Abstract
Hormonal control systems are complex in design and well integrated. Concern has been raised that these systems might act as evolutionary constraints when animals are subject to anthropogenic environmental change. Three systems are examined in vertebrates, especially birds, that are important for assessing this possibility: (i) the hypothalamic–pituitary–gonadal (HPG) axis, (ii) the activational effects of sex steroids on mating effort behaviour, and (iii) sexual differentiation. Consideration of how these systems actually work that takes adequate account of the brain's role and mechanisms suggests that the first two are unlikely to be impediments to evolution. The neural and molecular networks that regulate the HPG provide both phenotypic and evolutionary flexibility, and rapid evolutionary responses to selection have been documented in several species. The neuroendocrine and molecular cascades for behaviour provide many avenues for evolutionary change without requiring a change in peripheral hormone levels. Sexual differentiation has some potential to be a source of evolutionary inertia in birds and could contribute to the lack of diversity in certain reproductive (including life history) traits. It is unclear, however, whether that lack of diversity would impede adaptation to rapid environmental change given the role of behavioural flexibility in avian reproduction.
Keywords: hypothalamic–pituitary–gonadal axis, mating behaviour, hormonal activation, sexual differentiation, Japanese quail, constraint
1. Introduction
The notable achievements of modern biology include a sophisticated understanding of vertebrate endocrine systems. Much has been learned about how they work, their interactions with the nervous and immune systems, the ways they respond to the demands of the animal's internal and external environments, how they regulate behaviour and how they contribute to the development of sexual phenotypes (Norris 1996; Pfaff et al. 2002). Hormonal control systems are complex, with multiple levels of hierarchical control, negative and positive feedback loops and numerous signalling molecules. They are integrated systems capable of keeping hormone levels within certain limits. A perturbation in one hormone is likely to affect not only other levels of the same control system but also other hormonal systems altogether, as when an alteration in gonadal hormones affects adrenal hormone levels, and entire suites of correlated hormone-dependent traits (McGlothlin & Ketterson 2008).
Such integrated systems have been suggested to act as constraints; that is, sources of inertia slowing down evolution, when animals such as birds are subjected to a change in selective pressures (see McGlothlin & Ketterson 2008). In adaptive landscape terms, if a change in the environment produces a shift in the fitness landscape so that the population is no longer at an adaptive peak, hormonal control systems could conceivably reduce the probability that the population will make it to another adaptive peak in time, avoiding extinction. The rapidity of recent environmental change due to human activity has made knowing whether hormonal systems would significantly lower the chances of success a matter of some urgency. Ideally empirical evidence would be brought to bear on the question, especially from quantitative genetic studies of selection on hormone-dependent suites of traits in free-living populations, but such evidence is quite limited at this time (McGlothlin & Ketterson 2008). This essay will evaluate the question from the complementary perspective of behavioural neuroendocrinology. Three hormonal control systems will be examined in vertebrates, especially birds: (i) the hypothalamic–pituitary–gonadal (HPG) axis, (ii) the activational effects of sex steroids on fitness-promoting behaviour related to mating effort, and (iii) developmental cascades for sexual differentiation. It will be argued that such systems will not necessarily slow down adaptive walks.
2. The hypothalamic–pituitary–gonadal axis
The endocrine axis for the regulation of the gonads and their products is often part of discussions of the mechanistic underpinnings of life histories. The degree to which this axis is ‘on’ or ‘off’ is related to key life-history traits such as age at onset of reproductive maturity and timing of onset of seasonal breeding. This axis is also the basis for an animal's ability to respond hormonally to other individuals, for social influences on hormone levels.
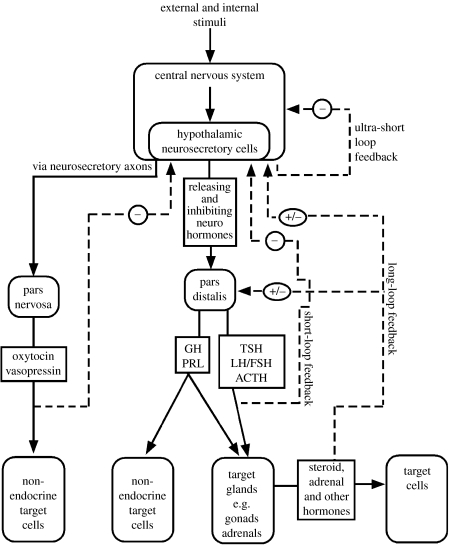
Figure 1 is a schematic of the HPG along with other major endocrine axes. Most of the major features shown here are present in birds and other vertebrates, including mammals. The axes function as multiple-tier hierarchies. For the HPG, the top tier includes the neurons in the hypothalamus that produce gonadotrophin-releasing hormone (GnRH). This peptide neurohormone is transported to the anterior pituitary (pars distalis), where it regulates the amount of luteinizing hormone (LH) and follicle-stimulating hormone (FSH) that will enter the general circulation. Upon reaching the gonads, these glycoprotein hormones then regulate the production of the sex steroid hormones that will enter the circulation.
Figure 1.
Endocrine axes, including the HPG axis. See text for description. (Adapted from Norris (1996). Copyright © Academic Press (Elsevier).)
These three tiers are linked in the reverse direction by feedback loops. In the case of the HPG, the circulating levels of the gonadal steroids are detected by the pars distalis and the hypothalamus, and appropriate adjustments occur to raise or lower the steroid levels according to age, season and other circumstances. Negative feedback is reflected in the observation that the testes shrink if males are given exogenous testosterone (see Wilhelms et al. 2005 for a recent example). Positive feedback can also occur, resulting in a continually increasing level, as when a sustained increase in oestradiol triggers an LH surge and ovulation in a mammal.
Figure 1 also outlines other axes involved in the regulation of hormones related to reproductive traits, including the axis involved in the regulation of the smaller peptides of the oxytocin family (mesotocin and vasotocin in birds instead of the mammalian oxytocin and vasopressin). These various axes interact with each other, with the hypothalamic–pituitary–adrenal axis (HPA) that regulates corticosterone, and with the thyroid hormone axis (Wilson 2001). Not shown in the figure is the complex array of neurochemicals and other molecules that impact the hypothalamic neurosecretory cells in the top tier (Romero & Sapolsky 1996; Kriegsfeld 2006). Also not shown is that the brain is a source of steroids as well as one of their targets.
This agglomerate system in figure 1 is impressively complex and nicely integrated. Does it follow that these mechanisms introduce inertia into the evolutionary process of responding to a changed environment? Would they slow down an adaptive response such as a change in the timing of breeding? Would a change in one component cause the whole system to malfunction? There are several reasons to think not, based on how the system works, its phylogenetic distribution and empirical evidence.
First, a critical operating principle of these systems is that the top tier consists of brain mechanisms (neurons, neurotransmitters and neuromodulators), not peripheral endocrine tissue. Endocrine control systems are neuroendocrine systems. The relevant part of the brain, the hypothalamus, is richly connected, neurally integrating several major brain systems (Butler & Hodos 2005). It receives (directly or indirectly) information about both external and internal environments. The indirect information includes an evaluation of the significance of what has been detected. This is what enables the endocrine axes to respond to complex and nuanced stimuli such as the quality of a male's song. The involvement of the brain in hormonal regulation also means that endocrine responses can be acquired through experience (Graham & Desjardins 1980). Hormonal regulation occurs in a top-down manner guided by a highly intelligent organ capable of adjustments over time through both evolution and learning.
With respect to genetic evolution, the complexity of the molecular networks that regulate GnRH opens many avenues for adaptive tinkering. What is likely to evolve is not so much the endocrinology of the axis itself (the hormones) but the neural mechanisms that assess the external world and the bird's condition and do the computational work for the HPG to be in the right state at the right time.
The phrase ‘tight integration’, when applied to hormonal control systems like the HPG, could easily be misinterpreted to mean that hormone levels themselves are tightly regulated. The concept of homeostatic regulation is certainly an important part of endocrinology, but there is a substantial difference in the tolerance limits between hormones required for health and life such as insulin or aldosterone and those for reproduction. In birds, the HPG axis is virtually off when not breeding (Dawson 2008). HPG hormone levels are dynamic and can respond to social encounters such as territorial intrusions (Wingfield et al. 1999). Peripheral steroid levels vary both within and across individuals to a considerable degree, and a substantial range of values has been found in animals that are reproducing successfully (Ketterson et al. 2005; Kempenaers et al. 2008). Yes, the feedback loops in the HPG allow the brain to set peripheral hormone levels fairly precisely (Kriegsfeld 2006). Precision does not mean rigidity, however, but rather temporary balance points that can undergo phenotypic change rapidly and flexibly.
A second reason to doubt that these hormonal control systems are inertial with respect to evolutionary change is that many of their features (those in figure 1) are characteristic of vertebrates generally. These features originated a very long time ago and have been working for those lineages that still exist for over 400 Myr. Mechanisms such as GnRH have already been filtered by selection to work in spite of environmental change and are currently accommodating a wider array of adaptive landscape peaks than those occupied by birds. It is heartening to realize that birds did make it through the mass extinction event that eliminated all the other dinosaurs, an event that may have involved climate change more sudden and extreme than what is occurring now (Clarke et al. 2005). Yet we would expect that some groups of birds would adapt through change better than others. For example, smaller birds with an earlier onset of reproduction and shorter generation intervals, and those that could evolve these characteristics rapidly, might be more likely to avoid extinction.
This brings us to the third reason, the empirical evidence for rapid responses to selection. Deermice (Peromyscus) populations contain a surprising amount of standing variation in the photoperiodic response, and artificial selection produces a significant change in only two generations (Desjardins et al. 1986; Avigdor et al. 2005). A comparative study of several cardueline finch species suggests considerable evolutionary lability in the photoperiodic response (Hahn et al. 2004). Avian migration has long been thought to involve hormonal regulation, and migratory tendencies, like seasonal schedules more generally, are flexible, responding to social cues and changing rapidly over generations (Helm & Gwinner 2006; Helm et al. 2006). In a population of blackcaps (Sylvia atricapilla), the onset of migratory activity was significantly heritable and responded rapidly to artificial selection (Pulido et al. 2001). The change in breeding onsets in Europe that has coincided with a warming trend (Winkler et al. 2002; Both et al. 2004), while alarming, can also be taken to mean that endocrine control systems involved in the photoperiodic response and in migration are not constraining birds from tracking temperature change due to human activities.
There are also reasons to be cautious about concluding that hormonal control systems would not prevent sufficiently rapid adaptation (see also Dawson 2008). One caveat is that the endocrinology of a critical life-history stage, sexual maturation, is not understood in most birds. Age at onset of reproduction is a major predictor of lifetime reproductive success (Roff 2001). In a few domestic or captive birds that breed at 1 year of age or earlier, the timing of reproductive onset has been shown to be regulated by increasing day lengths (e.g. quail) which may require prior breaking of photorefractoriness (e.g. starlings); regulation of onset in species with deferred maturity (e.g. seabirds) is much more mysterious (Follett 1992). In mammals, juvenile brain mechanisms seem to actively inhibit the HPG, and at puberty those inhibitory mechanisms are toned down (Plant 2001; Ojeda & Terasawa 2002). Much less is known about how the brain regulates sexual maturation in birds (see Dawson & Goldsmith 1989 and Fraley & Kuenzel 1993 for examples of some progress). Without knowing what the neural mechanisms are at that time the transition from juvenile life to reproductive adulthood in species with deferred maturity, it is hard to know how flexible they are, or how rapidly earlier onset of reproduction could evolve if selection for it increased. Living birds vary enormously in age at reproductive onset, but the phylogeny of that variation suggests that it has evolved over long time periods (Bennett & Owens 2002). Finch (2002) has argued that the hormonal architecture of life-history traits like puberty permits a great deal of evolutionary flexibility, and studies have shown that selection can alter hormone levels at one life-history stage without affecting others (Zera et al. 2007). Age at reproductive maturity is heritable in chickens (Craig et al. 1975), but it would be reassuring to have pertinent evidence from free-living birds. Would selection for earlier reproductive onset be constrained by trade-offs with other traits? A trade-off with growth does not seem likely, because unlike many other animals, birds, even those with very early puberty, reach adult body size prior to reproductive onset (Follett 1992). Trade-offs with immune function or experience-dependent parental foraging ability are possible, however.
Another caveat concerns the nature of the small sample of avian species whose endocrinology has been studied. Only a few clades are represented. Some species, such as chickens, are domesticated forms with a long history of artificial selection for reproduction. Those that are genetically wild, such as song and white-crowned sparrows (Melospiza melodia and Zonotrichia leucophrys, respectively), European starlings (Sturnus vulgaris) and house sparrows (Passer domesticus), have large ranges (the song and white-crowned sparrows) and are ‘weedy’ colonizers (starlings and house sparrows). These are generalists with sufficiently flexible neuroendocrine systems to adapt quickly to a wide array of latitudes and climates. Birds with more limited habitat preferences and ranges might not have this same pre-existing neuroendocrine flexibility.
A final caveat is the relative dearth of information about female birds. Female traits such as age at maturation and fecundity have a big impact on population size and risk of local extinction, and female–female aggressive interactions can be a major determinant of mating systems and social structure (Sandell 1998). Yet studies of hormones in wild species focus on males much more often than females.
3. Activation of behaviour related to mating effort
Adaptation to environmental change is behavioural as well as physiological. The important targets for gonadal hormones include the neural machinery responsible for the fitness-promoting behaviour of courtship and mating. How inflexible and constraining would this machinery be if, for example, environmental change selected for a change in mating frequency?
Major progress has been made discovering the neural circuits and hormone-initiated brain cascades responsible for the activation of mating (copulation), a critical fitness moment. The diverse array of vertebrates studied includes a bird, the Japanese quail (Coturnix japonica).
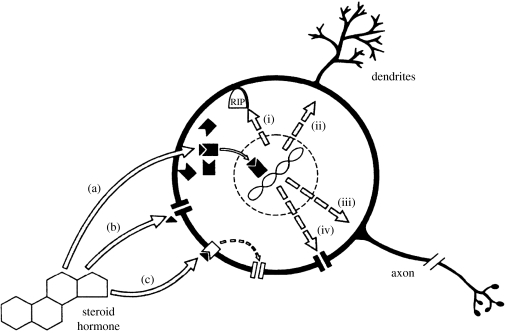
Steroids act through several mechanistic pathways to act on brain neurons to change behaviour probability. They are normally present in minuscule amounts, and their mechanisms of action involve cascades of events that amplify these tiny signals. Figure 2 summarizes steroid actions on neurons. The best known pathway, (a) in the figure, begins with intracellular receptors and involves the genome. The steroid receptors are ligand-dependent DNA-binding transcription factors. The steroid is the ligand. The steroid–receptor complex binds to hormone response elements located in the promoters of steroid-regulated genes. In combination with co-activators and co-repressors they alter gene transcription, regulating the amount of mRNA transcript emanating from the target genes. Those transcripts are in turn translated into peptides and proteins. Exactly what genes are affected, and how the target tissue will respond, depend on the steroid-receptor cofactors (co-regulatory activator or repressor proteins, not shown in the figure) and downstream mechanisms. In the brain's target neurons, the protein products can include enzymes for steroid synthesis and metabolism, steroid receptors (steroids often regulate their own receptors), enzymes for production or destruction of neurotransmitters, receptors for neurotransmitters, neuropeptides and their receptors, ion channels (pathway (iv)), proteins for building and repairing axons, dendrites and synapses (pathways (ii) and (iii)), and products that affect whether neurons will die (pathway (i)). The power of steroids to alter cellular structure and function by acting on the genome at multiple gene loci is impressive and helps to explain the diversity of steroid actions as well as their ability to coordinate multiple features of the behavioural and morphological phenotype.
Figure 2.
Mechanisms of action of steroid hormones on neurons. See text for description. (Reproduced with permission from Weeks & Levine (1995). Copyright © 2000 Elsevier Science).
Pathway (a) has been shown to be critical for the mating behaviour of the male Japanese quail (see Ball & Balthazart 2008). The behaviour is activated in part by oestrogen through a process in which some of the male's circulating testosterone is converted to oestradiol by the neurons in the preoptic area.
Other pathways of action ((b) and (c) in figure 2) have also been discovered. Some steroids can act on molecules in cell (neuron) membranes such as neurotransmitter receptors or ion channels (b). Steroids can bind not only to the intracellular receptors but also to steroid receptors located in cell membranes, thereby altering other proteins in the cell membrane via second messengers (c). These mechanisms of action have the potential to alter behaviour much more rapidly than pathway (a), in seconds or minutes instead of hours or days. These slow and fast pathways are thought to work together as two arms of an integrated signalling pathway, with the fast mechanisms setting up the slow ones (Lange 2007).
The relevance of pathways (b) and (c) for avian mating behaviour is just beginning to be studied, but there is already evidence from Japanese quail for a rapid effect of oestradiol on male mating (Balthazart & Ball 2006; Cornil et al. 2006). Further layers of complexity include a second oestrogen receptor (ERβ), dopaminergic and glutamatergic neurons, additional signalling molecules such as CREB, and other parallel cascades, including those involving the oxytocin family peptides and their receptors (Absil et al. 2001; Panzica et al. 2001; Ball & Balthazart 2008).
Even in a relatively well-worked out case like the mating behaviour of quail, there are still unsolved puzzles. For example, the male's behaviour is completely steroid dependent, and there are marked individual differences among males in mating frequency, yet thus far the mechanisms responsible for those individual differences have proven elusive (Yang et al. 1998; Ball & Balthazart 2008). As the raw material for evolutionary change, mechanisms behind individual variation are vitally important to discover yet seldom known.
This complexity of the molecular cascade for mating could be taken to mean that changing a hormonally regulated behaviour would be difficult. Yet there are three reasons to think that these activation cascades are permissive of evolutionary change rather than inertial.
First, this complexity provides many potential ways to ‘tweak’ the system on the receiving end (the steroid targets) through genetic change (Adkins-Regan 2005). Steroids and their receptors are not derived from the same genes (steroids are not direct gene products), neither are peptides and their receptors, nor the steroid and peptide receptors. The functional linkages occur through regulatory crosstalk, not genetic pleiotropism. In comparative endocrinology, it has long been appreciated that evolutionary change is more likely to occur at the target tissue and its properties, in the very mechanisms shown in figure 2, than in the hormones themselves (see Falcón et al. 2007 for a recent example). Artificial selection for hormone-dependent behaviour usually occurs without any change in the peripheral hormone level (see the work summarized by Yang et al. 1998 on long-term selection for mating frequency in male quail). Thus the possible costs of altered peripheral levels are not likely to act as constraints.
Second, modifications to organisms do not have to occur through mutations in structural DNA but can occur through alterations in gene expression across space and time, i.e. through changes in DNA regulatory elements (Britten & Davidson 1971; Lim et al. 2004). For example, all cells have the gene for ERα. Whether a neuron produces ERα is based on whether the ERα gene is expressed there. It is already known from studies of songbirds and voles that hormone-related gene expression patterns can evolve quickly enough to differ notably between related species, with interesting consequences for vocalization (Gahr & Balaban 1996; Shaw & Kennedy 2002), mating systems (Young et al. 1998) and sociality (Goodson et al. 2006). Variation in hormone-related gene expression has been discovered within species and populations that are associated with behavioural variation, providing a mechanistic basis for microevolutionary change (Phelps & Young 2003).
A third reason to resist interpreting these mechanisms as inertial is the existence of multiple parallel pathways involved both upstream (those shown in figure 2) and further downstream (the peptide cascades resulting from steroid interactions with DNA). For example, there are multiple receptor types for many hormones, peptides and neurotransmitters. This ensures that no one pathway is strictly necessary because together the rest will be sufficient. This is one reason gene knockouts designed to disable one hormone-related brain mechanism do not always have the expected behavioural impact (Nelson 1997). Naturally occurring genetic change could occur without disabling integrated hormonal activation systems.
4. The sexual differentiation cascades
The sexes generally share many life-history traits but differ quantitatively in some, for example, age at sexual maturation or qualitatively in others, e.g. clutch size, an exclusively female trait. How the two sexual phenotypes are produced is an important problem with a long history of research attention. How does the differentiation of male and female anatomy and behaviour occur from an initially undifferentiated embryo? What is the involvement of hormones in this process?
Quite a bit has been learned about the developmental processes and molecular cascades of sex determination and sexual differentiation in a few model species of birds, principally Japanese quail and chickens, both of which have precocial development, and zebra finches (Taeniopygia guttata), which have altricial development.
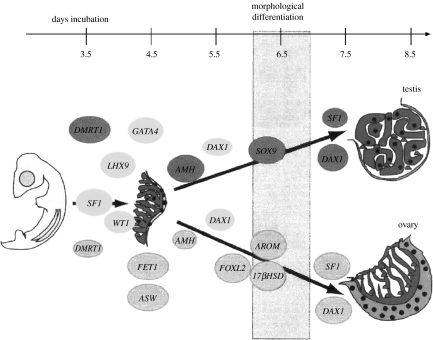
Birds have a ZZ/ZW system of sex chromosomes and the female (the ZW sex) is the sex-determining parent. If an ovum receives a Z chromosome, the embryo will be ZZ and will develop testes; if it receives a W, it will be ZW and will develop ovaries. Figure 3 summarizes some of what has been learned about the molecular cascades for gonadal sex determination. Some of these genes are the same or similar to those involved in mammals. That is, much of the cascade seems to be conserved even when the starting point (a ZZ/ZW versus XX/XY system) is not (Wilkins 2002). One interesting difference is that the aromatase enzyme and oestrogen are part of the cascade creating an ovary in birds and other vertebrates but not in mammals (Elbrecht & Smith 1992; Wade & Arnold 1996). Oestrogen is not only a product of an ovary but also part of the process that produces an ovary in the first place.
Figure 3.
Gene expression during gonadal sex differentiation in the chicken embryo. Circles indicate the onset of gene expression. (Reproduced with permission from Smith & Sinclair (2004). Copyright © 2004 Wiley Periodicals, Inc.)
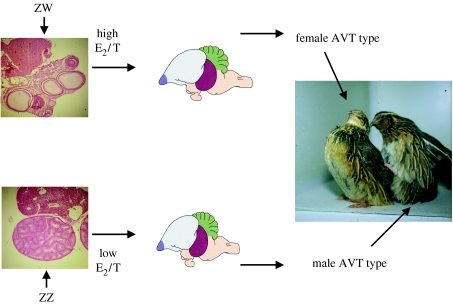
Sexual differentiation of the brain and mating behaviour in chickens and quail is a ‘bottom-up’ process directed by the embryo's own gonads according to an organizational principle (see Balthazart & Adkins-Regan 2002 for details). During a critical period before hatching, gonadal sex steroids determine whether the embryo will develop the capacity for an adult male or female behavioural phenotype. The process has been best explored in quail and is summarized in figure 4. An embryonic ovary will produce a high ratio of oestradiol to testosterone (Schumacher et al. 1988; Ottinger et al. 2001). Such steroid exposure will eliminate the capacity for male mounting behaviour and sexual interest in females. Embryonic testes will produce a low ratio of oestradiol to testosterone, resulting in an individual that will retain the ability to mate like a male and be sexually interested in females. The critical evidence for this scenario includes experiments in which birds hatched from eggs injected with oestradiol show a female behavioural profile as adults, regardless of their genetic sex, whereas birds hatched from eggs treated with oestrogen receptor antagonists or oestrogen synthesis inhibitors show a male behavioural profile, regardless of genetic sex. Among the advantages of this kind of early organization of adult behaviour are that a behaviour that promotes fitness in one sex can be permanently deleted from the other sex if its fitness effect there would be negative, eliminating a constraint on one sex due to a genetic correlation with the other.
Figure 4.
Organization by embryonic gonadal hormones of adult sex differences in the brain and behaviour of Japanese quail. See text for description.
What are the brain mechanisms responsible for such powerful and permanent effects of the embryonic steroid milieu? While this part of the story is still evolving, it is already clear that the neurons producing the peptide arginine vasotocin are an important chapter. There is a marked sex difference in quail in the vasotocinergic innervation of the medial preoptic area and bed nucleus of the stria terminalis (two brain regions for mating), with innervation in males but almost none in females. The same embryonic treatments that sex reverse mating behaviour also sex reverse this innervation pattern, a striking parallel that may reflect a causal relationship (Panzica et al. 1998).
Unfortunately, a satisfying picture of sexual differentiation of behaviour in songbirds (oscine passerines) such as zebra finches has not yet emerged. This is ironic given that the forebrain song system of songbirds contains the most striking brain sex differences of any vertebrates. Genetic females experimentally altered to develop testes still have a female song system, and genetic males given steroid synthesis inhibitors during the putative critical period still develop a male song system (Wade & Arnold 2004). The sex of the gonads does not predict the sex of the song system, ruling out a bottom-up organizational hormone principle altogether. The current alternative hypothesis is that sex chromosome gene products expressed in the brain are the signals for brain/behaviour sexual differentiation, but what those genes or signals are is unclear (Wade & Arnold 2004).
Meanwhile progress has been made towards discovering the cascades involved in sexual differentiation of the mammalian brain (De Vries & Simerly 2002; McCarthy et al. 2003; Forger 2006). Although the initiating signals originate differently in mammals than in birds (from testes in mammals, the ovary in chickens and quail and the brain in zebra finches), some of the downstream cascades could be conserved and generalize to bird brains. Figure 5 summarizes part of what has been learned.
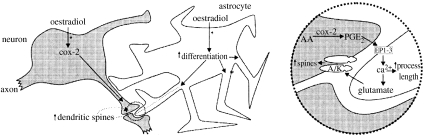
Figure 5.
Proposed mechanisms of sexual differentiation in the preoptic region of the male rat brain. The initiating signal is oestradiol (E2) derived from testosterone (T) by brain aromatization. COX-2, enzyme involved in the synthesis of PGE2 (prostaglandin-E2, which is both necessary and sufficient for masculinization of mating by E2); AA, arachidonic acid; EP1-3, prostaglandin receptor subtypes 1–3; A/K, AMPA/Kainate glutamate receptor. (Reproduced with permission from McCarthy et al. (2003). Copyright © 2003 New York Academy of Sciences.)
Once again, it is tempting to look at figures 3–5 and conclude that this complexity in developmental cascades must surely produce evolutionary inertia. How can anything about the differentiation process be altered except with difficulty (risk of maladaptive intersex states) and over a long period of time? Yet there are reasons to think that developmental complexity is not necessarily constraint.
One argument comes from evolutionary developmental biology (Gerhart & Kirschner 1997; Hall 1999; Wilkins 2002; West-Eberhard 2003). Developmental mechanisms are modular—networks of molecular interactions that are versatile and semi-discrete. Each module is well integrated within itself through regulatory linkage, not genetic linkage. Integration in turn provides stability, but stability is not constraint. Development is canalized owing to buffering and redundancy, not rigidity. New interactions within modules can evolve even if there are some seemingly awkward intermediate stages on the way to the new adaptive peak (Bridgham et al. 2006; Poelwijk et al. 2007).
Within-module integration allows sensibly coordinated change in an entire suite of characters. Surprisingly small genetic changes can produce a matched set of changes in all the products of the module, as in the evolution of vertebrate crania (Hanken & Gross 2005; Noden & Schneider 2006). With respect to brain circuits, separate genes are not needed to produce changes in each of the component parts. During development, groups of neurons (nuclei) affect those that project to them through trophic relationships and other regulatory signalling. A change in one nucleus will alter others to which it is connected (Striedter 2005).
These insights change how to think about the relation between molecular genetic and phenotypic evolution. There is no simple one-to-one relationship between the two. Furthermore, the only way to understand the true relationship between genotypic change and endocrine or behavioural change is to get the nervous system into the picture.
Modules are also dissociable. A sizable change in one does not have to perturb others. Males and females develop a very different set of reproductive organs without perturbing how the kidneys and other nearby organs develop. In seasonally breeding species, reproductive organs regress in the non-breeding season and recrudesce in the breeding season, again without ill effects on other organs. Nor are all the mechanisms for generating sex differences in response to natural and sexual selection part of the same module. A change in one kind of behaviour such as singing or parenting can occur without altering other behaviour. Males of species with sex role reversal, such as phalaropes, still mate like males, and female songbirds that sing still mate like females.
This leads to the second, more empirical, reason to doubt that the developmental cascades of sexual differentiation are inertial. Comparative analyses of evolutionary changes in sexual dimorphism tend to show that such changes have occurred frequently in birds (Szekely et al. 2000; Dunn et al. 2001). For example, Thryothorus wrens vary markedly in the degree of sexual dimorphism in both singing and the neural song system (Brenowitz & Kroodsma 1996).
On the other hand, even those evolutionary changes might have required more time than current anthropogenic environmental change will allow. Furthermore, some aspects of avian sexual differentiation might be hard to change, slowing down or preventing potentially adaptive phenotypic change. For example, in most birds the Z chromosome is much larger than the W. It is thought that once sex chromosomes have become highly heteromorphic, this process cannot be reversed, but instead goes to fixation (Charlesworth 1991). Suppose birds were to be subjected to some kind of selective pressure favouring a trait that could evolve with greater likelihood with an XX/XY system than a ZZ/ZW system. Such an adaptive walk might be impossible.
Developing birds are quite sensitive to exogenous oestrogens, both behaviourally, as when male brains are feminized by low doses, and in egg production, as when female embryos exposed to elevated levels have oviduct abnormalities and fail to lay eggs (Adkins-Regan et al. 1995). If pollution from endocrine disrupting chemicals continues, it may be difficult for birds to escape some fitness reducing consequences (Halldin 2005; Ottinger et al. 2005). How rapidly could a bird evolve resistance to environmental oestrogens without disrupting its normal sexual differentiation processes and essential egg production functions?
Another possible constraint is that birds have little potential for adaptive manipulation of primary sex ratios through either temperature sex determination (a method used by some reptiles) or ‘top-down’ socially induced adult sex change (a method used by some teleost fishes). Some female birds seem to produce adaptively biased primary or secondary offspring sex ratios. How they do this is not well understood, however, and those hormonal mechanisms for which there is evidence seem to come with a cost in fertilization failure or embryonic mortality (Correa et al. 2005; Love et al. 2005; Rutkowska & Badyaev 2008).
The reproductive mode of birds is relatively invariant. All lay eggs. Yet transitions between oviparity and viviparity have occurred many times in lizards and snakes. Why birds have never evolved viviparity is a longstanding evolutionary puzzle (Blackburn & Evans 1986; Anderson et al. 1987). Regardless of whether absence of viviparity reflects some interesting constraint, however, it is unlikely to prevent adaptation to climate warming, because viviparity is thought to be selected for when conditions are colder, rather than warmer (Shine 1995). Nonetheless, further consideration of hormonal constraints related to basic life-history features of birds seems warranted.
Instead of diverse reproductive modes, sex change and other tricks, birds, like some mammals, seem to rely on mobility and on behavioural flexibility in mating and parenting to cope with social and physical environments (Ligon 1999). Sexual behaviour arrangements vary from complete lifelong monogamy to females that pair with a close relative and seek extra-pair copulations for offspring production (Russell & Rowley 1996). Parenting arrangements include care by a group, males only, females only and every imaginable partitioning of effort between a male and a female including nest desertion by both (Bleeker et al. 2005). These are behavioural traits. Such traits tend to be relatively labile evolutionarily, and their phenotypic flexibility should not be assumed to slow down evolution (Böhning-Gaese & Oberrath 1999; Blomberg et al. 2003; Losos et al. 2004).
5. Conclusions
When and to what extent do hormonal control mechanisms affect the outcome of selection on life-history traits? I have described three complex integrated hormonal systems of birds. I have argued that of these, only one, sexual differentiation, is likely to be any source of evolutionary inertia, and even then only for certain kinds of change. The idea that the hormonal control systems would of necessity create inertia becomes less plausible once due consideration is given to the importance of behaviour in adaptation, the role of the brain in endocrine control systems and the developmental basis of evolutionary change. Integration is not inflexibility. Rather than invariably reducing the probability that a population will make it to another adaptive peak in time, hormonal control systems have the potential to influence which peak that will be if several are reasonably near, and what route will be taken to get there. This does not mean that living birds face no threat from anthropogenic environmental change. It simply means that if birds fail to adapt, it will probably not be because their endocrine systems have failed them.
Acknowledgments
The author is currently supported by National Science Foundation grant IBN-0130986.
Footnotes
One contribution of 12 to a Theme Issue ‘Integration of ecology and endocrinology in avian reproduction: a new synthesis’.
References
- Absil P, Baillien M, Ball G.F, Panzica G.C, Balthazart J. The control of preoptic aromatase activity by afferent inputs in Japanese quail. Brain Res. Rev. 2001;37:38–58. doi: 10.1016/s0165-0173(01)00122-9. doi:10.1016/S0165-0173(01)00122-9 [DOI] [PubMed] [Google Scholar]
- Adkins-Regan E. Princeton University Press; Princeton, NJ: 2005. Hormones and animal social behavior. [Google Scholar]
- Adkins-Regan E, Ottinger M.A, Park J. Maternal transfer of estradiol to egg yolks alters sexual differentiation of avian offspring. J. Exp. Zool. 1995;271:466–470. doi:10.1002/jez.1402710608 [Google Scholar]
- Anderson D.J, Stoyan N.C, Ricklefs R.E. Why are there no viviparous birds? A comment. Am. Nat. 1987;130:941–947. doi:10.1086/284757 [Google Scholar]
- Avigdor M, Sullivan S.D, Heideman P.D. Response to selection for photoperiod responsiveness on the density and location of mature GnRH-releasing neurons. Am. J. Physiol. 2005;288:R1226–R1236. doi: 10.1152/ajpregu.00562.2004. [DOI] [PubMed] [Google Scholar]
- Ball G, Balthazart J. Individual variation and the endocrine regulation of behavior and physiology in birds: a cellular/molecular perspective. Phil. Trans. R. Soc. B. 2008;363:1699–1710. doi: 10.1098/rstb.2007.0010. doi:10.1098/rstb.2007.0010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balthazart J, Adkins-Regan E. Sexual differentiation of brain and behavior in birds. In: Pfaff D.W, Arnold A.P, Etgen A.M, Fahrbach S.E, Rubin R.T, editors. Hormones, brain and behavior. vol. 4. Academic Press; San Diego, CA: 2002. pp. 223–301. [Google Scholar]
- Balthazart J, Ball G.F. Is brain estradiol a hormone or a neurotransmitter? Trends Neurosci. 2006;29:241–249. doi: 10.1016/j.tins.2006.03.004. doi:10.1016/j.tins.2006.03.004 [DOI] [PubMed] [Google Scholar]
- Bennett P.M, Owens I.P.F. Oxford University Press; Oxford, UK: 2002. Evolutionary ecology of birds. [Google Scholar]
- Blackburn D.G, Evans H.E. Why are there no viviparous birds? Am. Nat. 1986;128:165–190. doi:10.1086/284552 [Google Scholar]
- Bleeker M, Kingma S.A, Szentirmai I, Szekely T, Komdeur J. Body condition and clutch desertion in the penduline tit Remiz pendulinus. Behaviour. 2005;142:1465–1478. doi:10.1163/156853905774831855 [Google Scholar]
- Blomberg S.P, Garland T, Ives A.R. Testing for phylogenetic signal in comparative data: behavioral traits are more labile. Evolution. 2003;57:717–745. doi: 10.1111/j.0014-3820.2003.tb00285.x. [DOI] [PubMed] [Google Scholar]
- Böhning-Gaese K, Oberrath R. Phylogenetic effects on morphological, life-history, behavioural and ecological traits of birds. Evol. Ecol. Res. 1999;1:347–364. [Google Scholar]
- Both C, et al. Large-scale geographical variation confirms that climate change causes birds to lay earlier. Proc. R. Soc. B. 2004;271:1657–1662. doi: 10.1098/rspb.2004.2770. doi:10.1098/rspb.2004.2770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenowitz E.A, Kroodsma D.E. The neuroethology of birdsong. In: Kroodsma D.E, Miller E.H, editors. Ecology and evolution of acoustic communication in birds. Cornell University Press; Ithaca, NY: 1996. pp. 285–304. [Google Scholar]
- Bridgham J.T, Carroll S.M, Thornton J.W. Evolution of hormone-receptor complexity by molecular exploitation. Science. 2006;312:97–101. doi: 10.1126/science.1123348. doi:10.1126/science.1123348 [DOI] [PubMed] [Google Scholar]
- Britten R.J, Davidson E.H. Repetitive and non-repetitive DNA sequences and a speculation on the origins of evolutionary novelty. Q. Rev. Biol. 1971;46:111–138. doi: 10.1086/406830. doi:10.1086/406830 [DOI] [PubMed] [Google Scholar]
- Butler A.B, Hodos W. 2nd edn. Wiley; Hoboken, NJ: 2005. Comparative vertebrate neuroanatomy: evolution and adaptation. [Google Scholar]
- Charlesworth B. The evolution of sex chromosomes. Science. 1991;251:1030–1033. doi: 10.1126/science.1998119. doi:10.1126/science.1998119 [DOI] [PubMed] [Google Scholar]
- Clarke J.A, Tambussi C.P, Noriega J.I, Erickson G.M, Ketcham R.A. Definitive fossil evidence for the extant avian radiation in the Cretaceous. Nature. 2005;433:305–308. doi: 10.1038/nature03150. doi:10.1038/nature03150 [DOI] [PubMed] [Google Scholar]
- Cornil C.A, Dalla C, Papadopoulou-Daifoti Z, Baillien M, Balthazart J. Estradiol rapidly activates male sexual behavior and affects brain monoamine levels in the quail brain. Behav. Brain Res. 2006;166:110–123. doi: 10.1016/j.bbr.2005.07.017. doi:10.1016/j.bbr.2005.07.017 [DOI] [PubMed] [Google Scholar]
- Correa S.M, Adkins-Regan E, Johnson P.A. High progesterone during avian meiosis biases sex ratios toward females. Biol. Lett. 2005;1:215–218. doi: 10.1098/rsbl.2004.0283. doi:10.1098/rsbl.2004.0283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig J.V, Jan M.L, Polley C.R, Bhagwat A.L, Dayton A.D. Changes in relative aggressiveness and social dominance associated with selection for early egg production in chickens. Poult. Sci. 1975;54:1647–1658. doi: 10.3382/ps.0541647. [DOI] [PubMed] [Google Scholar]
- Dawson A. Control of the annual cycle in birds: endocrine constraints and plasticity in response to ecological variability. Phil. Trans. R. Soc. B. 2008;363:1621–1633. doi: 10.1098/rstb.2007.0004. doi:10.1098/rstb.2007.0004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson A, Goldsmith A.R. Sexual maturation in starlings raised on long or short days: changes in hypothalamic gonadotrophin-releasing hormone and plasma LH concentrations. J. Endocrinol. 1989;123:189–196. doi: 10.1677/joe.0.1230189. [DOI] [PubMed] [Google Scholar]
- Desjardins C, Bronson F.H, Blank J.L. Genetic selection for reproductive photoresponsiveness in deer mice. Nature. 1986;322:172–173. doi: 10.1038/322172a0. doi:10.1038/322172a0 [DOI] [PubMed] [Google Scholar]
- De Vries G.J, Simerly R.B. Anatomy, development, and function of sexually dimorphic neural circuits in the mammalian brain. In: Pfaff D.W, Arnold A.P, Etgen A.M, Fahrbach S.E, Rubin R.T, editors. Hormones, brain and behavior. vol. 4. Academic Press; San Diego, CA: 2002. pp. 137–192. [Google Scholar]
- Dunn P.O, Whittingham L.A, Pitcher T.E. Mating systems, sperm competition, and the evolution of sexual dimorphism in birds. Evolution. 2001;55:161–175. doi: 10.1111/j.0014-3820.2001.tb01281.x. [DOI] [PubMed] [Google Scholar]
- Elbrecht A, Smith R.G. Aromatase enzyme activity and sex determination in chickens. Science. 1992;255:467–470. doi: 10.1126/science.1734525. doi:10.1126/science.1734525 [DOI] [PubMed] [Google Scholar]
- Falcón J, Besseau L, Sauzet S, Boeuf G. Melatonin effects on the hypothalamo–pituitary axis in fish. Trends Endocrinol. Metab. 2007;18:81–88. doi: 10.1016/j.tem.2007.01.002. doi:10.1016/j.tem.2007.01.002 [DOI] [PubMed] [Google Scholar]
- Finch C.E. Evolution and plasticity of aging in the reproductive schedule in long-lived animals: the importance of genetic variation in neuroendocrine mechanisms. In: Pfaff D.W, Arnold A.P, Etgen A.M, Fahrbach S.E, Rubin R.T, editors. Hormones, brain and behavior. vol. 4. Academic Press; San Diego, CA: 2002. pp. 799–820. [Google Scholar]
- Follett B.K. The physiology of puberty in seasonally breeding birds. In: Hunzicker-Dunn M, Schwartz N.B, editors. Follicle stimulating hormone: regulation of secretion and molecular mechanisms of action. Springer; New York, NY: 1992. pp. 54–65. [Google Scholar]
- Forger N.G. Cell death and sexual differentiation of the nervous system. Neuroscience. 2006;138:929–938. doi: 10.1016/j.neuroscience.2005.07.006. doi:10.1016/j.neuroscience.2005.07.006 [DOI] [PubMed] [Google Scholar]
- Fraley G.S, Kuenzel W.J. Precocious puberty in chicks (Gallus domesticus) induced by central injections of neuropeptide Y. Life Sci. 1993;52:1649–1656. doi: 10.1016/0024-3205(93)90047-7. doi:10.1016/0024-3205(93)90047-7 [DOI] [PubMed] [Google Scholar]
- Gahr M, Balaban E. The development of a species difference in the local distribution of brain estrogen receptive cells. Dev. Brain Res. 1996;92:182–189. doi: 10.1016/0165-3806(95)00210-3. doi:10.1016/0165-3806(95)00210-3 [DOI] [PubMed] [Google Scholar]
- Gerhart J, Kirschner M. Blackwell Science; Malden, MA: 1997. Cells, embryos, and evolution. [Google Scholar]
- Goodson J.L, Evans A.K, Wang Y. Neuropeptide binding reflects convergent and divergent evolution in species-typical group sizes. Horm. Behav. 2006;50:223–236. doi: 10.1016/j.yhbeh.2006.03.005. doi:10.1016/j.yhbeh.2006.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham J.M, Desjardins C. Classical conditioning: induction of luteinizing hormone and testosterone secretion in anticipation of sexual activity. Science. 1980;210:1039–1041. doi: 10.1126/science.7434016. doi:10.1126/science.7434016 [DOI] [PubMed] [Google Scholar]
- Hahn T.P, Pereyra M.E, Sharbaugh S.M, Bentley G.E. Physiological responses to photoperiod in three cardueline finch species. Gen. Comp. Endocrinol. 2004;137:99–108. doi: 10.1016/j.ygcen.2004.02.014. doi:10.1016/j.ygcen.2004.02.014 [DOI] [PubMed] [Google Scholar]
- Hall B.K. Kluwer; Dordrecht, The Netherlands: 1999. Evolutionary developmental biology. [Google Scholar]
- Halldin K. Impact of endocrine disrupting chemicals on reproduction in Japanese quail. Domest. Anim. Endocrinol. 2005;29:420–429. doi: 10.1016/j.domaniend.2005.02.036. doi:10.1016/j.domaniend.2005.02.036 [DOI] [PubMed] [Google Scholar]
- Hanken J, Gross J.B. Evolution of cranial development and the role of neural crest: insights from amphibians. J. Anat. 2005;207:437–446. doi: 10.1111/j.1469-7580.2005.00481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helm B, Gwinner E. Migratory restlessness in an equatorial nonmigratory bird. PLoS Biol. 2006;4:e110. doi: 10.1371/journal.pbio.0040110. doi:10.1371/journal.pbio.0040110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helm B, Piersma T, van der Jeugd H. Sociable schedules: interplay between avian seasonal and social behavior. Anim. Behav. 2006;72:245–262. doi:10.1016/j.anbehav.2005.12.007 [Google Scholar]
- Kempenaers B, Peters A, Foerster K. Sources of individual variation in plasma testosterone levels. Phil. Trans. R. Soc. B. 2008;363:1711–1723. doi: 10.1098/rstb.2007.0001. doi:10.1098/rstb.2007.0001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ketterson E.D, Nolan V, Sandell M. Testosterone in females: mediator of adaptive traits, constraint on sexual dimorphism, or both? Am. Nat. 2005;166(Suppl. 4):S85–S98. doi: 10.1086/444602. doi:10.1086/444602 [DOI] [PubMed] [Google Scholar]
- Kriegsfeld L.J. Driving reproduction: RFamide peptides behind the wheel. Horm. Behav. 2006;50:655–666. doi: 10.1016/j.yhbeh.2006.06.004. doi:10.1016/j.yhbeh.2006.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange C.A. Membrane and nuclear steroid hormone receptors: two integrated arms of the same signaling pathway? Steroids. 2007;72:105–106. doi: 10.1016/j.steroids.2006.11.021. doi:10.1016/j.steroids.2006.11.021 [DOI] [PubMed] [Google Scholar]
- Ligon J.D. Oxford University Press; Oxford, UK: 1999. The evolution of avian breeding systems. [Google Scholar]
- Lim M.M, Wang Z, Olazabal D.E, Ren X, Terwilliger E.F, Young L.J. Enhanced partner preference in a promiscuous species by manipulating the expression of a single gene. Nature. 2004;429:754–757. doi: 10.1038/nature02539. doi:10.1038/nature02539 [DOI] [PubMed] [Google Scholar]
- Losos J.B, Schoener T.W, Spiller D.A. Predator-induced behaviour shifts and natural selection in field-experimental lizard populations. Nature. 2004;432:505–508. doi: 10.1038/nature03039. doi:10.1038/nature03039 [DOI] [PubMed] [Google Scholar]
- Love O.P, Chin E.H, Wynne-Edwards K.E, Williams T.D. Stress hormones: a link between maternal condition and sex-biased reproductive investment. Am. Nat. 2005;166:751–766. doi: 10.1086/497440. doi:10.1086/497440 [DOI] [PubMed] [Google Scholar]
- McCarthy M.M, Todd B.J, Amateau S.K. Estradiol modulation of astrocytes and the establishment of sex differences in the brain. Ann. NY Acad. Sci. 2003;1007:283–297. doi: 10.1196/annals.1286.027. doi:10.1196/annals.1286.027 [DOI] [PubMed] [Google Scholar]
- McGlothlin J.W, Ketterson E.D. Hormone-mediated suites as adaptations and evolutionary constraints. Phil. Trans. R. Soc. B. 2008;363:1611–1620. doi: 10.1098/rstb.2007.0002. doi:10.1098/rstb.2007.0002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson R.J. The use of genetic “knockout” mice in behavioral endocrinology research. Horm. Behav. 1997;31:188–196. doi: 10.1006/hbeh.1997.1381. doi:10.1006/hbeh.1997.1381 [DOI] [PubMed] [Google Scholar]
- Noden D.M, Schneider R.A. Neural crest cells and the community of plan for craniofacial development: historical debates and current perspectives. Adv. Exp. Med. Biol. 2006;589:1–23. doi: 10.1007/978-0-387-46954-6_1. [DOI] [PubMed] [Google Scholar]
- Norris D.O. 3rd edn. Academic Press; San Diego, CA: 1996. Vertebrate endocrinology. [Google Scholar]
- Ojeda S.R, Terasawa E. Neuroendocrine regulation of puberty. In: Pfaff D.W, Arnold A, Etgen A.M, Fahrbach S.E, Moss R.T, Rubin R, editors. Hormones, brain and behavior. vol. 4. Academic Press; San Diego, CA: 2002. pp. 589–660. [Google Scholar]
- Ottinger M.A, Pitts S, Abdelnabi M.A. Steroids hormones during embryonic development in Japanese quail: plasma, gonadal, and adrenal levels. Poult. Sci. 2001;80:795–799. doi: 10.1093/ps/80.6.795. [DOI] [PubMed] [Google Scholar]
- Ottinger M.A, Quinn M.J, Lavoie E, Abdelnabi M.A, Thompson N, Hazelton J.L, Wu J.M, Beavers J, Jaber M. Consequences of endocrine disrupting chemicals on reproductive endocrine function in birds: establishing reliable end points of exposure. Domest. Anim. Endocrinol. 2005;29:411–419. doi: 10.1016/j.domaniend.2005.02.038. doi:10.1016/j.domaniend.2005.02.038 [DOI] [PubMed] [Google Scholar]
- Panzica G.C, Castagna C, Viglietti-Panzica C, Russo C, Tlemçani O, Balthazart J. Organizational effects of estrogens on brain vasotocin and sexual behavior in quail. J. Neurobiol. 1998;37:684–699. doi: 10.1002/(sici)1097-4695(199812)37:4<684::aid-neu15>3.0.co;2-u. doi:10.1002/(SICI)1097-4695(199812)37:4<684::AID-NEU15>3.0.CO;2-U [DOI] [PubMed] [Google Scholar]
- Panzica G.C, Aste N, Castagna C, Viglietti-Panzica C, Balthazart J. Steroid-induced plasticity in the sexually dimorphic vasotocinergic innervation of the avian brain: behavioral implications. Brain Res. Rev. 2001;37:178–200. doi: 10.1016/s0165-0173(01)00118-7. doi:10.1016/S0165-0173(01)00118-7 [DOI] [PubMed] [Google Scholar]
- Pfaff D.W, Arnold A.P, Etgen A.M, Fahrbach S.E, Rubin R.T, editors. Hormones, brain and behavior. vol. 1–5. Academic Press; San Diego, CA: 2002. [Google Scholar]
- Phelps S.M, Young L.J. Extraordinary diversity in vasopressin (V1a) receptor distributions among wild prairie voles (Microtus ochrogaster): patterns of variation and covariation. J. Comp. Neurol. 2003;466:564–576. doi: 10.1002/cne.10902. doi:10.1002/cne.10902 [DOI] [PubMed] [Google Scholar]
- Plant T.M. Neurobiological bases underlying the control of the onset of puberty in the rhesus monkey: a representative higher primate. Front. Neuroendocrinol. 2001;22:1007–1139. doi: 10.1006/frne.2001.0211. doi:10.1006/frne.2001.0211 [DOI] [PubMed] [Google Scholar]
- Poelwijk F.J, Kiviet D.J, Weinreich D.M, Tans S.J. Empirical fitness landscapes reveal accessible evolutionary paths. Nature. 2007;445:383–386. doi: 10.1038/nature05451. doi:10.1038/nature05451 [DOI] [PubMed] [Google Scholar]
- Pulido F, Berthold P, Mohr G, Querner U. Heritability of the timing of autumn migration in a natural bird population. Proc. R. Soc. B. 2001;268:953–959. doi: 10.1098/rspb.2001.1602. doi:10.1098/rspb.2001.1602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roff D. Sinauer Associates; Sunderland, MA: 2001. Life history evolution. [Google Scholar]
- Romero L.M, Sapolsky R.M. Patterns of ACTH secretagog secretion in response to psychological stimuli. J. Neuroendocrinol. 1996;8:243–258. doi: 10.1046/j.1365-2826.1996.04441.x. doi:10.1046/j.1365-2826.1996.04441.x [DOI] [PubMed] [Google Scholar]
- Russell E, Rowley I. Partnerships in promiscuous splendid fairy-wrens. In: Black J.M, editor. Partnerships in birds: the study of monogamy. Oxford University Press; Oxford, UK: 1996. pp. 162–173. [Google Scholar]
- Rutkowska J, Badyaev A.V. Meiotic drive and sex determination: molecular and cytological mechanisms of sex ratio adjustment in birds. Phil. Trans. R. Soc. B. 2008;363:1675–1686. doi: 10.1098/rstb.2007.0006. doi:10.1098/rstb.2007.0006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandell M.I. Female aggression and the maintenance of monogamy: female behaviour predicts male mating status in European starlings. Proc. R. Soc. B. 1998;265:1307–1311. doi:10.1098/rspb.1998.0434 [Google Scholar]
- Schumacher M, Sulon J, Balthazart J. Changes in serum concentrations of steroids during embryonic and post-hatching development of male and female Japanese quail (Coturnix coturnix japonica) J. Endocrinol. 1988;118:127–134. doi: 10.1677/joe.0.1180127. [DOI] [PubMed] [Google Scholar]
- Shaw B.K, Kennedy G.G. Evidence for species differences in the pattern of androgen receptor distribution in relation to species differences in an androgen-dependent behavior. J. Neurobiol. 2002;52:203–220. doi: 10.1002/neu.10079. doi:10.1002/neu.10079 [DOI] [PubMed] [Google Scholar]
- Shine R. A new hypothesis for the evolution of viviparity in reptiles. Am. Nat. 1995;145:809–823. doi:10.1086/285769 [Google Scholar]
- Smith C.A, Sinclair A.H. Sex determination: insights from the chicken. BioEssays. 2004;26:120–132. doi: 10.1002/bies.10400. doi:10.1002/bies.10400 [DOI] [PubMed] [Google Scholar]
- Striedter G.F. Sinauer Associates; Sunderland, MA: 2005. Principles of brain evolution. [Google Scholar]
- Szekely T, Reynolds J.D, Figuerola J. Sexual size dimorphism in shorebirds, gulls, and alcids: the influence of sexual and natural selection. Evolution. 2000;54:1404–1413. doi: 10.1111/j.0014-3820.2000.tb00572.x. [DOI] [PubMed] [Google Scholar]
- Wade J, Arnold A.P. Functional testicular tissue does not masculinize development of the zebra finch song system. Proc. Natl Acad. Sci. USA. 1996;93:5264–5268. doi: 10.1073/pnas.93.11.5264. doi:10.1073/pnas.93.11.5264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade J, Arnold A.P. Sexual differentiation of the zebra finch song system. Ann. NY Acad. Sci. 2004;1016:540–559. doi: 10.1196/annals.1298.015. doi:10.1196/annals.1298.015 [DOI] [PubMed] [Google Scholar]
- Weeks J.C, Levine R.B. Steroid hormone effects on neurons subserving behavior. Curr. Opin. Neurobiol. 1995;5:809–815. doi: 10.1016/0959-4388(95)80110-3. doi:10.1016/0959-4388(95)80110-3 [DOI] [PubMed] [Google Scholar]
- West-Eberhard M.J. Oxford University Press; Oxford, UK: 2003. Developmental plasticity and evolution. [Google Scholar]
- Wilhelms K.W, Cutler S.A, Proudman J.A, Anderson L.L, Scanes C.G. Atrazine and the hypothalamo–pituitary–gonadal axis in sexually maturing precocial birds: studies in male Japanese quail. Toxicol. Sci. 2005;86:152–160. doi: 10.1093/toxsci/kfi170. doi:10.1093/toxsci/kfi170 [DOI] [PubMed] [Google Scholar]
- Wilkins A.S. Sinauer Associates; Sunderland, MA: 2002. The evolution of developmental pathways. [Google Scholar]
- Wilson F.E. A test of the hypothesis that T3 is the “seasonality” thyroid hormone in American tree sparrows (Spizella arborea): intracerebroventricular infusion of iopanoic acid, an inhibitor of T3 synthesis and degradation. J. Comp. Physiol. B. 2001;171:113–119. doi: 10.1007/s003600000155. doi:10.1007/s003600000155 [DOI] [PubMed] [Google Scholar]
- Wingfield J.C, Jacobs J.D, Soma K, Maney D.L, Hunt K, Wisti-Peterson D, Meddle S, Ramenofsky M, Sullivan K. Testosterone, aggression and communication: ecological bases of endocrine phenomena. In: Hauser M, Konishi M, editors. The design of animal communication. MIT Press; Cambridge, MA: 1999. pp. 255–284. [Google Scholar]
- Winkler D.W, Dunn P.O, McCulloch C.E. Predicting the effects of climate change on avian life-histories. Proc. Natl Acad. Sci. USA. 2002;99:13 595–13 599. doi: 10.1073/pnas.212251999. doi:10.1073/pnas.212251999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang N, Dunnington E.A, Siegel P.B. Forty generations of bidirectional selection for mating frequency in male Japanese quail. Poult. Sci. 1998;77:1469–1477. doi: 10.1093/ps/77.10.1469. [DOI] [PubMed] [Google Scholar]
- Young L.J, Wang Z, Insel T.R. Neuroendocrine bases of monogamy. Trends Neurosci. 1998;21:71–75. doi: 10.1016/s0166-2236(97)01167-3. doi:10.1016/S0166-2236(97)01167-3 [DOI] [PubMed] [Google Scholar]
- Zera A.J, Williams T.D, Harshman L.G. Evolutionary endocrinology: the developing synthesis between endocrinology and evolutionary genetics. Annu. Rev. Ecol. Evol. 2007;38:793–817. [Google Scholar]