Abstract
Differences in relative fitness of male and female offspring across ecological and social environments should favour the evolution of sex-determining mechanisms that enable adjustment of brood sex ratio to the context of breeding. Despite the expectation that genetic sex determination should not produce consistent bias in primary sex ratios, extensive and adaptive modifications of offspring sex ratio in relation to social and physiological conditions during reproduction are often documented. Such discordance emphasizes the need for empirical investigation of the proximate mechanisms for modifying primary sex ratios, and suggests epigenetic effects on sex-determining mechanisms as the most likely candidates. Birds, in particular, are thought to have an unusually direct opportunity to modify offspring sex ratio because avian females are heterogametic and because the sex-determining division in avian meiosis occurs prior to ovulation and fertilization. However, despite evidence of strong epigenetic effects on sex determination in pre-ovulatory avian oocytes, the mechanisms behind such effects remain elusive. Our review of molecular and cytological mechanisms of avian meiosis uncovers a multitude of potential targets for selection on biased segregation of sex chromosomes, which may reflect the diversity of mechanisms and levels on which such selection operates in birds. Our findings indicate that pronounced differences between sex chromosomes in size, shape, size of protein bodies, alignment at the meiotic plate, microtubule attachment and epigenetic markings should commonly produce biased segregation of sex chromosomes as the default state, with secondary evolution of compensatory mechanisms necessary to maintain unbiased meiosis. We suggest that it is the epigenetic effects that modify such compensatory mechanisms that enable context-dependent and precise adjustment of primary sex ratio in birds. Furthermore, we highlight the features of avian meiosis that can be influenced by maternal hormones in response to environmental stimuli and may account for the precise and adaptive patterns of offspring sex ratio adjustment observed in some species.
Keywords: epigenetic effects, hormones, segregation distortion, sex chromosomes, avian meiosis, maternal effects
1. Meiotic drive and adaptive sex ratio adjustment
The relationship between sex-determining genetic systems and selection for sex ratio bias is one of the most controversial and poorly understood topics in evolutionary biology. On the one hand, theoretical studies show that the evolution of genetic mechanisms producing consistent bias in sex ratio is unlikely (Fisher 1930; Trivers & Willard 1973; Charnov 1982; Bull 1983; Werren & Hatcher 2000; Le Galliard et al. 2005), and there is often no correlation between the genetic sex determination mechanisms and the magnitude of sex ratio adjustment (Morrish & Sinclair 2002; Janzen & Phillips 2006; Kozielska et al. 2006; Mank et al. 2006; Uller et al. 2007). On the other hand, the empirical studies frequently show strong parental modifications of offspring sex ratio, often in close concordance with the variable cost of production of different sex offspring across environments (Heinsohn et al. 1997; Komdeur et al. 1997; Badyaev et al. 2002; West & Sheldon 2002). The empirical findings of rapid and context-dependent adjustment of primary sex ratios have drawn attention to epigenetic effects on sex-determining mechanisms and have emphasized the value of understanding not only the proximate mechanisms behind sex determination (Johnstone et al. 1995; Mittwoch 1996; Clinton & Haines 1999; Kraak & Pen 2002; Pen & Weissing 2002) but also the level at which sex ratio selection on these mechanisms is likely to act (Kraak & de Looze 1993; Mittwoch 1993, 2006; Leimar 1996; Badyaev 2002; van Dooren & Leimar 2003; Munday et al. 2006; Schwanz et al. 2006; Freedberg & Taylor 2007). Indeed, among numerous empirical studies that document sex ratio adjustments, those that consider the exact context favouring sex ratio adjustment (West & Sheldon 2002; Sheldon & West 2004; Shuker & West 2004; Griffin et al. 2005; West et al. 2005; Badyaev et al. 2006b) and the precise proximate level at which the response to sex ratio selection should occur (e.g. meiosis, ovulation, implantation) document the most exact patterns of sex ratio adjustment (James 1996; Cameron 2004; Badyaev et al. 2005; Holand et al. 2006; Rutkowska & Cichoń 2006).
Among vertebrates, birds are thought to have an unusually direct control of offspring sex because the female is heterogametic, and both the peripheral location of meiotic plate in the oocyte and the timing of the first meiotic division (just prior to ovulation) make avian sex determination especially susceptible to maternal modifications (Krackow 1995; Pike & Petrie 2003; Alonso-Alvarez 2006). This suggestion is corroborated by the observations of precise sex bias in relation to ovulation sequence and oocyte growth patterns in birds (Blanco et al. 2002; Velando et al. 2002; Andersson et al. 2003; Young & Badyaev 2004; Badyaev et al. 2005, 2006a,b; Pike 2005), patterns that are difficult to explain by mechanisms other than epigenetic effects on sex chromosome drive. Furthermore, chromosomal interactions during meiosis in heterogametic avian females should facilitate rapid evolution of mechanisms that enhance the probability of chromosome transmission to the oocyte (Zwick et al. 1999; Padro-Manuel de Villena & Sapienza 2001a; Fishman & Willis 2005; Dawe & Henikoff 2006), and such mechanisms might be co-opted for sex ratio adjustment. Yet, whereas the references to meiotic drive as a potential mechanism of avian sex ratio adjustment are made frequently (Krackow 1995; Badyaev et al. 2005; Alonso-Alvarez 2006), no study to date have considered the molecular and cytological mechanisms behind sex-biased meiotic drive in birds or addressed whether known features of avian meiosis are susceptible to directional and, importantly, reversible segregation distortion of sex chromosomes favoured by context-dependent selection on primary sex ratio adjustment.
Whereas broad-sense meiotic drive is an unlikely mechanism of sex ratio adjustment in birds (box 1), avian meiosis nevertheless possesses a multitude of features that can significantly facilitate non-random segregation of sex chromosomes. Here, we first review what is known about avian oocyte maturation and the sex-determining first meiotic division (figure 1a–d). Second, we examine whether avian meiosis possesses the three general characteristics facilitating segregation distortion: asymmetrical meiotic division, asymmetry of the meiotic spindle pole, and expressed heterozygosity at a locus mediating chromosome attachment to the spindle (e.g. Padro-Manuel de Villena & Sapienza 2001b). Third, we examine the extent to which cytoskeleton-mediated mechanisms of sex chromosome movements during meiosis are susceptible to non-genomic effects of steroids (figure 2). Finally, we identify the potential targets of selection for precise sex ratio adjustment in variable environmental and social contexts of breeding.
Box 1. Broad-sense meiotic drive as a potential means of offspring sex adjustment in non-avian taxa.
Sex chromosome drive refers to the unequal transmission of the sex chromosomes from the individuals of the heterogametic sex, resulting in biased sex ratios among the progeny and within populations (Jaenike 2001). This phenomenon has been reported in plants, mammals and insects, and usually is based on the action of genes on autosomes or sex chromosomes that disable the development of functional gametes bearing one of the sex chromosomes. In all known cases, sex chromosome meiotic drive results in a pattern in which the same sex chromosome is favoured (see Jaenike 2001). In most cases, segregation distortion occurs during spermatogenesis and results from the non-disjunction of Y chromatids in meiosis II or X chromosome breakage leading to the failure of spermatids to develop (review in Taylor & Ingvarsson 2003). The valuable insight into the non-random chromosome segregation in meiosis comes from the spermatogenesis of heterogametic male flies Trichosia pubescens (Sciaridae) where, as in female meiosis, only a single functional gamete is produced and only the maternal set of chromosomes is transmitted into the spermatozoon. The formation of only one gamete in this system results from the presence of a monopolar and monocentric spindle in the first meiotic division (Fuge 1994). The non-random segregation of the genetic material in this case might be due to inactivation of the kinetochores of paternal chromosomes that are unable to capture the microtubules of the spindle. These chromosomes are, however, in contact with some microtubules, and thus the microtubules could be responsible for transporting parental chromosomes away from the functional pole (Fuge 1994).
Female meiotic drive has been reported less often, but the existing cases provide important insights into the mechanism of sex adjustment, especially when sex bias occurs in heterogametic females. In the butterfly Eucheria socialis westwoodi, females are heterogametic and produce strongly male-biased primary sex ratios (Underwood & Shapiro 1999), most likely as a result of non-random chromosome segregation in female meiosis. In some mammals, heterogametic females with X and Y chromosomes show meiotic drive. For example, in the wood lemming Myopus schisticolor, heterogametic females produce only oocytes bearing X chromosomes (Fredga et al. 1976), a likely result of a non-disjunction at the stage of primordial germ cells or oogonia of the foetal ovary, where the X chromosomes were doubled and the Y chromosome eliminated. Specifically, the failure of oocytes bearing Y chromosomes was due to non-homologous pairing (triple pairing, interchange, self-synapsis), numerical abnormalities and formation of univalents (Akhverdyan & Fredga 2001).
The other mechanisms of sex chromosome meiotic drive are based on the cytoplasmic elements responsible for female-biased sex ratio in progeny. The mechanisms behind this manipulation are often based on the maternally inherited cytoplasmic bacteria that induce parthenogenesis and feminize or kill genetic male offspring (reviewed in Jaenike 2001). Finally, the autosomal segregation distorters may act by preventing the condensation of heterochromatin in sperm nuclei and the formation of spermatids. In plants, meiotic drive is often caused by the production of non-viable male gametes (Taylor & Ingvarsson 2003).
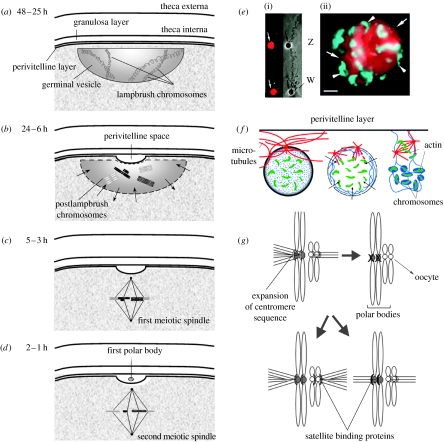
Figure 1.
Schematic of germinal disc during oocyte maturation in birds. (a) At the end of meiotic prophase I, germinal disc is at the periphery of the oocyte and germinal vesicle flattens against the follicular wall. Chromosomes, until this time in the lampbrush stage, now condense. (b) At prometaphase I, membrane of the germinal vesicle breaks enabling mixing of the protoplasm of the germinal disc and the content of the germinal vesicle indicated by small arrows. Perivitelline space forms between the surface of the oocyte and the perivitelline membrane. Condensed postlampbrush chromosomes (not shown to scale) are in the centre of the disintegrating germinal vesicle. (c) During metaphase I, the first meiotic spindle forms perpendicular to the surface of the oocyte with chromosomes aligned in the meiotic plate ready for segregation. (d) After meiotic division I, the first polar body is visible in the perivitelline space and the second meiotic spindle forms beneath it. (e) Immunofluorescent images of chaffinch showing chromosomes (stained lighter) and cohesion proteins (stained darker; modified from Krasikova et al. (2005)). (i) Sex chromosomes at the lampbrush stage; arrows indicate equal sized centromere protein bodies. Phase contrast highlights Z and W size difference. Scale bar, 10 μm. (ii) Vitellogenic stage of the oocyte, at which protein bodies form karyosphere; arrows point to cohesion proteins and arrowheads to chromosome bivalents. Scale bar, 20 μm. (f) Schematic of chromosome congression to the meiotic spindle in the starfish; modified from Maresca & Heald (2005). Actin filaments get polarized by substances (indicated by arrows) that invade the nucleus after the breakage of its membrane, embed chromosomes and deliver them to the metaphase plate of the first meiotic spindle. (g) Conceptual illustration of the centromere drive hypothesis is based on Dawe & Henikoff (2006) and Malik & Bayes (2006). Expansion of centromere sequence may lead to increased attachment of microtubules to chromosome, resulting in segregation distortion. Mutational effects on centromere proteins in the other sex chromosome can restore random chromosome segregation. Mutational effects in the protein that bind to centromere can either expand their size leading to increased number of captured microtubules, or restrict the size resulting in reduced microtubule attachment.
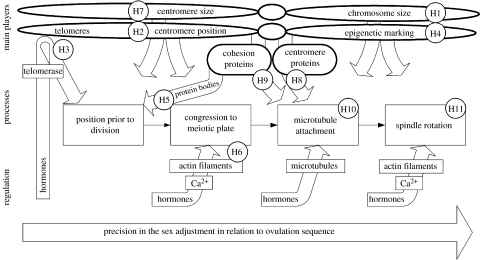
Figure 2.
Schematic of the relationships between elements of avian meiosis, which are crucial for non-random sex chromosome segregation and their possible regulation by maternal hormones. White arrows indicate characteristics of chromosomes that affect their behaviour at all stages of meiosis. Successive stages of meiosis are depicted by boxes at the centre of the figure and grey arrows show proposed pathways of hormonal manipulations of these stages. Hypotheses are listed in the text and are as follows: H1: non-random segregation of the sex chromosomes is facilitated by their dimorphism in size. H2: sex chromosome segregation distortion is facilitated by dimorphism in centromere position. H3: telomeres elongated during oocyte maturation affect movements of the chromosomes at several stages of meiosis, including their position in the nucleus prior to segregation. H4: epigenetic marking of the chromosomes enables identification of a particular sex chromosome and can influence its movement during segregation. H5: fusions of protein bodies that consist of cohesion proteins (figure 1d) affect chromosome position in the germinal vesicle before breakage of its membrane. H6: chromosome congression to the meiotic plate, the process directed by actin filaments is strongly affected by maternal hormones (figure 1b,f). H7: centromere size affects microtubule attachment to the kinetochore and therefore is responsible for non-random sex chromosome segregation by ‘centromere drive’ mechanism (figure 1g). H8 and H9: centromere and cohesion proteins modify kinetochore function, affect microtubule attachments and contribute to sex-biased segregation (figure 1g). H10: microtubule attachment to the kinetochore is altered due to checkpoint mechanism controlling their tension and allows for repeated capture and release of sex chromosomes. H11: change of direction of chromosomes segregation to the oocyte versus polar body is enabled by the rotation of the meiotic spindle, the process that can be influenced by maternal hormones. Mechanisms operating at the later stages produce more precise sex adjustment in relation to ovulation sequence (see text).
2. Background to avian meiosis
(a) The main players
Avian meiosis and oocyte maturation combines features that are generally conserved among taxa, such as stages of oocyte maturation (Harper 1904; Warren & Scott 1935; Bissonnette & Zujko 1936; Warren & Conrad 1939; Olsen 1942; Olsen & Fraps 1944; Chalana & Guraya 1979; Birrenkott et al. 1988; Yoshimura et al. 1993a,b, 1994) with features that are remarkably variable among and within species, such as size and shape dimorphism of sex chromosomes (Belterman & De Boer 1984; Solari 1993). Female birds hatch with most of their oocytes arrested in development, all chromosomes duplicated and synapsed with homologues forming pairs of lampbrush chromosomes soon after hatching (Solari 1993; Galkina et al. 2006). Birds have 52 to 98 chromosomes and, in most species, the Z chromosome is the fourth or fifth largest chromosome, representing approximately 10% of the genome. The W chromosome is much smaller (figure 1e; Solari 1993) and, in the lampbrush form, more condensed than other chromosomes (Solovei et al. 1993). In addition, avian sex chromosomes vary in centromere position (Panov & Bulatova 1972; Belterman & De Boer 1984; Solari 1993) and tandem repeats at terminal chromomeres (Matzke et al. 1990; Krasikova et al. 2006).
During the late stages of meiosis, homologue chromosomes are maintained as bivalents by the cohesion proteins located along the chromosome arms, and in some species, the cohesion proteins aggregate and form distinct round structures called protein bodies (Gaginskaya 1972; figure 1e) that play an important role in sex chromosome movements (see below). The size of protein bodies differs strongly among species, from 1 μm in diameter in the chicken (Gallus gallus domesticus; Krasikova et al. 2006) to 15 μm in the redwing Turdus iliacus (Gaginskaya 1972). Interestingly, there is an extensive within-species variation in protein body sizes; for example, in rock dove Columba livia, house sparrow Passer domesticus and chaffinch Fringilla coelebs, the protein bodies vary from 1 to 10 μm (Solovei et al. 1993). Importantly, protein bodies can also differ between sex chromosomes; for example, the protein body is larger on the W chromosome than on the Z chromosome in pigeons (Solovei et al. 1993). Although the protein bodies are typical of the lampbrush stage, the cohesion proteins remain attached to the centromeres throughout the subsequent chromosome contraction stages keeping homologous chromosomes paired until the second division of meiosis (Hagstrom & Meyer 2003; Krasikova et al. 2005, 2006). Only at the later stages of yolk formation, protein bodies fuse, forming a karyosphere with condensed chromosomes on its surface (Gaginskaya 1972; Saifitdinova et al. 2003; Krasikova et al. 2005, 2006).
(b) Place and time
During follicle growth and yellow yolk deposition, its nucleus—the germinal vesicle—lies in the centre of the germinal disc (Olsen 1942; Olsen & Fraps 1944). From 48 to 25 hours before ovulation, the germinal disc moves to the periphery of follicle and partially flattens against the vitelline membrane (figure 1a), while the inner surface remains hemispherical (Olsen 1942; Olsen & Fraps 1944). In several poultry species, at that time the germinal vesicle measures 90–110 μm in depth and 200–600 μm in diameter (Olsen 1942; Solari 1993; Yoshimura et al. 1993b). Twenty-four hours prior to ovulation, the wall of the vesicle begins to break at its upper surface (figure 1b) in response to an increased level of luteinizing hormone and, in some species, a surge of calcium (Homa 1995). The protoplasm of the germinal disc and the content of the germinal vesicle then mix and spread laterally beneath the vitelline membrane (Olsen 1942). At 6 hours prior to ovulation, the perivitelline space appears between the germinal disc and the perivitelline layer, and spherical chromosomes, characteristic of the postlampbrush stage, appear at the centre of the germinal vesicle (Yoshimura et al. 1993a,b). Finally, 2 hours prior to ovulation, bivalents of condensed chromosomes align in the division plate, and a meiotic spindle forms (figure 1c).
The meiotic spindle consists of two poles, set approximately 20 μm apart, and the microtubules. Once chromosomes are within the range of the microtubules, their kinetochores—spindle attachment sites at the centromeres—bind to the microtubules. Chromosome bivalents then move to the poles driven by microtubule shortening by kinetochore motor proteins. Importantly, the first meiotic spindle is located perpendicularly to the surface of the germinal disc (figure 1c), and it is this directionality that determines which chromosome bivalents remain in the ovum and, thus, the sex of the ova. The polar body, carrying bivalents of autosomes and either Z or W bivalent, appears in the perivitelline space approximately 1 or 2 hours prior to ovulation (figure 1d; Yoshimura et al. 1993a).
These features of avian meiosis present many potential targets for sex chromosome segregation distortion that might be favoured by selection for biased sex ratios. In §3, we propose several mechanisms that capitalize on these features.
3. Cytological and molecular mechanisms of offspring sex ratio adjustment
(a) Sex chromosome differences as a prerequisite for segregation distortion
Several features of avian sex chromosomes could account for sex specificity in chromosome movement patterns during female meiosis. One of the most striking features of bird karyotype is pronounced size and shape dimorphism in sex chromosomes (Solari 1993) that, in the absence of compensatory mechanisms, should always result in meiotic segregation distortion. Several important differences between the sex chromosomes should strongly facilitate their non-random segregation.
First, offspring sex adjustment can be facilitated by size difference of sex chromosomes; unlike autosomal pairs of bivalents, the W chromosome is smaller than the Z chromosome in most birds (Solari 1993). This dimorphism affects sex chromosome movement and the stability of chromosome dyads during meiosis because the polar wind—a force acting on the kinetochore as chromosomes approach a pole—is proportional to chromosome size (Carpenter 1991). Moreover, as a chromosome becomes smaller, the centromere protein domain responsible for capturing the microtubules becomes more compressed (Spence et al. 2006), leading to mis-segregation of dimorphic sex chromosomes. Thus, if chromosome size affects segregation distortion, then bird species with greater sexual dimorphism in chromosomes should have higher probability of offspring sex ratio adjustment. Because the sex chromosome size varies even within species (Panov & Bulatova 1972; Rebholz 1992), the Z/W ratio can be used to test the contribution of sex chromosome dimorphism to among-population and among-female variation in offspring sex bias, such that populations or individuals with greater dimorphism in sex chromosome size might be more likely to produce biased offspring sex ratios.
Second, offspring sex ratio adjustment might be facilitated by shape differences between sex chromosomes. Specifically, sex chromosomes in birds often differ in centromere position (e.g. Belterman & De Boer 1984), both within and among species (e.g. Panov & Bulatova 1972; Krasikova et al. 2006), and this affects chromosome movement and segregation during meiosis. The effects of centromere position on bias in sex chromosome segregation to the functional gamete are known in other taxa; the chromosome with the most centrally located centromere segregates preferentially to the gamete in Drosophila (Novitski 1967), whereas the acrocentric chromosome segregates to the gamete in common vole Microtus arvalis (Gileva & Rakitin 2006). More generally, the difference in chromosome morphology and specifically the evolution of centromere position might be driven by strong selection exerted by competition for preferential transmission of chromosomes during meiosis (Padro-Manuel de Villena & Sapienza 2001a). Thus, if the difference in centromere position between sex chromosomes affects segregation distortion in birds similarly to these effects in other taxa, then avian species with greater difference in centromere location should be more likely to manipulate offspring sex.
Furthermore, avian sex chromosomes differ in region-specific DNA sequences and epigenetic markings, which can enable chromosome recognition required for directional bias in segregation distortion. For example, telomeres with their highly repetitive DNA sequences at the end of the chromosomes (Zwick et al. 1999) are involved in chromosome pairing and segregation in mammalian male meiosis (Yogev et al. 2004). Interestingly, telomere involvement in chromosome recognition, and associated competition for preferential allocation into a functional gamete, is thought to account for the maintenance of high variability in telomere length (Zwick et al. 1999). Importantly, telomeres differ strongly between the sex chromosomes in chickens—the exceptionally long telomere array and other tandem repeats are present on the W, but not on the Z chromosome (Rodrigue et al. 2005; Krasikova et al. 2006), and this difference, in the absence of compensating factors, should facilitate biased segregation of sex chromosomes.
Apart from the differences in morphology and DNA sequences, distinct epigenetic marking of sex chromosomes may facilitate their segregation. The long-standing assumption that genomic imprinting does not occur in oviparous species (Haig & Graham 1991) is challenged by the recent documentation of epigenetic marking of avian chromosomes (Teranishi et al. 2001; Tuiskula-Haavisto et al. 2004; Gupta et al. 2006). For example, DNA methylation changes the conformation of chromatin from compact to loose in the promoter of the vitellogenin gene in Japanese quail Coturnix japonica (Gupta et al. 2006). In chickens, the presence of genomic imprinting is evident in differential expression of maternal and parental origin genes affecting egg production (Tuiskula-Haavisto et al. 2004). If offspring sex adjustment in birds is facilitated by sex chromosome recognition through genomic imprinting, then avian sex chromosomes should differ in methylation pattern. Indeed, there is evidence that hypermethylation of Z chromosome-based genes in avian males might be the mechanism of dosage compensation (Teranishi et al. 2001; Ellegren 2002; Nakagawa 2004). Such methylation, if retained until the Z chromosome reaches female meiosis, can bias segregation of methylated and unmethylated sex chromosomes.
(b) Position of the sex chromosome before nuclear envelope breakage as a factor affecting segregation distortion
Spatial organization of chromosome position in the germinal vesicle in relation to the future spindle poles might be an important determinant of chromosome fate upon segregation. Here, we describe two processes that affect chromosome orientation during breakage of nuclear membrane.
During meiotic prophase, chromosome telomeres move along the nuclear envelope and form ‘the bouquet’—the clustering of chromosome ends at the nuclear envelope—a pattern documented in fungi, plants, insects and mammals (Bass 2003). Two well-documented patterns in birds—the difference in telomere sequences and length of sex chromosomes—could influence the directionality of the sex chromosome movement along the nuclear envelope, and thus affect chromosome position during envelope breakage. Importantly, the telomeres are reconstructed by telomerase—an enzyme responsible for telomere elongation, which is highly expressed in developing oocytes, especially in the germinal vesicle and during metaphase of the first meiotic division (Bekaert et al. 2004). In turn, the activity of telomerase is affected by steroid hormones, oestrogen, progesterone and androgens, that act via cell cycle regulators and receptors (Bayne & Liu 2005). Such non-genomic hormonal regulation (see below) of telomerase is tissue specific, and depends closely on physiological condition, including health (Bayne & Liu 2005). For example, lower telomerase levels and shorter telomeres are associated with higher levels of stress hormones (Epel et al. 2006). Thus, under this mechanism, maternal hormonal status could affect telomere length in a context-dependent manner. However, it is not yet known whether the effect of hormones on telomerase activity differs between the sex chromosomes.
The second process involved in chromosome positioning before germinal vesicle breakdown is the fusion of protein bodies that carry condensed chromosomes on their surface (figure 1e; Gaginskaya 1972; Saifitdinova et al. 2003; Krasikova et al. 2005). Size difference in protein bodies between the sex chromosomes (see above) should affect the bivalent position and orientation before and during nuclear envelope breakage, and therefore bias sex chromosome segregation.
(c) Sex chromosome congression and segregation distortion
Among the chromosome bivalents aligned along the longer axis of the spindle, those closer to a pole should have higher probability of segregating towards that pole. Thus, regulation of chromosome congression—a delivery of chromosome bivalents to the meiotic spindle equator by the contraction of the actin network (Lénárt et al. 2005; figure 1f)—would directly affect chromosome segregation into a gamete or a polar body. Importantly, the actin network is activated by substances released into the nucleus at the rupture of the germinal vesicle membrane at the periphery of the oocyte (Lénárt et al. 2005; Sun & Schatten 2006; figure 1b,f). In turn, activated filaments of actin form shells around the chromosomes, and it is a contraction of the actin filaments that brings embedded chromosomes into the meiotic plate. Actin network sensitivity to external stimuli (see below) in combination with its effects on sex chromosome congression makes it a particularly likely mechanism for segregation distortion of sex chromosomes. Whereas dimorphism in sex chromosomes can affect the probability of their displacement from the spindle equator, the directionality of this displacement might depend on the immediate environment of the nucleus because the substances at the yolk side of the vesicle can differ from those at the side of the vitelline membrane. For example, a steroid hormone gradient in the vicinity of the germinal vesicle can affect the actin network, such that its behaviour will differ between the two sides of the spindle equator. In the following, we provide evidence for such hormonal gradients and the role of these gradients in modulating chromosome congression.
Although steroids commonly act as nuclear transcription factors (e.g. Rories & Spelsberg 1989), there is now evidence that steroid hormones can also exert rapid non-genomic reactions at cell surfaces, including modifications of cell cytoskeleton (e.g. Manavathi & Kumar 2006). Most of such non-genomic effects of steroids on cells described in fish, amphibian and mammalian oocytes are related to an increase in free Ca2+. For example, in maturing human oocytes, 17β-oestradiol triggers the release of free Ca2+ initially at the periphery of oocytes where it remains in the highest concentration (Tesarik & Mendoza 1995), ultimately forming an intra-oocyte gradient of free Ca2+ between the oocyte core and cortex. Because Ca2+ plays an important role in actin polymerization (e.g. Mooseker et al. 1980), such a gradient and its location leads to differential congression of chromosomes between the two sides of the meiotic plate. Moreover, there is a substantial evidence for the influence of hormones on chromosome congression (e.g. Hodges et al. 2002; Beker-van Woudenberg et al. 2004; Roberts et al. 2005). For example, during in vitro maturation of bovine oocytes, oestradiol causes abnormal dispersion of chromosomes (Beker-van Woudenberg et al. 2004) and high doses of follicle-stimulating hormone administrated in vitro to mouse oocytes lead to strong chromosome displacement and scattering across the spindle, such that some chromosome bivalents do not reach the plate before metaphase (Roberts et al. 2005). Similarly, elevated levels of lutenizing hormone and alterations of testosterone/oestradiol ratio during the final stages of oocyte growth and maturation results in the failure of chromosome congression (Hodges et al. 2002). An additional factor that might be involved in avian chromosome congression is chromokinesin—a protein associated with chromosome arms. First described in chickens (Wang & Adler 1995), this protein is now known to play an important role in chromosome congression in Xenopus laevis frogs and Drosophila flies (Endow 1999). More generally, the role of the actin network in segregation distortion of sex chromosomes holds great promise in the empirical studies of avian sex ratio adjustment, because it provides a precise mechanism by which external stimuli can modulate directional segregation of sex chromosomes.
(d) Attachment of sex chromosome microtubules and segregation distortion
Variability in microtubule capture by kinetochores, commonly assumed to be a random process (Nicklas 1997), is crucially important for sex determination. Non-random sex chromosome segregation can be enabled by the ‘centromere drive’ mechanism (figure 1g; Talbert et al. 2004; Malik & Bayes 2006), where the probability of chromosome segregating into the oocyte increases with the number of microtubules attached to that chromosome. When a centromere array of satellite repetitive DNA expands, it leads to a larger kinetochore size and its greater interaction with microtubules and, thus, greater chances of chromosome allocation into the oocyte (Malik & Bayes 2006). We show below that sex chromosome difference in the ability to capture microtubules may similarly facilitate offspring sex adjustment.
The factors influencing the number of captured microtubules and the effects of centromere drive on directionality of segregation vary between taxa. In chickens, chromosomal rearrangements with more centromeres segregate preferentially to the polar body (Dinkel et al. 1979). In mammals, the pattern varies across species and even among populations; chromosomes with more centromeres preferentially segregate to the oocyte in one race of mice and the polar body in the other (Padro-Manuel de Villena & Sapienza 2001b). This suggests that the effect of centromere number on sex chromosome segregation evolves rapidly and might be context dependent within a species. An avian sex chromosome typically has one centromere, and an increase in centromere size in one of the sex chromosomes can result in a greater number of microtubules captured by its kinetochore, leading to directional segregation of sex chromosomes (see also Zwick et al. 1999; Malik & Bayes 2006). Additionally, the probability of chromosome allocation into the gamete can increase with the array of highly repetitive heterochromatic domains on chromosomes, as has been shown for maize (Buckler et al. 1999).
If avian sex chromosomes differ in centromere size, then species with greater sex dimorphism in this trait should be more likely to bias offspring sex. Alternatively, segregation of sex chromosomes could be affected by changes in the centromere ability to capture microtubules, such as those observed in the differential ability of kinetochores to capture microtubules during meiosis, compared to mitosis (see below; Simchen & Hugerat 1993; Nicklas 1997). In the following, we first discuss the role of centromere proteins in the epigenetic regulation of centromere function; second, we suggest the potential role of the cohesion proteins in the modification of microtubule capturing by the kinetochore; and third, we introduce ‘spindle checkpoint’ as a potential mechanism for altering chromosome assignment to the spindle poles.
First, sex chromosome segregation distortion may depend on the centromere proteins that modify the number of microtubules captured by the kinetochore. Microtubule attachment to kinetochore is affected by the centromere proteins (Dawe & Henikoff 2006; Malik & Bayes 2006) that suppress meiotic drive of centromeres in several taxa (Talbert et al. 2004). Interestingly, one of the centromere proteins, CENP-C, is present in chicken in a single copy on the fourth chromosome, and this protein is also attached to kinetochores during mitotic metaphase (Okamura et al. 2001), thus warranting further investigation of CENP-C role in segregation distortion of the avian sex chromosomes.
Second, sex chromosome segregation can be modified by the cohesion proteins that affect microtubule attachment to kinetochore and influence orientation of the centromere towards the poles (Hagstrom & Meyer 2003; Morrison et al. 2003). In birds, the cohesion proteins are aggregated into protein bodies (figure 1e) that vary in size among and within species (see above, Gaginskaya 1972; Solovei et al. 1993; Krasikova et al. 2006). The differences in the size of protein bodies on the Z and W chromosomes (Solovei et al. 1993) can modify the attachment of microtubules to the kinetochore, and thus facilitate non-random sex chromosome segregation due to centromere drive (see above). Furthermore, even though the protein bodies fuse before the microtubule attaches to the kinetochores (figure 1e), the cohesion proteins remain at the centromeres and affect their accessibility to microtubules. Thus, a bigger protein body of one of the sex chromosome should result in restricted attachment of the microtubules and less likely allocation to the oocyte. Indeed, pronounced within-species variation in the size of protein bodies may account for the differences among females in the strategies and precision of offspring sex adjustment.
In addition to their function of capturing microtubules, kinetochores also contain molecular motor proteins that coordinate chromosome movements and are responsible for generating a ‘mitotic spindle checkpoint’—a mechanism signalling proper chromosome attachment and alignment on the division plate (e.g. Nicklas 1997; Biggins & Walczak 2003). Mitosis can proceed only when the forces in the mitotic spindle generate tension at the kinetochores (Reddy et al. 2007; Stegmeier et al. 2007). Such a checkpoint was assumed to be absent in female meiosis, but recent evidence suggests that it might function in mammals—the first spindle checkpoint protein responsible for the regulation of chromosome alignment was recently described in mouse oocytes (Yin et al. 2006). If a similar mechanism exists in avian meiosis, then it would allow repeated capture and release of the microtubules and provide a mechanism for non-random attachment and segregation of the specific sex bivalent to the gamete. Furthermore, the attachment of different sized bivalents could be stabilized by a local hormonal gradient; maternal hormones at this stage of meiosis act via direct binding of 17β-oestradiol to the microtubules and affect the shape and functioning of the metaphase spindle (Beker-van Woudenberg et al. 2004). Such binding would not only counterbalance the effect of different sized bivalents but also differentially interfere with the CENP (see above) and the cohesion proteins of Z and W chromosomes, and ultimately modulate the directionality of sex chromosome segregation.
Finally, spindle positioning in relation to the cortex and core of the oocyte strongly affects its polarity, although evidence for spindle polarity in birds is mixed. Whereas spindle asymmetry in either the number or length of the microtubules emerging from the two poles has not been described in cytological preparations of avian oocytes (Yoshimura et al. 1993a,b), non-random segregation of chromosomal rearrangements to the polar body versus oocyte in the chicken (Dinkel et al. 1979) provides some support for the asymmetry of avian first meiotic spindle.
(e) Rotation of the meiotic spindle as a mechanism for segregation distortion of sex chromosomes
After the chromosome bivalents attach to the opposite spindle poles but before segregation, ‘the last minute’ mechanism to alter the sex determination is a rotation of the meiotic spindle. In X. laevis oocytes, the meiotic spindle is oriented parallel to the cell cortex initially, but right before the chromosome bivalents start to separate, the spindle turns 90°, thus determining the fate of the chromosome bivalents (Gard et al. 1995). Similarly, rotation of the first meiotic spindle accompanies chromosome segregation and formation of the first polar body in the marsupial Sminthopsis macroura (Merry et al. 1995) and the horse (Tremoleda et al. 2001). Because spindle rotation is controlled by cytoskeleton elements—such as the actin filaments and the microtubules (Merry et al. 1995; Tremoleda et al. 2001; Sun & Schatten 2006)—the rotation might similarly be affected by the intra-germinal vesicle gradient of maternal hormones in combination with the differences in size and epigenetic markings between the sex chromosomes (see above). If these traits of a given sex chromosome exhibit higher affinity for one side of the dividing cell versus the other, for example, as a result of a difference in exposure to maternal hormone gradients, then the rotation of the meiotic spindle can play a key role in offspring sex manipulation in birds.
4. Hormonal integration of external cues and molecular mechanism of offspring sex adjustment
In the above discussion, we identified several stages of meiosis at which adjustment of the sex ratio can be affected by hormones. For example, non-genomic actions of steroids can influence cell cytoskeleton by both triggering the release of Ca2+ that activates the actin network and chromosome congression and binding to the microtubules that affects orientation and shape of the meiotic spindle. Similarly, a link between oocyte growth rate and segregation distortion of sex chromosomes in birds (Young & Badyaev 2004) and other taxa (Hodges et al. 2002) deserves further studies. More generally, the levels of maternal hormones in the yolk can reflect maternal hormonal profile at different reproductive stages and breeding contexts (Schwabl 1996; Badyaev et al. 2005; Williams et al. 2005; Sockman et al. 2006) and hormone allocation differs systematically among concentric lipid layers of the oocyte (Lipar et al. 1999; Johnson 2000; Hackl et al. 2003). Because the germinal disc is located in the layer deposited during the last day of vitellogenesis, changes in a female's circulating hormones at that time are reflected on the oocyte surface generating a hormonal gradient in the immediate vicinity of the germinal disc, ultimately leading to differential segregation of the sex chromosomes (R. Marshall 2003, unpublished MS; Badyaev et al. 2006a). Thus, hormonal effects on the mechanisms of sex chromosome segregation distortion can provide an important mediator between environmental factors experienced by the female and offspring sex adjustment (Badyaev & Oh 2008).
5. Conclusions
We integrated classical studies of oocyte maturation in birds with novel findings of chromosome segregation during meiosis (figure 1) to propose testable hypotheses for epigenetic regulation and disruption of meiotic segregation of sex chromosomes. We show that several features of avian sex chromosomes, including size dimorphism and sequence and epigenetic modifications, can affect their interactions with the cell cytoskeleton and influence chromosome movement patterns and segregation. Furthermore, we present evidence for strong effects of maternal hormones on cell cytoskeleton and its interactions with chromosomes, which provides an opportunity for the modulation of sex chromosome segregation in relation to external factors (figure 2). The multitude of potential targets of selection on sex ratio adjustment probably reflects the diversity of mechanisms and levels on which selection for sex ratio adjustment can operate in birds. Our review also suggests directions for future research; the hypotheses outlined in this review can be tested empirically with the comparative approach and with direct hormonal manipulations of growing follicles (Warren & Scott 1935) or follicles cultured in vitro (Olszańska et al. 1996).
Comparison of the proposed mechanisms suggests that the later the mechanisms operate in the meiotic sequence, the more precise will be the sex ratio adjustment (figure 2). Moreover, if the offspring sex is determined at the early stages of meiosis, then it would be more likely to covary with the egg content, providing the mechanism for proposed association of sex determination and sex-specific maternal allocation of substances into eggs (Badyaev 2005; Badyaev & Oh in press). For example, substances present in the nucleus can affect elongation of the telomeres before the breakage of nuclear envelope (Bekaert et al. 2004), influencing offspring sex adjustment at the early stages of meiosis, whereas hormones secreted shortly before ovulation can affect the behaviour of the microtubules (Beker-van Woudenberg et al. 2004) and spindle rotation and influence offspring sex adjustment at the late stages of meiosis.
We identified several meiotic mechanisms that produce distinct directionality of segregation depending on the contexts of breeding. Such mechanisms are likely to allow for rapid phenotypic adjustment of sex allocation strategies to breeding context and can explain some of the most remarkable patterns of adaptive adjustment of primary sex ratio in birds in relation to the egg content and laying sequence.
Acknowledgments
We thank two anonymous reviewers, John Wingfield, Renee Duckworth, and the members of Badyaev Lab for critical comments on the previous versions of this manuscript and useful discussions. A.V.B. is grateful to Rupert Marshall for generously sharing his extensive knowledge of classical studies on avian meiosis. This work was supported, in part, by the Kościuszko Foundation postdoctoral fellowship to J.R. and the grants from the National Science Foundation (USA) and the David and Lucille Packard Fellowship to A.V.B.
Footnotes
One contribution of 12 to a Theme Issue ‘Integration of ecology and endocrinology in avian reproduction: a new synthesis’.
References
- Akhverdyan M, Fredga K. EM studies of female meiosis in wood lemmings with different sex chromosome constitutions. J. Exp. Zool. 2001;290:504–516. doi: 10.1002/jez.1094. doi:10.1002/jez.1094 [DOI] [PubMed] [Google Scholar]
- Alonso-Alvarez C. Manipulation of primary sex-ratio: an updated review. Avian Poult. Biol. Rev. 2006;17:1–20. [Google Scholar]
- Andersson M, Wallander J, Oring L, Akst E, Reed J.M, Fleischer R.C. Adaptive seasonal trend in brood sex ratio: test in two sister species with contrasting breeding systems. J. Evol. Biol. 2003;16:510–515. doi: 10.1046/j.1420-9101.2003.00533.x. doi:10.1046/j.1420-9101.2003.00533.x [DOI] [PubMed] [Google Scholar]
- Badyaev A.V. Growing apart: an ontogenetic perspective on the evolution of sexual size dimorphism. Trends Ecol. Evol. 2002;17:369–378. doi:10.1016/S0169-5347(02)02569-7 [Google Scholar]
- Badyaev A.V. Maternal inheritance and rapid evolution of sexual size dimorphism: passive effects or active strategies? Am. Nat. 2005;166:S17–S30. doi: 10.1086/444601. doi:10.1086/444601 [DOI] [PubMed] [Google Scholar]
- Badyaev A.V, Oh K.P. Environmental induction and phenotypic retention of adaptive maternal effects. BMC Evol. Biol. 2008;8:3. doi: 10.1186/1471-2148-8-3. doi:10.1186/1471-2148-8-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badyaev A.V, Hill G.E, Beck M.L, Dervan A.A, Duckworth R.A, McGraw K.J, Nolan P.M, Whittingham L.A. Sex-biased hatching order and adaptive population divergence in a passerine bird. Science. 2002;295:316–318. doi: 10.1126/science.1066651. doi:10.1126/science.1066651 [DOI] [PubMed] [Google Scholar]
- Badyaev A.V, Schwabl H, Young R.L, Duckworth R.A, Navara K, Parlow A.F. Adaptive sex differences in growth of pre-ovulation oocytes in a passerine bird. Proc. R. Soc. B. 2005;272:2165–2172. doi: 10.1098/rspb.2005.3194. doi:10.1098/rspb.2005.3194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badyaev A.V, Acevedo Seaman D, Navara K.J, Hill G.E, Mendonça M.T. Evolution of sex-biased maternal effects in birds: III. Adjustment of ovulation order can enable sex-specific allocation of hormones, carotenoids, and vitamins. J. Evol. Biol. 2006a;19:1044–1057. doi: 10.1111/j.1420-9101.2006.01106.x. doi:10.1111/j.1420-9101.2006.01106.x [DOI] [PubMed] [Google Scholar]
- Badyaev A.V, Hamstra T.L, Oh K.P, Acevedo Seaman D. Sex-biased maternal effects reduce ectoparasite-induced mortality in a passerine bird. Proc. Natl Acad. Sci. USA. 2006b;103:14 406–14 411. doi: 10.1073/pnas.0602452103. doi:10.1073/pnas.0602452103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bass H.W. Telomere dynamics unique to meiotic prophase: formation and significance of the bouquet. Cell. Mol. Life Sci. 2003;60:2319–2324. doi: 10.1007/s00018-003-3312-4. doi:10.1007/s00018-003-3312-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayne S, Liu J.P. Hormones and growth factors regulate telomerase activity in ageing and cancer. Mol. Cell. Endocrinol. 2005;240:11–22. doi: 10.1016/j.mce.2005.05.009. doi:10.1016/j.mce.2005.05.009 [DOI] [PubMed] [Google Scholar]
- Bekaert S, Derradji H, Baatout S. Telomere biology in mammalian germ cells and during development. Dev. Biol. 2004;274:15–30. doi: 10.1016/j.ydbio.2004.06.023. doi:10.1016/j.ydbio.2004.06.023 [DOI] [PubMed] [Google Scholar]
- Beker-van Woudenberg A.R, van Tol H.T.A, Roelen B.A.J, Colenbrander B, Bevers M.M. Estradiol and its membrane-impermeable conjugate (estradiol–bovine serum albumin) during in vitro maturation of bovine oocytes: effects on nuclear and cytoplasmic maturation, cytoskeleton, and embryo quality. Biol. Reprod. 2004;70:1465–1474. doi: 10.1095/biolreprod.103.025684. doi:10.1095/biolreprod.103.025684 [DOI] [PubMed] [Google Scholar]
- Belterman R.H.R, De Boer L.E.M. A karyological study of 55 species of birds, including karyotypes of 39 species new to cytology. Genetica. 1984;65:39–82. doi:10.1007/BF00056765 [Google Scholar]
- Biggins S, Walczak C.E. Captivating capture: how microtubules attach to kinetochores. Curr. Biol. 2003;13:R449–R460. doi: 10.1016/s0960-9822(03)00369-5. doi:10.1016/S0960-9822(03)00369-5 [DOI] [PubMed] [Google Scholar]
- Birrenkott G.P, Shoop M.A, Cooper K, Wiggens M. Ovarian follicular growth and maturation in the domestic pigeon and guinea fowl (Numida meleagris) Poult. Sci. 1988;67:1783–1786. doi: 10.3382/ps.0671783. [DOI] [PubMed] [Google Scholar]
- Bissonnette T.H, Zujko A.J. Normal progressive changes in the ovary of the starling (Sturnus vulgaris) from December to April. Auk. 1936;53:30–50. [Google Scholar]
- Blanco G, Dávila J.A, López Septiem J.A, Rodríguez R, Martínez F. Sex-biased initial eggs favour sons in the slightly size-dimorphic Scops owl (Otus scops) Biol. J. Linn. Soc. 2002;76:1–7. doi:10.1046/j.1095-8312.2002.00036.x [Google Scholar]
- Buckler E.S, Phelps-Durr T.L, Buckler C.S.K, Dawe R.K, Doebley J.F, Holtsford T.P. Meiotic drive of chromosomal knobs reshaped the maize genome. Genetics. 1999;153:415–426. doi: 10.1093/genetics/153.1.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bull J.J. Benjamin/Cummings; Menlo Park, CA: 1983. Evolution of sex determining mechanisms. [Google Scholar]
- Cameron E. Facultative adjustment of mammalian sex ratios in support of the Trivers–Willard hypothesis: evidence for a mechanism. Proc. R. Soc. B. 2004;271:1723–1728. doi: 10.1098/rspb.2004.2773. doi:10.1098/rspb.2004.2773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter A.T.C. Distributive segregation—motors in the polar wind. Cell. 1991;64:885–890. doi: 10.1016/0092-8674(91)90313-n. doi:10.1016/0092-8674(91)90313-N [DOI] [PubMed] [Google Scholar]
- Chalana R.K, Guraya S.S. Morphological and histochemical observations on the primordial and early growing oocytes of crow (Corvus spendens) and myna (Acridotheres tristis) Poult. Sci. 1979;58:225–231. doi: 10.3382/ps.0580225. [DOI] [PubMed] [Google Scholar]
- Charnov, E. L. 1982 The theory of sex allocation Monographs in population biology. Princeton, NJ: Princeton University Press. [PubMed]
- Clinton M, Haines L.C. An overview of factors influencing sex determination and gonadal development in birds. Cell. Mol. Life Sci. 1999;55:876–886. doi: 10.1007/978-3-0348-7781-7_6. doi:10.1007/s000180050341 [DOI] [PubMed] [Google Scholar]
- Dawe R.K, Henikoff S. Centromeres put epigenetics in the driver's seat. Trends Biochem. Sci. 2006;31:662–669. doi: 10.1016/j.tibs.2006.10.004. doi:10.1016/j.tibs.2006.10.004 [DOI] [PubMed] [Google Scholar]
- Dinkel B.J, O'Laughlin-Phillips E.A, Fechheimer N.S, Jaap R.G. Gametic products transmitted by chickens heterozygous for chromosomal rearrangements. Cytogenet. Cell Genet. 1979;23:124–136. doi: 10.1159/000131313. [DOI] [PubMed] [Google Scholar]
- Ellegren H. Dosage compensation: do birds do it as well? Trends Genet. 2002;18:25–28. doi: 10.1016/s0168-9525(01)02553-7. doi:10.1016/S0168-9525(01)02553-7 [DOI] [PubMed] [Google Scholar]
- Endow S.A. Microtubule motors in spindle and chromosome motility. Eur. J. Biochem. 1999;262:12–18. doi: 10.1046/j.1432-1327.1999.00339.x. doi:10.1046/j.1432-1327.1999.00339.x [DOI] [PubMed] [Google Scholar]
- Epel E.S, Lin J, Wilhelm F.H, Wolkowitz O.M, Cawthon R, Adler N.E, Dolbier C, Mendes W.B, Blackburn E.H. Cell aging in relation to stress arousal and cardiovascular disease risk factors. Psychoneuroendocrinology. 2006;31:277–287. doi: 10.1016/j.psyneuen.2005.08.011. doi:10.1016/j.psyneuen.2005.08.011 [DOI] [PubMed] [Google Scholar]
- Fisher R.A. Clarendon Press; Oxford, UK: 1930. The genetical theory of natural selection. [Google Scholar]
- Fishman L, Willis J.H. A novel meiotic drive locus almost completely distorts segregation in mimulus (monkeyflower) hybrids. Genetics. 2005;169:347–353. doi: 10.1534/genetics.104.032789. doi:10.1534/genetics.104.032789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredga K, Gropp A, Winking H, Frank F. Fertile Xx-type and Xy-type females in wood lemming Myopus schisticolor. Nature. 1976;261:225–227. doi: 10.1038/261225a0. doi:10.1038/261225a0 [DOI] [PubMed] [Google Scholar]
- Freedberg S, Taylor D.R. Sex ratio variance and the maintenance of environmental sex determination. J. Evol. Biol. 2007;20:213–220. doi: 10.1111/j.1420-9101.2006.01209.x. doi:10.1111/j.1420-9101.2006.01209.x [DOI] [PubMed] [Google Scholar]
- Fuge H. Unorthodox male meiosis in Trichosia pubescens (Sciaridae)—chromosome elimination involves polar organelle degeneration and monocentric spindles in first and 2nd division. J. Cell Sci. 1994;107:299–312. doi: 10.1242/jcs.107.1.299. [DOI] [PubMed] [Google Scholar]
- Gaginskaya E.R. Nuclear structures in oocytes of adult birds. II. Protein bodies and karyosphere. Tsitologiia. 1972;14:568–578. [PubMed] [Google Scholar]
- Galkina S, Deryusheva S, Fillon V, Vignal A, Crooijmans R, Groenen M, Rodionov A, Gaginskaya E. FISH on avian lampbrush chromosomes produces higher resolution gene mapping. Genetica. 2006;128:241–251. doi: 10.1007/s10709-005-5776-7. doi:10.1007/s10709-005-5776-7 [DOI] [PubMed] [Google Scholar]
- Gard D.L, Cha B.-J, Roeder A.D. F-actin is required for spindle anchoring and rotation in Xenopus oocytes: a re-examination of the effects of cytochalasin B on oocyte maturation. Zygote. 1995;3:17–26. doi: 10.1017/s0967199400002331. [DOI] [PubMed] [Google Scholar]
- Gileva E.A, Rakitin S.B. Factors of maintaining chromosome polymorphism in common vole Microtus arvalis Pallas, 1779: reduced fertility and meiotic drive. Russ. J. Genet. 2006;42:498–504. doi:10.1134/S1022795406050061 [PubMed] [Google Scholar]
- Griffin A.S, Sheldon B.C, West S.A. Cooperative breeders adjust offspring sex ratios to produce helpful helpers. Am. Nat. 2005;166:628–632. doi: 10.1086/491662. doi:10.1086/491662 [DOI] [PubMed] [Google Scholar]
- Gupta S, Pathak R.U, Kanungo M.S. DNA methylation induced changes in chromatin conformation of the promoter of the vitellogenin II gene of Japanese quail during aging. Gene. 2006;377:159–168. doi: 10.1016/j.gene.2006.04.020. doi:10.1016/j.gene.2006.04.020 [DOI] [PubMed] [Google Scholar]
- Hackl R, Bromundt V, Daisley J, Kotrschal K, Möstl E. Distribution and origin of steroid hormones in the yolk of Japanese quail eggs (Coturnix coturnix japonica) J. Comp. Physiol. B: Biochem. Syst. Environ. Physiol. 2003;173:327–331. doi: 10.1007/s00360-003-0339-7. doi:10.1007/s00360-003-0339-7 [DOI] [PubMed] [Google Scholar]
- Hagstrom K.A, Meyer B.J. Condensin and cohesin: more than chromosome compactor and glue. Nat. Rev. Genet. 2003;4:520–534. doi: 10.1038/nrg1110. doi:10.1038/nrg1110 [DOI] [PubMed] [Google Scholar]
- Haig D, Graham C. Genomic imprinting and the strange case of the insulin-like growth factor-II receptor. Cell. 1991;64:1045–1046. doi: 10.1016/0092-8674(91)90256-x. doi:10.1016/0092-8674(91)90256-X [DOI] [PubMed] [Google Scholar]
- Harper E.H. The fertilization and early development of the pigeon's egg. Am. J. Anat. 1904;3:349–386. doi:10.1002/aja.1000030402 [Google Scholar]
- Heinsohn R, Legge S, Barry S. Extreme bias in sex allocation in Eclectus parrots. Proc. R. Soc. B. 1997;264:1325–1329. doi:10.1098/rspb.1997.0183 [Google Scholar]
- Hodges C.A, Hagan A, Jennings D, Keri R, Nilson J, Hunt P.A. Experimental evidence that changes in oocyte growth influence meiotic chromosome segregation. Hum. Reprod. 2002;17:1171–1180. doi: 10.1093/humrep/17.5.1171. doi:10.1093/humrep/17.5.1171 [DOI] [PubMed] [Google Scholar]
- Holand Ø, Mysterud A, Røed N.H, Coulson T, Gjøstein H, Weladji R.B, Nieminen M. Adaptive adjustment of offspring sex ratio and maternal reproductive effort in an iteroparous mammal. Proc. R. Soc. B. 2006;273:293–299. doi: 10.1098/rspb.2005.3330. doi:10.1098/rspb.2005.3330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homa S.T. Calcium and meiotic maturation of the mammalian oocyte. Mol. Reprod. Dev. 1995;40:122–134. doi: 10.1002/mrd.1080400116. doi:10.1002/mrd.1080400116 [DOI] [PubMed] [Google Scholar]
- Jaenike J. Sex chromosome meiotic drive. Annu. Rev. Ecol. Syst. 2001;32:25–49. doi:10.1146/annurev.ecolsys.32.081501.113958 [Google Scholar]
- James W.H. Evidence that mammalian sex ratios at birth are partially controlled by parental hormone levels at the time of conception. J. Theor. Biol. 1996;180:271–286. doi: 10.1006/jtbi.1996.0102. doi:10.1006/jtbi.1996.0102 [DOI] [PubMed] [Google Scholar]
- Janzen F.J, Phillips P.C. Exploring the evolution of environmental sex determination, especially in reptiles. J. Evol. Biol. 2006;19:1775–1784. doi: 10.1111/j.1420-9101.2006.01138.x. doi:10.1111/j.1420-9101.2006.01138.x [DOI] [PubMed] [Google Scholar]
- Johnson A.L. Reproduction in the female. In: Whittow G.C, editor. Sturkie's avian physiology. Academic Press; San Diego, CA: 2000. pp. 569–596. [Google Scholar]
- Johnstone C.M, Barnett M, Sharpe P.T. The molecular biology of temperature-dependent sex determination. Phil. Trans. R. Soc. B. 1995;350:297–303. doi: 10.1098/rstb.1995.0165. doi:10.1098/rstb.1995.0165 [DOI] [PubMed] [Google Scholar]
- Komdeur J, Daan S, Tinbergen J, Mateman C. Extreme adaptive modification in sex ratio of the Seychelles warbler's eggs. Nature. 1997;385:522–525. doi:10.1038/385522a0 [Google Scholar]
- Kozielska M, Pen I, Beukeboom L.W, Weissing F.J. Sex ratio selection and multi-factorial sex determination in the housefly: a dynamic model. J. Evol. Biol. 2006;19:879–888. doi: 10.1111/j.1420-9101.2005.01040.x. doi:10.1111/j.1420-9101.2005.01040.x [DOI] [PubMed] [Google Scholar]
- Kraak S.B.M, de Looze E.M.A. A new hypothesis on the evolution of sex determination in vertebrates; big females ZW, big males XY. Neth. J. Zool. 1993;43:260–273. [Google Scholar]
- Kraak S.B.M, Pen I. Sex-determining mechanisms in vertebrates. In: Hardy I.C.M, editor. Sex ratios: concepts and research methods. Cambridge University Press; Cambridge, UK: 2002. pp. 158–177. [Google Scholar]
- Krackow S. Potential mechanisms for sex ratio adjustment in mammals and birds. Biol. Rev. 1995;70:225–241. doi: 10.1111/j.1469-185x.1995.tb01066.x. [DOI] [PubMed] [Google Scholar]
- Krasikova A, Barbero J.L, Gaginskaya E. Cohesion proteins are present in centromere protein bodies associated with avian lampbrush chromosomes. Chromosome Res. 2005;13:675–685. doi: 10.1007/s10577-005-1005-6. doi:10.1007/s10577-005-1005-6 [DOI] [PubMed] [Google Scholar]
- Krasikova A, Deryusheva S, Galkina S, Kurganova A, Evteev A, Gaginskaya E. On the positions of centromeres in chicken lampbrush chromosomes. Chromosome Res. 2006;14:777–789. doi: 10.1007/s10577-006-1085-y. doi:10.1007/s10577-006-1085-y [DOI] [PubMed] [Google Scholar]
- Le Galliard J.-F, Fitze P.S, Ferriere R, Clobert J. Sex ratio bias, male aggression, and population collapse in lizards. Proc. Natl Acad. Sci. USA. 2005;102:18 231–18 236. doi: 10.1073/pnas.0505172102. doi:10.1073/pnas.0505172102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leimar O. Life-history analysis of the Trivers and Willard sex-ratio problem. Behav. Ecol. 1996;7:316–325. doi:10.1093/beheco/7.3.316 [Google Scholar]
- Lénárt P, Bacher C.P, Daigle N, Hand A.R, Eils R, Terasaki M, Ellenberg J. A contractile nuclear actin network drives chromosome congression in oocytes. Nature. 2005;436:812–818. doi: 10.1038/nature03810. doi:10.1038/nature03810 [DOI] [PubMed] [Google Scholar]
- Lipar J.L, Ketterson E.D, Nolan V, Casto J.M. Egg yolk layers vary in the concentration of steroid hormones in two avian species. Gen. Comp. Endocrinol. 1999;115:220–227. doi: 10.1006/gcen.1999.7296. doi:10.1006/gcen.1999.7296 [DOI] [PubMed] [Google Scholar]
- Malik H.S, Bayes J.J. Genetic conflicts during meiosis and the evolutionary origins of centromere complexity. Biochem. Soc. Trans. 2006;34:569–573. doi: 10.1042/BST0340569. doi:10.1042/BST0340569 [DOI] [PubMed] [Google Scholar]
- Manavathi B, Kumar R. Steering estrogen signals from the plasma membrane to the nucleus: two sides of the same coin. J. Cell. Phys. 2006;207:594–604. doi: 10.1002/jcp.20551. doi:10.1002/jcp.20551 [DOI] [PubMed] [Google Scholar]
- Mank J.E, Promislow D.E.L, Avise J.C. Evolution of alternative sex-determining mechanisms in teleost fishes. Biol. J. Linn. Soc. 2006;87:83–93. doi:10.1111/j.1095-8312.2006.00558.x [Google Scholar]
- Maresca T.J, Heald R. Chromosome congression: another fine mesh we've gotten into. Dev. Cell. 2005;9:314–315. doi: 10.1016/j.devcel.2005.08.005. doi:10.1016/j.devcel.2005.08.005 [DOI] [PubMed] [Google Scholar]
- Matzke M.A, Varga F, Berger H, Schernthaner J, Schweizer D, Mayr B, Matzke A.J.M. A 41–42 bp tandemly repeated sequence isolated from nuclear envelopes of chicken erythrocytes is located predominantly on microchromosomes. Chromosoma. 1990;99:131–137. doi: 10.1007/BF01735329. doi:10.1007/BF01735329 [DOI] [PubMed] [Google Scholar]
- Merry N.E, Johnson M.H, Gehring C.A, Selwood L. Cytoskeletal organization in the oocyte, zygote, and early cleaving embryo of the stripe-faced dunnart (Sminthopsis macroura) Mol. Reprod. Dev. 1995;41:212–224. doi: 10.1002/mrd.1080410212. doi:10.1002/mrd.1080410212 [DOI] [PubMed] [Google Scholar]
- Mittwoch U. Blastocysts prepare for the race to be male. Hum. Reprod. 1993;8:1550–1555. doi: 10.1093/oxfordjournals.humrep.a137889. [DOI] [PubMed] [Google Scholar]
- Mittwoch U. Sex-determining mechanisms in animals. Trends Ecol. Evol. 1996;11:63–67. doi: 10.1016/0169-5347(96)81044-5. doi:10.1016/0169-5347(96)81044-5 [DOI] [PubMed] [Google Scholar]
- Mittwoch U. Sex is a threshold dichotomy mimicking a single gene effect. Trends Genet. 2006;22:96–100. doi: 10.1016/j.tig.2005.12.003. doi:10.1016/j.tig.2005.12.003 [DOI] [PubMed] [Google Scholar]
- Mooseker M.S, Graves T.A, Wharton K.A, Falco N, Howe C.L. Regulation of microvillus structure: calcium-dependent solation and cross-linking of actin filaments in the microvilli of intestinal epithelial cells. J. Cell Biol. 1980;87:809–822. doi: 10.1083/jcb.87.3.809. doi:10.1083/jcb.87.3.809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrish B.C, Sinclair A.H. Vertebrate sex determination: many means to an end. Reproduction. 2002;124:447–457. doi: 10.1530/rep.0.1240447. doi:10.1530/rep.0.1240447 [DOI] [PubMed] [Google Scholar]
- Morrison C, Vagnarelli P, Sonoda E, Takeda S, Earnshaw W.C. Sister chromatid cohesion and genome stability in vertebrate cells. Biochem. Soc. Trans. 2003;31:263–265. doi: 10.1042/bst0310263. [DOI] [PubMed] [Google Scholar]
- Munday P.L, Buston P.M, Warner R.R. Diversity and flexibility of sex-change strategies in animals. Trends Ecol. Evol. 2006;21:89–95. doi: 10.1016/j.tree.2005.10.020. doi:10.1016/j.tree.2005.10.020 [DOI] [PubMed] [Google Scholar]
- Nakagawa S. Is avian sex determination unique?: clues from a warbler and from chickens. Trends Genet. 2004;20:479–480. doi: 10.1016/j.tig.2004.07.010. doi:10.1016/j.tig.2004.07.010 [DOI] [PubMed] [Google Scholar]
- Nicklas R.B. How cells get the right chromosomes. Science. 1997;275:632–637. doi: 10.1126/science.275.5300.632. doi:10.1126/science.275.5300.632 [DOI] [PubMed] [Google Scholar]
- Novitski E. Nonrandom disjunction in Drosophila. Annu. Rev. Genet. 1967;1:71–86. doi:10.1146/annurev.ge.01.120167.000443 [Google Scholar]
- Okamura A, Pendon C, Valdivia M.M, Ikemura T, Fukagawa T. Gene structure, chromosomal localization and immunolocalization of chicken centromere proteins CENP-C and ZW10. Gene. 2001;262:283–290. doi: 10.1016/s0378-1119(00)00517-5. doi:10.1016/S0378-1119(00)00517-5 [DOI] [PubMed] [Google Scholar]
- Olsen M.W. Maturation, fertilization, and early cleavage in the hen's egg. J. Morphol. 1942;70:513–528. doi:10.1002/jmor.1050700307 [Google Scholar]
- Olsen M.W, Fraps R.M. Maturation, fertilization, and early cleavage of the egg of the domestic turkey. J. Morphol. 1944;74:297–309. doi:10.1002/jmor.1050740204 [Google Scholar]
- Olszańska B, Malewska A, Stępińska U. Maturation and ovulation of Japanese quail oocytes under in vitro conditions. Br. Poult. Sci. 1996;37:929–935. doi: 10.1080/00071669608417924. [DOI] [PubMed] [Google Scholar]
- Padro-Manuel de Villena F, Sapienza C. Female meiosis drives karyotypic evolution in mammals. Genetics. 2001a;159:1179–1189. doi: 10.1093/genetics/159.3.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padro-Manuel de Villena F, Sapienza C. Nonrandom segregation during meiosis: the unfairness of females. Mamm. Genome. 2001b;12:331–339. doi: 10.1007/s003350040003. doi:10.1007/s003350040003 [DOI] [PubMed] [Google Scholar]
- Panov E.N, Bulatova N.S. A comparative analysis of karyotypes of 18 species from the family Turdidae (Aves) Zool. Zhu. 1972;51:1371–1380. [Google Scholar]
- Pen I, Weissing F.J. Optimal sex allocation: steps towards a mechanistic theory. In: Hardy I.C.W, editor. Sex ratios: concepts and research methods. Cambridge University Press; Cambridge, UK: 2002. pp. 26–47. [Google Scholar]
- Pike T.W. Sex ratio manipulation in response to maternal condition in pigeons: evidence for pre-ovulatory follicle selection. Behav. Ecol. Sociobiol. 2005;58:407–413. doi:10.1007/s00265-005-0931-9 [Google Scholar]
- Pike T.W, Petrie M. Potential mechanisms of avian sex manipulation. Biol. Rev. 2003;78:553–574. doi: 10.1017/s1464793103006146. doi:10.1017/S1464793103006146 [DOI] [PubMed] [Google Scholar]
- Rebholz W.E.R. Z-chromosome dimorphism in eagle owls. Zoo Biol. 1992;11:291–295. doi:10.1002/zoo.1430110409 [Google Scholar]
- Reddy S.K, Rape M, Margansky W.A, Kirschner M.W. Ubiquitination by the anaphase-promoting complex drives spindle checkpoint inactivation. Nature. 2007;446:921–925. doi: 10.1038/nature05734. doi:10.1038/nature05734 [DOI] [PubMed] [Google Scholar]
- Roberts R, Iatropoulou A, Ciantar D, Stark J, Becker D.L, Franks S, Hardy K. Follicle-stimulating hormone affects metaphase I of chromosome alignment and increases aneuploidy in mouse oocytes matured in vitro. Biol. Reprod. 2005;72:107–118. doi: 10.1095/biolreprod.104.032003. doi:10.1095/biolreprod.104.032003 [DOI] [PubMed] [Google Scholar]
- Rodrigue K.L, May B.P, Famula T.R, Delany M.E. Meiotic instability of chicken ultra-long telomeres and mapping of a 2.8 megabase array to the W-sex chromosome. Chromosome Res. 2005;13:581–591. doi: 10.1007/s10577-005-0984-7. doi:10.1007/s10577-005-0984-7 [DOI] [PubMed] [Google Scholar]
- Rories C, Spelsberg T.C. Ovarian steroid action on gene expression: mechanisms and models. Annu. Rev. Physiol. 1989;51:653–681. doi: 10.1146/annurev.ph.51.030189.003253. doi:10.1146/annurev.ph.51.030189.003253 [DOI] [PubMed] [Google Scholar]
- Rutkowska J, Cichoń M. Maternal testosterone affects the primary sex ratio and offspring survival in zebra finches. Anim. Behav. 2006;71:1283–1288. doi:10.1016/j.anbehav.2005.07.025 [Google Scholar]
- Saifitdinova A, Derjusheva S, Krasikova A, Gaginskaya E. Lampbrush chromosomes of the chaffinch (Fringilla coelebs L.) Chromosome Res. 2003;11:99–113. doi: 10.1023/a:1022859713777. doi:10.1023/A:1022859713777 [DOI] [PubMed] [Google Scholar]
- Schwabl H. Environment modifies the testosterone levels of a female bird and its eggs. J. Exp. Zool. 1996;276:157–163. doi: 10.1002/(SICI)1097-010X(19961001)276:2<157::AID-JEZ9>3.0.CO;2-N. doi:10.1002/(SICI)1097-010X(19961001)276:2<157::AID-JEZ9>3.0.CO;2-N [DOI] [PubMed] [Google Scholar]
- Schwanz L.E, Bragg J.G, Charnov E.L. Maternal condition and facultative sex ratios in populations with overlapping generations. Am. Nat. 2006;168:521–530. doi: 10.1086/507993. doi:10.1086/507993 [DOI] [PubMed] [Google Scholar]
- Sheldon B.C, West S.A. Maternal dominance, maternal condition, and offspring sex ratio in ungulate mammals. Am. Nat. 2004;163:40–54. doi: 10.1086/381003. doi:10.1086/381003 [DOI] [PubMed] [Google Scholar]
- Shuker D.M, West S.A. Information constraints and the precision of adaptation: sex ratio manipulation in wasps. Proc. Natl Acad. Sci. USA. 2004;101:10 363–10 367. doi: 10.1073/pnas.0308034101. doi:10.1073/pnas.0308034101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simchen G, Hugerat Y. What determines whether chromosomes segregate reductionally or equationally in meiosis. Bioessays. 1993;15:1–8. doi: 10.1002/bies.950150102. doi:10.1002/bies.950150102 [DOI] [PubMed] [Google Scholar]
- Sockman K.W, Sharp P.J, Schwabl H. Orchestration of avian reproductive effort: an integration of the ultimate and proximate bases for flexibility of clutch size, incubation behavior, and yolk-androgen deposition. Biol. Rev. 2006;81:629–666. doi: 10.1017/S1464793106007147. doi:10.1017/S1464793106007147 [DOI] [PubMed] [Google Scholar]
- Solari A.J. CRC Press; Boca Raton, FL: 1993. Sex chromosomes and sex determination in vertebrates. [Google Scholar]
- Solovei I, Gaginskaya E, Hutchison N, Macgregor H. Avian sex chromosomes in the lampbrush form: the ZW lampbrush bivalents from six species of bird. Chromosome Res. 1993;1:153–166. doi: 10.1007/BF00710769. doi:10.1007/BF00710769 [DOI] [PubMed] [Google Scholar]
- Spence J.M, Mills W, Mann K, Huxley C, Farr C.J. Increased missegregation and chromosome loss with decreasing chromosome size in vertebrate cells. Chromosoma. 2006;115:60–74. doi: 10.1007/s00412-005-0032-6. doi:10.1007/s00412-005-0032-6 [DOI] [PubMed] [Google Scholar]
- Stegmeier F, et al. Anaphase initiation is regulated by antagonistic ubiquitination and deubiquitination activities. Nature. 2007;446:876–881. doi: 10.1038/nature05694. doi:10.1038/nature05694 [DOI] [PubMed] [Google Scholar]
- Sun Q.Y, Schatten H. Regulation of dynamic events by microfilaments during oocyte maturation and fertilization. Reproduction. 2006;131:193–205. doi: 10.1530/rep.1.00847. doi:10.1530/rep.1.00847 [DOI] [PubMed] [Google Scholar]
- Talbert P, Bryson T, Henikoff S. Adaptive evolution of centromere proteins in plants and animals. J. Biol. 2004;3:18. doi: 10.1186/jbiol11. doi:10.1186/jbiol11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor D.R, Ingvarsson P.K. Common features of segregation distortion in plants and animals. Genetica. 2003;117:27–35. doi: 10.1023/a:1022308414864. doi:10.1023/A:1022308414864 [DOI] [PubMed] [Google Scholar]
- Teranishi M, et al. Transcripts of the MHM region on the chicken Z chromosome accumulate as non-coding RNA in the nucleus of female cells adjacent to the DMRT1 locus. Chromosome Res. 2001;9:147–165. doi: 10.1023/a:1009235120741. doi:10.1023/A:1009235120741 [DOI] [PubMed] [Google Scholar]
- Tesarik J, Mendoza C. Nongenomic effects of 17-beta-estradiol on maturing human oocytes—relationship to oocyte developmental potential. J. Clin. Endocrinol. Metab. 1995;80:1438–1443. doi: 10.1210/jcem.80.4.7714121. doi:10.1210/jc.80.4.1438 [DOI] [PubMed] [Google Scholar]
- Tremoleda J.L, Schoevers E.J, Stout T.A.E, Colenbrander B, Bevers M.M. Organisation of the cytoskeleton during in vitro maturation of horse oocytes. Mol. Reprod. Dev. 2001;60:260–269. doi: 10.1002/mrd.1086. doi:10.1002/mrd.1086 [DOI] [PubMed] [Google Scholar]
- Trivers R.L, Willard D.E. Natural selection of parental ability to vary the sex ratio of offspring. Science. 1973;179:90–91. doi: 10.1126/science.179.4068.90. doi:10.1126/science.179.4068.90 [DOI] [PubMed] [Google Scholar]
- Tuiskula-Haavisto M, De Koning D.J, Honkatukia M, Schulman N.F, Maki-Tanila A, Vilkki J. Quantitative trait loci with parent-of-origin effects in chicken. Genet. Res. 2004;84:57–66. doi: 10.1017/s0016672304006950. doi:10.1017/S0016672304006950 [DOI] [PubMed] [Google Scholar]
- Uller, T., Pen, I., Wapstra, E., Beukeboom, L. W. & Komdeur, J. 2007 The evolution of sex ratios and sex-determining systems. Trends Ecol. Evol.22, 292–297. (doi:10.1016/j.tree.2007.03.008) [DOI] [PubMed]
- Underwood D.L.A, Shapiro A.M. A male-biased primary sex ratio and larval mortality in Eucheira socialis (Lepidoptera: Pieridae) Evol. Ecol. Res. 1999;1:703–717. [Google Scholar]
- van Dooren T.J.M, Leimar O. The evolution of environmental and genetic sex determination in fluctuating environments. Evolution. 2003;57:2667–2677. [PubMed] [Google Scholar]
- Velando A, Graves J, Ortega-Ruano J. Sex ratio in relation to timing of breeding, and laying sequence in a dimorphic seabird. Ibis. 2002;144:9–16. doi:10.1046/j.0019-1019.2001.00002.x [Google Scholar]
- Wang S.Z, Adler R. Chromokinesin: a DNA-binding, kinesin-like nuclear protein. J. Cell Biol. 1995;128:761–768. doi: 10.1083/jcb.128.5.761. doi:10.1083/jcb.128.5.761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren D.C, Conrad R.M. Growth of the hen's ovum. J. Agric. Res. 1939;58:875–893. [Google Scholar]
- Warren D.C, Scott H.M. The time factor in egg formation. Poult. Sci. 1935;14:195–207. [Google Scholar]
- Werren J.H, Hatcher M.J. Maternal-zygotic gene conflict over sex determination: effects of inbreeding. Genetics. 2000;155:1469–1479. doi: 10.1093/genetics/155.3.1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West S.A, Sheldon B.C. Constraints in the evolution of sex ratio adjustment. Science. 2002;295:1685–1688. doi: 10.1126/science.1069043. doi:10.1126/science.1069043 [DOI] [PubMed] [Google Scholar]
- West S.A, Shuker D.M, Sheldon B.C. Sex-ratio adjustment when relatives interact: a test of contraints on adaptation. Evolution. 2005;59:1211–1228. [PubMed] [Google Scholar]
- Williams T.D, Ames C.E, Kiparissis Y, Wynne-Edwards K.E. Laying-sequence-specific variation in yolk oestrogen levels, and relationship to plasma oestrogen in female zebra finches (Taeniopygia guttata) Proc. R. Soc. B. 2005;272:173–177. doi: 10.1098/rspb.2004.2935. doi:10.1098/rspb.2004.2935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin S, Wang Q, Liu J.H, Ai J.S, Liang C.G, Hou Y, Chen D.Y, Schatten H, Sun Q.Y. Bub1 prevents chromosome misalignment and precocious anaphase during mouse oocyte meiosis. Cell Cycle. 2006;5:2130–2137. doi: 10.4161/cc.5.18.3170. [DOI] [PubMed] [Google Scholar]
- Yogev L, et al. Sex chromosome alignment at meiosis of azoospermic men with azoospermia factor microdeletion. J. Androl. 2004;25:110–116. doi: 10.1002/j.1939-4640.2004.tb02765.x. [DOI] [PubMed] [Google Scholar]
- Yoshimura Y, Okamotoa T, Tamura T. Electron microsope observations on LH-induced oocyte maturation in Japanese quail (Coturnix coturnix japonica) J. Reprod. Fertil. 1993a;98:401–407. doi: 10.1530/jrf.0.0980401. [DOI] [PubMed] [Google Scholar]
- Yoshimura Y, Okamotoa T, Tamura T. Ultrastructural changes of oocyte and follicular wall during oocyte maturation in the Japanese quail (Coturnix coturnix japonica) J. Reprod. Fertil. 1993b;97:189–196. doi: 10.1530/jrf.0.0970189. [DOI] [PubMed] [Google Scholar]
- Yoshimura Y, Tischkau S.A, Bahr J.M. Destruction of the germinal disc region of an immature preovulatory follicle suppresses follicular maturation and ovulation. Biol. Reprod. 1994;51:229–233. doi: 10.1095/biolreprod51.2.229. doi:10.1095/biolreprod51.2.229 [DOI] [PubMed] [Google Scholar]
- Young R.L, Badyaev A.V. Evolution of sex-biased maternal effects in birds: I. Sex-specific resource allocation among simultaneously growing oocytes. J. Evol. Biol. 2004;17:1355–1366. doi: 10.1111/j.1420-9101.2004.00762.x. doi:10.1111/j.1420-9101.2004.00762.x [DOI] [PubMed] [Google Scholar]
- Zwick M.E, Salstrom J.L, Langley C.H. Genetic variation in rates of nondisjunction: association of two naturally occurring polymorphisms in the chromokinesin nod with increased rates of nondisjunction in Drosophila melanogaster. Genetics. 1999;152:1605–1614. doi: 10.1093/genetics/152.4.1605. [DOI] [PMC free article] [PubMed] [Google Scholar]