Abstract
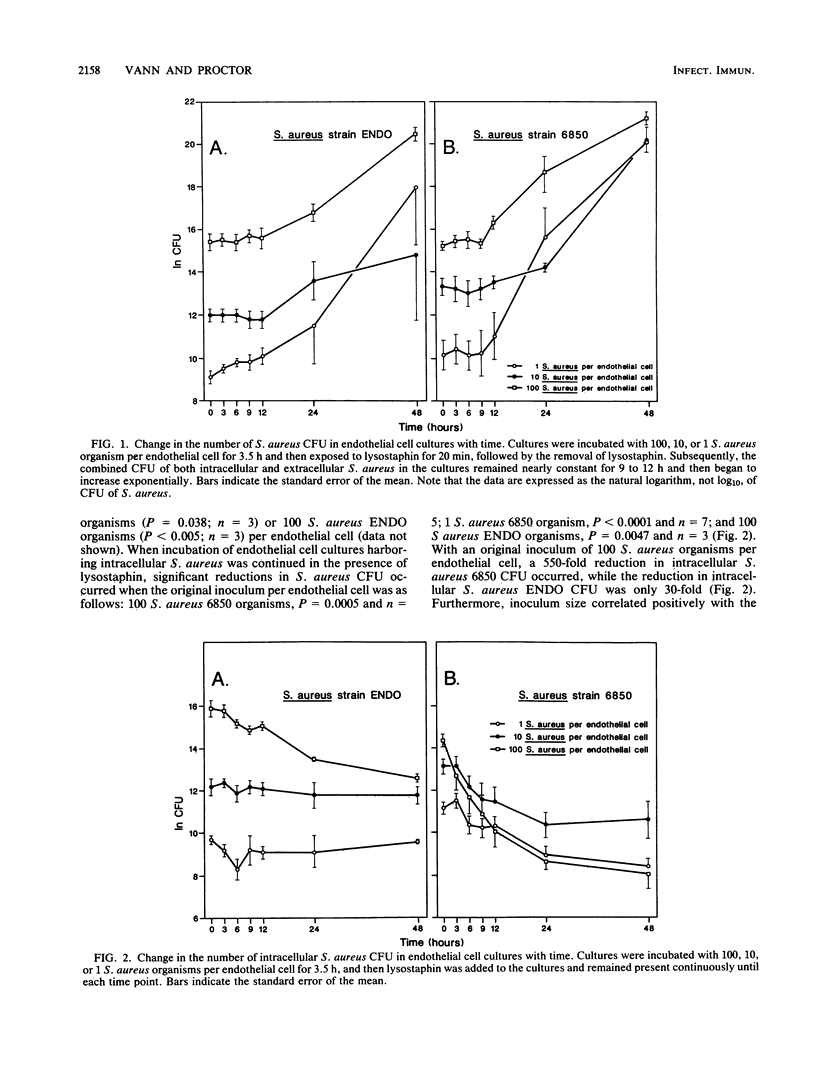
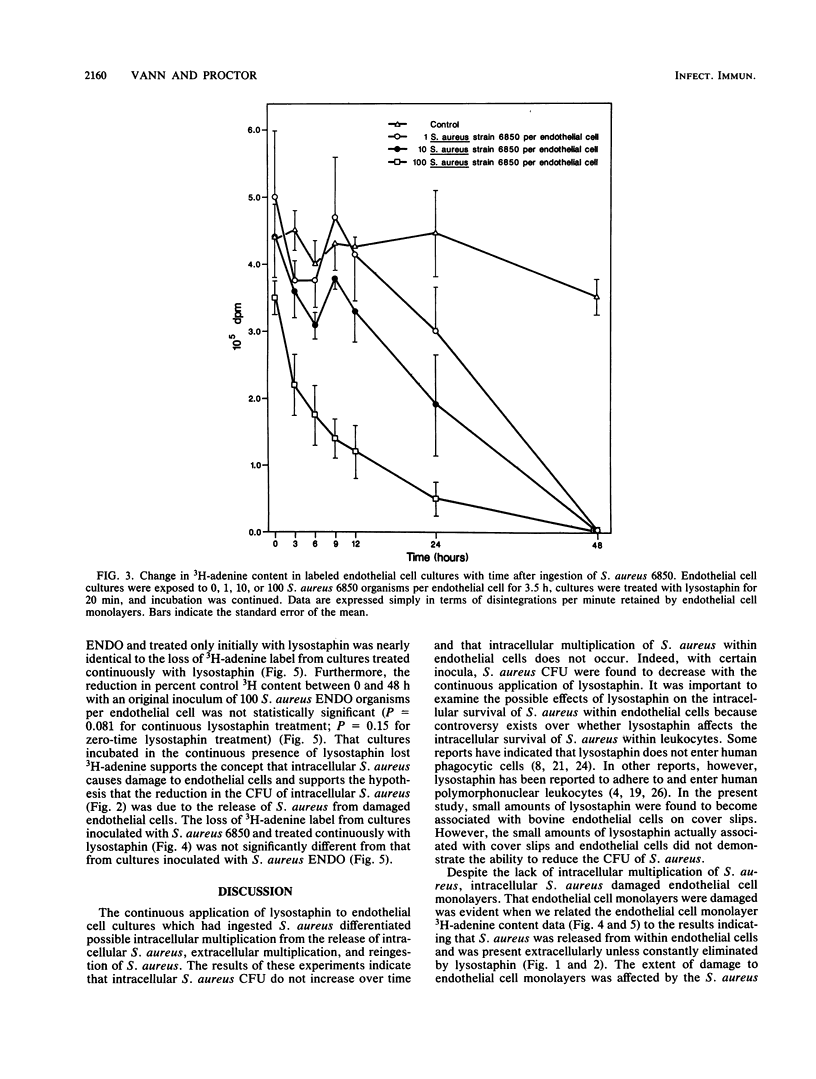
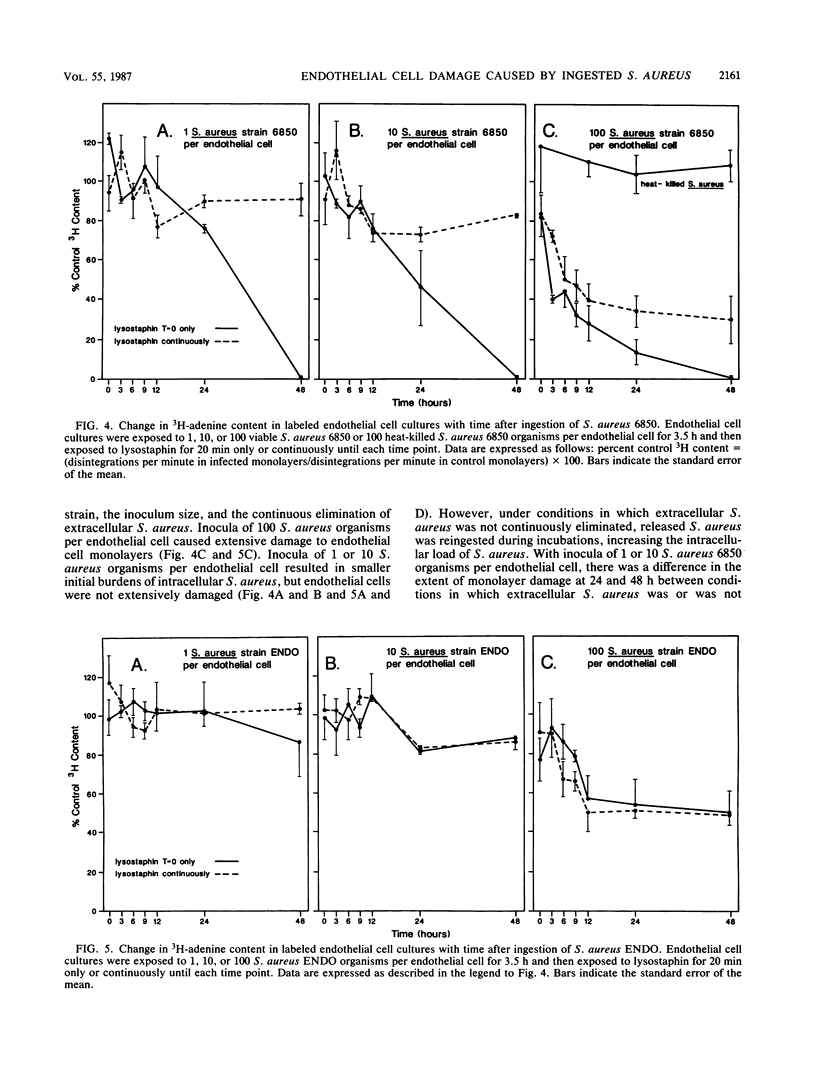
Cultured endothelial cells phagocytize Staphylococcus aureus, but the resultant effects are unknown. Monolayers of cultured bovine endothelial cells with or without [3H]adenine label were exposed to 100, 10, or 1 S. aureus organism per endothelial cell for 3.5 h. Lysostaphin was then applied to all cultures to destroy extracellular but not phagocytized S. aureus. In cultures treated for only 20 min with lysostaphin, S. aureus multiplied exponentially after a 9- to 12-h lag period. In cultures treated continuously with lysostaphin, numbers of S. aureus remained constant or decreased. These results indicate that S. aureus became extracellular and multiplied but did not multiply intracellularly. In parallel experiments, the release of 3H-adenine from prelabeled endothelial cell monolayers was assayed to indicate cytotoxicity. Results indicated that the loss of 3H-adenine from endothelial cell monolayers depended on the following: (i) the size of the S. aureus inoculum, (ii) the strain of S. aureus, and (iii) the length of time after exposure to S. aureus. S. aureus endocarditis and persistent septicemia could arise, at least in part, from ingestion of S. aureus by host endothelium. The intracellular location would afford S. aureus protection from host defenses and antibiotics. Eventual damage to endothelial cells could expose collagen, thus resulting in platelet adherence and vegetation formation. Intracellular S. aureus would be continuously released into the circulation, possibly accounting for the persistent bacteremia that is found in S. aureus endovascular infections.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andreoli S. P., Baehner R. L., Bergstein J. M. In vitro detection of endothelial cell damage using 2-deoxy-D-3H-glucose: comparison with chromium 51, 3H-leucine, 3H-adenine, and lactate dehydrogenase. J Lab Clin Med. 1985 Sep;106(3):253–261. [PubMed] [Google Scholar]
- Baughn R., Bonventre P. F. Phagocytosis and intracellular killing of Staphylococcus aureus by normal mouse peritoneal macrophages. Infect Immun. 1975 Aug;12(2):346–352. doi: 10.1128/iai.12.2.346-352.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper M. D., Jeffery C., Gall D. L., Anderson A. S. Scanning electron microscopy studies of staphylococcal adherence to heart valve endothelial cells in organ culture: an in vitro model of acute endocarditis. Scan Electron Microsc. 1985;(Pt 3):1231–1237. [PubMed] [Google Scholar]
- Easmon C. S., Lanyon H., Cole P. J. Use of lysostaphin to remove cell-adherent staphylococci during in vitro assays of phagocyte function. Br J Exp Pathol. 1978 Aug;59(4):381–385. [PMC free article] [PubMed] [Google Scholar]
- Faville R. J., Jr, Zaske D. E., Kaplan E. L., Crossley K., Sabath L. D., Quie P. G. Staphylococcus aureus endocarditis. Combined therapy with vancomycin and rifampin. JAMA. 1978 Oct 27;240(18):1963–1965. doi: 10.1001/jama.240.18.1963. [DOI] [PubMed] [Google Scholar]
- Hamill R. J., Vann J. M., Proctor R. A. Phagocytosis of Staphylococcus aureus by cultured bovine aortic endothelial cells: model for postadherence events in endovascular infections. Infect Immun. 1986 Dec;54(3):833–836. doi: 10.1128/iai.54.3.833-836.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hand W. L., King-Thompson N. L. Contrasts between phagocyte antibiotic uptake and subsequent intracellular bactericidal activity. Antimicrob Agents Chemother. 1986 Jan;29(1):135–140. doi: 10.1128/aac.29.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe E. A., Hoyer L. W., Nachman R. L. Synthesis of antihemophilic factor antigen by cultured human endothelial cells. J Clin Invest. 1973 Nov;52(11):2757–2764. doi: 10.1172/JCI107471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan E. L., Rich H., Gersony W., Manning J. A collaborative study of infective endocarditis in the 1970s. Emphasis on infections in patients who have undergone cardiovascular surgery. Circulation. 1979 Feb;59(2):327–335. doi: 10.1161/01.cir.59.2.327. [DOI] [PubMed] [Google Scholar]
- Karchmer A. W. Staphylococcal endocarditis. Laboratory and clinical basis for antibiotic therapy. Am J Med. 1985 Jun 28;78(6B):116–127. doi: 10.1016/0002-9343(85)90374-2. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Macneal W. J., Spence M. J., Slavkin A. E. Early Lesions of Experimental Endocarditis Lenta. Am J Pathol. 1943 Sep;19(5):735–749. [PMC free article] [PubMed] [Google Scholar]
- Mandell G. L., Vest T. K. Killing of intraleukocytic Staphylococcus aureus by rifampin: in-vitro and in-vivo studies. J Infect Dis. 1972 May;125(5):486–490. doi: 10.1093/infdis/125.5.486. [DOI] [PubMed] [Google Scholar]
- Mirimanoff R. O., Glauser M. P. Endocarditis during Staphylococcus aureus septicemia in a population of non-drug addicts. Arch Intern Med. 1982 Jul;142(7):1311–1313. [PubMed] [Google Scholar]
- Ogawa S. K., Yurberg E. R., Hatcher V. B., Levitt M. A., Lowy F. D. Bacterial adherence to human endothelial cells in vitro. Infect Immun. 1985 Oct;50(1):218–224. doi: 10.1128/iai.50.1.218-224.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruzanski W., Saito S., Nitzan D. W. The influence of lysostaphin on phagocytosis, intracellular bactericidal activity, and chemotaxis of human polymorphonuclear cells. J Lab Clin Med. 1983 Aug;102(2):298–305. [PubMed] [Google Scholar]
- Rajashekaraiah K. R., Rice T., Rao V. S., Marsh D., Ramakrishna B., Kallick C. A. Clinical significance of tolerant strains of Staphylococcus aureus in patients with endocarditis. Ann Intern Med. 1980 Dec;93(6):796–801. doi: 10.7326/0003-4819-93-6-796. [DOI] [PubMed] [Google Scholar]
- Schaffner W., Melly M. A., Hash J. H., Koenig M. G. Lysostaphin: an enzymatic approach to staphylococcal disease. I. In vitro studies. Yale J Biol Med. 1967 Feb;39(4):215–229. [PMC free article] [PubMed] [Google Scholar]
- Scheld W. M., Valone J. A., Sande M. A. Bacterial adherence in the pathogenesis of endocarditis. Interaction of bacterial dextran, platelets, and fibrin. J Clin Invest. 1978 May;61(5):1394–1404. doi: 10.1172/JCI109057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan J. S., Watanakunakorn C., Phair J. P. A modified assay of neutrophil function: use of lysostaphin to differentiate defective phagocytosis from impaired intracellular killing. J Lab Clin Med. 1971 Aug;78(2):316–322. [PubMed] [Google Scholar]
- Thompson R. L. Staphylococcal infective endocarditis. Mayo Clin Proc. 1982 Feb;57(2):106–114. [PubMed] [Google Scholar]
- Vercellotti G. M., Lussenhop D., Peterson P. K., Furcht L. T., McCarthy J. B., Jacob H. S., Moldow C. F. Bacterial adherence to fibronectin and endothelial cells: a possible mechanism for bacterial tissue tropism. J Lab Clin Med. 1984 Jan;103(1):34–43. [PubMed] [Google Scholar]
- Watanakunakorn C., Tan J. S., Phair J. P. Some salient features of Staphylococcus aureus endocarditis. Am J Med. 1973 Apr;54(4):473–481. doi: 10.1016/0002-9343(73)90043-0. [DOI] [PubMed] [Google Scholar]
- van den Broek P. J., Dehue F. A., Leijh P. C., van den Barselaar M. T., van Furth R. The use of lysostaphin in in vitro assays of phagocyte function: adherence to and penetration into granulocytes. Scand J Immunol. 1981 May;15(5):467–473. doi: 10.1111/j.1365-3083.1982.tb00672.x. [DOI] [PubMed] [Google Scholar]



