and found an environmentally mediated role of parental AB on behavioural problems in male offspring. By contrast, common genetic risk confounds the entire intergenerational transmission in female offspring. The potential of this design is great, but there are also some noteworthy limitations. For example, this study design requires large sample sizes for more detailed analyses of family relationships and CoT models that include only one parent present potential problems (e.g. divorce: Eaves et al. 2005). Adoption designs could also be used to demonstrate passive rGE. Passive rGE exists if biological parents' parenting predicts that their adopted away child will also parent poorly. This study design would show that negative parenting is genetically transmitted, in the absence of social transmission. Currently, no such studies exist due to difficulty in obtaining intergenerational parenting data in adoption studies.
If we were to ask whether evocative rGE exists, we would be asking whether a genetic child effect confounds the association between bad parenting and children's AB. A first step to assess evocative rGE is to compare MZ and DZ twins' ratings of the parental treatment they receive. Such studies have demonstrated that genetic child effects on parenting exist (Pike et al. 1996; Neiderhiser et al. 2004), although the finding may partly be due to the MZ twins' more similar perception of parenting. These studies did not, however, explore what it is that children do to provoke negative parenting. Twin studies that also include children's AB as a measured variable are needed to more directly assess evocative rGE. Such multivariate twin designs assess to what extent Twin A's AB predicts the negative parenting Twin B' receives and vice versa. If the correlation between Twin A's AB and Twin B's experience of negative parenting is higher among MZ pairs than DZ pairs, then this would support evocative rGE processes. Existing research indicates that children's genes account for most of the relationship between negative parenting within the normal range and AB (Neiderhiser et al. 1999; Larsson et al. 2008), but not between more extreme forms of parenting (i.e. maltreatment) and AB (Jaffee et al. 2004).
Adoptee designs provide a different opportunity to explore the potential importance of evocative rGE for AB (Ge et al. 1996; O'Connor et al. 1998). One example of such a study found that children at higher genetic risk for AB (i.e. those with antisocial biological parents) are more likely to receive punitive parenting from their adoptive parents than those children without genetic risk for AB (O'Connor et al. 1998). However, the results also showed an association between negative parenting and AB, after the impact of evocative rGE was controlled for, which highlights the role of environmentally mediated parent effects.
The G×E refers to genetically influenced individual differences in the sensitivity to specific environmental factors (Rutter 2005). Within twin design, G×E can be demonstrated when response to environmental risk occurs as a function of genetic vulnerability (Kendler et al. 1995). Those MZ twins whose co-twin is affected with a disorder are at the highest risk of developing any disorder that has a genetic component, which responds to environmental risk. The DZ twins whose co-twin is affected are at the second highest risk to develop the disorder. The DZ twins with an unaffected co-twin are less vulnerable, but show a higher genetic risk than the MZ twins with an unaffected co-twin. Comparing twin pairs with different levels of genetic vulnerability, Jaffee et al. (2005) studied child conduct problems in physically maltreated and non-maltreated individuals. In line with the G×E hypothesis they found that maltreated MZ twins with an affected (i.e. antisocial) co-twin were at the highest risk of developing conduct problems after physical maltreatment, while the MZ twins with unaffected co-twins showed the lowest risk of developing conduct problems. The DZ twins with affected and unaffected co-twins fell in between the two types of MZ pairs, as predicted. Adoption studies generally demonstrate that the combination of a genetic predisposition (antisocial personality disorder and/or alcohol abuse/dependence in biological parents) and a high-risk environment (adverse adoptive home environment with stress and discord) leads to greater pathology than what would be expected from either factor acting alone or both in an additive combination (Cadoret et al. 1995).
In short, twin and adoption designs have demonstrated that genetic effects on individual differences in liability to exposure to environmental risk (rGE) and genetically influenced individual differences in the sensitivity to specific environmental factors (G×E) are important in explaining variance in AB. The G×E research on AB is now moving beyond quantitative genetic designs to studying the effects of specific genes (e.g. monoamine oxidase A) in response to environmental risk (e.g. Caspi et al. 2002; Kim-Cohen et al. 2006). This research suggests that the main effects of individual genes and environmental factors can be small, but that the G×E effects are bigger. The challenge for social scientists is to perfect environmental measurement, which is always going to be more ambiguous than determining someone's genotype or twin zygosity status. More studies of environmental effects in the abnormal range are required and environmental influences beyond the family need to be studied in more detail both within a twin/adoption framework and in study designs including measured genotypes. These are the future challenges of quantitative genetic research.
6. Translational implications of quantitative genetic research
Publication of results from twin and adoption data can often make sensational headlines, largely due to the misunderstanding of what the heritability statistic represents. A recent Academy of Medical Sciences working group report highlights that science should be integrated into policy making by, for example, embedding researchers into policy teams and providing senior civil servants with scientific training (Academy of Medical Sciences 2007). It is important to engage with policy makers to ensure that the nature of the heritability statistic is understood. A heritability estimate of 70% does not mean that a single individual is at a 70% risk of developing a disorder or that a disorder outcome is nearly inevitable. It merely means that 70% of the individual or group differences in the population (at that particular time) are influenced by genetic differences among individuals. It is also important to highlight that no genes directly code for AB. Instead, genes code for proteins that influence characteristics such as neurocognitive vulnerabilities that may in turn increase risk for AB.
In this section we will focus our discussion to prevention and treatment of AB in child populations. Antisocial personality disorder does not suddenly manifest itself as a complete problem in adulthood; rather it has its origins and early indicators in childhood and adolescence. Research has demonstrated robust trajectories of AB, and strong links between delinquency, conduct disorder and later antisocial personality disorder have been reported (Moffitt 2003). Given the high burden and cost of AB, novel prevention and treatment approaches for at risk children are imperative. Early intervention strategies have potentially substantial cost benefits for the tax payer. High-quality early intervention has been shown to be cost effective in the US (Schweinhart & Weikart 1998) and has been highly recommended by Romeo et al. (2006) in a study explicitly investigating the economic cost of severe AB in children.
Research into the environmental risk factors within quantitative genetic designs has highlighted a number of important issues. First of all, the implicit assumption within social sciences research that risk environments have a main effect on behaviour appears to be true for some important social risk factors, such as parenting and maltreatment. It has also been shown that for children with vulnerable genotype, this genotype will react with risk environments, the phenomenon known as G×E. Furthermore, at least one of the parents will share the risk genes for AB and thus the parental genotype can also contribute to a less-than optimal rearing environment, a phenomenon known as passive rGE. It is also possible that a child with a certain genotype is more likely to evoke negative parenting, a phenomenon known as evocative rGE. Parenting programmes, such as those outlined in the UK National Institute for Health and Clinical Excellence (NICE) guidelines, are targeted to changing parental response to difficult behaviours that children with conduct problems exhibit (http://www.nice.org.uk/guidance/index.jsp?action=byID&o=11584). Mapping out the environmental risk profile and the potential challenges to ameliorating such a profile is extremely important for successful intervention. This notion has also been implemented with results in programmes such as multisystemic therapy (Schaeffer & Borduin 2005). It is also noteworthy that simple interventions, such as nurse-visit programmes, can be extremely successful in breaking the association between maltreatment and AB (Olds et al. 1997; Eckenrode et al. 2001). This suggests that genetic risk can be effectively moderated by environmental intervention and enables us to think about treatment as a positive form of G×E. However, at-risk families should be monitored and helped regardless of the child's or parent's genotype. Does the genetic information therefore add anything? We would argue that even if environmental interventions are already provided, the scope to make them better lies within better understanding of the mechanisms of gene–environment interplay.
Quantitative genetic research can also help in isolating disorders or patterns that may require distinct forms of intervention. For example, current quantitative genetic research supports the notion of subtyping antisocial children on callous–unemotional traits (Viding et al. 2005, 2008). Aetiologically heterogeneous samples may explain why intervention programmes can sometimes have mixed results on their success (Frick 2001; Hawes & Dadds 2005). Some children seem to respond to well-timed early prevention and treatment while others do not. We would suggest that the root of this may lie in aetiological differences and concomitant differences in cognitive profile of distinct subtypes of children with AB. It is particularly challenging to map out the cognitive profiles and make predictions about treatment approaches that capitalize on what is known about cognitive strengths and weaknesses. For example, children with CU traits are strong on self-interest and get motivated by rewards, but do not characteristically process others' distress or react to punishment (Blair et al. 2006). These are cognitive strengths and limitations that have to be worked with to produce change in behaviour. There may also eventually be scope for pharmacogenetic interventions (i.e. medical interventions tailored to suit genotype-driven response to drugs) that support cognitive behavioural and family approaches, provided that the research suggests that the pharmacogenetic interventions enhance the success of these therapeutic programmes.
7. Summary
Both genetic vulnerability and environmental factors account for variance in AB. Of the environmental risk factors, those that act in a child-specific manner, such as negative parenting, appear to be most important for the development of AB. Genetic effects on individual differences in liability to exposure to environmental risk (rGE) and genetically influenced individual differences in the sensitivity to specific environmental factors (G×E) both operate to increase liability to AB. Future research must concentrate on better understanding the mechanisms of gene–environment interplay and on translating such understanding to improved intervention programmes. Quantitative genetic research can also improve the understanding of different subtypes of AB and resultant scope and limitations for tailored interventions.
Acknowledgments
This work was supported by grants from the Medical Research Council (G0401170) and the Department of Health FMH Programme (MRD 12-37) to E.V. and H.L. was supported by a postdoctoral stipend from the Swedish Brain Foundation.
Footnotes
One contribution of 11 to a Discussion Meeting Issue ‘The neurobiology of violence: implications for prevention and treatment’.
References
- Academy of Medical Sciences 2007 Identifying the environmental causes of disease: how should we decide what to believe and when to take action? See http://www.acmedsci.ac.uk/p47prid50.html
- Arseneault L, Moffitt T.E, Caspi A, Taylor A, Rijsdijk F.V, Jaffee S.R, Ablow J.C, Measelle J.R. Strong genetic effects on cross-situational antisocial behaviour among 5-year-old children according to mothers, teachers, examiner-observers, and twins' self-reports. J. Child Psychol. Psychiatry. 2003;44:832–848. doi: 10.1111/1469-7610.00168. doi:10.1111/1469-7610.00168 [DOI] [PubMed] [Google Scholar]
- Asbury K, Dunn J.F, Pike A, Plomin R. Nonshared environmental influences on individual differences in early behavioral development: a monozygotic twin differences study. Child Dev. 2003;74:933–943. doi: 10.1111/1467-8624.00577. doi:10.1111/1467-8624.00577 [DOI] [PubMed] [Google Scholar]
- Bailey S. Treatment of delinquents. In: Rutter M, Taylor E, editors. Child and adolescent psychiatry: modern approaches. Blackwell Scientific; Oxford, UK: 2002. pp. 1019–1037. [Google Scholar]
- Blair R.J.R, Peschardt K.S, Budhani S, Mitchell D.G.V, Pine D.S. The development of psychopathy. J. Child Psychol. Psychiatry. 2006;47:262–275. doi: 10.1111/j.1469-7610.2006.01596.x. doi:10.1111/j.1469-7610.2006.01596.x [DOI] [PubMed] [Google Scholar]
- Burt S.A, McGue M, Iacono W.G, Krueger R.F. Differential parent–child relationships and adolescent externalizing symptoms: cross-lagged analyses within a monozygotic twin differences design. Dev. Psychol. 2006;42:1289–1298. doi: 10.1037/0012-1649.42.6.1289. doi:10.1037/0012-1649.42.6.1289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadoret R.J, Yates W.R, Troughton E, Woodworth G, Stewart M.A. Genetic–environmental interaction in the genesis of aggressivity and conduct disorders. Arch. Gen. Psychiatry. 1995;52:916–924. doi: 10.1001/archpsyc.1995.03950230030006. [DOI] [PubMed] [Google Scholar]
- Caspi A, McClay J, Moffitt T.E, Mill J, Martin J, Craig I.W, Taylor A, Poulton R. Role of genotype in the cycle of violence in maltreated childern. Science. 2002;297:851–854. doi: 10.1126/science.1072290. doi:10.1126/science.1072290 [DOI] [PubMed] [Google Scholar]
- Caspi A, et al. Maternal expressed emotion predicts children's antisocial behaviour problems: using MZ-twin differences to identify environmental effects on behavioural development. Dev. Psychol. 2004;40:149–161. doi: 10.1037/0012-1649.40.2.149. doi:10.1037/0012-1649.40.2.149 [DOI] [PubMed] [Google Scholar]
- Cronk N.J, Slutske W.S, Madden P.A, Bucholz K.K, Reich W, Heath A.C. Emotional and behavioral problems among female twins: an evaluation of the equal environments assumption. J. Am. Acad. Child Adolesc. Psychiatry. 2002;41:829–837. doi: 10.1097/00004583-200207000-00016. doi:10.1097/00004583-200207000-00016 [DOI] [PubMed] [Google Scholar]
- Department for Education and Skills. HM Stationery Office; London, UK: 2003. Every child matters green paper. [Google Scholar]
- Dick D.M, Viken R.J, Kaprio J, Pulkkinen L, Rose R.J. Understanding the covariation among childhood externalizing symptoms: genetic and environmental influences on conduct disorder, attention deficit hyperactivity disorder, and oppositional defiant disorder symptoms. J. Abnorm. Child Psychol. 2005;33:219–229. doi: 10.1007/s10802-005-1829-8. doi:10.1007/s10802-005-1829-8 [DOI] [PubMed] [Google Scholar]
- D'Onofrio B.M, Turkheimer E, Eaves L.J, Corey L.A, Berg K, Solaas M.H, Emery R.E. The role of the children of twins design in elucidating causal relations between parent characteristics and child outcomes. J. Child Psychol. Psychiatry. 2003;44:1130–1144. doi: 10.1111/1469-7610.00196. doi:10.1111/1469-7610.00196 [DOI] [PubMed] [Google Scholar]
- D'Onofrio B.M, Slutske W.S, Turkheimer E, Emery R.E, Harden K.P, Heath A.C, Madden P.A, Martin N.G. Intergenerational transmission of childhood conduct problems: a children of twins study. Arch. Gen. Psychiatry. 2007;64:820–829. doi: 10.1001/archpsyc.64.7.820. doi:10.1001/archpsyc.64.7.820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaves L.J, Silberg J.L, Maes H.H. Revisiting the children of twins: can they be used to resolve the environmental effects of dyadic parental treatment on child behavior? Twin Res. Hum. Genet. 2005;8:283–290. doi: 10.1375/1832427054936736. doi:10.1375/twin.8.4.283 [DOI] [PubMed] [Google Scholar]
- Eckenrode J, Zielinski D, Smith E, Marcynyszyn L.A, Henderson C.R, Jr, Kitzman H, Cole R, Powers J, Olds D.L. Child maltreatment and the early onset of problem behaviors: can a program of nurse home visitation break the link? Dev. Psychopathol. 2001;13:873–890. [PubMed] [Google Scholar]
- Eley T.C, Lichtenstein P, Stevenson J. Sex differences in the etiology of aggressive and nonaggressive antisocial behavior: results from two twin studies. Child Dev. 1999;70:155–168. doi: 10.1111/1467-8624.00012. doi:10.1111/1467-8624.00012 [DOI] [PubMed] [Google Scholar]
- Eley T.C, Lichtenstein P, Moffitt T.E. A longitudinal behavioral genetic analysis of the etiology of aggressive and non-aggressive antisocial behavior. Dev. Psychopathol. 2003;15:383–402. doi: 10.1017/s095457940300021x. doi:10.1017/S095457940300021X [DOI] [PubMed] [Google Scholar]
- Frick P.J. Effective interventions for children and adolescents with conduct disorder. Can. J. Psychiatry. 2001;46:597–608. doi: 10.1177/070674370104600703. [DOI] [PubMed] [Google Scholar]
- Ge X, Conger R.D, Cadoret R.J, Neiderhiser J.M, Yates W, Troughton E, Stewart M.A. The developmental interface between nature and nurture: a mutual influence model of child antisocial behavior and parent behaviors. Dev. Psychol. 1996;32:574–589. doi:10.1037/0012-1649.32.4.574 [Google Scholar]
- Harden K.P, Turkheimer E, Emery R.E, D'Onofrio B.M, Slutske W.S, Heath A.C, Martin N.G. Marital conflict and conduct problems in children of twins. Child Dev. 2007;78:1–18. doi: 10.1111/j.1467-8624.2007.00982.x. doi:10.1111/j.1467-8624.2007.00982.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawes D.J, Dadds M.R. The treatment of conduct problems in children with callous–unemotional traits. J. Consult. Clin. Psychol. 2005;73:737–741. doi: 10.1037/0022-006X.73.4.737. doi:10.1037/0022-006X.73.4.737 [DOI] [PubMed] [Google Scholar]
- Hinshaw S.P. Process, mechanism, and explanation related to externalizing behavior in developmental psychopathology. J. Abnorm. Child Psychol. 2002;30:431–446. doi: 10.1023/a:1019808712868. doi:10.1023/A:1019808712868 [DOI] [PubMed] [Google Scholar]
- Jaffee S.R, Caspi A, Moffitt T.E, Polo-Tomas M, Price T.S, Taylor A. The limits of child effects: evidence for genetically mediated child effects on corporal punishment but not on physical maltreatment. Dev. Psychol. 2004;40:1047–1058. doi: 10.1037/0012-1649.40.6.1047. doi:10.1037/0012-1649.40.6.1047 [DOI] [PubMed] [Google Scholar]
- Jaffee S.R, Caspi A, Moffitt T.E, Dodge K.A, Rutter M, Taylor A, Tully L.A. Nature X nurture: genetic vulnerabilities interact with physical maltreatment to promote conduct problems. Dev. Psychopathol. 2005;17:67–84. doi: 10.1017/s0954579405050042. doi:10.1017/S0954579405050042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler K.S, Kessler R.C, Walters E.E, MacLean C, Neale M.C, Heath A.C, Eaves L.J. Stressful life events, genetic liability, and onset of an episode of major depression in women. Am. J. Psychiatry. 1995;152:833–842. doi: 10.1176/ajp.152.6.833. [DOI] [PubMed] [Google Scholar]
- Kim-Cohen J, Caspi A, Taylor A, Williams B, Newcombe R, Craig I.W, Moffitt T.E. MAOA, maltreatment, and gene–environment interaction predicting childern's mental health: new evidence and a meta-analysis. Mol. Psychiatry. 2006;11:903–913. doi: 10.1038/sj.mp.4001851. doi:10.1038/sj.mp.4001851 [DOI] [PubMed] [Google Scholar]
- Krueger R.F, Hicks B.M, Patrick C.J, Carlson S.R, Iacono W.G, McGue M. Etiologic connections among substance dependence, antisocial behavior, and personality: modeling the externalizing spectrum. J. Abnorm. Psychol. 2002;111:411–424. doi:10.1037/0021-843X.111.3.411 [PubMed] [Google Scholar]
- Krug E.G, Dahlberg L, Mercy J, Zwi A, Lozano R. WHO Library; Geneva, Switerzland: 2002. World report on violence and health. [Google Scholar]
- Larsson H, Tuvblad C, Rijsdijk F, Andershed H, Grann M, Lichtenstein P. A common genetic factor explains the association between psychopathic personality and antisocial behaviour. Psychol. Med. 2007;37:15–26. doi: 10.1017/S003329170600907X. doi:10.1017/S003329170600907X [DOI] [PubMed] [Google Scholar]
- Larsson, H., Viding, E., Rijsdijk, F. V. & Plomin, R. 2008 Relationships between parental negativity and childhood antisocial behavior over time: a bidirectional effect model in a longitudinal genetically informative design. J. Abnorm. Child Psychol 36, 633–645. (doi:10.1007/s10802-007-9151-2) [DOI] [PubMed]
- Moffitt T.E. Life-course-persistent and adolescence-limited antisocial behavior. In: Lahey B.B, Moffitt T.E, Caspi A, editors. Causes of conduct disorder and juvenile delinquency. Guilford Press; New York, NY: 2003. pp. 49–75. [Google Scholar]
- Moffitt T.E. The new look of behavioral genetics in developmental psychopathology: gene–environment interplay in antisocial behaviors. Psychol. Bull. 2005;131:533–554. doi: 10.1037/0033-2909.131.4.533. doi:10.1037/0033-2909.131.4.533 [DOI] [PubMed] [Google Scholar]
- Nadder T.S, Rutter M, Silberg J.L, Maes H.H, Eaves L.J. Genetic effects on the variation and covariation of attention deficit-hyperactivity disorder (ADHD) and oppositional-defiant disorder/conduct disorder (ODD/CD) symptomatologies across informant and occasion of measurement. Psychol. Med. 2002;32:39–53. doi: 10.1017/s0033291701004792. doi:10.1017/S0033291701004792 [DOI] [PubMed] [Google Scholar]
- Neiderhiser J.M, Reiss D, Hetherington E.M, Plomin R. Relationships between parenting and adolescent adjustment over time: genetic and environmental contributions. Dev. Psychol. 1999;35:680–692. doi: 10.1037//0012-1649.35.3.680. doi:10.1037/0012-1649.35.3.680 [DOI] [PubMed] [Google Scholar]
- Neiderhiser J.M, Reiss D, Pedersen N, Lichtenstein P, Spotts E.L, Hansson K, Cederblad M, Elthammer O. Genetic and environmental influences on mothering of adolescents: a comparison of two samples. Dev. Psychol. 2004;40:335–351. doi: 10.1037/0012-1649.40.3.335. doi:10.1037/0012-1649.40.3.335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor T.G, Deater-Deckard K, Fulker D, Rutter M, Plomin R. Genotype–environment correlations in late childhood and early adolescence: antisocial behavioral problems and coercive parenting. Dev. Psychol. 1998;34:970–981. doi: 10.1037//0012-1649.34.5.970. doi:10.1037/0012-1649.34.5.970 [DOI] [PubMed] [Google Scholar]
- Olds D.L, et al. Long-term effects of home visitation on maternal life course and child abuse and neglect. Fifteen-year follow-up of a randomized trial. JAMA. 1997;278:637–643. doi:10.1001/jama.278.8.637 [PubMed] [Google Scholar]
- Pike A, McGuire S, Hetherington E.M, Reiss D, Plomin R. Family environment and adolescent depression and antisocial behavior: a multivariate genetic analysis. Dev. Psychol. 1996;32:590–603. doi:10.1037/0012-1649.32.4.590 [Google Scholar]
- Plomin R, McClearn G.E, Pedersen N.L, Nesselroade J.R, Bergeman C.S. Genetic influence on adults' ratings of their current family environment. J. Marriage Fam. 1989;51:791–803. doi:10.2307/352177 [Google Scholar]
- Plomin R, DeFries J, McClearn G, McGuffin P. 5th edn. Worth Publishers; New York, NY: 2008. Behavioral genetics. [Google Scholar]
- Rhee S.H, Waldman I.D. Genetic and environmental influences on antisocial behavior: a meta-analysis of twin and adoption studies. Psychol. Bull. 2002;128:490–529. doi:10.1037/0033-2909.128.3.490 [PubMed] [Google Scholar]
- Romeo R, Knapp M, Scott S. Economic cost of severe antisocial behaviour in children—and who pays for it. Br. J. Psychiatry. 2006;188:547–553. doi: 10.1192/bjp.bp.104.007625. doi:10.1192/bjp.bp.104.007625 [DOI] [PubMed] [Google Scholar]
- Rutter M. Blackwell Publishing; Oxford, UK: 2005. Genes and behavior: nature–nurture interplay explained. [Google Scholar]
- Schaeffer C.M, Borduin C.M. Long-term follow-up to a randomized clinical trial of multisystemic therapy with serious and violent juvenile offenders. J. Consult. Clin. Psychol. 2005;73:445–453. doi: 10.1037/0022-006X.73.3.445. doi:10.1037/0022-006X.73.3.445 [DOI] [PubMed] [Google Scholar]
- Schweinhart L.J, Weikart D.P. High/Scope Perry Preschool Program effects at age twenty-seven. In: Crane J, editor. Social programs that work. Russell Sage Foundation; New York, NY: 1998. pp. 148–162. [Google Scholar]
- Silberg J, Meyer J, Pickles A, Simonoff E, Eaves L, Hewitt J, Maes H, Rutter M. Heterogeneity among juvenile antisocial behaviours: findings from the Virginia twin study of adolescent behavioural development. Ciba Found. Symp. 1996a;194:76–86. doi: 10.1002/9780470514825.ch5. [DOI] [PubMed] [Google Scholar]
- Silberg J, Rutter M, Meyer J, Maes H, Hewitt J, Simonoff E, Pickles A, Loeber R, Eaves L. Genetic and environmental influences on the covariation between hyperactivity and conduct disturbance in juvenile twins. J. Child Psychol. Psychiatry. 1996b;37:803–816. doi: 10.1111/j.1469-7610.1996.tb01476.x. doi:10.1111/j.1469-7610.1996.tb01476.x [DOI] [PubMed] [Google Scholar]
- Taylor J, Iacono W.G, McGue M. Evidence for a genetic etiology of early-onset delinquency. J. Abnorm. Psychol. 2000a;109:634–643. doi: 10.1037//0021-843x.109.4.634. doi:10.1037/0021-843X.109.4.634 [DOI] [PubMed] [Google Scholar]
- Taylor J, McGue M, Iacono W.G. Sex differences, assortative mating, and cultural transmission effects on adolescent delinquency: a twin family study. J. Child Psychol. Psychiatry. 2000b;41:433–440. doi:10.1017/S0021963000005692 [PubMed] [Google Scholar]
- Taylor J, Loney B.R, Bobadilla I, Iacono W.G, McGue M. Genetic and environmental influences on psychopathy traits dimensions in a community sample of male twins. J. Abnorm. Child Psychol. 2003;31:633–645. doi: 10.1023/a:1026262207449. doi:10.1023/A:1026262207449 [DOI] [PubMed] [Google Scholar]
- Thapar A, Harrington R, McGuffin P. Examining the comorbidity of ADHD-related behaviours and conduct problems using a twin study design. Br. J. Psychiatry. 2001;179:224–229. doi: 10.1192/bjp.179.3.224. doi:10.1192/bjp.179.3.224 [DOI] [PubMed] [Google Scholar]
- Van Hulle C.A, Rodgers J.L, D'Ononfriou B.M, Waldman I.D, Lahey B.B. Sex differences in the causes of self-reported adolescent delinquency. J. Abnorm. Psychol. 2007;116:236–248. doi: 10.1037/0021-843X.116.2.236. doi:10.1037/0021-843X.116.2.236 [DOI] [PubMed] [Google Scholar]
- Viding E, Blair R.J.R, Moffitt T.E, Plomin R. Evidence for substantial genetic risk for psychopathy in 7-year-olds. J. Child Psychol. Psychiatry. 2005;46:592–597. doi: 10.1111/j.1469-7610.2004.00393.x. doi:10.1111/j.1469-7610.2004.00393.x [DOI] [PubMed] [Google Scholar]
- Viding E, Frick P.J, Plomin R. Aetiology of the relationship between callous–unemotional traits and conduct problems in childhood. Br. J. Psychiatry. 2007;190:s33–s38. doi: 10.1192/bjp.190.5.s33. doi:10.1192/bjp.190.5.s33 [DOI] [PubMed] [Google Scholar]
- Viding E, Jones A.P, Frick P, Moffitt T.E, Plomin R. Genetic and phenotypic investigation to early risk factors for conduct problems in children with and without psychopathic tendencies. Dev. Sci. 2008;11:17–22. [Google Scholar]
- Waldman I.D, Rhee S.H. Genetic and environmental influences on psychopathy and antisocial behavior. In: Patrick C.J, editor. Handbook of psychopathy. Guilford; New York, NY: 2006. pp. 205–228. [Google Scholar]