Abstract
This paper reviews information from ecological and physiological studies to assess how extrinsic factors can modulate intrinsic physiological processes. The annual cycle of birds is made up of a sequence of life-history stages: breeding, moult and migration. Each stage has evolved to occur at the optimum time and to last for the whole duration of time available. Some species have predictable breeding seasons, others are more flexible and some breed opportunistically in response to unpredictable food availability. Photoperiod is the principal environmental cue used to time each stage, allowing birds to adapt their physiology in advance of predictable environmental changes. Physiological (neuroendocrine and endocrine) plasticity allows non-photoperiodic cues to modulate timing to enable individuals to cope with, and benefit from, short-term environmental variability. Although the timing and duration of the period of full gonadal maturation is principally controlled by photoperiod, non-photoperiodic cues, such as temperature, rainfall or food availability, could potentially modulate the exact time of breeding either by fine-tuning the time of egg-laying within the period of full gonadal maturity or, more fundamentally, by modulating gonadal maturation and/or regression. The timing of gonadal regression affects the time of the start of moult, which in turn may affect the duration of the moult. There are many areas of uncertainty. Future integrated studies are required to assess the scope for flexibility in life-history strategies as this will have a critical bearing on whether birds can adapt sufficiently rapidly to anthropogenic environmental changes, in particular climate change.
Keywords: annual cycle in birds, photoperiodism, breeding season, moult, temperature, rainfall
1. Introduction
Life-history strategies evolve in response to extrinsic (biotic and abiotic) constraints. In the context of this review, they explain why breeding seasons occur at a particular time, last a particular duration, the degree of flexibility in these parameters, and why birds moult at a particular time and in a particular relationship with the breeding season. Intrinsic physiological mechanisms control each life-history stage. Habitats are diverse with respect to seasonality and in the degree of predictability of this seasonality, i.e. the amount of intra- and inter-annual variation. Predictability of seasonal change is important because, in order to match their behaviour to the seasons, birds need to predict the optimal time for each life-history stage to complete the necessary changes in physiology in advance (Colwell 1974; Wingfield et al. 1992; Wingfield 2007). Comparatively rigid physiological mechanisms have evolved in response to predictable ecological constraints, but physiological plasticity also exists to enable individuals to cope with, and benefit from, short-term environmental variability. This review examines how predictable external cues (principally photoperiod) are used to time physiological processes, and how unpredictable cues (e.g. temperature) may modulate these. Trying to explain the control of life-history stages without understanding the potentially important physiological processes can lead to erroneous conclusions. Furthermore, adaptive responses to environmental change (natural or anthropogenic) must act initially through physiology.
2. Basic life-history strategies
Seasonal changes in climate during the year impose constraints on life-history strategies. In particular, there is intense selective pressure to restrict breeding attempts to the time of year when food on which young are dependent is most abundant. Furthermore, seasonal differences in food availability often lead to a life-history strategy that includes migration (Ramenofsky & Wingfield 2007). Most species of birds also need to completely replace their plumage each year, and so a period for moult needs to be included in the annual cycle. Detailed annual cycles for many species can be found in Cramp (1998). Each of the stages, breeding, moult, spring and autumn migrations, has evolved to occur at the optimum time and in the optimum sequence. Also, an overlap between successive stages tends to be minimized. Clearly, breeding cannot start until the spring migration is complete. Moult is normally separated from breeding because both are energetically demanding (Newton 1966; Morton et al. 1969; Murphy & King 1992; Newton & Rothery 2005). Often, moult starts after breeding, although in some species, it may take place on the wintering grounds after migration (Barta et al. in press). Some species may have two moults, one immediately after breeding and before the autumnal migration, and the other after migration (Underhill et al. 1992). This may be owing to time constraints on the immediate post-nuptial moult resulting in poor quality plumage that then needs to be replaced later. Yet, other species have a slow protracted moult that continues, albeit at a slower rate, during the breeding season. Moult and migration are normally temporally distinct because birds migrating with incomplete plumage would clearly be at a disadvantage. In species that moult before autumn migration, moult tends to be completed before migration starts (Pulido & Coppack 2004), but in some species moulting may be interrupted by migration, and completed after migration (Hall & Fransson 2001).
Temporal overlap between successive stages tends to be minimized, while at the same time there will also be selection pressure to use the whole of the time available for each stage. Breeding activity often starts as soon as migration to the breeding grounds is complete, particularly at more northerly latitudes (Wingfield & Farner 1978; Bauchinger & Klaassen 2005). Birds attempt to breed during the whole period when it is probable that young can be successfully raised, so species or individuals for which appropriate food reserves are available for a long period or where time constraints imposed by latitude are less, will tend to be multi-brooded (Cooper et al. 2005; Weggler 2006). Post-nuptial moult starts as soon as breeding is finished, if not earlier, and progresses until migration. Consequently, in individuals that breed late, raise more broods, or breed at higher latitudes, moult progresses more rapidly (Hall & Fransson 2000).
3. Predictability and variability
Many aspects of environmental change during the year are broadly the same each year and hence predictable. Change in photoperiod is entirely predictable. That temperature will increase in spring and decrease in autumn, outside the tropics, is predictable, and the approximate time and duration of the food resources required for raising young tends to be predictable. Physiological processes do not happen instantly. So birds, as other animals, use reliable environmental cues to anticipate the onset of predictable environmental changes. Because change in photoperiod is entirely predictable at any particular latitude, both within and between years, it is used as the fundamental cue to time the physiological preparations for the three major life-history stages: breeding, moult and migration. However, whether absolute photoperiod or change in photoperiod acts directly to control physiology, or if photoperiod acts to synchronize an innate circannual rhythm of life-history changes, is still unclear (Hamner 1971; Wingfield 1993; Gwinner 2003; Dawson 2007).
In the shorter term, changes in environment and habitat can show variability within and between years. The degree of variability to which birds will be exposed differs between individuals and species depending on habitat, latitude and the type of food resource on which they depend. At one extreme, tropical seabirds may have little variability in climate or food resources during the year to cope with (Chapin & Wing 1959). At the other, migrants to the high arctic have a highly predictable breeding season but can be challenged with dramatic short-term changes in weather (Wingfield & Farner 1982; Wingfield 1985). Many species have unpredictable breeding seasons because they rely on ephemeral food resources, for example desert species that depend on unpredictable rainfall patterns (Immelmann 1973), or crossbills that rely on the unpredictable availability of pine cones (Newton 1972). To respond appropriately to short-term and unpredictable environmental and ecological changes, birds can use a suite of non-photoperiodic cues (Wingfield 1980). Which cues are used and their relative importance will vary between species and at different stages of the annual cycle.
The timing and duration of the breeding season is ultimately dictated by the availability of food on which young depend. Consequently, there are marked differences between species as to when breeding starts and ends. Furthermore, breeding seasons will rarely be symmetrical with the annual cycle in photoperiod, and yet photoperiod is the primary proximate cue used to time gonadal maturation. Asymmetry of breeding seasons relative to photoperiod is imposed by photorefractoriness (Nicholls et al. 1988). Although gonadal maturation in spring is a response to an increase in, or increasing, photoperiod, later in the year long photoperiods induce a state of photorefractoriness. This exists in two forms, absolute and relative. Absolute photorefractoriness is characterized by an apparently spontaneous gonadal regression during continued exposure to long photoperiods and that, following regression, a further increase in photoperiod does not induce renewed gonadal maturation (Hamner 1968). Relative photorefractoriness requires a decrease in the photoperiod to induce regression, but regression occurs under a photoperiod that is still longer than that which induced maturation earlier in the year (Robinson & Follett 1982). In general, it is thought that birds with short predictable breeding seasons, particularly those with breeding seasons relatively early in the year, become absolutely photorefractory. Those with later, longer or less predictable breeding seasons are thought more likely to show relative photorefractoriness (Hahn et al. 2004).
Species relying on unpredictable food resources require particularly plastic reproductive physiology. Desert species such as zebra finches (Taeniopygia guttata) breed after rainfall, and this can occur at any time of year (Immelmann 1973). Similarly, crossbills (genus Loxia) can breed opportunistically in most months of the year (Newton 1972). They breed in response to their food supply, conifer seeds, which are produced in different amounts in different years and at unpredictable times of the year.
Tropical seabirds are possibly faced with fewer environmental constraints than other groups. Photoperiod, climate and, most importantly, food resources, remain largely consistent throughout the year. Possibly as a consequence of this, wideawake terns (Sterna fuscata) on Ascension Island (8° S) do not breed at the same time each calendar year. Individual birds breed at intervals of 9.5 months (Chapin & Wing 1959; Ashmole 1963). They do not face ecological constraints, but do have a physiological constraint—the need to moult. The interval between successive breeding attempts is the sum of the time required to breed and to complete a moult. It may be significant that if starlings are kept experimentally on an equatorial photoperiod (a constant 12 h of light per day) they too undergo cycles of gonadal maturation, regression and moult that last approximately 9.5 months (Schwab 1971).
Most species of birds are constrained by the need to breed when food that their young require is abundant. Columbiformes adopt a different strategy; they feed their young on crop milk. Thus, as long as the mother can obtain sufficient food, breeding can be successful. Consequently, breeding seasons can be very long and probably symmetrical with photoperiod. Such species apparently lack any form of photorefractoriness (Murton & Westwood 1977).
4. The basic physiological template
This focus of this review is to assess which extrinsic cues may modulate intrinsic control mechanisms. Hence, a brief description of the neuroendocrine and endocrine mechanisms involved will suffice here; for in-depth reviews see Sharp et al. (1998) and Dawson et al. (2001). The rate and degree of gonadal maturation is ultimately determined by the rate of secretion of chicken gonadotrophin-releasing hormone-I (cGnRH-I) and possible antagonistic effects of gonadotrophin-inhibiting hormone (GnIH; Bentley et al. 2003; Ukena et al. 2003) and/or prolactin (Dawson & Sharp 1998; Sharp & Blache 2003). cGnRH-I is a decapeptide which is synthesized in the cell bodies of specialist neurosecretory neurons. It passes down the axons of these neurons and is secreted from the median eminence at the base of the hypothalamus, where it passes via a blood portal system to the pituitary gland. In the pituitary, cGnRH-I stimulates the synthesis of the two gonadotrophic hormones, luteinizing hormone and follicle stimulating hormone. These two hormones are secreted into the circulation and induce gonadal maturation. The activity of the cGnRH-I neurons is primarily controlled by photoperiodic information received from encephalic photoreceptors integrated in some way with a circadian clock. An increase in the photoperiod stimulates conversion of thyroxine to its active form, triiodothyronine (Yasuo et al. 2005), and this, in part at least (Takagi et al. 2007), leads to an increased secretion of cGnRH-I (Yamamura et al. 2006). Other neural inputs may allow non-photoperiodic information to modulate photoperiodically controlled cGnRH-I secretion. The conversion of thyroxine to triiodothyronine may act as a general ‘long-photoperiod’ signal and so may also ultimately downregulate GnRH-I synthesis as birds become photorefractory (Watanabe et al. 2007).
(a) Photoperiodic control of gonadal maturation
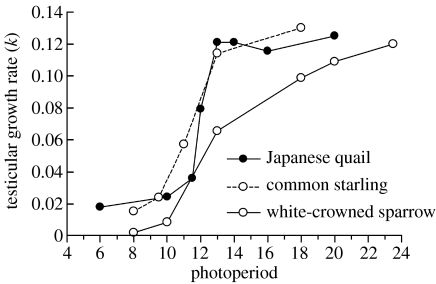
In most species at mid and high latitudes, gonadal maturation occurs during spring, as photoperiod increases. The exact time and rate of maturation varies between species, depending on their breeding seasons, and it can vary within species depending upon latitude and local non-photoperiodic factors. Rowan (1925) was the first to demonstrate that an increase in the photoperiod could advance gonadal maturation and Burger (1947) showed that the longer the (constant) photoperiod to which birds were exposed, the greater the rate of maturation. The relationship between the initial rate of gonadal maturation, denoted as k (defined as the rate of increase in log gonadal mass; Farner & Wilson 1957) and photoperiod differs between species (figure 1). In Japanese quail (Coturnix coturnix japonica), the rate of maturation is low when the photoperiod is less than 11.5 h of light per day, somewhat greater on 11.5 h and rapid on 12 h (Follett & Maung 1978). Data such as this led to the concept of a critical photoperiod; in the case of quail this is 11.5 h. As the photoperiod increases during spring, gonadal maturation begins when it reaches this critical threshold. However, the concept of a critical photoperiod should be treated with caution. In quail, the difference in gonadal growth rate changes rapidly over a short range of photoperiods, so a critical photoperiod is easy to define. In starlings (Dawson 1989) and white-crowned sparrows (Farner & Wilson 1957), gonadal growth rate varies over a wider range of photoperiods (figure 1). Moreover, many species show substantial testicular development under short photoperiods (e.g. Schwab & Rutledge 1978; Dawson 1989; Wingfield 1993; Hahn et al. 2004).
Figure 1.
The rate of testicular growth as a function of photoperiod for three species. Each point represents the initial rate of growth following transfer from a short photoperiod to each particular longer photoperiod. In Japanese quail, there is a rapid transition from non-stimulatory photoperiods to maximally stimulating photoperiods. In Gambel's white-crowned sparrow, which breeds at high latitudes, longer photoperiods are needed to induce testicular growth. Redrawn from data in Farner & Wilson (1957), Follett & Maung (1978) and Dawson (1989).
The concept of a critical photoperiod also assumes that it is an absolute photoperiod that birds are responding to rather than a change in the photoperiod. This difference is difficult to distinguish because it is not possible to have an increase in the photoperiod without changing the absolute photoperiod and vice versa. Many species of birds have breeding ranges that span a wide range of latitudes. Several species even have transequatorial breeding ranges, extending from high northern latitudes to well south of the Equator (Lofts & Murton 1968). Individuals at different latitudes will experience very different changes in photoperiod during the year. If birds simply respond to absolute photoperiod, then within each species, a marked consistent latitudinal gradation in the timing of breeding and moult would be expected. Great tits (Parus major) have probably been studied more than any other species. Data reviewed in birds of the Western Palaearctic (Cramp 1998) show that breeding starts earlier in the year at lower latitudes (and see Sanz 1998). Data on the end of breeding are scarce, but because the start of moult is associated with the end of breeding, this may be used as an indicator. Moult also tends to start sooner at lower latitudes, but the difference is much less than in the start of breeding. The moult rate is greater at higher latitudes such that there is little difference in the timing of the end of moult at different latitudes. The comparative lack of differences in the timing of breeding and moult at different latitudes may be the result of genetic differences in photoperiodic responses in populations at different latitudes. Silverin et al. (1993) showed that Italian Great tits appeared to initiate gonadal maturation at a shorter photoperiod than Scandinavian birds, but there was a little difference between birds from southern Sweden and northern Norway, even though these would naturally be exposed to a greater difference in natural photoperiods. In a recent study on common starlings (Sturnus vulgaris; Dawson 2007), birds were held under annual changes in photoperiod that simulated very different latitudes (52° and 9°). Testicular maturation started slightly earlier at the lower latitude but the timing of regression and moult were the same, a situation similar to that occurring naturally. This suggests that birds may use subtle photoperiodic cues relating to change in photoperiod rather than absolute photoperiod to time physiological responses. Certainly, birds retain a memory of photoperiod and so could perceive change in photoperiod (Dawson & King 1994; Brandstätter et al. 2000).
(b) Photoperiodic control of gonadal regression
As mentioned earlier, there are two different mechanisms by which photoperiod controls gonadal regression and so ends the breeding season: absolute and relative photorefractoriness. The different mechanisms reflect fundamental differences in the pattern of the annual cycle in the activity of cGnRH-I neurons. The absolutely photorefractory state is characterized by low hypothalamic cGnRH-I content (see references in Dawson et al. (2001) and subsequently Dawson et al. (2002), Marsh et al. (2002) and Stevenson & MacDougall-Shackleton (2005)). Because the cGnRH-I content is low, and cGnRH-I secretion is assumed to be minimal (since gonadal size is minimal), it is presumed that synthesis of cGnRH-I is also minimal or entirely absent. Unfortunately, this cannot yet be verified because absolute photorefractoriness has only been formally characterized in passerine species, and the cGnRH-I gene has not yet been cloned for any passerine. Turkeys (Meleagris gallopavo) show a form of absolute photorefractoriness, and in photorefractory turkeys cGnRH-I gene expression is decreased (Kang et al. 2006). During short photoperiods in autumn, the hypothalamic cGnRH-I content increases in passerines (Williams et al. 1987; Dawson & Goldsmith 1997). Presumably, there is no decrease in the cGnRH-I secretion at this time, so again, the presumption must be that synthesis of cGnRH-I resumes, or at least increases.
Relative photorefractoriness has only been formally characterized in one domesticated species, the Japanese quail (Robinson & Follett 1982); the wild subspecies, the European quail (Coturnix coturnix), appears to show identical photoperiodic responses (Boswell et al. 1993). In quail, the gonads remain mature indefinitely if birds are kept on a long photoperiod. Gonadal regression, induced by a decrease in photoperiod, is not associated with a decrease in hypothalamic cGnRH-I (Foster et al. 1988). It can be reasonably assumed therefore that cGnRH-I is synthesized continuously throughout the year and that seasonal changes in gonadal maturity are consequent entirely on changes in secretion.
Many species of birds appear to show elements of both absolute and relative photorefractoriness. Song sparrows (Melospiza melodia morphna) exposed to a long photoperiod (18 h light) eventually showed spontaneous regression; birds exposed to 16 h light did not show regression, but a decrease to 14 h light did precipitate regression and moult (Wingfield 1993). Among three species of cardueline finches the degree of absolute to relative photorefractoriness varied with the degree of their reproductive flexibility (Hahn et al. 2004). Seasonally breeding common redpolls (Carduelis flammea) and flexibly breeding pine siskins (Carduelis pinus) did show spontaneous gonadal regression, one criterion for absolute photorefractoriness, but the opportunistically breeding white-winged crossbills (Loxia leucoptera) did not. In a further study (MacDougall-Shackleton et al. 2006) which included other cardueline finches, only one species, Cassin's finch (Carpodacus cassinii), showed no renewed testicular maturation when challenged with a long photoperiod following regression (the other criterion for absolute photorefractoriness). The other species therefore show elements of both absolute and relative photorefractoriness. These differences are reflected in the changes in cGnRH-I (Pereyra et al. 2005). Similarly, house sparrows (Passer domesticus) become absolutely photorefractory, but a decrease in the photoperiod immediately before regression also accelerates regression and moult, as in relative photorefractoriness (Dawson 1991). Changes in hypothalamic cGnRH-I in house sparrows suggested that relative photorefractoriness was associated with decreased secretion, and absolute photorefractoriness with decreased synthesis, of cGnRH-I (Hahn & Ball 1995).
(c) Photoperiodic control of moult
Moult is a critically important life-history stage within the annual cycle and yet little is known about its environmental or physiological control. Photostimulation is required to induce moult but it also induces gonadal maturation and then regression. Therefore, it is unclear whether photoperiod has a direct effect on moult, or whether it is a secondary consequence of photoperiodic control of the gonadal cycle and a physiological link between gonadal regression and moult, satisfying the ecological requirement for moult to immediately follow breeding. Because the prebasic moult normally follows breeding, and overlap between breeding and moult is minimized, it is plausible that a reproductive hormone may have an inhibitory effect on moult. And indeed, many studies have shown that implants of testosterone can delay, prevent or interrupt moult (Schleussner et al. 1985; Nolan et al. 1992; Dawson 1994, 2004a). Such a mechanism would delay the start of moult until the time of gonadal regression. However, castration has a little effect on the timing of moult in starlings (Dawson & Goldsmith 1984).
It is more likely that prolactin is involved. Several studies have investigated seasonal cycles in prolactin in free-living birds and, in each of these, peak prolactin concentrations were found at or just before the start of moult (see Dawson 2006). In starlings under natural and experimental photoperiods, peak prolactin coincides fairly closely with the start of moult (Dawson & Goldsmith 1982, 1983). Importantly, immunization against vasoactive intestinal peptide, the prolactin-releasing hormone in birds, inhibited photoperiodically induced prolactin secretion and prevented moult (Dawson & Sharp 1998), and moult can be disengaged from gonadal regression but not from changes in prolactin (Dawson 2006). The timing of moult was related to the time of peak prolactin concentrations, but not to the magnitude of that peak. In other words, moult started not once a particular threshold prolactin concentration had been attained, but when prolactin started to decrease.
5. Physiological plasticity
If birds rely entirely on photoperiod to control the time of gonadal maturation and regression then these events would occur at the same time every year. The exact time of breeding clearly can differ between years, and it can differ within years between different habitats at the same latitude. This leaves two possibilities. Firstly, that photoperiod alone controls the time of gonadal maturation and regression and so sets the limits to a physiological window within which breeding can occur. Flexibility exists within this window so that non-photoperiodic cues can affect the exact timing of egg-laying. Certainly, female birds often require a range of non-photoperiodic cues to initiate the latter stages of ovarian maturation and ovulation. (Females often need behavioural cues from a mate to induce ovulation, but this is outside the scope of this review.) The second possibility is that non-photoperiodic cues can themselves modulate photoperiodic control of maturation and regression and so directly affect the window within which egg-laying can occur. In reality, it is probable that both operate.
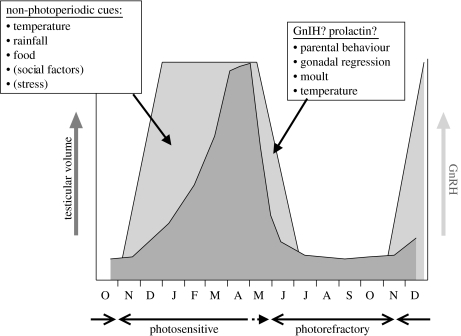
Figure 2 is a schematic diagram based on data from starlings. cGnRH-I synthesis begins during the autumn and signals the end of the period of absolute photorefractoriness and the start of photosensitivity. cGnRH-I synthesis ceases during late spring or early summer. In starlings, photosensitivity and photorefractoriness each happen to last for approximately six months. cGnRH-I synthesis resumes as a result of exposure to short photoperiods, and ceases as a result of exposure to long photoperiods. There is no evidence that any factor other than photoperiod has a bearing on these two fundamental events, although the possibility cannot be excluded. In males, gonadal maturation begins almost immediately after birds become photosensitive. Maturation rate increases as photoperiod increases during spring and full maturation is attained at the end of March. In captive birds in outdoor aviaries, testicular regression happens during May, so the testes are fully mature only during April, corresponding to the time of first clutches in free-living birds. However, in free-living birds, clutches can be laid later than the time of regression in captive birds, suggesting a possible effect of non-photoperiodic cues on the timing of regression. In females, the start and end of photorefractoriness is the same, but a suite of non-photoperiodic cues is required in addition to photoperiod to attain full ovarian maturation. At the end of the breeding season, testicular regression occurs just before the decrease in hypothalamic stores of cGnRH-I (Dawson et al. 2002; Dawson 2005b). Similarly, in Gambel's white-crowned sparrows (Zonotrichia leucophrys gambelii: Meddle et al. 1999, 2006a) and juncos (Junco hyemalis: Meddle et al. 2006b), cGnRH-I remains high immediately after testicular regression.
Figure 2.
Changes in reproductive physiology in male common starlings during the year. Starlings are photosensitive for six months each year and photorefractory for the other six months. The photorefractory period is associated with low hypothalamic levels of cGnRH-I, and the photosensitive period with high levels, as indicated by the light grey area. The timing of the switch between these two states, i.e. the switching on and off of cGnRH-I synthesis, is assumed to be entirely photoperiodically controlled. The darker grey area represents changes in testicular maturity. Non-photoperiodic cues could modulate the exact time of breeding (i.e. egg-laying) by affecting the exact time of laying within the period of full gonadal maturity (the top of the dark grey area) or by modulating the timing or rate of gonadal maturation and/or regression within the period of photosensitivity (the light grey area).
In the case of starlings, there are therefore two periods during which non-photoperiodic cues have the potential to modulate photoperiodic control of gonadal maturity: a long period during winter and early spring, between the renewal of cGnRH-I synthesis and full photoperiodically induced maturation, and a shorter period at the end of the breeding season, immediately preceding the decrease in releasable cGnRH-I. If non-photoperiodic cues can modulate the time or rate of photoperiodically induced gonadal maturation and regression, then there is considerable scope for plasticity during maturation and less so during regression. However, starlings have only a brief period of full photoperiodically induced gonadal maturation, so the capacity for flexibility in the exact time of egg-laying within this physiological window is limited. Species which have a longer period of full photoperiodically induced gonadal maturation, particularly those that show elements of relative photorefractoriness, will have a greater capacity for flexibility within this physiological window in addition to non-photoperiodic cues potentially modulating photoperiodically induced maturation and regression.
(a) Plasticity in gonadal maturation
There are many examples of birds breeding at different times in different habitats at the same latitude, and in the same habitat between different years. For example, in song sparrows breeding over a range of altitudes but at the same latitude, gonadal vernal maturation is delayed at higher altitudes (Perfito et al. 2004). There were no differences in response to photoperiodic cues in the laboratory. Clearly, non-photoperiodic cues must be important. Blue tits (Parus caeruleus) in deciduous oaks in Corsica breed one month earlier than birds breeding in evergreen oaks even though the two populations are only 25 km apart (Caro et al. 2005a). The development of song nuclei and initial testicular development occurred at approximately the same time, but the subsequent rate of testicular development was faster in the early breeding population (Caro et al. 2004, 2005b, 2006). Two tropical populations of Rufous-collared sparrows (Zonotrichia capensis), separated by only 25 km, have highly asynchronous breeding seasons (Moore et al. 2005) and reproductive physiology (Moore et al. 2006) associated with different local rainfall patterns. Clearly, non-photoperiodic cues must be important. There is a range of non-photoperiodic cues that could potentially modulate the timing of breeding, including temperature, rainfall and food availability. Of course, these may not be entirely independent—for example, temperature and rainfall may alter food availability. What is the evidence that these cues act directly; do they modulate the time and rate of photoperiodically induced gonadal maturation or rather the exact time of egg-laying within a physiological window dictated by photoperiodic cues?
(i) Temperature
There is abundant evidence in many, but not all, species that the time of egg-laying varies with spring temperature (e.g. Korpimaki 1978; Perrins & McCleery 1989; McCleery & Perrins 1998; Crick & Sparks 1999; Meijer et al. 1999; Visser et al. 2003; Both et al. 2004, 2005; Torti & Dunn 2005). It is less clear that this is a consequence of a direct effect of temperature on photoperiodically induced gonadal maturation. Several experimental studies have assessed the effects of temperature on gonadal maturation following an acute move from a short to a long photoperiod. In the Gambel's white-crowned sparrow, which has a short predictable breeding season at high latitudes, temperature had no effect on the rate of testicular or ovarian maturation (Wingfield et al. 1996). In Zonotrichia leucophrys pugetensis, which breeds at mid-latitudes, temperature did not affect testicular growth but did affect ovarian development. And in Zonotrichia leucophrys oriantha, which breeds at lower latitudes but high altitudes and has a flexible breeding season, low temperature inhibited gonadal maturation in both the sexes and delayed the onset of photorefractoriness (Wingfield et al. 2003). These studies suggest that temperature is likely to have a greater effect on the rate of gonadal maturation in species with longer and more flexible breeding seasons (Wingfield et al. 1992). In willow tits (Parus montanus), high temperature accelerated testicular maturation, but in Great tits there was little effect (Silverin & Viebke 1994). In canaries kept at very different temperatures (0 and 25°C) and moved to a 20 h photoperiod, testicular growth was slightly slower and birds became photorefractory later at the lower temperature (Storey & Nicholls 1982). However, in all of these studies, the experimental paradigm was an acute transfer from a short to a long photoperiod, rather than a natural increase in photoperiod. Perhaps surprisingly, there have been few studies addressing whether temperature directly affects physiological responses to naturally increasing photoperiod during spring. In an early experiment on Great tits, Suomalainen (1938) showed no apparent effect of increased temperatures on testicular size during early spring. When song sparrows were exposed to naturally increasing photoperiods, higher temperature slightly enhanced testicular growth in birds from a mountain population, but had no effect in birds from a coastal population (Perfito et al. 2005). In starlings, temperature had no effect on the timing or rate of testicular maturation, but a higher temperature advanced the onset of testicular regression and the start of moult (Dawson 2005a). Higher temperatures inside nest boxes advanced egg-laying in starlings (Meijer et al. 1999) and tits (Dhondt 1979), suggesting that temperature may more directly control the latter stages of ovarian maturation. However, preliminary results from a study by Visser (2006) suggest that higher temperature did not advance the date of first egg-laying in Great tits but did shorten the period of egg-laying. This is similar to changes in some free-living populations (Visser et al. 2003)—increasing temperatures are associated with a decrease in the proportion of birds starting a second clutch.
(ii) Rainfall
Tropical species often have fixed breeding seasons related to predictable periods of rainfall (Wikelski et al. 2000). Although the annual change in photoperiod is slight within the tropics, this is sufficient to entrain gonadal maturation (Hau et al. 1998; Beebe et al. 2005; Dawson 2007). Rainfall itself may act only as a short-term cue to fine-tune the time of breeding (Wikelski et al. 2000).
Many species inhabiting arid habitats breed following rain, the timing of which can be unpredictable. The physiological mechanisms underlying this, i.e. the nature of the cue and how it interacts with reproductive physiology, are unclear. It may be that the cue is a result of rainfall, i.e. the appearance of green vegetation or an improved food supply, rather than rainfall itself. However, some species begin nest-building as soon as rain starts (Immelmann 1973), which implies that the reproductive system must have already been fairly mature in order to be able to respond so quickly. This may be true for zebra finches. This species does respond to changes in photoperiod (Bentley et al. 2000). It is a seasonal breeder in predictable habitats but breeds opportunistically where rainfall patterns are unpredictable. In predictable habitats, seasonal changes in reproductive physiology are similar to other species, with regressed gonads outside the breeding season. However, in unpredictable habitats, more non-breeding birds maintained an active reproductive system, presumably so that breeding could start at short notice (Perfito et al. 2007). Experimental water deprivation resulted in short-term inhibition, but not in full regression, of the reproductive system so that it remained active and able to respond to subsequent stimulation (Perfito et al. 2006). Similarly, the Rufous-winged sparrow (Aimophila carpalis) shows photoperiodically controlled gonadal seasonality, but the exact time of breeding within this physiological window occurs in response to rainfall (Deviche et al. 2006). Likewise, canaries (Serinus canarius) are photoperiodic (Storey & Nicholls 1976) but also breed in response to rainfall (Leitner et al. 2003). Several neotropical passerines show full gonadal regression for several months following the end of their breeding seasons at the end of the rainy season, but whether they show renewed gonadal maturation in advance of the next rainy season is unclear (Wikelski et al. 2003). However, Darwin's finches (Geospiza fuliginosa) undergo gonadal maturation at very different times in different years in response to rainfall and the gonads appear to remain fully regressed between breeding seasons (Hau et al. 2004).
(iii) Food
Although availability of food on which the young depend is frequently the most important ultimate factor controlling the timing of breeding (Lack 1968), because females require sufficient food for production of eggs, food can also act as a proximate factor (Perrins 1970). Studies in which supplemental food have been provided sometimes, but not always, result in an advance in laying date (see data reviewed in Meijer et al. (1990) and subsequently Hornfeldt & Eklund (1990), Simmons (1993), Nakamura (1995), Schoech (1996), Korpimaki & Wiehn (1998), Scheuerlein & Gwinner (2002), Reynolds et al. (2003)). Advances are normally in the range of a few days, which tallies with the time taken for egg production. In starlings, supplementary food advanced egg-laying by 5 days in free-living birds (Källander & Karlsson 1993) and food restriction delayed laying in captive breeding starlings (Meijer & Langer 1995). Yet, restricting food availability had little or no effect on photoperiodically controlled gonadal maturation in either sex (Dawson 1986; Meijer 1989, 1991). This implies that the modulating effect of food availability is restricted to the latter stages of ovarian maturation and that the effect of food as a proximate factor is to fine-tune the exact date of laying within the physiological window defined by photoperiodically controlled gonadal maturation and regression.
Some opportunistically breeding species breed more directly in response to food. Crossbills (genus Loxia) were thought to breed opportunistically at almost any time of the year when food happened to become abundant. However, even in these species, gonadal maturation and regression appear to be regular seasonal events, and opportunism is confined within this underlying seasonality (Deviche 1997; Hahn 1998; Deviche & Sharp 2001). In other words, the response to annual change in photoperiod in these species is such that there is a long physiological window within which breeding can occur. The exact timing depends on food availability. Outside this window, during gonadal regression and moult, breeding cannot occur even if food happens to become available (Hahn 1995).
(iv) Plasticity in gonadal maturation: conclusions
Of the three non-photoperiodic cues discussed, only in the case of temperature is there firm evidence for a direct effect on gonadal maturation. In studies where higher temperature increases gonadal maturation rate following acute photostimulation, it also advances the onset of photorefractoriness. In other words, the higher temperature appears to enhance all of the consequences of the long photoperiod. On the other hand, in studies where photoperiod was increased to follow the natural changes, the consequences appear to have been restricted to the time of regression. In either case, the physiological mechanism remains obscure. Photoperiod is known to act through encephalic photoreceptors. How external temperature influences gonadal maturation in an endotherm remains to be established. The other non-photoperiodic cues may control the exact time of egg-laying by modulating the final stages of ovarian maturation and ovulation. Temperature may also operate in this way, in addition to possible effects on the earlier stages of maturation, since increasing the temperature in nest boxes was particularly effective in advancing egg-laying. Effects on the exact time of laying may operate through neural input to the cGnRH-I neurons inducing the surge of cGnRH-I secretion required for ovulation. Females may require social stimuli from their mates, such as a courtship song, to induce ovulation and neural links involved in this, linking auditory centres and cGnRH-I neurons, have been demonstrated (Cheng 2005).
(b) Plasticity in gonadal regression and moult
Species that become absolutely photorefractory show a decrease in cGnRH-I. However, gonadal regression precedes this and so is not a direct consequence of it. Some other factor(s) must initiate gonadal regression, possibly by decreasing GnRH-I secretion in advance of the decrease in synthesis. Two potential candidates for this are GnIH (Bentley et al. 2003; Ukena et al. 2003) and prolactin (Dawson & Sharp 1998; Sharp & Blache 2003). The period between gonadal regression and the decrease in cGnRH-I may be equivalent to relative photorefractoriness and offer a degree of plasticity in the exact time of regression, and hence the end of the physiological window within which egg-laying can occur. The converse situation, the increase in cGnRH-I in advance of gonadal maturation during autumn, does not appear to be analogous to relative photorefractoriness (Dawson 2004b).
There is a close relationship between the end of breeding and the start of moult, and prolactin may play a key role in this relationship. The timing of moult is related to the time at which prolactin starts to decrease (Dawson 2006). Prolactin secretion is photoperiodically controlled, but secretion increases much more in breeding birds than in non-breeding birds (Dawson & Goldsmith 1985; Hiatt et al. 1987; Wingfield & Goldsmith 1990; Goldsmith 1991). Breeding activity can delay the onset of moult (Morton 1992; Hemborg & Merila 1999; Newton & Rothery 2005) possibly because prolactin does not decrease until breeding activity ceases. This mechanism would minimize moult/breeding overlap but at the same time ensure that moult starts as soon as possible after breeding. This would allow flexibility in the timing of the start of moult to accommodate differences in the end of breeding.
Although moult frequently starts as soon as breeding activity is completed, there is also flexibility in this relationship. There are many examples of moult starting before breeding has finished; this may be more common in males than in females, more common in late-breeding individuals and in birds breeding at higher latitudes (e.g. Hemborg 1999a–c; Hemborg et al. 2001).
Flexibility in the timing of the end of breeding necessitates further flexibility in the duration of moult. A delay in the start of moult leads to a more rapid moult (Dawson 2004a) presumably because moult needs to be completed before migration. The increase in moult rate is a photoperiodic effect and again may be mediated by prolactin.
6. Consequences of anthropogenic environmental change
There are many anthropogenic changes to which birds will need to adapt if populations are not going to decline, e.g. habitat degradation, urbanization, tourism and, of course, climate change. Some changes will have a direct effect on reproductive success, for example by decreasing the area of suitable habitat, nest sites and food abundance. Some anthropogenic changes will alter the timing of food abundance, and in these cases the scope for flexibility in life-history strategies will have a critical bearing on whether birds can adapt successfully and sufficiently rapidly (see Ghalambor et al. 2007). The major effect of climate change is the breakdown of natural phenological schedules (Visser et al. 2004; Laaksonen et al. 2006). Many species of birds require invertebrate food resources to feed their young. The timing of such food resources will tend to advance as temperature increases, both as a direct result of the effect of temperature on developmental rate of the invertebrates and also possibly as a secondary consequence of an effect of temperature on botanical phenology. Photoperiod is the principal environmental cue used to time the physiology underlying the annual cycle in birds, and, obviously, the annual cycle in photoperiod will remain constant. Whether birds can adapt to a change in the timing of food availability will depend on the plasticity of their physiology in response to non-photoperiodic cues. Hence, birds with historically predictable breeding seasons may be particularly at risk. Those with flexible breeding seasons may have a greater capacity to adapt. However, if the effect of non-photoperiodic cues is restricted to controlling the timing of egg-laying within a comparatively inflexible photoperiodically controlled physiological window, then there will still be limits to the degree of their adaptability. The fact that birds may not be responding to selection pressure for earlier breeding (Gienapp et al. 2006) may reflect the lack of flexibility in their photoperiodic responses (Coppack & Pulido 2004).
Furthermore, the effects will not be restricted to the timing of breeding. In long-distance migrants, the timing of migration is probably based on photoperiod, and so will remain constant (e.g. Both & Visser 2001; Coppack & Both 2002; Sanz 2003; Battley 2006). This will restrict the degree of flexibility in the timing of breeding (Both et al. 2006). On the other hand, short-distance migrants may be more flexible in their migratory schedules. Several such species seem to be migrating back to their breeding grounds earlier in the year. The advance in migration dates is greater than any advance in breeding, reflecting less plasticity in reproductive physiology (Sparks et al. 2005).
In addition to climate change, urbanization and habitat degradation may alter the timing of food availability. In species that have successfully adapted to urban environments, food may be available earlier in the year than in rural habitats (Fleischer et al. 2003; Partecke et al. 2004). Street lighting may, in theory, affect photoperiodic responses, although in practice it is probable that light intensity is too low to have significant effects. In degraded habitats, such as woodland fragments, the peak in food availability may be later (Hinsley et al. 2003). Any effect on the timing of the end of breeding will also have consequences on the time and rate of moult, and hence on over-winter survival.
7. Uncertainties and future directions
This review highlights many areas of uncertainty. The exact time that eggs are laid determines whether the period of greatest demand for food by the young exactly corresponds to the time of maximum food availability, and this is a major determinant of breeding success. The cues and physiology controlling when birds decide to lay remain obscure. Clearly, there is phenotypic plasticity and involvement of non-photoperiodic cues, but whether non-photoperiodic cues simply influence the exact time of laying within a fixed physiological window, or whether non-photoperiodic cues can directly control this window, is an important unknown. This stems from historically different approaches. Physiological studies on captive birds have, as their endpoint, changes in hormone concentrations or gonadal size. Ecological studies on free-living birds record egg-laying dates. An integrated approach is required. For example, captive birds can be exposed to controlled environmental conditions and yet given sufficient space and environmental enrichment so that egg-laying is not inhibited. This is not easy and is expensive also. However, such data are important in assessing the ability of birds to adapt to global change.
Footnotes
One contribution of 12 to a Theme Issue ‘Integration of ecology and endocrinology in avian reproduction: a new synthesis’.
References
- Ashmole N.P. The biology of the wideawake or sooty tern Sterna fuscata on Ascension Island. Ibis. 1963;103:297–364. [Google Scholar]
- Barta, Z., McNamara, J. M., Houston, A. I., Weber, T. P., Hedenström, A. & Feró, O. In press. Optimal moult strategies in migratory birds. Phil. Trans. R. Soc. B (doi:10.1098/rstb.2007.2136) [DOI] [PMC free article] [PubMed]
- Battley P.F. Consistent annual schedules in a migratory shorebird. Biol. Lett. 2006;2:517–520. doi: 10.1098/rsbl.2006.0535. doi:10.1098/rsbl.2006.0535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauchinger U, Klaassen M. Longer days in spring than in autumn accelerate migration speed of passerine birds. J. Avian Biol. 2005;36:3–5. doi:10.1111/j.0908-8857.2005.03444.x [Google Scholar]
- Beebe K, Bentley G.E, Hau M. A seasonally breeding tropical bird lacks absolute photorefractoriness in the wild, despite high photoperiodic sensitivity. Funct. Ecol. 2005;19:505–512. doi:10.1111/j.1365-2435.2005.00994.x [Google Scholar]
- Bentley G.E, Spar B.D, MacDougall-Shackleton S.A, Hahn T.P, Ball G.F. Photoperiodic regulation of the reproductive axis in male zebra finches, Taeniopygia guttata. Gen. Comp. Endocrinol. 2000;117:449–455. doi: 10.1006/gcen.1999.7430. doi:10.1006/gcen.1999.7430 [DOI] [PubMed] [Google Scholar]
- Bentley G.E, Perfito N, Ukena K, Tsutsui K, Wingfield J.C. Gonadotropin-inhibitory peptide in song sparrows (Melospiza melodia) in different reproductive conditions, and in house sparrows (Passer domesticus) relative to chicken-gonadotropin-releasing hormone. J. Neuroendocrinol. 2003;15:794–802. doi: 10.1046/j.1365-2826.2003.01062.x. [DOI] [PubMed] [Google Scholar]
- Boswell T, Hall M.R, Goldsmith A.R. Annual cycles of migratory fattening, reproduction and moult in European quail (Coturnix coturnix) J. Zool. Lond. 1993;231:627–644. [Google Scholar]
- Both C, Visser M.E. Adjustment to climate change is constrained by arrival date in a long-distance migrant bird. Nature. 2001;411:296–298. doi: 10.1038/35077063. doi:10.1038/35077063 [DOI] [PubMed] [Google Scholar]
- Both C, et al. Large-scale geographical variation confirms that climate change causes birds to lay earlier. Proc. R. Soc. B. 2004;271:1657–1662. doi: 10.1098/rspb.2004.2770. doi:10.1098/rspb.2004.2770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Both C, Piersma T, Roodbergen S.P. Climatic change explains much of the 20th century advance in laying date of Northern Lapwing Vanellus vanellus in The Netherlands. Ardea. 2005;93:79–88. [Google Scholar]
- Both C, Bouwhuis S, Lessells C.M, Visser M.E. Climate change and population declines in a long-distance migratory bird. Nature. 2006;441:81–83. doi: 10.1038/nature04539. doi:10.1038/nature04539 [DOI] [PubMed] [Google Scholar]
- Brandstätter R, Kumar V, Abraham U, Gwinner E. Photoperiodic information acquired and stored in vivo is retained in vitro by a circadian oscillator, the avian pineal gland. Proc. Natl Acad. Sci. USA. 2000;97:12 324–12 328. doi: 10.1073/pnas.200354997. doi:10.1073/pnas.200354997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger J.W. On the relation of daylength to the phase of testicular involution and inactivity of the spermatogenic cycle of the starling. J. Exp. Zool. 1947;124:227–239. doi: 10.1002/jez.1401050207. doi:10.1002/jez.1401240203 [DOI] [PubMed] [Google Scholar]
- Caro S, Lambrechts M.M, Balthazart J. Seasonal development of song control nuclei HVC and RA preceeds peaks in plasma testosterone and singing activity in male Corsican blue tits. Horm. Behav. 2004;46:108. [Google Scholar]
- Caro S.P, Balthazart J, Thomas D.W, Lacroix A, Chastel O, Lambrechts M.M. Endocrine correlates of the breeding asynchrony between two Corsican populations of blue tits (Parus caeruleus) Gen. Comp. Endocrinol. 2005a;140:52–60. doi: 10.1016/j.ygcen.2004.09.016. doi:10.1016/j.ygcen.2004.09.016 [DOI] [PubMed] [Google Scholar]
- Caro S.P, Lambrechts M.A, Balthazart J.B. Early seasonal development of brain song control nuclei in male blue tits. Neurosci. Lett. 2005b;386:139–144. doi: 10.1016/j.neulet.2005.03.074. doi:10.1016/j.neulet.2005.03.074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caro S.P, Lambrechts M.M, Chastel O, Sharp P.J, Thomas D.W, Balthazart J. Simultaneous pituitary-gonadal recrudescence in two Corsican populations of male blue tits with asynchronous breeding dates. Horm. Behav. 2006;50:347–360. doi: 10.1016/j.yhbeh.2006.03.001. doi:10.1016/j.yhbeh.2006.03.001 [DOI] [PubMed] [Google Scholar]
- Chapin J.P, Wing L.W. The wideawake calendar, 1953–1958. Auk. 1959;76:153–158. [Google Scholar]
- Cheng M.-F. Audio-vocal pathways controlling GnRH release. In: Dawson A, Sharp P.J, editors. Functional avian endocrinology. Narosa Publishing House; New Delhi, India: 2005. pp. 113–128. [Google Scholar]
- Colwell R.K. Periodicity, constancy and contingency of periodic phenomena. Ecology. 1974;55:1148–1153. doi:10.2307/1940366 [Google Scholar]
- Cooper C.B, Hochachka W.M, Dhondt A.A. Latitudinal trends in within-year reoccupation of nest boxes and their implications. J. Avian Biol. 2005;36:31–39. doi:10.1111/j.0908-8857.2005.03319.x [Google Scholar]
- Coppack T, Both C. Predicting life-cycle adaptation of migratory birds to global climate change. Ardea. 2002;90:369–378. [Google Scholar]
- Coppack, T. & Pulido, F. 2004 Photoperiodic response and the adaptability of avian life cycles to environmental change. Adv. Ecol. Res.35, 131–150.
- Cramp S. Oxford University Press; Oxford, UK: 1998. The complete birds of the Western Palearctic on CD-ROM. [Google Scholar]
- Crick H.Q.P, Sparks T.H. Climate change related to egg-laying trends. Nature (Lond.) 1999;399:423–424. doi:10.1038/20839 [Google Scholar]
- Dawson A. The effect of restricting the daily period of food availability on testicular growth in starlings, Sturnus vulgaris. Ibis. 1986;128:572–575. [Google Scholar]
- Dawson A. Pharmacological doses of thyroxine simulate the effects of increased daylength, and thyroidectomy, decreased daylength, on the reproductive system of European starlings. J. Exp. Zool. 1989;249:62–67. doi:10.1002/jez.1402490112 [Google Scholar]
- Dawson A. Photoperiodic control of testicular regression and moult in male house sparrows, Passer domesticus. Ibis. 1991;133:312–316. [Google Scholar]
- Dawson A. The effects of daylength and testosterone on the initiation and progress of moult in starlings, Sturnus vulgaris. Ibis. 1994;136:335–340. [Google Scholar]
- Dawson A. The effects of delaying the start of moult on the duration of moult, primary feather growth rates and feather mass in common starlings, Sturnus vulgaris. Ibis. 2004a;146:493–500. doi:10.1111/j.1474-919x.2004.00290.x [Google Scholar]
- Dawson A. Evidence against a period of relative photorefractoriness during the recovery of photosensitivity in common starlings. Gen. Comp. Endocrinol. 2004b;136:117–121. doi: 10.1016/j.ygcen.2003.12.008. doi:10.1016/j.ygcen.2003.12.008 [DOI] [PubMed] [Google Scholar]
- Dawson A. The effect of temperature on photoperiodically regulated gonadal maturation, regression and moult in starlings—potential consequences of climate change. Funct. Ecol. 2005a;19:995–1000. doi:10.1111/j.1365-2435.2005.01061.x [Google Scholar]
- Dawson A. Seasonal differences in the secretion of luteinising hormone and prolactin in response to N-methyl-dl-aspartate in starlings (Sturnus vulgaris) J. Neuroendocrinol. 2005b;17:105–110. doi: 10.1111/j.1365-2826.2005.01284.x. doi:10.1111/j.1365-2826.2005.01284.x [DOI] [PubMed] [Google Scholar]
- Dawson A. Control of molt in birds: association with prolactin and gonadal regression in starlings. Gen. Comp. Endocrinol. 2006;147:314–322. doi: 10.1016/j.ygcen.2006.02.001. doi:10.1016/j.ygcen.2006.02.001 [DOI] [PubMed] [Google Scholar]
- Dawson A. Seasonality in a temperate zone bird can be entrained by near equatorial photoperiods. Proc. R. Soc. B. 2007;274:721–725. doi: 10.1098/rspb.2006.0067. doi:10.1098/rspb.2006.0067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson A, Goldsmith A.R. Prolactin and gonadotrophin secretion in wild starlings (Sturnus vulgaris) during the annual cycle and in relation to nesting, incubation, and rearing young. Gen. Comp. Endocrinol. 1982;48:213–221. doi: 10.1016/0016-6480(82)90019-3. doi:10.1016/0016-6480(82)90019-3 [DOI] [PubMed] [Google Scholar]
- Dawson A, Goldsmith A.R. Plasma prolactin and gonadotrophins during gonadal development and the onset of photorefractoriness in male and female starlings (Sturnus vulgaris) on artificial photoperiods. J. Endocrinol. 1983;97:253–260. doi: 10.1677/joe.0.0970253. [DOI] [PubMed] [Google Scholar]
- Dawson A, Goldsmith A.R. Effects of gonadectomy on seasonal changes in plasma LH and prolactin concentrations in male and female starlings (Sturnus vulgaris) J. Endocrinol. 1984;100:213–218. doi: 10.1677/joe.0.1000213. [DOI] [PubMed] [Google Scholar]
- Dawson A, Goldsmith A.R. Modulation of gonadotrophin and prolactin secretion by daylength and breeding behaviour in free-living starlings, Sturnus vulgaris. J. Zool. Lond. 1985;206:241–252. [Google Scholar]
- Dawson A, Goldsmith A.R. Changes in gonadotrophin-releasing hormone (GnRH-I) in the pre-optic area and median eminence of starlings (Sturnus vulgaris) during the recovery of photosensitivity and during photostimulation. J. Reprod. Fertil. 1997;111:1–6. doi: 10.1530/jrf.0.1110001. [DOI] [PubMed] [Google Scholar]
- Dawson A, King V. Thyroidectomy does not affect the daily or free-running rhythms of plasma melatonin in European starlings. J. Biol. Rhythms. 1994;9:137–144. doi: 10.1177/074873049400900204. doi:10.1177/074873049400900204 [DOI] [PubMed] [Google Scholar]
- Dawson A, Sharp P.J. The role of prolactin in the development of reproductive photorefractoriness and postnuptial molt in the European starling (Sturnus vulgaris) Endocrinology. 1998;139:485–490. doi: 10.1210/endo.139.2.5701. doi:10.1210/en.139.2.485 [DOI] [PubMed] [Google Scholar]
- Dawson A, King V.M, Bentley G.E, Ball G.F. Photoperiodic control of seasonality in birds. J. Biol. Rhythms. 2001;16:366–381. doi: 10.1177/074873001129002079. doi:10.1177/074873001129002079 [DOI] [PubMed] [Google Scholar]
- Dawson A, Talbot R.T, Dunn I.C, Sharp P.J. Changes in basal hypothalamic chicken gonadotropin-releasing hormone-I and vasoactive intestinal polypeptide associated with a photo-induced cycle in gonadal maturation and prolactin secretion in intact and thyroidectomized starlings (Sturnus vulgaris) J. Neuroendocrinol. 2002;14:533–539. doi: 10.1046/j.1365-2826.2002.00807.x. doi:10.1046/j.1365-2826.2002.00807.x [DOI] [PubMed] [Google Scholar]
- Deviche P. Seasonal reproductive pattern of white-winged crossbills in interior Alaska. J. Field Ornithol. 1997;68:613–621. [Google Scholar]
- Deviche P, Sharp P.J. Reproductive endocrinology of a free-living, opportunistically breeding passerine (white-winged crossbill, Loxia leucoptera) Gen. Comp. Endocrinol. 2001;123:268–279. doi: 10.1006/gcen.2001.7675. doi:10.1006/gcen.2001.7675 [DOI] [PubMed] [Google Scholar]
- Deviche P, Small T, Sharp P, Tsutsui K. Control of luteinizing hormone and testosterone secretion in a flexibly breeding male passerine, the Rufous-winged sparrow, Aimophila carpalis. Gen. Comp. Endocrinol. 2006;149:226–235. doi: 10.1016/j.ygcen.2006.06.004. doi:10.1016/j.ygcen.2006.06.004 [DOI] [PubMed] [Google Scholar]
- Dhondt A.A. Temperature and date of laying by tits Parus spp. Ibis. 1979;121:329–331. [Google Scholar]
- Farner D.S, Wilson A.C. A quantitative examination of testicular growth in the white-crowned sparrow. Biol. Bull. 1957;113:254–267. doi: 10.2307/1539953. doi:10.2307/1539083 [DOI] [PubMed] [Google Scholar]
- Fleischer A.L, Bowman R, Woolfenden G.E. Variation in foraging behavior, diet, and time of breeding of Florida scrub-jays in suburban and wildland habitats. Condor. 2003;105:515–527. doi:10.1650/7224 [Google Scholar]
- Follett B.K, Maung S.L. Rate of testicular maturation, in relation to gonadotrophin and testosterone levels, in quail exposed to various artificial photoperiods and to natural daylengths. J. Endocrinol. 1978;78:267–280. doi: 10.1677/joe.0.0780267. [DOI] [PubMed] [Google Scholar]
- Foster R.G, Panzica G.C, Parry D.M, Viglietti-Panzica C. Immunocytochemical studies on the LHRH system of the Japanese quail: influence by photoperiod and aspects of sexual differentiation. Cell Tissue Res. 1988;253:327–335. doi: 10.1007/BF00222289. doi:10.1007/BF00222289 [DOI] [PubMed] [Google Scholar]
- Ghalambor C.K, Mckay J.K, Carroll S.P, Reznick D.N. Adaptive versus non-adaptive phenotypic plasticity and the potential for contemporary adaptation in new environments. Funct. Ecol. 2007;21:394–407. doi:10.1111/j.1365-2435.2007.01283.x [Google Scholar]
- Gienapp P, Postma E, Visser M.E. Why breeding time has not responded to selection for earlier breeding in a songbird population. Evolution. 2006;60:2381–2388. [PubMed] [Google Scholar]
- Goldsmith, A. R. 1991 Prolactin and avian reproductive strategies. In Acta XX Congressus Internationalis Ornithologici, pp. 2063–2071. Wellington, New Zealand: New Zealand Ornithological Congress Trust Board.
- Gwinner E. Circannual rhythms in birds. Curr. Opin. Neurobiol. 2003;13:770–778. doi: 10.1016/j.conb.2003.10.010. doi:10.1016/j.conb.2003.10.010 [DOI] [PubMed] [Google Scholar]
- Hahn T.P. Integration of photoperiodic and food cues to time changes in reproductive physiology by an opportunistic breeder, the red crossbill, Loxia curvirostra (Aves: Carduelinae) J. Exp. Zool. 1995;272:213–226. doi:10.1002/jez.1402720306 [Google Scholar]
- Hahn T.P. Reproductive seasonality in an opportunistic breeder, the red crossbill, Loxia curvirostra. Ecology. 1998;79:2365–2375. [Google Scholar]
- Hahn T.P, Ball G.F. Changes in brain GnRH associated with photorefractoriness in house sparrows (Passer domesticus) Gen. Comp. Endocrinol. 1995;99:349–363. doi: 10.1006/gcen.1995.1119. doi:10.1006/gcen.1995.1119 [DOI] [PubMed] [Google Scholar]
- Hahn T.P, Pereyra M.E, Sharbaugh S.M, Bentley G.E. Physiological responses to photoperiod in three cardueline finch species. Gen. Comp. Endocrinol. 2004;137:99–108. doi: 10.1016/j.ygcen.2004.02.014. doi:10.1016/j.ygcen.2004.02.014 [DOI] [PubMed] [Google Scholar]
- Hall K.S.S, Fransson T. Lesser whitethroats under time-constraint moult more rapidly and grow shorter wing feathers. J. Avian Biol. 2000;31:583–587. doi:10.1034/j.1600-048X.2000.310419.x [Google Scholar]
- Hall K.S.S, Fransson T. Wing moult in relation to autumn migration in adult common whitethroats, Sylvia communis communis. Ibis. 2001;143:580–586. [Google Scholar]
- Hamner W.M. The photorefractory period of the house finch. Ecology. 1968;49:211–227. doi:10.2307/1934450 [Google Scholar]
- Hamner W.M. On seeking an alternative to the endogenous reproductive rhythm hypothesis in birds. In: Menaker M, editor. Biochronometry. National Academy of Sciences; Washington, DC: 1971. pp. 448–462. [Google Scholar]
- Hau M, Wikelski M, Wingfield J.C. A neotropical forest bird can measure the slight changes in tropical photoperiod. Proc. R. Soc. B. 1998;1391:89–95. doi:10.1098/rspb.1998.0268 [Google Scholar]
- Hau M, Wikelski M, Gwinner H, Gwinner E. Timing of reproduction in a Darwin's finch: temporal opportunism under spatial constraints. Oikos. 2004;106:489–500. doi:10.1111/j.0030-1299.2004.13206.x [Google Scholar]
- Hemborg C. Annual variation in the timing of breeding and moulting in male and female pied flycatchers, Ficedula hypoleuca. Ibis. 1999a;141:226–232. [Google Scholar]
- Hemborg C. Sexual differences in moult-breeding overlap and female reproductive costs in pied flycatchers, Ficedula hypoleuca. J. Anim. Ecol. 1999b;68:429–436. doi:10.1046/j.1365-2656.1999.00295.x [Google Scholar]
- Hemborg C. Annual variation in the timing of breeding and moult in male and female pied flycatchers, Ficedula hypoleuca. Ibis. 1999c;141:226–232. [Google Scholar]
- Hemborg C, Merila J. Reproductive investment and moult-breeding overlap in the collared flycatcher Ficedula albicollis: an experimental approach. Ann. Zool. Fenn. 1999;36:1–9. [Google Scholar]
- Hemborg C, Sanz J.J, Lundberg A. Effects of latitude on the trade-off between reproduction and moult: a long-term study with pied flycatcher. Oecologia. 2001;129:206–212. doi: 10.1007/s004420100710. doi:10.1007/s004420100710 [DOI] [PubMed] [Google Scholar]
- Hiatt E.S, Goldsmith A.R, Farner D.S. Plasma levels of prolactin and gonadotrophins during the reproductive cycle of white-crowned sparrows (Zonotrichia leucophrys) Auk. 1987;104:208–217. [Google Scholar]
- Hinsley S.A, Rothery P, Ferns P.N, Bellamy P.E, Dawson A. Wood size and timing of moult in birds: potential consequences for plumage quality and bird survival. Ibis. 2003;145:337–340. doi:10.1046/j.1474-919X.2003.00167.x [Google Scholar]
- Hornfeldt B, Eklund U. The effect of food on laying date and clutch-size in Tengmalms owl, Aegolius funereus. Ibis. 1990;132:395–406. [Google Scholar]
- Immelmann K. Ecological aspects of periodic reproduction. In: Farner D.S, King J.R, editors. Avian biology. Academic Press; New York, NY: 1973. pp. 341–389. [Google Scholar]
- Källander H, Karlsson J. Supplemental food and laying date in the European starling. Condor. 1993;95:1031–1034. doi:10.2307/1369440 [Google Scholar]
- Kang S.W, Thayananuphat A, Rozenbolm I, Millam J.R, Proudman J.A, El Halawani M.E. Expression of hypothalamic GnRH-I mRNA in the female turkey at different reproductive states and following photo stimulation. Gen. Comp. Endocrinol. 2006;146:91–99. doi: 10.1016/j.ygcen.2005.10.017. doi:10.1016/j.ygcen.2005.10.017 [DOI] [PubMed] [Google Scholar]
- Korpimaki E. Breeding biology of the starling Sturnus vulgaris in western Finland. Ornis Fenn. 1978;55:93–104. [Google Scholar]
- Korpimaki E, Wiehn J. Clutch size of kestrels: seasonal decline and experimental evidence for food limitation under fluctuating food conditions. Oikos. 1998;83:259–272. doi:10.2307/3546837 [Google Scholar]
- Laaksonen T, Ahola M, Eeva T, Vaisanen R.A, Lehikoinen E. Climate change, migratory connectivity and changes in laying date and clutch size of the pied flycatcher. Oikos. 2006;114:277–290. doi:10.1111/j.2006.0030-1299.14652.x [Google Scholar]
- Lack D. Methuen; London, UK: 1968. Ecological adaptations for breeding in birds. [Google Scholar]
- Leitner S, Van't Hof T.J, Gahr M. Flexible reproduction in wild canaries is independent of photoperiod. Gen. Comp. Endocrinol. 2003;130:102–108. doi: 10.1016/s0016-6480(02)00574-9. doi:10.1016/S0016-6480(02)00574-9 [DOI] [PubMed] [Google Scholar]
- Lofts B, Murton R.K. Photoperiodic and physiological adaptations regulating avian breeding cycles and their ecological significance. J. Zool. Lond. 1968;155:327–394. [Google Scholar]
- MacDougall-Shackleton S.A, Katti M, Hahn T.P. Tests of absolute photorefractoriness in four species of cardueline finch that differ in reproductive state. J. Exp. Biol. 2006;209:3786–3794. doi: 10.1242/jeb.02447. doi:10.1242/jeb.02447 [DOI] [PubMed] [Google Scholar]
- Marsh R.H, MacDougall-Shackleton S.A, Hahn T.P. Photorefractoriness and seasonal changes in the brain in response to changes in day length in American goldfinches (Carduelis tristis) Can. J. Zool. 2002;80:2100–2107. doi:10.1139/z02-208 [Google Scholar]
- McCleery R.H, Perrins C.M. Temperature and egg-laying trends. Nature. 1998;391:30–31. doi:10.1038/34073 [Google Scholar]
- Meddle S.L, Maney D.L, Wingfield J.C. Effects of N-methyl-d-aspartate on luteinizing hormone release and Fos-like immunoreactivity in the male white-crowned sparrow (Zonotrichia leucophrys gambelii) Endocrinology. 1999;140:5922–5928. doi: 10.1210/endo.140.12.7206. doi:10.1210/en.140.12.5922 [DOI] [PubMed] [Google Scholar]
- Meddle S.L, Bush S, Sharp P.J, Millar R.P, Wingfield J.C. Hypothalamic pro-GnRH-GAP, GnRH-I and GnRH-II during the onset of photorefractoriness in the white-crowned sparrow (Zonotrichia leucophrys gambelii) J. Neuroendocrinol. 2006a;18:217–226. doi: 10.1111/j.1365-2826.2005.01403.x. doi:10.1111/j.1365-2826.2005.01403.x [DOI] [PubMed] [Google Scholar]
- Meddle S.L, Wingfield J.C, Millar R.P, Deviche P.J. Hypothalamic GnRH-I and its precursor during photorefractoriness onset in free-living male dark-eyed juncos (Junco hyemalis) of different year classes. Gen. Comp. Endocrinol. 2006b;145:148–156. doi: 10.1016/j.ygcen.2005.08.013. doi:10.1016/j.ygcen.2005.08.013 [DOI] [PubMed] [Google Scholar]
- Meijer T. Photoperiodic control of reproduction and molt in the kestrel, Falco tinnunculus. J. Biol. Rhythms. 1989;4:351–364. doi: 10.1177/074873048900400304. doi:10.1177/074873048900400304 [DOI] [PubMed] [Google Scholar]
- Meijer T. The effect of a period of food restriction on gonad size and moult of female starlings Sturnus vulgaris under constant photoperiod. Ibis. 1991;133:80–84. [Google Scholar]
- Meijer T, Langer U. Food availability and egg laying of captive European starlings. Condor. 1995;97:718–728. doi:10.2307/1369180 [Google Scholar]
- Meijer T, Daan S, Hall M.R. Family planning in the kestrel (Falco tinnunculus): the proximate control of laying date and clutch size. Behaviour. 1990;114:117–136. [Google Scholar]
- Meijer T, Nienaber U, Langer U, Trillmich F. Temperature and timing of egg-laying of European starlings. Condor. 1999;101:124–132. doi:10.2307/1370453 [Google Scholar]
- Moore I.T, Bonier F, Wingfield J.C. Reproductive asynchrony and population divergence between two tropical bird populations. Behav. Ecol. 2005;16:755–762. doi:10.1093/beheco/ari049 [Google Scholar]
- Moore I.T, Bentley G.E, Wotus C, Wingfield J.C. Photoperiod-independent changes in immunoreactive brain gonadotropin-releasing hormone (GnRH) in a free-living, tropical bird. Brain Behav. Evol. 2006;68:37–44. doi: 10.1159/000093059. doi:10.1159/000093059 [DOI] [PubMed] [Google Scholar]
- Morton M.L. Control of postnuptial molt in the mountain white-crowned sparrow: a perspective from field data. Ornis Scand. 1992;23:322–327. doi:10.2307/3676656 [Google Scholar]
- Morton M.L, King J.R, Farner D.S. Postnuptial and postjuvenal molt in white-crowned sparrows in central Alaska. Condor. 1969;71:376–385. doi:10.2307/1365736 [Google Scholar]
- Murphy M.E, King J.R. Energy and nutrient use during molt by white crowned sparrows, Zonotrichia leucophrys gambelii. Ornis Scand. 1992;23:304–313. doi:10.2307/3676654 [Google Scholar]
- Murton R.K, Westwood N.J. Clarendon; Oxford, UK: 1977. Avian breeding cycles. [Google Scholar]
- Nakamura M. Effect of supplemental feeding and female age on timing of breeding in the alpine accentor. Ibis. 1995;137:56–63. [Google Scholar]
- Newton I. The moult of the bullfinch Pyrrhula pyrrhula. Ibis. 1966;108:41–67. [Google Scholar]
- Newton I. Collins; London, UK: 1972. Finches. [Google Scholar]
- Newton I, Rothery P. The timing, duration and pattern of moult and its relationship to breeding in a population of the European greenfinch Carduelis chloris. Ibis. 2005;147:667–679. doi:10.1111/j.1474-919X.2005.00439.x [Google Scholar]
- Nicholls T.J, Goldsmith A.R, Dawson A. Photorefractoriness in birds and comparison with mammals. Physiol. Rev. 1988;68:133–176. doi: 10.1152/physrev.1988.68.1.133. [DOI] [PubMed] [Google Scholar]
- Nolan V, Ketterson E.D, Ziegenfus C, Cullen D.P, Chandler C.R. Testosterone and avian life histories—effects of experimentally elevated testosterone on prebasic molt and survival in male dark-eyed juncos. Condor. 1992;94:364–370. doi:10.2307/1369209 [Google Scholar]
- Partecke J, Van't Hof T, Gwinner E. Differences in the timing of reproduction between urban and forest blackbirds (Turdus merula): result of phenotypic flexibility or genetic differences? Proc. R. Soc. B. 2004;271:1995–2000. doi: 10.1098/rspb.2004.2821. doi:10.1098/rspb.2004.2821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereyra M.E, Sharbaugh S.M, Hahn T.P. Interspecific variation in photo-induced GnRH plasticity among nomadic cardueline finches. Brain Behav. Evol. 2005;66:35–49. doi: 10.1159/000085046. doi:10.1159/000085046 [DOI] [PubMed] [Google Scholar]
- Perfito N, Tramontin A.D, Meddle S, Sharp P, Afik D, Gee J, Ishii S, Kikuchi M, Wingfield J.C. Reproductive development according to elevation in a seasonally breeding male songbird. Oecologia. 2004;140:201–210. doi: 10.1007/s00442-004-1576-5. doi:10.1007/s00442-004-1576-5 [DOI] [PubMed] [Google Scholar]
- Perfito N, Meddle S, Tramontin A.D, Sharp P, Wingfield J.C. Seasonal gonadal recrudescence in song sparrows: response to temperature cues. Gen. Comp. Endocrinol. 2005;143:121–128. doi: 10.1016/j.ygcen.2005.03.004. doi:10.1016/j.ygcen.2005.03.004 [DOI] [PubMed] [Google Scholar]
- Perfito N, Bentley G, Hau M. Tonic activation of brain GnRH immunoreactivity despite reduction of peripheral reproductive parameters in opportunistically breeding zebra finches. Brain Behav. Evol. 2006;67:123–134. doi: 10.1159/000090977. doi:10.1159/000090977 [DOI] [PubMed] [Google Scholar]
- Perfito N, Zann R.A, Bentley G.E, Hau M. Opportunism at work: habitat predictability affects reproductive readiness in free-living zebra finches. Funct. Ecol. 2007;21:291–301. doi:10.1111/j.1365-2435.2006.01237.x [Google Scholar]
- Perrins C.M. The timing of birds' breeding seasons. Ibis. 1970;112:242–255. [Google Scholar]
- Perrins C.M, McCleery R.H. Laying dates and clutch size in the Great tit. Wilson Bull. 1989;101:236–253. [Google Scholar]
- Pulido F, Coppack T. Correlation between timing of juvenile moult and onset of migration in the blackcap, Sylvia atricapilla. Anim. Behav. 2004;68:167–173. doi:10.1016/j.anbehav.2003.11.006 [Google Scholar]
- Ramenofsky M, Wingfield J.C. Regulation of migration. Bioscience. 2007;57:135–143. doi:10.1641/B570208 [Google Scholar]
- Reynolds S.J, Schoech S.J, Bowman R. Nutritional quality of prebreeding diet influences breeding performance of the Florida scrub-jay. Oecologia. 2003;134:308–316. doi: 10.1007/s00442-002-1126-y. [DOI] [PubMed] [Google Scholar]
- Robinson J.E, Follett B.K. Photoperiodism in Japanese quail: the termination of seasonal breeding by photorefractoriness. Proc. R. Soc. B. 1982;215:95–116. doi: 10.1098/rspb.1982.0030. [DOI] [PubMed] [Google Scholar]
- Rowan W. Relation of light to bird migration and developmental changes. Nature. 1925;115:494–495. [Google Scholar]
- Sanz J.J. Effects of geographic location and habitat on breeding parameters of Great tits. Auk. 1998;115:1034–1051. [Google Scholar]
- Sanz J.J. Large-scale effect of climate change on breeding parameters of pied flycatchers in Western Europe. Ecography. 2003;26:45–50. doi:10.1034/j.1600-0587.2003.03251.x [Google Scholar]
- Scheuerlein A, Gwinner E. Is food availability a circannual zeitgeber in tropical birds? A field experiment on stonechats in tropical Africa. J. Biol. Rhythms. 2002;17:171–180. doi: 10.1177/074873002129002465. doi:10.1177/074873002129002465 [DOI] [PubMed] [Google Scholar]
- Schleussner G, Dittami J.P, Gwinner E. Testosterone implants affect molt in male European starlings, Sturnus vulgaris. Physiol. Zool. 1985;58:597–604. [Google Scholar]
- Schoech S.J. The effect of supplemental food on body condition and the timing of reproduction in a cooperative breeder, the Florida scrub-jay. Condor. 1996;98:234–244. doi:10.2307/1369141 [Google Scholar]
- Schwab R.G. Circannian testicular periodicity in the European starling in the absence of photoperiodic change. In: Menaker M, editor. Biochronometry. National Academy of Sciences; Washington, DC: 1971. pp. 428–447. [Google Scholar]
- Schwab R.G, Rutledge J.T. Testicular metamorphosis rates in European starlings maintained under short daily photophases. Int. J. Biometeorol. 1978;22:116–122. doi: 10.1007/BF01552891. doi:10.1007/BF01552891 [DOI] [PubMed] [Google Scholar]
- Sharp P.J, Blache D. A neuroendorcine model for prolactin as the key mediator of seasonal breeding in birds under long- and short-day photoperiods. Can. J. Physiol. Pharmacol. 2003;81:350–358. doi: 10.1139/y03-025. doi:10.1139/y03-025 [DOI] [PubMed] [Google Scholar]
- Sharp P.J, Dawson A, Lea R.W. Control of luteinizing hormone and prolactin secretion in birds. Comp. Biochem. Physiol. C. 1998;119:275–282. doi: 10.1016/s0742-8413(98)00016-4. [DOI] [PubMed] [Google Scholar]
- Silverin B, Viebke P.A. Low temperatures affect the photoperiodically induced LH and testicular cycles differently in closely related species of tits (Parus spp.) Horm. Behav. 1994;28:199–206. doi: 10.1006/hbeh.1994.1017. doi:10.1006/hbeh.1994.1017 [DOI] [PubMed] [Google Scholar]
- Silverin B, Massa R, Stokkan K.-A. Photoperiodic adaptation to breeding at different latitudes in Great tits. Gen. Comp. Endocrinol. 1993;90:14–22. doi: 10.1006/gcen.1993.1055. doi:10.1006/gcen.1993.1055 [DOI] [PubMed] [Google Scholar]
- Simmons R.E. Effects of supplementary food on density-reduced breeding in an African eagle—adaptive restraint or ecological constraint. Ibis. 1993;135:394–402. [Google Scholar]
- Sparks T.H, Bairlein F, Bojarinova J.G, Huppop O, Lehikoinen E.A, Rainio K, Sokolov L.V, Walker D. Examining the total arrival distribution of migratory birds. Glob. Change Biol. 2005;11:22–30. doi:10.1111/j.1365-2486.2004.00887.x [Google Scholar]
- Stevenson T.J, MacDougall-Shackleton S.A. Season- and age-related variation in neural cGnRH-I and cGnRH-II immunoreactivity in house sparrows (Passer domesticus) Gen. Comp. Endocrinol. 2005;143:33–39. doi: 10.1016/j.ygcen.2005.02.019. doi:10.1016/j.ygcen.2005.02.019 [DOI] [PubMed] [Google Scholar]
- Storey C.R, Nicholls T.J. Some effects of manipulation of daily photoperiod on the rate of onset of a photorefractory state in canaries (Serinus canarius) Gen. Comp. Endocrinol. 1976;30:204–208. doi: 10.1016/0016-6480(76)90101-5. doi:10.1016/0016-6480(76)90101-5 [DOI] [PubMed] [Google Scholar]
- Storey C.R, Nicholls T.J. Low environmental temperature delays photoperiodic induction of avian testicular maturation and the onset of post-nuptial photorefractoriness. Ibis. 1982;124:172–174. [Google Scholar]
- Suomalainen H. The effect of temperature on the sexual activity of non-migratory birds, stimulated by artificial lighting. Ornis Fenn. 1938;14:108–112. [Google Scholar]
- Takagi T, Yamamura T, Anraku T, Yasuo S, Nakao N, Watanabe M, Iigo M, Ebihara S, Yoshimura T. Involvement of transforming growth factor alpha in the photoperiodic regulation of reproduction in birds. Endocrinology. 2007;148:2788–2792. doi: 10.1210/en.2007-0112. doi:10.1210/en.2007-0112 [DOI] [PubMed] [Google Scholar]
- Torti V.M, Dunn P.O. Variable effects of climate change on six species of North American birds. Oecologia. 2005;145:486–495. doi: 10.1007/s00442-005-0175-4. doi:10.1007/s00442-005-0175-4 [DOI] [PubMed] [Google Scholar]
- Ukena K, Ubuka T, Tsutsui K. Distribution of a novel avian gonadotropin-inhibitory hormone in the quail brain. Cell Tissue Res. 2003;312:73–79. doi: 10.1007/s00441-003-0700-x. [DOI] [PubMed] [Google Scholar]
- Underhill L.G, Prys-Jones R.P, Dowsett R.J, Herroelen P, Johnson D.N, Lawn M.R, Norman S.C, Pearson D.J, Tree J. The biannual primary moult of willow warblers Phylloscopus trochilus in Europe and Africa. Ibis. 1992;134:286–297. [Google Scholar]
- Visser M.E. Timing of reproduction: genetic variation in the mechanism underlying phenotypic plasticity. J. Ornithol. 2006;147:108–109. [Google Scholar]
- Visser M.E, et al. Variable responses to large-scale climate change in European Parus populations. Proc. R. Soc. B. 2003;270:367–372. doi: 10.1098/rspb.2002.2244. doi:10.1098/rspb.2002.2244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser M.E, Both C, Lambrechts M.M. Global climate change leads to mistimed avian reproduction. Adv. Ecol. Res. 2004;35:89–110. [Google Scholar]
- Watanabe T, Yamamura T, Watanabe M, Yasuo S, Nakao N, Dawson A, Ebihara S, Yoshimura T. Hypothalamic expression of thyroid-activating and -inactivating enzyme genes in relation to photorefractoriness in birds and mammals. Am. J. Physiol. Reg. Int. Comp. Physiol. 2007;292:R568–R572. doi: 10.1152/ajpregu.00521.2006. [DOI] [PubMed] [Google Scholar]
- Weggler M. Constraints on, and determinants of, the annual number of breeding attempts in the multi-brooded black redstart, Phoenicurus ochruros. Ibis. 2006;148:273–284. [Google Scholar]
- Wikelski M, Hau M, Wingfield J.C. Seasonality of reproduction in a neotropical rain forest bird. Ecology. 2000;81:2458–2472. [Google Scholar]
- Wikelski M, Hau M, Robinson W.D, Wingfield J.C. Reproductive seasonality of seven neotropical passerine species. Condor. 2003;105:683–695. doi:10.1650/7251 [Google Scholar]
- Williams T.D, Dawson A, Nicholls T.J, Goldsmith A.R. Short days induce premature reproductive maturation in juvenile starlings, Sturnus vulgaris. J. Reprod. Fertil. 1987;80:327–333. doi: 10.1530/jrf.0.0800327. [DOI] [PubMed] [Google Scholar]
- Wingfield J.C. Fine temporal adjustment of reproductive functions. In: Epple A, Stetson M.H, editors. Avian endocrinology. Academic Press; New York, NY: 1980. pp. 367–389. [Google Scholar]
- Wingfield J.C. Influences of weather on reproductive function in female song sparrows, Melospiza melodia. J. Zool. Lond. A. 1985;205:545–558. [Google Scholar]
- Wingfield J.C. Control of testicular cycles in the song sparrow, Melospiza melodia: interaction of photoperiod and an endogenous program? Gen. Comp. Endocrinol. 1993;92:388–401. doi: 10.1006/gcen.1993.1176. doi:10.1006/gcen.1993.1176 [DOI] [PubMed] [Google Scholar]
- Wingfield, J. C. In press. Organization of vertebrate annual cycles: implications for control mechanisms. Phil. Trans. R. Soc. B (doi:10.1098/rstb.2007.2149) [DOI] [PMC free article] [PubMed]
- Wingfield J.C, Farner D.S. The annual cycle of plasma irLH and steroid hormones in feral populations of the white-crowned sparrow, Zonotrichia leucophrys gambelii. Biol. Reprod. 1978;19:1046–1056. doi: 10.1095/biolreprod19.5.1046. doi:10.1095/biolreprod19.5.1046 [DOI] [PubMed] [Google Scholar]
- Wingfield J.C, Farner D.S. Endocrine responses of white-crowned sparrows to environmental stress. Condor. 1982;84:399–409. doi:10.2307/1367443 [Google Scholar]
- Wingfield J.C, Goldsmith A.R. Plasma levels of prolactin and gonadal steroids in relation to multiple-brooding and renesting in free-living populations of the song sparrow, Melospiza melodia. Horm. Behav. 1990;24:89–103. doi: 10.1016/0018-506x(90)90029-w. doi:10.1016/0018-506X(90)90029-W [DOI] [PubMed] [Google Scholar]
- Wingfield J.C, Hahn T.P, Levin R, Honey P. Environmental predictability and control of gonadal cycles in birds. J. Exp. Zool. 1992;261:214–231. doi:10.1002/jez.1402610212 [Google Scholar]
- Wingfield J.C, Hahn T.P, Wada M, Astheimer L.B, Schoech S. Interrelationship of day length and temperature on the control of gonadal development, body mass, and fat score in white-crowned sparrows, Zonotrichia leucophrys gambelii. Gen. Comp. Endocrinol. 1996;101:242–255. doi: 10.1006/gcen.1996.0027. doi:10.1006/gcen.1996.0027 [DOI] [PubMed] [Google Scholar]
- Wingfield J.C, Hahn T.P, Maney D.L, Schoech S.J, Wada M, Morton M.L. Effects of temperature on photoperiodically induced reproductive development, circulating plasma luteinizing hormone and thyroid hormones, body mass, fat deposition and molt in mountain white-crowned sparrows, Zonotrichia leucophrys oriantha. Gen. Comp. Endocrinol. 2003;131:143–158. doi: 10.1016/s0016-6480(02)00648-2. doi:10.1016/S0016-6480(02)00648-2 [DOI] [PubMed] [Google Scholar]
- Yamamura T, Yasuo S, Hirunagi K, Ebihara S, Yoshimura T. T-3 implantation mimics photoperiodically reduced encasement of nerve terminals by glial processes in the median eminence of Japanese quail. Cell Tissue Res. 2006;324:175–179. doi: 10.1007/s00441-005-0126-8. doi:10.1007/s00441-005-0126-8 [DOI] [PubMed] [Google Scholar]
- Yasuo S, Watanabe M, Nakao N, Takagi T, Follett B.K, Ebihara S, Yoshimura T. The reciprocal switching of two thyroid hormone-activating and -inactivating enzyme genes is involved in the photoperiodic gonadal response of Japanese quail. Endocrinology. 2005;146:2551–2554. doi: 10.1210/en.2005-0057. doi:10.1210/en.2005-0057 [DOI] [PubMed] [Google Scholar]